Summary
Endovascular coil treatment of intracranial aneurysms is now widely accepted. We discuss some of the arguments for the relative roles of electrothrombosis, spontaneous thrombosis, mechanical filling, haemodynamic effects and surface properties in successful coil treatment. Despite an enormous body of literature, with many theories and much data, there is limited evidence for, or understanding of, the mechanisms by which coil treatment protects against aneurysm rupture. It seems likely that electrothrombosis plays no part. Dense packing is probably important in preventing recurrence. New technologies aiming to encourage endothelialisation and increased connective tissue formation appear promising.
Key words: aneurysm, endovascular treatment, coils, -electrolytic, -mechanical, -thermal, -hydrostatic, electrothrombosis, thrombosis
Introduction
The concept of exclusion of the lumen of an aneurysm by filling it with exogenous material to prevent rupture and intracranial haemorrhage is not new. However, it first began to gain widespread acceptance with the introduction of detachable coils, allowing retrieval of a coil if its position was felt to be unacceptable.
Various techniques involving coils, balloons and assorted other materials have been used to achieve interruption of flow within intracranial aneurysms. In general, it has been assumed that the essential goal of treatment is to fill the aneurysm with exogenous material and thrombus. In the case of electrolytically detached coils, it has been hypothesised that the electrostatically mediated attraction of blood components to the coil surface may also play a part in exclusion of the aneurysm. The term “electrothrombosis” has been applied to this process for over a century. This term describes a course of events in which a positive charge is applied to an introduced conductor (in the context of aneurysms, usually a platinum coil), to encourage the formation of thrombus on the coil surface by attraction of red blood cells, platelets and fibrinogen (whose split product fibrin forms one of the main components of a blood clot). There is histological evidence that reactive changes in the aneurysm wall and on the surface of the coil mass also play a role.
Although this hypothesis is intuitively appealing, as yet there is no convincing evidence that there is any difference in the efficacy of treatment of intracranial aneurysms with coils detached by mechanisms involving electrical current or coils detached by other mechanisms. The purpose of this paper is to discuss the underlying principles of endovascular aneurysm exclusion, and in particular, the evidence for a role of electrothrombosis.
Historical overview
It stands without question that the work of Guglielmi, Sepetka, Viñuela et Al (1991a,b)31,32 that culminated in the introduction of the GDC system of endovascularly deployed retrievable platinum coils irrevocably altered the treatment of intracranial aneurysms (Strother 2001) 103.
The bases of this coil system had previously been described in the publications of Piton et Al (1978a,b, 1979)75,76,77, but many of the underlying principles had begun to be explored in the early 19th century.
In 1824, Scudamore 95 observed that blood thrombosed at a positive but not a negative electrode. Velpeau (1831)110 and Philips (1831)70 described the blockage of arterial vessels by temporary puncture with a pin. In 1847, Ciniselli 15 described the use of direct current applied through pins in an aneurysm to induce thrombosis. Buressi et Al (1879) 8 applied current to a wire that had been introduced through a needle into an aneurysm. Poore (1895) 78 again pointed out that coagulation can be accelerated through electric current. Moore et Al (1864) 55 employed steel wire and Power et Al (1903,1921)79,80 used silver wire to fill thoracic aortic aneurysms. In their publications they discuss the use of direct current to intensify thrombus formation. Eshner (1910) 22 reviewed 27 publications with a total of 32 case reports in which aneurysms had been treated by the introduction of wire and application of direct current and added two of their own cases. Colt (1925) 16 and Thompson et Al (1935)107 introduced gold-coated steel wire with a rough surface to encourage thrombus formation through a trocar into saccular aneurysms. Linton et Al (1951,1952) 45,46 used stainless steel wire (18-8, 0,01 inch diameter) to fill thoracic and abdominal aortic aneurysms.
Early descriptions of application of these principles to an intracranial aneurysm come from Blakemore et Al (1938) 6 and Werner et Al (1941)111, who developed a procedure in which a wire of silver-copper alloy was introduced through a needle into an aneurysm, and to which electrical current was applied. The aneurysm of the internal carotid artery was reached by percutaneous puncture through the orbit.
Since then, a variety of different procedures and materials have been used for the endovascular treatment of intracranial aneurysms. Gallagher (1963, 1964) 26,27 injected animal hairs into surgically exposed intracranial aneurysms. He discussed the hypothesis that thrombosis results through the penetration of hairs through the aneurysm wall and reduction of the negative polarity of the aneurysmal lumen. Genest (1965) 28 carried out animal experiments in which he injected methyl methacrylate through needles in aneurysms. Although he did not attempt the procedure, he described the possibility of the injection of this agent into the aneurysm through a catheter. Mullan et Al (1965, 1969, 1974) 57,58,59 described the treatment of intracranial aneurysms with Berrylium-Copper coils. Abe et Al (1973) 1 treated a patient with bilateral giant aneurysms of the internal carotid artery, by puncturing the aneurysms with a needle and filling them with copper wire. Alksne et Al (1980) 3 used stereotactic guidance to puncture intracranial aneurysms and then inject fluid methyl methylacrylate mixed with iron particles. Inadvertent embolization of the parent vessel was prevented by an external magnet. Copper wire (Fujita et Al 1987, Sakakibara et Al 1983) 25,87 or a copper coated steel needle (Mullan et Al 1969) 58 have also been inserted into intracranial aneurysms. However, Fischer et Al (1957) 23 and Cooper et Al (1966) 17 raised concerns about the intracranial use of silver and copper. Many of the underlying principles of current coil-occlusion techniques had been described in detail by Mullan et Al (1974) 59: “It was thus concluded that the speed of thrombosis could be regulated by the volume of copper (thrombogenic) and beryllium copper (packing material) introduced into an aneurysm. Beryllium copper can be preformed in any convenient spring shape before insertion. It can even be made to take the form of a coil immediately after entering the aneurysm; this is too large to pass through the neck into the arterial lumen. Copper cannot be formed into a spring, and it is conceivable that it could find its way into the artery; thus it seems desirable to insert beryllium copper first to form a mass that would trap the copper within the aneurysm lumen”. In this same article, Mullan also deals with the principle of electro-thrombosis. He used direct current of 0,5-1 mA applied for several minutes.
Nishijima et Al (1984)62 concluded that similar results could be achieved without the application of an external current.
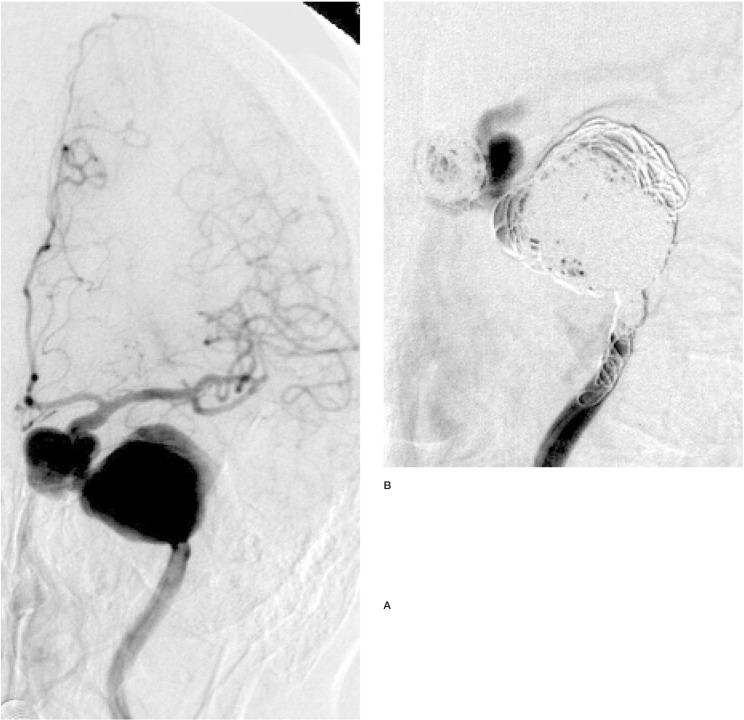
Figure 1.
Two aneurysms of the left internal carotid artery, treated by parent vessel occlusion with electrolytically detachable coils. Note that despite the electrolytic detachment of innumerable coils within both aneurysms and the parent vessel, there is persistent flow in the aneurysms and parent vessel, and no evident thrombus formation.
They say “... electrothrombosis by copper needle insertion was done. This surgical method is based on the attraction of negatively charged intravascular elements, such as blood cells or fibrinogen or positively charged metallic ions, such as Fe++ or Cu++, resulting in the formation of a thrombus.... Direct anodal current is sometimes applied to the cut end of the wire or needle to initiate thrombus..., but in this series we did not use it. On the basis of both experimentally study and clinical experience, it is known that a thrombus starts to form around the inserted needle within 5 minutes after insertion”.
The work of Piton et Al (1978a,b, 1979)75,76,77 already described all the essential elements of what would subsequently be called GDC. Platinum wires, among others, were employed to induce intravascular thrombosis. With 9 Volts, a current of 10 mA was applied for up to 20 minutes. The authors report: “...partial or complete electrolysis of the tip of the wire occurred, fragments of the wire coming to lie free in the region of the thrombus”. This did not appear to be of practical importance, however, and was even somewhat advantageous, since these fragments were visible on the subsequent radiographs. The possible future importanceof this technique was obviously recognised by the authors; “Experiments are being done to apply this technique to aneurysms” (Piton et Al 1979)77.
Physiological basis of intravascular electrothrombosis
“Electrothrombosis” is a fundamentally different process to “electrocoagulation”. During electrocoagulation, a temperature rise is induced by the supply of electric current (for example high-frequency alternating current), causing thermal damage to blood components and the vessel wall. In contrast to this thermal mechanism, in electrothrombosis coagulation is induced exclusively by bio-electric mechanisms.
The mechanism of electrothrombosis is dependent on reduction of the normal transluminal potential difference. Normally, the inner (intimal) surface of intact blood vessels shows a negative charge with respect to the external (adventitial) surface of the vessel (Sawyer et Al 1953c)92.
The cellular blood components also have a negative charge on their surface and are therefore repelled by the intact vessel wall.
Injury to the intima, or placement of a positive electrode (anode) into the lumen decreases the repulsion of the cellular elements and therefore promotes thrombosis (Sawyer et Al 1953 a,b) 90,91.
The amount of thrombus induced by direct current is dependent on the applied current intensity and the duration of application (Araki et Al 1965, Miller et Al 1978, Sawyer et Al 1961,1964, Thompson et Al 1977) 4,52,93,94,108. Its formation is opposed by Heparin (Araki et Al 1965)4. It has been postulated by Lamb et Al (1965) 44 that the process of electrothrombosis is also voltage-dependent, only occurring with potential differences of greater than 2V. During the endovascular treatment of intracranial aneurysms, direct current with the currently used parameters (3-5 V, 1-2 mA, application time < 60 sec) may induce thrombus formation on the platinum coil surface, which does not significantly differ from the spontaneous thrombus formation and which is so thin that little or no effect on the aneurysm can be expected (Byrne et Al 1997a)10.
Animal studies and their relevance to clinical practice
The current concept of electrothrombosis for the cure of intracranial aneurysms is largely based on observations in animal models of surgically created aneurysms and in vitro models using stagnant blood. Neither model is directly applicable to clinical practice. Coagulation occurs far more slowly in the human being than in the pig or dog. This has been confirmed in several studies both in stagnant blood and in thrombosis induced by foreign bodies (e.g., guide-wires) (Ovitt et Al 1974)65.
Comparative animal experimental studies showed that aneurysms created with the same experimental technique in pigs and dogs had different tendencies to formation of a neointima over the aneurysm neck (Raymond et Al 1999b)82. In this study, the histological findings in pigs were similar if GDC coils or collagen sponge was used to exclude the aneurysm. In general, there is a high incidence of spontaneous thrombosis in surgically created aneurysms in animals (Pile-Spellman et Al 1997) 74. This is rare in humans.
A rabbit model of bifurcation aneurysms may have advantages over the side-wall aneurysm model in the pig, used by Guglielmi et Al (1991a,b)31,32. The haemodynamic patterns and the vessel dimensions are similar to those seen in human intracranial aneurysms (Kwan et Al 1993)43.
In the primate model of Tenjin et Al (1995)106, 26% of the “aneurysms” had spontaneously thrombosed two weeks after the operation (venous side-wall pouch aneurysms on the carotid artery). Three months after coil placement, a thick membrane was found at the neck in the three aneurysms examined. This study does not make any direct statements about the possible importance of electrothrombosis, however, early endothelialisation at the aneurysm neck contradicts most comparable experiences in the human being.
Mawad et Al (1995) 51 in an animal model (dog) examined the histological changes in previously surgically created aneurysms occluded with GDC. The constant demonstration of a neointima across the ostium of the aneurysms at six months after coil placement contradicts comparable observations in the human being (Bavinzski et Al 1999)5, showing that observations from this model regarding the behaviour of coils under clinical conditions should be made with care. Similar findings, in particular the frequent formation of a neointima, were already available from Graves et Al (1990)29 in this animal model using non-retrievable coils with and without fibres.
In the study of Dawson et Al (1995)19, 19 venous side-wall pouch aneurysms on the carotid artery were created in ten pigs. Of those, five aneurysms spontaneously thrombosed within three weeks. Szikora et Al (1997)104, in dogs, observed thrombosis of two of 26 aneurysms after four weeks. In an animal study (pig) by Byrne et Al (1997b)11, 23 aneurysms were created and 18 were filled with coils. All five aneurysms not filled with coils occluded spontaneously in the following weeks. It would seem likely that there are significant differences in the behaviour of coil systems in clinical practice, animal models and in vitro studies. We believe that data derived from non-clinical scenarios should be applied with great caution.
Clinical applications of electrically-mediated aneurysm-and vessel exclusion
In clinical practice, a wide range of combinations of voltage, current (both alternating and direct) and application time have been utilised. Much of this work was performed on extracranial aneurysms or vessels, using parameters quite different from those now adopted for endovascular treatment of intracranial aneurysms. It is not even always clear if the clinicians were aiming for electrothrombosis or electrocoagulation.
Occlusion of extracranial target vessels:
Finney (1912) 24 treated aortic aneurysms by the introduction of wire and application of direct current at 10-100 mA, as a rule for longer than one hour. Hare (1927) 33 used a platinumgold alloy and initially 5 mA of direct current, raised stepwise to 45 mA over half an hour and then reduced again slowly so that total application times were about one hour. Blakemore (1951) 7 introduced silver wire into aortic aneurysms and heated it to 80° centigrade with 100 V direct current for ten seconds. Sawyer et Al (1961) 93 produced intravascular thrombosis in arteries of various organs of 15 patients with direct current at a usual current intensity between 20-40 mA (though up to 80 mA), usually for a duration of a few minutes (though up to two hours). Salazar (1961)89 used direct current in an animal experiment for the induction of coronary artery thrombosis. Anodes were placed within the coronary arteries with 3 V at a current intensity of 1-9 mA, applied for 18-93 minutes. Miller et Al (1978) 52 applied 10-15 mA over 30 minutes through an intravascular guidewire of high-grade steel to block vessels. Sedlarik (1977,1981)96,97 applied 30 mA over 15 minutes in an animal experiment, leading to the formation of thrombus obliterating the vessel lumen. Ogawa et Al (1982) 63 simultaneously employed up to 40 copper beryllium needles to induce thrombosis of extracranial vessels using a direct current of 0.3-5 mA, applied for a duration of 30-60 minutes. The pins were left in-situ for three to four days. Dado et Al (1987) 18 could not achieve a similar result with comparable current intensity and application duration.
Occlusion of intracranial aneurysms
An early application of this technique to intracranial aneurysms by Araki et Al (1965) 4 used a combined technique for anterior communicating artery aneurysm treatment, applying temporary vessel occlusion proximal and distal to the aneurysm, then puncturing the aneurysm up to nine times with a platinum needle and applying a direct current at 3 mA for one minute. The puncture sites were sealed for some distance from the needle. The treatment led only to a partial obliteration of the aneurysm; approximately one third remained perfused. Mullan et Al (1965) 57 describe the electrothrombotic treatment of intracranial aneurysms using direct puncture with a thin steel needle and a direct current of 0.2-2 mA, applied for 62-390 minutes. Because of the high complication rates, they modified their procedure in the following years (Mullan et Al 1969) 58 to use copper-coated steel needles with a length of 10 mm placed at 1mm intervals in the aneurysm neck. These were detached by application of 1 mA direct current for five minutes. Mori (1967)56 required 3-5 mA in animal experiments for the electrothrombosis of aneurysms with an intravascular anode of platinum and an application time of 20 minutes. In a clinical application of this technique to an anterior communicating artery aneurysm, the parent artery was temporarily clipped, however, only 60% thrombosis was produced after application of 3mA for nine minutes.
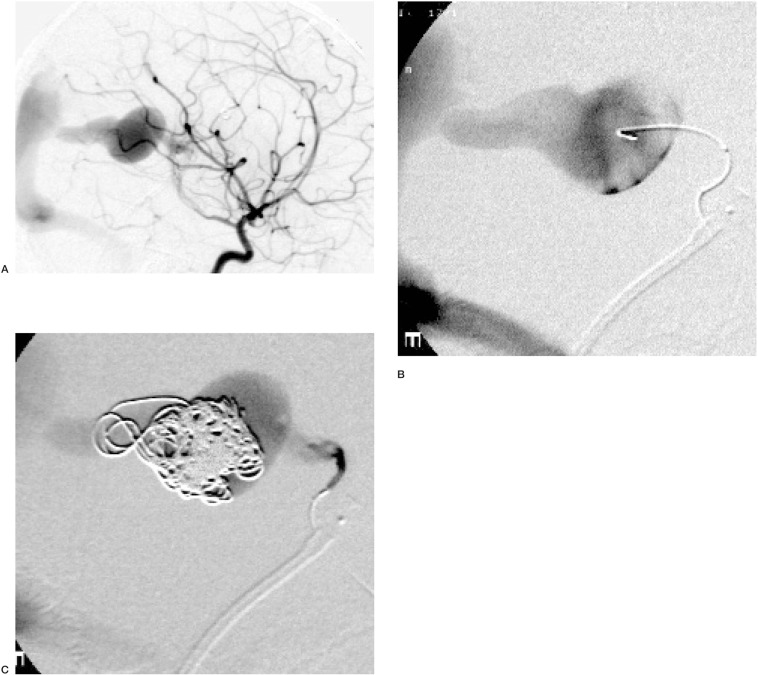
Figure 2.
Treatment of a Vein of Galen Malformation in a four year old child, by partial filling of the varix with electrolytically detached coils. Note that despite a dense coil mass, extending into the falcine sinus, there is persistent flow and no evident thrombus. Subsequently, the choroidal arterial pedicle in which the microcatheter is evident was embolized with Histoacryl.
Occlusion of intracranial non-aneurysmal target vessels
Peterson et al (1969) 69 used a copper wire which was introduced into the cavernous sinus and a current of 2 mA applied for four hours to close a direct carotid-cavernous fistula.
Hosobuchi (1975)38, Sakurada et Al (1975)88, Ishikawa et Al (1982) 39 and Nishijima et Al (1983,1984)61,62 treated carotid-cavernous fistulae by filling the superior ophthalmic vein with copper wire or copper needles. Direct current was used (0,2-0,8 mA, 10-30 minutes), however, thrombosis was also observed without the use of any electricity (Nishijima et Al 1984) 62. Phillips et Al (1973,1975)71,72 also treated arteriovenous fistulae in an animal model with a current intensity of 10 mA, at 50 Volts, however, the application duration was 40-80 minutes. Thompson et Al (1977)108 carried out electrothrombosis with comparable parameters. Yoneda et Al (1977)112 induced thrombosis of the vessels of cerebral arteriovenous malformations by the direct introduction of up to 60 electrodes of copper-beryllium and application of 5-10 mA over 30-60 minutes.
Roles of electrothrombosis, mechanical filling, coil surface and spontaneous thrombosis in aneurysm treatment
The effectiveness of electrothrombosis for treatment of intracranial aneurysms is not proven. At the typical voltage, current intensity and duration used for some effective coil systems (Henkes et Al 2002, Pierot et Al 2002) 34,73, little, if any, electrothrombosis occurs. Effective coil systems exist that do not use any electrical mechanism for detachment (Tournade et Al 2001) 109. Electrothrombosis without complete filling of the lumen of the vascular structure concerned have in general not resulted in effective exclusion of the lumen (Byrne et Al 1994) 9.
There is some evidence that where electrothrombosis has contributed to filling of an aneurysm lumen with thrombus, without dense mechanical filling, recanilization rates may be unacceptably high. Mullan et Al (1965) 57 performed angiographic follow-up in six out of 12 patients in whom intracranial aneurysms had been treated by electrothrombosis. They demonstrated that the occlusion induced by electrothrombosis was only temporary and had not led to permanent obliteration of the aneurysm. In a subsequent publication, Mullan et Al (1969)58 stated: “...that partial thrombosis of the aneurysm is not only unsuccessful in that it does not extend to complete thrombosis within a reasonable time, but it is even possible that it may precipitate hemorrhage by setting up irregularities and eddies in the previously smoothly flowing intra-aneurysmal circulation.”
Marks et Al (1994) 50 examined mechanically and electrolytically detached coil systems and observed: “It is not clear how much of the observed thrombosis [in GDC] is the result of electrothrombosis or vascular stasis from packing of coils into a vascular space. It has been observed that intra-aneurysmal thrombosis progresses after detachment using this [GDC] coil system ... Endovascular coil procedures are generally performed using systemic heparinization to impede blood clot formation at the time of placement; it is not clear whether there is an advantage to having thrombus form at the time of each coil placement.”
Murayama et Al (2001) 60 developed a modified coil system in which a thin, electrolytically detachable platinum coil is coated with an absorbable plastic (Matrix-CoilTM, Target Therapeutics/Boston Scientific). The formation of connective tissue is supposed to be encouraged by this coil surface-coating. A more permanent filling of the aneurysm lumen may result. The coating is, according to the authors, approximately 50% less thrombogenic than the uncoated surface of traditional GDC coils. Clearly the surface-coating will reduce the importance of both electrothrombosis and spontaneous thrombus formation in aneurysm exclusion. Shrinkage of the aneurysm may occur by loss of volume due to degradation of the surface coating. On the other hand, Bavinzsky et Al (1999) 5, on the basis of histological examination of aneurysms treated with traditional GDC, suggested that aneurysm recurrence may be partly due to the volume loss that occurs as connective tissue within the lumen shrinks, pulling coils away from the neck. This mechanism is likely to be even more significant if the Matrix-CoilTM does encourage connective tissue formation.
Horowitz et Al (1997b) 37 described the intraoperative findings in a patient, in which a ruptured aneurysm of the distal anterior cerebral artery had been filled with two GDC platinum coils. The procedure was carried out without heparinisation. The detachment times were 2.09 and 1.36 minutes. The microcatheter perforated the aneurysm wall. Subsequent angiography did not show any further opacification of the aneurysm lumen. The patient deteriorated clinically and the aneurysm was operated on two hours after coil occlusion. The aneurysm was not filled with thrombus. The authors therefore postulate that the GDC coils had prevented aneurysm filling through mechanical attenuation of the pulse wave rather than by electrothrombosis. The transmural pressure gradient would be reduced by such an attenuation of the pulse wave. Nevertheless, thrombosis in the aneurysm could be induced by turbulent blood flow, which can activate the coagulation cascade.
Padolecchia et Al (2001) 68 examined the formation of thrombus on the surface of platinum coils in heparinized blood, with and without application of current. Even though the blood was stagnant, presumably encouraging thrombus formation, there was only a film of fibrin and platelets on the coil surface, even in those to which current had been applied.
It is questionable whether significant electrothrombosis actually occurs in the human being during the endovascular treatment of an intracranial aneurysm at typically utilised parameters of voltage, current and application time. Anticoagulation with Heparin, often in combination anti-platelet agents, probably further reduces its occurrence.
Mechanical effects of coil deposition
In clinical conditions, the amount of thrombosis occurring on the surface of freshly inserted platinum coils is probably so small that it is quantitatively unimportant in terms of volume of the aneurysm lumen filled. For this reason, we believe that maximally dense filling of the aneurysm is necessary. The effect of coil-occlusion on the probability of rupture of an aneurysm is related, in the initial stage, to mechanical filling of the aneurysm lumen.
Ahuja et Al (1993) 2, using electron-microscopy, examined the surface of GDC coils that had been coated with different materials to increase their thrombogenicity. It is assumed that with such a system electrothrombosis is inhibited since the surface of the coils is electrically insulated by the coating. The authors stated: “As currently used, the major mechanism for obliteration of aneurysms with the Guglielmi detachable coil (GDC) is dense packing of the aneurysm with platinum coil. ... Increasing the thrombogenicity of the GDC is one approach that might make it possible to achieve successful aneurysm obliteration with less need for complete mechanical packing of the aneurysm lumen.”
Dawson et Al (1996) 20 compared in an animal model (pig) the effectiveness of conventional and collagen-laden mechanically detached coils. Ten weeks after coil incorporation they found that conventional coil-occluded aneurysms had loose, cell-poor connective tissue while in those occluded with collagen coils, aneurysms had cell-rich connective tissue probably containing additional collagen deposition. The authors stated: “The theoretical approach to endovascular therapy for cerebral aneurysms has heretofore relied primarily on the creation of a thrombus within the aneurysmal lumen. This approach is inherently flawed, as thrombus has no permanency. Thrombus, as a dynamic biological material, may and should lyse over time, with secondary recanalization occuring as a natural and expected event. This combination of events often leads to recanalization and regrowth of the aneurysm.”
Szikora et Al (1997) 104 in an animal model, examined whether endovascular aneurysm treatment can be improved when collagen-containing threads are incorporated into platinum microcoils. In the discussion of their results the authors wrote: “Platinum has an inherently low thrombogenicity, and thrombus generated by the electrolytic detachment process may not occlude the aneurysm sufficiently unless the cavity is tightly packed with coils. Further, fresh thrombus and platinum coils create a relatively soft mass, that may be incapable of resisting continuous forces of pulsatile blood flow upon the coil surface at the aneurysm orifice, ... Packing density was the single determinant of aneurysmal occlusion.” Stiver et Al (1998) 102 describing endo-vascular aneurysm treatment said: “Dense packing with coils is thought to be the principal mechanism by which GDC therapy leads to aneurysm obliteration... “.
Spontaneous thrombosis, independent of electrothrombosis, probably forms in stagnant blood between coil loops inserted in an aneurysm. It is doubtful that it has any permanent occlusive effect as fibrin is removed within days by innate thrombolytic activity, at least for multiple emboli of autologous thrombus (Deaton et Al 1960, Osterman et Al 1976)21,64.
The histologic investigation by Mizoi et Al. (1996) 53 of an aneurysm that had been resected 18 months after endovascular occlusion with platinum coils (GDC), showed unorganized thrombus between the loops of the platinum coils, without connective tissue and without endothelialisation of the luminal surface of the thrombus. In an autopsy study (Horowitz et al 1997a) 36, electron-microscopic investigation of an aneurysm of the basilar artery four weeks after its endovascular treatment demonstrated the platinum coils with a thin fibrin film, surrounded by thrombus. At the aneurysm neck, cellular proliferation was found but the luminal surface was not covered with neointima after four weeks. Romeike et al (1999) 86 found, two weeks after the GDC treatment of an aneurysm of the basilar tip, that unorganized thrombus surrounded the coil loops. The authors were of the opinion that under these circumstances there is inadequate protection against re-rupture of the aneurysm. The formation of unorganized thrombus on the coil-surface and between the coil-loops is probably independent of the electrolytic coil-detach-ment mechanism. Ozawa et Al (1998)66 found similar changes to those described above by electron microscopic examination of a platinum coil that had been placed eight months before by mechanical detachment. Manabe et al (1998)49 histologically examined an aneurysm treated eight months previously with mechanically detachable platinum coils (IDC), after a fatal second hemorrhage. They found unorganized thrombus within the aneurysm without endothelialisation. The aneurysm wall had a thin layer of granulation tissue and fibrotic modification, similar to those findings described for GDC-treated aneurysms.
Mechanisms of long-term aneurysm exclusion
For the process of morphological cure of an aneurysm, the formation of early intra-aneurysmal thrombus is neither necessary nor sufficient. The following steps may be postulated: the formation of a new intima at the ostium of the aneurysm, resorption of any unorganised intra-aneurysmal thrombus and the formation of scarring-fibrous connective tissue between the coil-loops and eventually, reactive thickening of the aneurysm wall.
With regard to the formation of a new intima at the ostium
Tamatani et al (1997) 105 found, in vitro, that cultivated endothelial cells do not spread on uncoated implant surfaces. Endothelialisation was facilitated if the coil surface was coated with type I collagen. Stiver et Al (1998)102 observed a fibrin membrane within an aneurysm that had been filled by GDC treatment 36 hours before death. The formation of such a fibrin membrane between the lumen of the parent vessel and the aneurysm sac itself could precede endothelialisation. Kinugasa et Al (1994) 40 made similar observations ten days after filling of an aneurysm with a liquid embolic material (cellulose acetate polymer, CAP). Koizumi et Al (1997) 42 found at an autopsy four weeks after the treatment of an unruptured aneurysm with mechanically detachable platinum coils that the ostium of the occluded aneurysm had covered over with a membrane designated by the authors as endothelium. On the other hand, only in one of 18 GDC-treated aneurysms with narrow necks could Bavinzski et Al (1999) 5 histologically verify complete organization of the thrombus and complete endothelialisation of the cell-rich intra-aneurysmal connective tissue.
With regard the resorption of early intra-aneurysmal thrombus and the formation of scarring-fibrous connective tissue between the coil-loops
Molyneux et Al (1995) 54 examined two large, wide-necked aneurysms histologically two and six months after the patients had been treated with GDC. In both aneurysms, the coils contained in the aneurysmal sac were surrounded by unorganized thrombus. There was little new vessel formation or formation of connective tissue at the aneurysm neck. Dawson et Al (1995) 19 examined the formation of intra-aneurysmal connective tissue in an animal experiment (pig). Collagen-covered coils and coils with attached Dacron fibres were compared. The formation of intra-aneurysmal connective tissue was far more marked with the collagen-covered coils. A comparison with GDC coils is not possible by means of this study, but the authors point out explicitly that the formation of an intra-aneurysmal thrombus, if it can be achieved by electrothrombosis, may hinder the filling of the aneurysm by connective tissue.
Szikora et Al (1997) 104 histologically examined experimentally created aneurysms in the dog, finding that coils with collagen were surrounded by more fibroblast proliferation and had more collagen formation than traditional GDC coils. The favourable effect was limited to the immediate neighbourhood of the coils while the remaining intra-aneurysmal thrombus was not significantly different.
Reul et Al (1998) 85 found in the rabbit that within a few days of coil insertion, complete filling of the aneurysm with fresh thrombus occurred. However, by 3-7 days, recanalisation and fibrinolysis led to partial re-opening at the neck and perhaps also in the sac of the aneurysm. After two to three weeks, the initial thrombus was largely dissolved. Connective tissues with fibroblast proliferation and new vessel formation were observed in the sac. In the neck of the aneurysm no thrombus was evident between the coil-loops. In the same experimental model (Spetzger et Al, 1996) 101, three to six months after GDC placement, recanalisation of the aneurysm had occurred with open spaces between the coil loops without remaining thrombus. In 13 of 17 aneurysms, the coil-surface was devoid of any tissue, and only in four aneurysms was a thin layer of granulation tissue found. No endothelial cells were evident.
Padolecchia et Al (1999) 67 examined an aneurysm that had been treated with GDC 16 hours before death of the patient. The aneurysm was filled completely with thrombus into which the platinum coils were imbedded. A thin fibrin layer was found by electron-microscopic examination of the coil-surface. Castro et Al (1999) 13 examined a patient who had two intracranial aneurysms treated with platinum coils (GDC) 33 months before his death. He observed complete replacement of intraaneurysmal thrombus with scarring and connective tissue. In one of the two aneurysms, the ostium was covered with endothelium. New capillaries seemed to arise from the parent artery, not from the aneurysm wall. The authors presumed that dense filling of an aneurysm favoured the formation of scar tissue. Bavinzski et Al (1999) 5 examined six patients with aneurysms that had been treated by GDC three to seven days before histological examination. They found unorganized thrombus with few fibroblasts. In a further six aneurysms in which GDC coils had been inserted 9-14 days earlier, granulation tissue was forming at the periphery of the thrombus. In three giant aneurysms with wide necks in which the GDC coils had been placed 17-22 days earlier, thrombus was still evident. Fibroblasts covered the coil surface, however, endothelialisation had not occurred.
With regard to modifications to the aneurysm wall
Romeike et Al (1999) 86 histologically examined an aneurysm 6.5 months after coil occlusion with GDC. The aneurysm had been surgically clipped after angiography revealed residual filling. The aneurysm wall consisted of dense connective tissue and collagen into which the coil loops were embedded. Shimizu et Al (1999) 98 histologically examined an aneurysm 42 days after partial coil occlusion. There was unorganized thrombus in the centre of the aneurysm, but between the platinum coils and the aneurysm wall they found capillaries and organization of the thrombus with in-growth of fibre-rich connective tissue. This course of events, independent of initial thrombosis, is probably significant for the long-term stabilization of an aneurysm. Bavinzski et Al (1999) 5 examined an aneurysm 54 months after GDC coil placement. The coils were embedded into fibrotic tissue rich in collagen. The aneurysm wall was thickened and fibrosed, and had incorporated coil loops.
These studies argue that modifications of the surface properties of coils may increase our success in achieving long-term exclusion of intracranial aneurysms.
Coils detached by non-electrolytic mechanisms do not appear to be less efficacious
The clinical effectiveness of GDC has been verified in numerous publications (Graves et Al 1995) 30. There is no convincing evidence that coil systems with non-electrolytic detachment mechanisms are less than comparably effective in any publication we know of. Favourable results were achieved by Higashida et Al (1991) 35 and Knuckey et Al (1992) 41 by endovascular treatment of intracranial aneurysms with free platinum coils. Casasco et Al (1993) 12 treated 71 aneurysms with free fibred platinum coils. All their complications (four cases of parent vessel occlusion) could have been avoided through the use of retrievable coils. Complete exclusion of 94 % of aneurysms with a diameter under 10 mm and of 82% of aneurysms with a diameter of 1025mm is comparable to the efficacy of the published GDC studies. In terms of recurrence, their results of 5.5% (3 of 54) after an average of 13 months in the initially completely filled aneurysms are better than those in several GDC series. Cekirge et Al (1996)14 treated five and Manabe et Al(1997) 48 treated 12 intracranial aneurysms with mechanically retrievable platinum coils (Interlocking Detachable Coil, Target Therapeutics). The results are comparable to those achieved with GDC. Koizumi et Al (1997)42, in a patient who had coil exclusion of an aneurysm with a mechanical IDC system, demonstrated endothelialisation around the ostium histologically. These findings suggest that non-electrolytic coil systems are able to achieve similar morphological results to electrolytically detached coils.
Reul et Al (1997a,b) 83,84 filled eight aneurysms with GDC and nine aneurysms with mechanically detachable Tungsten coils in rabbits. Histologic examination after three and six months found no organized intra-aneurysmal thrombus in either group. Slightly more favourable results were found in the mechanically removable Tungsten coils, said to be due to the more strongly thrombogenic surface of these coils. The permanence of the exclusion of the aneurysms was favourably influenced by an initial dense filling. The administration of Heparin did not have any recognizable disadvantageous effect, also pointing toward the dubious significance of electrothrombosis, which is suppressed by heparinisation.
Byrne et Al (1997b) 11 in an animal model (pig), compared the effectiveness of GDC coils and mechanically detachable Tungsten coils. They compared intra-aneurysmal thrombus formation, connective tissue formation, the inflammatory reaction and connective tissue formation at the ostium. No difference was found between the coil types. The authors stated “ ... the MDS [tungsten] coils were more thrombogenic than the GDC coils... Electrothrombosis, which potentially enhances GDC thrombogenicity, appears to have little effect on the induction of thrombus or its nature...”. Tournade et Al (2001) 109 described the treatment of 40 aneurysms using a total of 242 coil systems from Cook, mechanically detached by manual rotation of an attaching thread. The safety and efficacy corresponded to those of GDC studies. Pierot et Al (2002)73 treated 78 patients with 80 intracranial aneurysms using 457 coil systems from Micrus (detached by electrothermal interruption of a polymer thread). The angiograhic and clinical results corresponded to those of the published GDC studies. Lylyk et Al (2002)- compared the non-electrolytic coil systems from Cook (mechanical detachment by tortion on a thread) GDC (electrolytic), Micrus (electro-thermal interruption of a polymer thread) and Microvention (liberation of the coil attached to a thin metal small tube by an epoxy hose, through liquid injection into an air-free small tube). No significant differences were found with regard to the effectiveness and safety of these treatments.
The experimental investigations of Sorteberg et Al (2001, 2002) 99,100 explain the effective mechanism of coil occlusion of intracranial aneurysms through haemodynamic modifications within the aneurysm lumen. By placement of coils into the lumen of an aneurysm there is:
— a decrease of the pulse pressure amplitude
— a decrease of the rate of change of pressure
— a significant flow reduction in the aneurysm.
The mean pressure in the aneurysm remains constant or even increases. In both studies, GDC were employed. The authors do not refer to possible effects of electrothrombosis in any manner. The fact that an effect on blood flow was demonstrable after the incorporation only of one coil argues for a simple haemodynamic effect of the coils.
Evidence from clinical cases
In our experience, when electrolytically detached endovascular coils are used in circumstances of free flow, such as fusiform aneurysms, varices, arteriovenous shunts or for vessel occlusion, electrothrombosis is not clinically evident on detachment. In these circumstances, there is no clinically evident difference in behaviour between electrolytically detached coils and other coil detachment systems. On the other hand, there is clearly greater occlusive effect from fibred coils, either electrolytically or otherwise deployed under these circumstances.
Conclusion
The concept of electrothrombosis for the treatment of aneurysms has been explored for over one hundred years. Neither the principles from its historical application, nor the evidence derived from animal studies can be applied to current electrolytically detached coil systems. It would seem unlikely that electrothrombosis plays any part in modern endovascular intracranial aneurysm treatment as whatever thrombus formation occurs on the coil surface by this mechanism is likely to pale into insignificance when compared to spontaneous thrombus formation and the direct effects of the coil mass. The long-term goal of endovascular treatment of aneurysms is to promote aneurysm healing by encouraging formation of connective tissue and ideally a new intima over the ostium. It is doubtful that whatever intraaneurysmal thrombus does form at the time of coil placement contributes to achievement of this long-term goal. New technologies aiming to promote connective tissue formation or a greater fraction of volumetric filling of the aneurysm may result in superior long-term occlusion rates.
References
- 1.Abe Y, Ehara K, et al. Bilateral giant aneurysms. Copper wire thrombosis of unruptured giant aneurysms. Excerpta Medica International Congress Series. 1973;293:151. [Google Scholar]
- 2.Ahuja A, Hergenrother RW, et al. Platinum coil coating to increase thrombogenicity: a preliminary study in rabbits. Am J Neuroradiol. 1993;14:794–798. [PMC free article] [PubMed] [Google Scholar]
- 3.Alksne JF, Smith RW. Stereotactic occlusion of 22 consecutive anterior communicating artery aneurysms. J Neurosurg. 1980;52:790–793. doi: 10.3171/jns.1980.52.6.0790. [DOI] [PubMed] [Google Scholar]
- 4.Araki C, Handa H, et al. Electrically induced thrombosis for the treatment of intracranial aneurysms and angiomas. Excerpta Medica International Congress Series. 1965;110:651–654. [Google Scholar]
- 5.Bavinzski G, Talazoglu V, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg. 1999;91:284–293. doi: 10.3171/jns.1999.91.2.0284. [DOI] [PubMed] [Google Scholar]
- 6.Blakemore AH, King BG. Electrothermic coagulation of aortic aneurysms. JAMA. 1938;111:1821–1827. [Google Scholar]
- 7.Blakemore AH. Progressive constrictive occlusion of the abdominal aorta with wiring and electrothermic coagulation. One stage operation for arteriosclerotic aneurysms of the abdominal aorta. Ann Surg. 1951;133:447–462. doi: 10.1097/00000658-195113340-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buressi P, Corradi G. Lo sperimentale. 1879;1:445. [Google Scholar]
- 9.Byrne JV, Hubbard N, et al. Endovascular coil occlusion of experimental aneurysms: partial treatment does not prevent subsequent rupture. Neurol Res. 1994;16:425–427. doi: 10.1080/01616412.1994.11740267. [DOI] [PubMed] [Google Scholar]
- 10.Byrne JV, Guglielmi G. Endovascular treat-ment of intracranial aneurysms. Berlin, Germany: Springer; 1997a. [Google Scholar]
- 11.Byrne JV, Hope JKA, et al. The nature of thrombosis induced by platinum and tungsten coils in saccular aneurysms. Am J Neuroradiol. 1997b;18:29–33. [PMC free article] [PubMed] [Google Scholar]
- 12.Casasco AE, Aymard A, et al. Selective endovascular treatment of 71 intracranial aneurysms with platinum coils. J Neurosurg. 1993;79:3–10. doi: 10.3171/jns.1993.79.1.0003. [DOI] [PubMed] [Google Scholar]
- 13.Castro E, Fortea F, et al. Long-term histopathologic findings in two cerebral aneurysms embolized with Guglielmi detachable coils. Am J Neuroradiol. 1999;20:549–552. [PMC free article] [PubMed] [Google Scholar]
- 14.Cekirge HS, Saatci I, et al. Interlocking detachable coil occlusion in the endovascular treatment of intracranial aneurysms: preliminary results. Am J Neuroradiol. 1996;17:1651–1657. [PMC free article] [PubMed] [Google Scholar]
- 15.Ciniselli L. Sulla electro-punctura nella cura degli aneurismi. Gazz Med Ital. 1847;6:9–14. [Google Scholar]
- 16.Colt GH. Three cases of aneurysm of the aorta treated by wiring. Brit J Surg. 1925;13:109–119. [Google Scholar]
- 17.Cooper R, Crow HJ. Toxic effects of intra-cerebral electrodes. Med Biol Eng Comput. 1966;4:575–581. doi: 10.1007/BF02474827. [DOI] [PubMed] [Google Scholar]
- 18.Dado DV, Stalnecker MC, et al. Experience with electrothrombosis in the treatment of angiomas. Ann Plast Surg. 1987;18:12–16. doi: 10.1097/00000637-198701000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Dawson RC, III, Krisht AF, et al. Treatment of experimental aneurysms using collagen-coated microcoils. Neurosurgery. 1995;36:133–140. doi: 10.1227/00006123-199501000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Dawson RC, III, et al. Histologic effects of collagen-filled interlocking detachable coils in the ablation of experimental aneurysms in swine. Am J Neuroradiol. 1996;17:853–858. [PMC free article] [PubMed] [Google Scholar]
- 21.Deaton HL, Anlyan WG. A study of experimental method for producing thrombosis in small arteries. Surgery Gynecology Obstetrics. 1960;111:131–134. [PubMed] [Google Scholar]
- 22.Eshner AA. Treatment of aneurysm of the aorta by introduction of wire and passage of a galvanic current. Am J M Sc. 1910;140:496–505. [Google Scholar]
- 23.Fischer G, Sayre GP, et al. Histological changes in the cat´s brain after introduction of metallic and plasticcoated wire. Proc Staff Meet Mayo Clin. 1957;32:14–22. [PubMed] [Google Scholar]
- 24.Finney JMT. Wiring of otherwise inoperable aneurysm with report of cases. Ann Surg. 1912;55:661–681. doi: 10.1097/00000658-191205000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujita K, Kondo T, et al. [Experience with electrothrombosis of a giant aneurysm with copper wire] [Article in Japanese] No Shinkei Geka. 1987;15:75–79. [PubMed] [Google Scholar]
- 26.Gallagher JP. Obliteration of intracranial aneurysms by pilojection. JAMA. 1963;183:231–236. doi: 10.1001/jama.1963.03700040033008. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher JP. Pilojection for intracranial aneurysms. Report of progress. J Neurosurg. 1964;21:129–134. doi: 10.3171/jns.1964.21.2.0129. [DOI] [PubMed] [Google Scholar]
- 28.Genest AS. Experimental use of intraluminal plastics in the treatment of carotid aneurysms. J Neurosurg. 1965;22:136–140. doi: 10.3171/jns.1965.22.2.0136. [DOI] [PubMed] [Google Scholar]
- 29.Graves VB, Partington CR, et al. Treatment of carotid artery aneurysms with platinum coils: an experimental study in dogs. Am J Neuroradiol. 1990;11:249–252. [PMC free article] [PubMed] [Google Scholar]
- 30.Graves VB, Strother CM, et al. Early treatment of ruptured aneurysms with Guglielmi detachable coils: effect on subsequent bleeding. Neurosurgery. 1995;37:640–648. doi: 10.1227/00006123-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Guglielmi G, Viñuela F, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: Electrochemical basis, technique, and experimental results. J Neurosurg. 1991a;75:1–7. doi: 10.3171/jns.1991.75.1.0001. [DOI] [PubMed] [Google Scholar]
- 32.Guglielmi G, Viñuela F, et al. Electrothrombosis of sac-cular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991b;75:814. doi: 10.3171/jns.1991.75.1.0008. [DOI] [PubMed] [Google Scholar]
- 33.Hare HA. Wiring with electrolysis in saccular aneurysm. JAMA. 1927;88:230–232. [Google Scholar]
- 34.Henkes H, Drepper P, et al. Technical note on VDS. A system for the endovascular electrolytical detachment of platinum coils at variable length. Interv Neuroradiol. 2002;8:197–200. doi: 10.1177/159101990200800212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higashida RT, Halbach VV, et al. Interventional neurovascular treatment of a giant intracranial aneurysm using platinum microcoils. Surg Neurol. 1991;35:54–68. doi: 10.1016/0090-3019(91)90205-n. [DOI] [PubMed] [Google Scholar]
- 36.Horowitz M, Purdy P, et al. Scanning electron microscopic findings in a basilar tip aneurysm embolized with Guglielmi detachable coils. Am J Neuroradiol. 1997a;18:688–690. [PMC free article] [PubMed] [Google Scholar]
- 37.Horowitz M, Samson D, et al. Does electrothrombosis occur immediately after embolization of an aneurysm with Guglielmi detachable coils? Am J Neuroradiol. 1997b;18:510–513. [PMC free article] [PubMed] [Google Scholar]
- 38.Hosobuchi Y. Electrothrombosis of carotid-cavernous fistula. J Neurosurg. 1975;42:76–85. doi: 10.3171/jns.1975.42.1.0076. [DOI] [PubMed] [Google Scholar]
- 39.Ishikawa M, Handa H, et al. Management of spontaneous carotid-cavernous fistulae. Surg Neurol. 1982;18:131–139. doi: 10.1016/0090-3019(82)90372-x. [DOI] [PubMed] [Google Scholar]
- 40.Kinugasa K, Mandai S, et al. Cellulose acetate polymer thrombosis for the emergency treatment of aneurysms: angiographic findings, clinical experience, and histopathological study. Neurosurgery. 1994;34:694–701. doi: 10.1227/00006123-199404000-00019. [DOI] [PubMed] [Google Scholar]
- 41.Knuckey NW, Haas R, et al. Thrombosis of difficult intracranial aneurysms by the endovascular placement of platinum-Dacron mircocoils. J Neurosurg. 1992;77:43–50. doi: 10.3171/jns.1992.77.1.0043. [DOI] [PubMed] [Google Scholar]
- 42.Koizumi T, Kawano T, et al. [Histological findings in aneurysm treated with IDC: scanning electron microscopical study] Surg Neurol. 1997;25:1027–1031. (Japanese English abstract.) [PubMed] [Google Scholar]
- 43.Kwan ESK, Heilman CB, et al. Endovascular packing of carotid bifurcation aneurysm with polyester fibercoated platinum coils in a rabbit model. Am J Neuroradiol. 1993;14:323–333. [PMC free article] [PubMed] [Google Scholar]
- 44.Lamb JC, Isaacs JP, et al. Electrical thrombosis of blood vessels: a voltage-dependent phenomenon. Am J Physiol. 1965;208:1006–1008. doi: 10.1152/ajplegacy.1965.208.5.1006. [DOI] [PubMed] [Google Scholar]
- 45.Linton RR. Intrasaccular wiring of abdominal arteriosclerotic aortic aneurysms by the „pack” method. Angiology. 1951;2:485–498. doi: 10.1177/000331975100200606. [DOI] [PubMed] [Google Scholar]
- 46.Linton RR, Hardy IB. Treatment of thoracic aortic aneurysms by the „pack” method of intrasaccular wiring. New Engl J Med. 1952;246:847–855. doi: 10.1056/NEJM195205292462202. [DOI] [PubMed] [Google Scholar]
- 47.Lylyk P, Cerrato R, et al. The 5th Joint Annual Meeting of the AANS/CNS Section on Cerebrovascular Surgery and the American Society of Interventional and Therapeutic Neuroradiology, Program Book 2002. Dallas, TX, USA: Addison; 2002. Feb 3-6, Evolution of the mechanical treatment of intracranial aneurysms: the contribution of different coils; p. 78. [Google Scholar]
- 48.Manabe H, Fujita S, et al. Embolisation of ruptured cerebral aneurysms with interlocking detachable coils in acute stage. Interv Neuroradiol. 1997;3:49–63. doi: 10.1177/159101999700300106. [DOI] [PubMed] [Google Scholar]
- 49.Manabe H, Fujita S, et al. Rerupture of coil-embolized aneurysm during long-term observation. Case report. J Neurosurg. 1998;88:1096–1098. doi: 10.3171/jns.1998.88.6.1096. [DOI] [PubMed] [Google Scholar]
- 50.Marks MP, Chee H, et al. A mechanically detachable coil for the treatment of aneurysms and occlusions of blood vessels. Am J Neuroradiol. 1994;15:821–827. [PMC free article] [PubMed] [Google Scholar]
- 51.Mawad ME, Mawad JK, et al. Long-term histopathologic changes in canine aneurysms embolized with Guglielmi detachable coils. Am J Neuroradiol. 1995;16:7–13. [PMC free article] [PubMed] [Google Scholar]
- 52.Miller MD, Johnsrude IS, et al. Clinical use of transcatheter electrocoagulation. Radiology. 1978;129:211–214. doi: 10.1148/129.1.211. [DOI] [PubMed] [Google Scholar]
- 53.Mizoi K, Yoshimoto T, et al. A pitfall in the surgery of a recurrent aneurysm after coil embolization and its histological observation: technical case report. Neurosurgery. 1996;39:165–168. doi: 10.1097/00006123-199607000-00035. [DOI] [PubMed] [Google Scholar]
- 54.Molyneux AJ, Ellison DW, et al. Histological findings in giant aneurysms treated with Guglielmi detachable coils. Report of two cases with autopsy correlation. J Neurosurg. 1995;83:129–132. doi: 10.3171/jns.1995.83.1.0129. [DOI] [PubMed] [Google Scholar]
- 55.Moore CH, Murchison C. New method of procuring consolidation of fibrin in certain incurable aneurysms. Medico Chirurg Tr. 1864;47:129–149. doi: 10.1177/095952876404700115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mori K. Experimental studies on electrically induced arterial thrombosis in dogs, with special reference to the treatment of intracranial aneurysms and arteriovenous malformations. Arch Jap Chir. 1967;36:35–61. [Google Scholar]
- 57.Mullan S, Raimondi AJ, et al. Electrically induced thrombosis in intracranial aneurysms. J Neurosurg. 1965;22:539–547. doi: 10.3171/jns.1965.22.6.0539. [DOI] [PubMed] [Google Scholar]
- 58.Mullan S, Reyes C, et al. Stereotactic copper electric thrombosis of intracranial aneurysms. In: Krayenbühl H, Maspes PE, Sweet WH, editors. Progress in Neurological Surgery. Vol. 3. Basel and Chicago: Karger and Yearbook; 1969. pp. 193–211. [Google Scholar]
- 59.Mullan S. Experiences with surgical thrombosis of intracranial berry aneurysms and carotid cavernous fistulas. J Neurosurg. 1974;41:657–670. doi: 10.3171/jns.1974.41.6.0657. [DOI] [PubMed] [Google Scholar]
- 60.Murayama Y, Viñuela F, et al. Bioabsorbable polymeric material coils for embolization of intracranial aneurysms: a preliminary experimental study. J Neurosurg. 2001;94:454–463. doi: 10.3171/jns.2001.94.3.0454. [DOI] [PubMed] [Google Scholar]
- 61.Nishijima M, Kamiyama K, et al. [Copper needle inser-tion for the treatment of spontaneous carotid caver-nous fistula]. [Japanese] No Shinkei Geka. 1983;11:505–511. [PubMed] [Google Scholar]
- 62.Nishijima M, Kamiyama K, et al. Electrothrombosis of spontaneous carotid-cavernous fistula by copper needle insertion. Neurosurgery. 1984;14:400–405. doi: 10.1227/00006123-198404000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Ogawa Y, Inoue K. Electrothrombosis as a treatment of cirsoid angioma in the face and scalp and varicosis of the leg. Plast Reconstr Surg. 1982;70:310–318. doi: 10.1097/00006534-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Osterman FA, Bell WR, et al. Natural history of autologous blood clot embolization in swine. Invest Radiol. 1976;11:267–276. doi: 10.1097/00004424-197607000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Ovitt TW, Durst S, et al. Guide wire thrombogenicity and its reduction. Radiology. 1974;111:43–46. doi: 10.1148/111.1.43. [DOI] [PubMed] [Google Scholar]
- 66.Ozawa T, Koike T, et al. Scanning electron microscopic study of the migrated platinum coil after endovascular embolization of a giant cerebral aneurysm. Am J Neuroradiol. 1998;19:594–595. [PMC free article] [PubMed] [Google Scholar]
- 67.Padolecchia R, Puglioli M, et al. Acute histologic and ultrastructural study in one case of human basilar tip aneurysm embolised with Guglielmi detachable coils. Interv Neuroradiol. 1999;5:257–260. doi: 10.1177/159101999900500309. [DOI] [PubMed] [Google Scholar]
- 68.Padolecchia R, Guglielmi G, et al. Role of electrothrombosis in aneurysm treatment with Guglielmi detachable coils: an in vitro scanning electron microscopic study. Am J Neuroradiol. 2001;22:1757–1760. [PMC free article] [PubMed] [Google Scholar]
- 69.Peterson EW, Valberg J, et al. Electrically induced thrombosis of the cavernous sinus in the treatment of carotid cavernous fistula. Excerpta Medica International Congress Series. 1969;193:105. [Google Scholar]
- 70.Philips B. Series of experiments for purpose of showing that arteries may be obliterated without ligature, compression, or knife. Med Gaz. 1831:499. [Google Scholar]
- 71.Phillips JF. Transcatheter electrocoagulation of blood vessels. Invest Radiol. 1973;8:295–304. doi: 10.1097/00004424-197309000-00001. [DOI] [PubMed] [Google Scholar]
- 72.Phillips JF, Robinson AE, et al. Experimental closure of arteriovenous fistulae by transcatheter electro-coagulation. Radiology. 1975;115:319–321. doi: 10.1148/115.2.319. [DOI] [PubMed] [Google Scholar]
- 73.Pierot L, Flandroy P, et al. Selective endovascular treatment of intracranial aneurysms using Micrus microcoils: preliminary results in a series of 78 patients. J Neuroradiol. 2002;29:114–121. [PubMed] [Google Scholar]
- 74.Pile-Spellman J, Wu J. Coil embolization of aneurysms: angiographic and histologic changes. Am J Neuroradiol. 1997;18:43–44. [PMC free article] [PubMed] [Google Scholar]
- 75.Piton J, Billerey J, et al. Vascular thrombosis induced by direct electric current. Neuroradiology. 1978a;16:385–388. doi: 10.1007/BF00395312. [DOI] [PubMed] [Google Scholar]
- 76.Piton J, Billerey J, et al. Thromboses vasculaires sélectives par courant électrique continu; expérimentation chez l´animal. [Selective vascular thrombosis induced by a direct electrical current: animal experiments.] J Neuroradiol. 1978b;5:139–152. [PubMed] [Google Scholar]
- 77.Piton J, Billerey J, et al. Embolisation par courant électrique continu: ECEC. Applications théra-peutiques. J Radiol. 1979;80:799–808. [PubMed] [Google Scholar]
- 78.Poore G. Electricity in medicine. Quain´s Medical Dictionary. Vol I. London: Longmans, Green & Co., Inc; 1895. [Google Scholar]
- 79.Power D, Colt GH. A case of aneurysm of abdominal aorta treated by introduction of silver wire. Lancet. 1903;2:808–813. [PMC free article] [PubMed] [Google Scholar]
- 80.Power D. The palliative treatment of aneurysm by “wiring” with Colt´s apparatus. Brit J Surg. 1921;9:27–36. [Google Scholar]
- 81.Raymond J, Desfaits AC, et al. Fibrinogen and vascular smooth muscle cell grafts promote healing of experimental aneurysms treated by embolization. Stroke. 1999;30:513–518. doi: 10.1161/01.str.30.8.1657. [DOI] [PubMed] [Google Scholar]
- 82.Raymond J, Venne D, et al. Healing mechanisms in experimental aneurysms. I. Vascular smooth cells and neointima formation. J Neuroradiol. 1999b;26:7–20. [PubMed] [Google Scholar]
- 83.Reul J, Weis J, et al. Long-term angiographic and histopathologic findings in experimental aneurysms of the carotid bifurcation embolized with platinum and tungsten coils. Am J Neuroradiol. 1997a;18:35–42. [PMC free article] [PubMed] [Google Scholar]
- 84.Reul J, Spetzger U, et al. Endovascular occlusion of experimental aneurysms with detachable coils: influence of packing density and perioperative anticoagulation. Neurosurgery. 1997b;41:1160–1168. doi: 10.1097/00006123-199711000-00028. [DOI] [PubMed] [Google Scholar]
- 85.Reul J, Spetzger U, et al. The nature of early intraluminal thrombosis in terminal aneurysms occluded with Guglielmi detachable coils. Interv Neuroradiol. 1998;4:39–48. doi: 10.1177/159101999800400104. [DOI] [PubMed] [Google Scholar]
- 86.Romeike BF, Niedermayer I, et al. Histopathologische Befunde in Hirnbasisaneurysmen nach Embolisation mit Guglielmi detachable platinum coils (GDC) [Histopathologic findings in cerebral artery aneurysms after embolization with Guglielmi detachable platinum coils (GDC): report of two cases.] Radiologe. 1999;39:900–903. doi: 10.1007/s001170050729. [DOI] [PubMed] [Google Scholar]
- 87.Sakakibara T, Nitta T, et al. [Experience with electrothrombosis of intracranial aneurysms inappropriate for clipping]. (in Japanese) Neurol Med Chir (Tokyo) 1983;23:783–788. doi: 10.2176/nmc.23.783. [DOI] [PubMed] [Google Scholar]
- 88.Sakurada O, Imai Y, et al. [An attempt of electrothrombosis of carotid-cavernous fistula] (in Japanese) No Shinkei Geka / Neurol Surg. 1975;3:757–761. [PubMed] [Google Scholar]
- 89.Salazar AE. Experimental myocardial infarction. Induction of coronary thrombosis in the intact closedchest dog. Circ Res. 1961;9:1351–1356. doi: 10.1161/01.res.9.6.1351. [DOI] [PubMed] [Google Scholar]
- 90.Sawyer PN, Pate JW. Bio-electric phenomena as an etiologic factor in intravascular thrombosis. Am J Physiol. 1953a;175:103–107. doi: 10.1152/ajplegacy.1953.175.1.103. [DOI] [PubMed] [Google Scholar]
- 91.Sawyer PN, Pate JW, et al. Relations of abnormal and injury electric potential differences to intravascular thrombosis. Am J Physiol. 1953b;175:108–112. doi: 10.1152/ajplegacy.1953.175.1.108. [DOI] [PubMed] [Google Scholar]
- 92.Sawyer PN, Pate JW. Electric potential differences across the normal aorta and aortic grafts of dogs. Am J Physiol. 1953c;175:113–117. doi: 10.1152/ajplegacy.1953.175.1.113. [DOI] [PubMed] [Google Scholar]
- 93.Sawyer PN, Wesolowski SA. Studies in direct-current coagulation. Surg. 1961;49:486–491. [PubMed] [Google Scholar]
- 94.Sawyer PN, Wesolowski SA. Bioelectric phenomena and intravascular thrombosis. The first 12 years. Surg. 1964;56:1020–1026. [PubMed] [Google Scholar]
- 95.Scudamore C. Essay on the blood. London: Longmans, Hurst, Rees, Orme, Brown, and Green; 1824. [Google Scholar]
- 96.Sedlarik K. Die elektrisch induzierte Thrombose als Therapiemaßnahme bei einer lebensbedrohlichen Nierenblutung. Eine experimentelle Studie. [Electrically induced thrombosis as therapeutic measure in life-threatening renal hemorrhage. An experimental study] Zschr Urol. 1977;70:277–282. [PubMed] [Google Scholar]
- 97.Sedlarik K. Die Elektrothrombose als eine TherapieMaßnahme [Electrothrombosis as a treatment measure] Z Exp Chir. 1981;14:107–115. [PubMed] [Google Scholar]
- 98.Shimizu S, Kurata A, et al. Tissue response of a small saccular aneurysm after incomplete occlusion with a Guglielmi detachable coil. Am J Neuroradiol. 1999;20:546–548. [PMC free article] [PubMed] [Google Scholar]
- 99.Sorteberg A, Sorteberg W, et al. Effect of Guglielmi detachable coil placement on intraaneurysmal pressure: experimental study in canines. Am J Neuroradiol. 2001;22:1750–1756. [PMC free article] [PubMed] [Google Scholar]
- 100.Sorteberg A, Sorteberg W, et al. Effect of Guglielmi detachable coils on intraaneurysmal flow: experimental study in canines. Am J Neuroradiol. 2002;23:288–294. [PMC free article] [PubMed] [Google Scholar]
- 101.Spetzger U, Reul J, et al. Microsurgically produced bifurcation aneurysms in a rabbit model for endovascular coil embolization. J Neurosurg. 1996;85:488–495. doi: 10.3171/jns.1996.85.3.0488. [DOI] [PubMed] [Google Scholar]
- 102.Stiver SI, Porter PJ, et al. Acute human histopatho-logy of an intracranial aneurysm treated using Guglielmi detachable coils: case report and review of the literature. Neurosurgery. 1998;43:1203–1208. doi: 10.1097/00006123-199811000-00106. [DOI] [PubMed] [Google Scholar]
- 103.Strother CM. Historical perspectiv. Electro-thrombosis of saccular aneurysm via endovascular approach: part 1 and part 2. Am J Neuroradiol. 2001;22:1010–1012. [PubMed] [Google Scholar]
- 104.Szikora I, Wakhloo AK, et al. Initial experience with collagen-filled Guglielmi detachable coils for endovascular treatment of experimental aneurysms. Am J Neuroradiol. 1997;18:667–672. [PMC free article] [PubMed] [Google Scholar]
- 105.Tamatani S, Ozawa T, et al. Histological interaction of cultured endothelial cells and endovascular embolic materials coated with extracellular matrix. J Neurosurg. 1997;86:109–112. doi: 10.3171/jns.1997.86.1.0109. [DOI] [PubMed] [Google Scholar]
- 106.Tenjin H, Fushiki S, et al. Effect of Guglielmi detachable coils on experimental carotid artery aneurysms in primates. Stroke. 1995;26:2075–2080. doi: 10.1161/01.str.26.11.2075. [DOI] [PubMed] [Google Scholar]
- 107.Thompson T, Souttar HS, et al. Saccular aneurysm of thoracic aorta treated by wiring with Colt´s apparatus. Lancet. 1935;1:11–14. [Google Scholar]
- 108.Thompson WM, Pizzo SV, et al. Transcatheter electrocoagulation: a therapeutic angiographic technique for vessel occlusion. Invest Radiol. 1977;12:146–153. doi: 10.1097/00004424-197703000-00008. [DOI] [PubMed] [Google Scholar]
- 109.Tournade A, Riquelme C, et al. Endovascular treatment of berry intracranial aneurysms using a new detachable coil system (DCS® - Detachable Coil System Cook) Interventional Neuroradiology. 2001;7:93–102. doi: 10.1177/159101990100700201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Velpeau A. Mémoir sur la piqûre ou l´acupuncture des artères dans le traitement des anévrismes. Gaz Méd De Par. 1831;2:1. [Google Scholar]
- 111.Werner SC, Blakemore AH, et al. Aneurysm of the internal carotid artery within the skull: wiring and electrothermic coagulation. JAMA. 1941;116:578–582. [Google Scholar]
- 112.Yoneda S, Matsuda M, et al. Electrothrombosis of arteriovenous malformation. Neurol Med Chir (Tokyo) 1977;17:19–28. doi: 10.2176/nmc.17pt1.19. [DOI] [PubMed] [Google Scholar]