Abstract
The serotonin 5-HT2A receptor is the major target of psychedelic drugs such as lysergic acid diethylamide (LSD), mescaline, and psilocybin. Serotonergic psychedelics induce profound effects on cognition, emotion, and sensory processing that often seem uniquely human. This raises questions about the validity of animal models of psychedelic drug action. Nonetheless, recent findings suggest behavioral abnormalities elicited by psychedelics in rodents that predict such effects in humans. Here we review the behavioral effects induced by psychedelic drugs in rodent models, discuss the translational potential of these findings, and define areas where further research is needed to better understand the molecular mechanisms and neuronal circuits underlying their neuropsychological effects.
Keywords: Psychedelic, hallucinogenic, schizophrenia, psychosis, serotonin 5-HT2A receptor, G protein-coupled receptor (GPCR), lysergic acid diethylamide (LSD), mouse behavior models
Elucidating the mechanisms by which psychedelics induce their unique neuropsychological effects has important implications for a better understanding of behavioral processes such as cognition, perception, emotion, and sense of self.1−5 The term psychedelic was coined in 1957 by the British psychiatrist Humphry Osmond to describe the effects of psychoactive drugs such as psilocybin, mescaline, and lysergic acid diethylamide (LSD).6 These drugs belong to a larger group of substances known as hallucinogens, which also includes dissociatives (e.g., ketamine and phencyclidine), and deliriants (e.g., scopolamine and atropine), as well as compounds such as salvinorin A. Psychedelics all behave as agonists or partial agonists at the serotonin 5-HT2A receptor, whereas dissociatives and deliriants have been identified as noncompetitive NMDA receptor antagonists, and competitive muscarinic receptor antagonists, respectively. Salvinorin A is a potent κ-opioid receptor agonist.7−12
Although all of these hallucinogenic drugs profoundly alter perception, according to the Hallucinogen Rating Scale (HRS) and the Five-Dimensional Altered States of Consciousness (5D-ASC) rating scale, there are also features that are unique to each of these groups.13−15 Research using behavioral and cognitive tasks indicates that different groups of hallucinogens induce overlapping, yet distinct sets of changes in sensory processing. Recent findings regarding the molecular mechanism of action of psychedelic and other hallucinogenic drugs have been reviewed elsewhere.7,8,11,16−24 In this review, we will discuss the effects of psychedelics in a range of animal behavioral assays, and their utility as preclinical models of the effects of these drugs in humans.
Modeling Psychosis in Animals
Modeling in rodents the neuropsychological effects induced by psychedelic drugs remains controversial. The above-mentioned psychometric rating scales HRS and 5D-ASC measure aspects of subjective experience such as “oceanic boundlessness”, “dread of ego dissolution”, and “spiritual experience” that are difficult to evaluate in the absence of verbalization.13,25 Furthermore, rodent sensory systems differ from those of humans, with relatively poor vision and comparatively well developed olfactory and somatosensory abilities.26 Given these limitations, one of the priorities in molecular pharmacology research is to determine which behaviors in rodents predict specific types of neuropsychological effects in humans. Ideally, suitable rodent models that are analogous to specific behavioral features induced by psychedelic drugs in humans may be used as tools to investigate the anatomy and molecular mechanisms of action underlying such behavioral outcomes. However, every rodent behavioral model has certain limitations.
Drug-Induced Head-Twitch Behavior
Although it lacks face validity, head-twitch behavioral response is useful as a mouse behavioral proxy of human psychedelic action, mostly due to its predictive validity. Head-twitch behavior is induced in mice by all psychedelic 5-HT2A receptor agonists studied, and is not induced by nonpsychedelic 5-HT2A agonists such as lisuride and ergotamine.27,28 Head-twitch is distinct from other behavioral responses in rodents, such as head-weaving (slow, side-to-side lateral head movement) and wet-dog shakes (repetitive shaking of the body), which are observed after administration of dissociative drugs and during morphine withdrawal, respectively.23,29 To our knowledge, the first study reporting that LSD produces abnormal behavior in mice was published in 1955.30 It was shown that injection of LSD affected locomotor behavior and induced tremor in mice placed on an inclined glass plate. In the search of a behavioral response that was more reliable and easier to quantify, Keller and Umbreit reported the head-twitch behavior induced by LSD as a rapid and violent head shaking.31 Following these initial studies, it was shown that a large dose of the serotonin precursor 5-hydroxytryptophan (5-HTP) induces head-twitch behavior in mice.32 However, to our knowledge, equivalent doses of 5-HTP have not been tested in healthy volunteers, and therefore, it remains unknown whether 5-HTP is psychedelic in humans. Subsequently, numerous psychedelic compounds were shown to induce head-twitch behavior.27,33−36 Head-twitch behavior is occasionally observed at baseline, but at a much lower frequency than that observed in the presence of psychedelic treatment.
Before the first G protein-coupled receptors (including β2-adrenergic, 5-HT1A, and 5-HT2A) were cloned, pharmacological assays had shown that antiserotonergic drugs, such as methysergide, methiothepin, and mianserin, antagonize the head-twitch behavior induced by 5-HTP and LSD.37−41 Although these findings suggested that serotonin receptors were involved in the head-twitch response, it took more than a decade before LSD and other psychedelics were shown to bind with high affinity to the 5-HT2A receptor in rat cortex, after which the human 5-HT2A receptor was cloned and expressed heterologously in murine fibroblasts.42,43 Pharmacological inactivation of the 5-HT2A receptor blocks the head-twitch response induced by systemic administration of psychedelics such as 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM), 1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane (DOB), psilocybin, and mescaline2,35,36,44−46 (see also Table 1). Consistent with these studies are observations that 5-HT2A receptor knockout mice do not exhibit head-twitch response to psychedelics (see also Figure 1).27,28,47 The dose-dependent blockage of the effects of psilocybin in humans by the 5-HT2A receptor antagonist ketanserin further supports the relevance of these findings.5 It should also be noted that activation of 5-HT2C receptors attenuates the head-twitch behavior induced by psychedelics.48 Since the psychedelic drug DOI has a high affinity for the 5-HT2A receptor, and a lower affinity for the 5-HT2C receptor,49 together these findings suggest that the 5-HT2C receptor might be involved in the inverted U-shaped dose–response of DOI on head-twitch behavior.48 On the other hand, recent findings showed attenuated head-twitch behavior induced by DOI in 5-HT2C receptor knockout mice,50 suggesting that 5-HT2A and 5-HT2C receptors interact to influence psychosis-like behavior in a complex way that requires further investigation.
Table 1. Summary of the Effects Hallucinogens in a Range of Behavioral Paradigms.
| psychedelics (5-HT2A agonists) | dissociatives (NMDA antagonists) | deliriants (muscarinic antagonists) | Salvinorin A (κ-opioid agonist) | amphetamine (DA releaser/DAT inhibitor)a | |
|---|---|---|---|---|---|
| head-twitch | yes27 | unknown | unknown | unknown | unknown |
| drug discrimination (LSD-like) | yes19 | no (but potentiates LSD-like effects)116 | unknown | no68,116 | no116,117 |
| FR-40 ″pause″ | yes76 | yes75 | unknown | unknown | no118 |
| locomotion/exploration | Inverted U-shaped77,79 | increased119,120 | increased121 | decreased122 | yes123,124 |
| PPI disruption | yes89,90,92 | yes125 | yes126 | conflicting results127,128 | yes129,130 |
| conflict anxiety tests | anxiolytic-like94,131 | anxiolytic-like132 | anxiogenic-like133 | anxiolytic-like134 | conflicting results135,136 |
| impulsivity | increased137,138 | increased139,140 | increased141 | unknown | conflicting results140,142 |
| peak interval timing | leftward shift106 | rightward shift109 | nonspecific decrease in accuracy143 | unknown | leftward shift106 |
| fear conditioning | enhanced114 | impairment144 | impairment145 | unknown | increased at low doses146 |
| fear extinction | enhanced114 | impairment under certain conditions147 | impairment148 | unknown | no effect149 |
DA: dopamine; DAT: dopamine transporter.
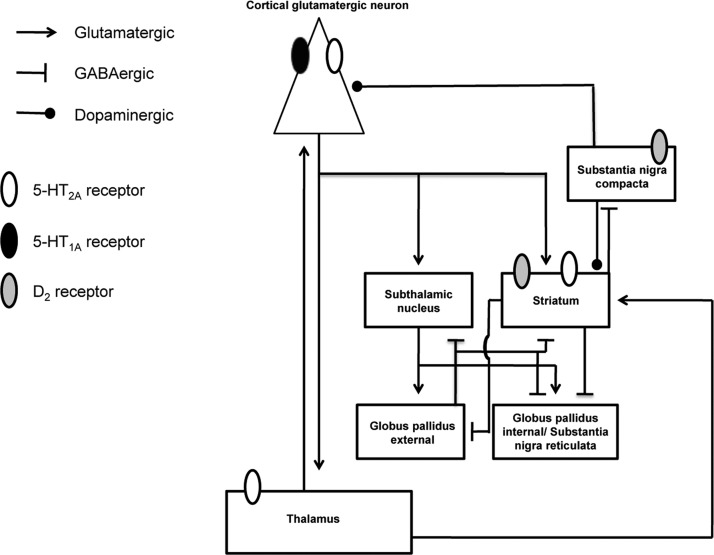
Figure 1.
Neurotransmitter receptor subtypes and neuronal circuits involved in the mechanism of action of psychedelic drugs.
There are few examples of false positives obtained with head-twitch behavior as rodent model of psychedelic action. Among these, cannabinoid CB1 receptor antagonists and α2-adrenergic receptor antagonists induce head-twitch response in rodents.51−53 It has been suggested that CB1 receptor antagonists induce head-twitch behavior through a mechanism that requires serotonin release and activation of 5-HT2A receptors.51,52 An additional report found that the stimulation of [35S]GTPγS binding (an assay to measure receptor-G protein coupling) by the psychedelic drug DOI was decreased in frontal cortex and hippocampal regions of CB1 receptor knockout mice, suggesting an adaptive change that attenuates 5-HT2A receptor-dependent signaling.54 Since the head-twitch behavior induced by psychedelic drugs is either attenuated or induced by CB1 receptor agonists (e.g., tetrahydrocannabinol, THC) and antagonists, respectively, together these findings point toward a close functional crosstalk between 5-HT2A and CB1 receptors.51,52,55
The head-twitch behavioral response induced by psychedelic 5-HT2A agonists is decreased in knockout mice for the metabotropic glutamate 2 (mGlu2) receptor.56 This glutamate receptor has been shown to be expressed in close molecular proximity with the 5-HT2A receptor in tissue culture and mouse frontal cortex.57,58 Although activation of the 5-HT2A receptor in cortical pyramidal neurons is necessary to induce head-twitch by psychedelics,27,45,46 these data suggest that other receptors, including CB1 and mGlu2, are also involved in the modulation of their behavioral effects.
As a summary, head-twitch behavior in mouse is predictive of psychedelic potential in humans with a high degree of reliability.
Drug Discrimination
In the two-lever drug discrimination paradigm, laboratory animals are trained to recognize the internal state (discriminative stimulus) induced by a specific dose of a particular drug (training drug).19 Discriminative responses between two operant devices (usually levers) are maintained by food reward.35 During training sessions, if the training drug is administered, lever presses on the drug-designated lever produce reinforcement (food reward). If vehicle is administered, responses on the alternate, vehicle-designated lever produce reinforcement. After the training period, the animal is injected with the drug for the test session, and drug-appropriate responding is presumed to be the result of similar interoceptive cues to those induced by the training drug.
With respect to the serotonergic receptor subtype involved in the responses following psychedelic drug administration, the use of selective antagonists indicates that discriminative stimuli of the effects of psychedelics are mediated by their agonist activity at the 5-HT2A receptor.59−64 More specifically, assays using selective 5-HT2A receptor antagonists and intracerebral microinjection of LSD suggest the 5-HT2A receptor in the anterior cingulate cortex as responsible for the discriminative stimulus properties of LSD in rats (Figure 1).65
Drug-appropriate responding in the test phase is generally observed when the training and the test drugs are both psychedelic 5-HT2A receptor agonists.66 Substitution for LSD in drug discrimination assays does not occur with other psychoactive drugs such as phenyclidine (PCP),67 salvinorin A, (68) cocaine, or amphetamine.69 There are a few false positives such as the nonpsychedelic lisuride, which mimics LSD in traditional two-lever drug discrimination assays. However, it has also been shown that lisuride can be discriminated from LSD in three-lever drug discrimination paradigms,19,59,67 suggesting that this paradigm may be able to distinguish between psychedelic and nonpsychedelic 5-HT2A receptor agonists. However, it is still unknown whether a three-lever paradigm discriminates between different psychedelics, and further work is needed to determine whether the three-lever paradigm may be used to categorize psychedelic versus nonpsychedelic 5-HT2A receptor agonists.
Pauses on Fixed-Ratio Schedules of Reinforcement
In the fixed-ratio schedule of reinforcement, reinforcements are given only after the animal has emitted a specified number of responses.70 In a fixed-ratio 40 (FR-40) task, food restricted rats are trained to press a lever 40 times in order to obtain a reward.70 Two parameters have been used to study the effects of psychedelics in this task: the number of reinforcements obtained, and number of periods of nonresponding lasting 10 s or more (i.e., “pauses”). Psychedelics increase the number of pauses, and, importantly, these periods of nonresponding are interspersed with periods of responses that are similar to those of the control group.71−73 Increases in pausing are not observed in animals injected with other psychoactive drugs, such as pentobarbital and amphetamine.71,74 In addition to reducing the rate of responding, noncompetitive NMDA antagonists have been shown to induce a pause-like effect in monkeys.75 Similar effects on FR-40 behavior are also induced by the nonpsychedelic 5-HT2A agonist lisuride in rats.73 Importantly, the atypical antipsychotic and 5-HT2A/dopamine D2 receptor antagonist pipamperone does not affect the effects of lisuride on the number of pauses, but reverses the behavioral effect of psychedelics on FR-40 behavior.76 Whether pauses on fixed-ratio schedules of reinforcement in rodents model a behavioral effect induced in humans by psychedelics remains unknown.
Locomotor Response and Exploratory Behavior
Mark Geyer and his laboratory have extensively studied the effects of psychedelics on locomotion and exploratory behavior in the open field. In rats, they found that LSD and DOI induce 5-HT2A receptor-dependent decreases in the amount of activity (horizontal locomotion), exploratory behavior (number of nose-pokes in a holeboard, and rears), and the tendency to repeatedly follow a similar path (referred to as “path stereotypy” or “spatial scaling exponent d”, a paradigm that describes the geometrical properties of movements).77 When the effects of LSD and lisuride were compared, they found that lisuride induced a biphasic dose–response curve in rats, with suppression and enhancement of horizontal activity at low and high doses, respectively.78 However, LSD decreased horizontal locomotion, number of nose-pokes, and path stereotypy at all but the lowest dose tested.78 More recent findings suggest that DOI induces an inverted U-shaped dose–response curve on locomotor activity in mice.79 Interestingly, the increase in the locomotor activity induced by the lower doses of DOI was absent in 5-HT2A receptor knockout mice, whereas the reduction in the locomotor activity induced by the higher doses of DOI was reversed by pretreatment with the 5-HT2C receptor antagonist (+)-cis-4,5,7a,8,9,10,11,11a-octahydro-7H-10-methylindolo[1,7-bc][2,6]-naphthyridine (SER-082). The authors concluded that 5-HT2A and 5-HT2C receptors are responsible for the opposite effects of DOI on the locomotor activity in mice.79 These findings based on the use of the 5-HT2A/2C agonist DOI are further supported by the inverted U-shaped dose–response of DOI on head-twitch behavior described above.48
Psychotomimetic drugs such as noncompetitive NMDA antagonists (PCP, ketamine and MK-801) and amphetamine also induce hyperlocomotion in rodents, an effect that is attenuated by 5-HT2A receptor antagonists and inverse agonists such as (R)-(+)-a-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-pipidinemethanol (M100907) and clozapine.58,80−83 Recent findings suggest that the 5-HT2A receptor is involved in the locomotor suppressive effects of clozapine.84,85
Prepulse Inhibition of Startle
Prepulse inhibition (PPI) of startle is a measure of sensorimotor gating that refers to the reduction in the startle response produced by a low-intensity nonstartling stimulus (the prepulse) presented shortly before the startle stimulus.86 In humans, psilocybin disrupts PPI at short interstimulus intervals (ISIs), but has no effect at medium ISIs, and, notably, enhances PPI at long ISIs.87,88 In rodents, some, but not all, of these studies suggest that psychedelics disrupt PPI at short, medium and long ISIs.89−91 It is also worth noting that, while both lisuride and LSD disrupt PPI in rats, pretreatment with the 5-HT2A antagonist α-phenyl-1-(2-phenylethyl)-4-piperidinemethanol (MDL11939) reverses the effect of LSD, but not that of lisuride. The effects of lisuride on PPI are prevented by the dopamine D2/D3 receptor antagonist raclopride.92 The neuronal circuits through which 5-HT2A- and/or D2/D3-dependent signaling affects sensorimotor gating in rodents remain unknown.
Anxiety-like Behavior
The molecular mechanisms responsible for anxiety-like behavior in rodents have been the focus of many studies and models. Several studies have reported anxiolytic-like effects of the hallucinogen DOI in conflict-based anxiety tests, with an increase in punished-drinking behavior and punished-passages in the four-plate test, as well as increased open-arm activity in the elevated plus-maze test.93,94 Additionally, in rat pups, systemic administration of DOI induces anxiolytic-like behavior, including decreases in ultrasonic vocalizations.95 These findings contrast with the feelings of fear and anxiety that are frequently precipitated by psychedelics in humans.96 On the other hand, in rats, DOI induces freezing behavior in the open field exploration test, which suggests that activation of 5-HT2A receptors by psychedelics affects different measures of anxiety-like behavior differently.97
Recent findings indicate absence of effect of a low dose of DOI alone on anxiety-like behavior in mice. Fergusson and collaborators showed that preadministration of corticotropin-releasing factor (CRF) into the frontal cortex of mice induced 5-HT2A receptor-dependent anxiety-like behavior in response to the same dose of DOI.98 They also demonstrated that CRF receptor 1 (CRFR1) affects 5-HT2A receptor endocytosis and recycling, a mechanism proposed as involved in anxiety-like behavior.
That 5-HT2A receptor-dependent signaling plays a role in anxiety-like behavior is further supported by recent findings in 5-HT2A receptor knockout mice.99 These mice show decreased anxiety-like behavior, and rescuing 5-HT2A receptor expression in cortical neurons normalizes anxiety-like behavior.99 It has also been suggested that stimulation of 5-HT2A receptors in the dorsal periaqueductal gray matter induces panicolytic-like behavior in rats.100 Together, these findings suggest that 5-HT2A receptors expressed in cortical and midbrain regions exert opposite effects on anxiety-like behavior. They also raise the possibility that psychedelics may serve as a research tool to better understand the molecular basis of mood disorders.22
Impulsivity and Response Inhibition
Impulsivity is broadly defined as the inability to withhold a behavioral response when such delay would produce a more favorable outcome. It is commonly divided into “impulsive action”, which is the inability to withhold a motor response until an appropriate time, and “impulsive choice”, which is the selection of a smaller reward more quickly instead of a larger reward that requires a delayed response.101 Impulsive behaviors are often seen in association with psychiatric disorders, such as schizophrenia, and bipolar disorder.101 Using motor and impulsive choice paradigms, several studies have reported that psychedelics increase impulsivity in a 5-HT2A receptor-dependent manner.102−104 When considering the translational relevance of these preclinical findings, it is important to note that the doses tested were much lower than those used to study head-twitch behavior (see above).
Time Perception
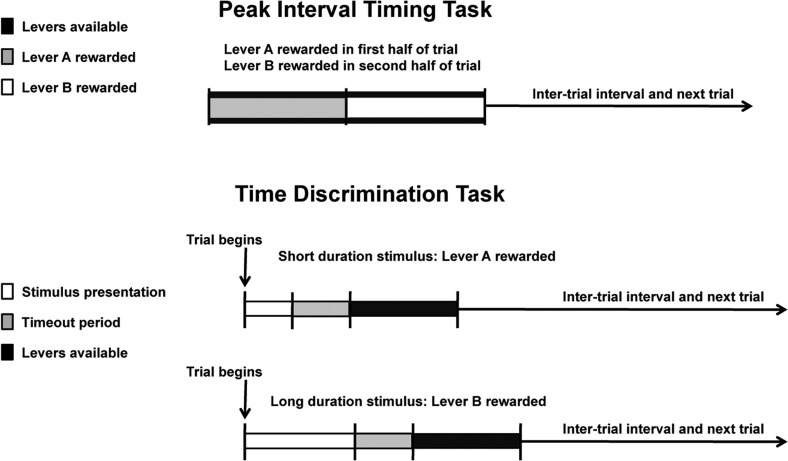
Time is a fundamental construct for determining the actions we take. Time perception has been demonstrated in a variety of species, such as bees, fish, and rodents. “Peak interval timing” is one of the behavior models used to investigate time perception in rodents (Figure 2)105 In this task, test subjects have a choice of responding on either of two levers (A or B) during a trial of a fixed duration (e.g., 50 s). In a 50 s trial, pressing on lever A in the first 25 s will result in reward delivery, while, in the second 25 s, only responses on lever B will be rewarded. Once the animal has switched from the first lever (A) to the second lever (B), lever A is withdrawn. Thus, the maximum number of rewards is obtained by switching from lever A to lever B at exactly 25 s into the trial. In short, this task trains animals to indicate with their behavioral response when they perceive a particular duration of time to have passed.
Figure 2.
Schematic representation of two paradigms of time perception in mice: peak interval timing task and time discrimination task.
Another task to investigate time perception in rodents is “time discrimination”, which is a retrospective timing task (Figure 2). In this task, a stimulus, such as light, is presented for a variable duration (t) (e.g., 2.5–50 s). During stimulus presentation, no levers are available and no reward can be obtained. Following stimulus presentation, there is a timeout period after which the two levers are presented. If (t) was less than 25 s, a press on lever A, but not B, is rewarded. If (t) was greater than 25 s, lever B, but not A, is rewarded.
In a “peak interval timing” task, the psychedelic 5-HT2A agonist DOI and amphetamine both elicit an overestimate of time intervals in rats, as they tend to press the second lever earlier in time, and, therefore, the curve is shifted to the left.106 The effects of both DOI and amphetamine on timing performance are reversed by the 5-HT2A receptor antagonist MDL-100907. However, the selective dopamine D1 receptor antagonist SKF-83566 blocks the effect of amphetamine, but not DOI, on this task.106
An opposite pattern of errors was observed in the time discrimination task (which is a retrospective timing task), as DOI decreased the percentage of responses on lever B at longer durations.107 This highlights how similar tasks my produce varying and seemingly contradictory conclusions. Using brightness discrimination as a control, it was also found that amphetamine, but not DOI, impaired brightness discrimination. Thus, the effects of DOI on time perception may not be attributed to a general deficit in discrimination tasks. Clozapine induces similar alterations in peak-interval timing to those observed after injection of DOI or amphetamine.108 Another interesting finding is that MK-801 (although not ketamine) elicits an opposite effect on peak-interval timing as compared to psychedelic 5-HT2A agonists.109,110
The effect of psilocybin on time perception has been studied in humans, and similar results to the peak interval task were found.111 Volunteers were presented with a test tone of variable duration, followed by a short time-out period, after which a second tone followed. Subjects were instructed to press a key to switch off the stimulus when they believed that second tone had been on for the same duration as the previously presented stimulus. Although interesting, this paradigm is based on both retrospective judgements of the duration of the stimulus and the timing of the responses, which may confound the different types of judgments made in the “retrospective timing” and “peak interval timing” tasks described in rodents. Further investigation is needed regarding the effects of psychoactive 5-HT2A receptor ligands on time perception in rodents and humans.
Memory
In some of the few studies in which the effects of psychedelic 5-HT2A receptor agonists on memory have been investigated, it was reported that LSD and DOI, but not lisuride, enhance trace conditioning of the nictitating membrane response in rabbits (a simple associative learning of a motor response), and this effect was reversed by 5-HT2A/2C receptor antagonists.112,113 Fear memory in a trace conditioning paradigm was also affected by activation of the 5-HT2A receptor in rats,114 as post-training administration of the 5-HT2A receptor agonist (4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine hydrobromide (TCB-2)115 enhanced subsequent freezing in a trace fear conditioning test.
Conclusion
One of the main limitations in molecular psychiatry is the development of convincing animal models of psychiatric disorders. While modeling all the behavioral responses induced by psychedelic drugs is highly unlikely, rodent models recapitulate some individual aspects of the behavioral effects induced by psychedelic drugs in humans. The use of pharmacological and genetic tools in rodent models provides compelling evidence that the serotonin 5-HT2A receptor is the primary target responsible for psychedelic effects. Nevertheless, GPCRs such as serotonin 5-HT2C, dopamine D2, dopamine D1, glutamate mGlu2, cannabinoid CB1, adenosine A1, and μ-opioid, to name a few, have been involved in the modulation (enhancement or attenuation) of 5-HT2A receptor-dependent cellular signaling pathways and behaviors. Further work is necessary to better understand the molecular mechanisms and neuronal circuits through which these receptors modulate the behavioral effects of psychedelics.
Rodent behavioral assays, such as head-twitch response, drug discrimination assay, and pauses on fixed-ratio schedules of reinforcement, represent valuable tools to predict psychedelic potential in humans. Other behaviors, such as disruption of PPI of startle, changes in locomotor response and exploratory behavior, and alterations in time perception, are affected by various groups of psychoactive drugs, such as psychedelics, dissociatives, and deliriants, and these may be used to obtain a more fine-grained understanding of the similarities and differences between these drugs. We look forward to models that will be used for unraveling the neurochemical events that converge on shared patterns of behavioral alterations, or distinguish those that differ.
Since studies in healthy volunteers are limited in their ability to probe neurochemical mechanisms, the use of translational animal models will enable to better understand the molecular basis through which psychedelics affect cognition, perception, and mood in humans. Elucidating the neuronal and signaling mechanisms underlying psychedelic effects should also help advance our understanding and treatment of endogenous psychoses such as schizophrenia.
Acknowledgments
The authors would like to thank Terrell Holloway and Caitlin McOmish for critical review of the manuscript.
Author Contributions
J.B.H and J.G.-M. searched the literature, conceived the topic of the review, and wrote the manuscript.
J.G.-M. is supported by a grant from NIH (R01 MH084894).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Hofmann A. (1980) LSD: My Problem Child, McGraw-Hill, New York. [Google Scholar]
- Shulgin A. S. A. (1995) PIHKAL: Phenethylamines I have known and loved: a chemical love story, Transform Press, Berkeley. [Google Scholar]
- Griffiths R. R.; Richards W. A.; McCann U.; Jesse R. (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology 187, 268–283discussion 284–292. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E.; Heekeren K.; Neukirch a.; Stoll M.; Stock C.; Obradovic M.; Kovar K.-a. (2005) Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry 38, 301–311. [DOI] [PubMed] [Google Scholar]
- Vollenweider F. X.; Vollenweider-Scherpenhuyzen M. F.; Babler A.; Vogel H.; Hell D. (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport 9, 3897–3902. [DOI] [PubMed] [Google Scholar]
- Osmond H. (1957) A review of the clinical effects of psychotomimetic agents. Ann. N.Y. Acad. Sci. 66, 418–434. [DOI] [PubMed] [Google Scholar]
- Nichols D. E. (2004) Hallucinogens. Pharmacol Ther. 101, 131–81. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J.; Sealfon S. C. (2009) Agonist-trafficking and hallucinogens. Curr. Med. Chem. 16, 1017–1027. [DOI] [PubMed] [Google Scholar]
- Morris B. J.; Cochran S. M.; Pratt J. A. (2005) PCP: from pharmacology to modelling schizophrenia. Curr. Opin. Pharmacol. 5, 101–106. [DOI] [PubMed] [Google Scholar]
- Kristiansen L. V.; Huerta I.; Beneyto M.; Meador-Woodruff J. H. (2007) NMDA receptors and schizophrenia. Curr. Opin. Pharmacol. 7, 48–55. [DOI] [PubMed] [Google Scholar]
- Halpern J. H. (2004) Hallucinogens and dissociative agents naturally growing in the United States. Pharmacol. Ther. 102, 131–138. [DOI] [PubMed] [Google Scholar]
- Roth B. L.; Baner K.; Westkaemper R.; Siebert D.; Rice K. C.; Steinberg S.; Ernsberger P.; Rothman R. B. (2002) Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc. Natl. Acad. Sci. U.S.A. 99, 11934–11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman R. J.; Qualls C. R.; Uhlenhuth E. H.; Kellner R. (1994) Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch. Gen. Psychiatry 51, 98–108. [DOI] [PubMed] [Google Scholar]
- Studerus E.; Gamma A.; Kometer M.; Vollenweider F. X. (2012) Prediction of psilocybin response in healthy volunteers. PloS One 7, e30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E.; Gamma A.; Vollenweider F. X. (2010) Psychometric evaluation of the altered states of consciousness rating scale (OAV). PloS One 5, e12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein H. (2005) Hallucinogen actions on 5-HT receptors reveal distinct mechanisms of activation and signaling by G protein-coupled receptors. AAPS J. 7, E871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D. E.; Nichols C. D. (2008) Serotonin receptors. Chem. Rev. 108, 1614–1641. [DOI] [PubMed] [Google Scholar]
- Aghajanian G. K. (2009) Modeling ″psychosis″ in vitro by inducing disordered neuronal network activity in cortical brain slices. Psychopharmacology (Berlin, Ger.) 206, 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel J. B.; West W. B.; Buggy J. (2004) LSD, 5-HT (serotonin), and the evolution of a behavioral assay. Neurosci. Biobehav. Rev. 27, 693–701. [DOI] [PubMed] [Google Scholar]
- Fantegrossi W. E.; Murnane K. S.; Reissig C. J. (2008) The behavioral pharmacology of hallucinogens. Biochem. Pharmacol. 75, 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse R. M.; Berridge K. C. (1997) Psychoactive drug use in evolutionary perspective. Science 278, 63–66. [DOI] [PubMed] [Google Scholar]
- Vollenweider F. X.; Kometer M. (2010) The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat. Rev. Neurosci. 11, 642–651. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J.; Sealfon S. C. (2009) Psychedelics and schizophrenia. Trends Neurosci. 32, 225–232. [DOI] [PubMed] [Google Scholar]
- Geyer M. A.; Vollenweider F. X. (2008) Serotonin research: contributions to understanding psychoses. Trends Pharmacol. Sci. 29, 445–453. [DOI] [PubMed] [Google Scholar]
- Dittrich A. (1998) The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry 31(Suppl 2), 80–84. [DOI] [PubMed] [Google Scholar]
- Crawley J. N. (2007) What’s Wrong With My Mouse?: Behavioral Phenotyping of Transgenic and Knockout Mice, 2nd ed., Wiley, John & Sons. [Google Scholar]
- Gonzalez-Maeso J.; Weisstaub N. V.; Zhou M.; Chan P.; Ivic L.; Ang R.; Lira A.; Bradley-Moore M.; Ge Y.; Zhou Q.; Sealfon S. C.; Gingrich J. A. (2007) Hallucinogens Recruit Specific Cortical 5-HT(2A) Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 53, 439–452. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J.; Yuen T.; Ebersole B. J.; Wurmbach E.; Lira A.; Zhou M.; Weisstaub N.; Hen R.; Gingrich J. A.; Sealfon S. C. (2003) Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J. Neurosci. 23, 8836–8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. R.; Wikler A.; Eades C. G.; Pescor F. T. (1963) Tolerance to and Physical Dependence on Morphine in Rats. Psychopharmacologia 4, 247–260. [DOI] [PubMed] [Google Scholar]
- Woolley D. W. (1955) Production of Abnormal (Psychotic?) Behavior in Mice with Lysergic Acid Diethylamide, and Its Partial Prevention with Cholinergic Drugs and Serotonin. Proc. Natl. Acad. Sci. U.S.A. 41, 338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller D. L.; Umbreit W. W. (1956) Permanent alteration of behavior in mice by chemical and psychological means. Science 124, 723–724. [DOI] [PubMed] [Google Scholar]
- Corne S. J.; Pickering R. W.; Warner B. T. (1963) A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Br. J. Pharmacol. Chemother. 20, 106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T.; Calil H. M. (1975) Screening hallucinogenic drugs: systematic study of three behavioral tests. Psychopharmacologia 42, 163–171. [DOI] [PubMed] [Google Scholar]
- Yamamoto T.; Ueki S. (1981) The role of central serotonergic mechanisms on head-twitch and backward locomotion induced by hallucinogenic drugs. Pharmacol., Biochem. Behav. 14, 89–95. [DOI] [PubMed] [Google Scholar]
- Fantegrossi W. E.; Harrington A. W.; Eckler J. R.; Arshad S.; Rabin R. A.; Winter J. C.; Coop A.; Rice K. C.; Woods J. H. (2005) Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology (Berlin, Ger.) 181, 496–503. [DOI] [PubMed] [Google Scholar]
- Fantegrossi W. E.; Reissig C. J.; Katz E. B.; Yarosh H. L.; Rice K. C.; Winter J. C. (2008) Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol., Biochem. Behav. 88, 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A.; Kobilka B. K.; Strader D. J.; Benovic J. L.; Dohlman H. G.; Frielle T.; Bolanowski M. A.; Bennett C. D.; Rands E.; Diehl R. E.; et al. (1986) Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature 321, 75–79. [DOI] [PubMed] [Google Scholar]
- Fargin A.; Raymond J. R.; Lohse M. J.; Kobilka B. K.; Caron M. G.; Lefkowitz R. J. (1988) The genomic clone G-21 which resembles a beta-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature 335, 358–360. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B.; Bach A. W.; Wozny M.; Taleb O.; Dal Toso R.; Shih J. C.; Seeburg P. H. (1988) Structure and functional expression of cloned rat serotonin 5HT-2 receptor. EMBO J. 7, 4135–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malick J. B.; Doren E.; Barnett A. (1977) Quipazine-induced head-twitch in mice. Pharmacol., Biochem. Behav. 6, 325–329. [DOI] [PubMed] [Google Scholar]
- Maj J.; Sowinska H.; Baran L.; Gancarczyk L.; Rawlow A. (1978) The central antiserotonergic action of mianserin. Psychopharmacology (Berlin, Ger.) 59, 79–84. [DOI] [PubMed] [Google Scholar]
- McKenna D. J.; Peroutka S. J. (1989) Differentiation of 5-hydroxytryptamine2 receptor subtypes using 125I-R-(−)2,5-dimethoxy-4-iodo-phenylisopropylamine and 3H-ketanserin. J. Neurosci. 9, 3482–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchek T.; Adham N.; Macchi M.; Kao H. T.; Hartig P. R. (1990) [3H]-DOB(4-bromo-2,5-dimethoxyphenylisopropylamine) and [3H] ketanserin label two affinity states of the cloned human 5-hydroxytryptamine2 receptor. Mol. Pharmacol. 38, 604–609. [PubMed] [Google Scholar]
- Colpaert F. C.; Janssen P. A. (1983) The head-twitch response to intraperitoneal injection of 5-hydroxytryptophan in the rat: antagonist effects of purported 5-hydroxytryptamine antagonists and of pirenperone, an LSD antagonist. Neuropharmacology 22, 993–1000. [DOI] [PubMed] [Google Scholar]
- Schmid C. L.; Raehal K. M.; Bohn L. M. (2008) Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc. Natl. Acad. Sci. U.S.A. 105, 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins D. L.; Meltzer H. Y. (1997) Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J. Pharmacol. Exp. Ther. 282, 699–706. [PubMed] [Google Scholar]
- Keiser M. J.; Setola V.; Irwin J. J.; Laggner C.; Abbas A. I.; Hufeisen S. J.; Jensen N. H.; Kuijer M. B.; Matos R. C.; Tran T. B.; Whaley R.; Glennon R. A.; Hert J.; Thomas K. L.; Edwards D. D.; Shoichet B. K.; Roth B. L. (2009) Predicting new molecular targets for known drugs. Nature 462, 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi W. E.; Simoneau J.; Cohen M. S.; Zimmerman S. M.; Henson C. M.; Rice K. C.; Woods J. H. (2010) Interaction of 5-HT2A and 5-HT2C receptors in R(-)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J .Pharmacol. Exp. Ther. 335, 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D.; Clarke D. E.; Fozard J. R.; Hartig P. R.; Martin G. R.; Mylecharane E. J.; Saxena P. R.; Humphrey P. P. (1994) International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 46, 157–203. [PubMed] [Google Scholar]
- Canal C. E.; Olaghere da Silva U. B.; Gresch P. J.; Watt E. E.; Sanders-Bush E.; Airey D. C. (2010) The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology (Berlin, Ger.) 209, 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani N. a.; Pandya D. K. (2000) Involvement of other neurotransmitters in behaviors induced by the cannabinoid CB1 receptor antagonist SR 141716A in naive mice. J. Neural Transm. 107, 931–945. [DOI] [PubMed] [Google Scholar]
- Darmani N. A.; Janoyan J. J.; Kumar N.; Crim J. L. (2003) Behaviorally active doses of the CB1 receptor antagonist SR 141716A increase brain serotonin and dopamine levels and turnover. Pharmacol., Biochem. Behav. 75, 777–787. [DOI] [PubMed] [Google Scholar]
- Matsumoto K.; Mizowaki M.; Thongpraditchote S.; Murakami Y.; Watanabe H. (1997) alpha2-Adrenoceptor antagonists reverse the 5-HT2 receptor antagonist suppression of head-twitch behavior in mice. Pharmacol., Biochem. Behav. 56, 417–422. [DOI] [PubMed] [Google Scholar]
- Mato S.; Aso E.; Castro E.; Martín M.; Valverde O.; Maldonado R.; Pazos A. (2007) CB1 knockout mice display impaired functionality of 5-HT1A and 5-HT2A/C receptors. J. Neurochem. 103, 2111–2120. [DOI] [PubMed] [Google Scholar]
- Darmani N. A. (2001) Cannabinoids of diverse structure inhibit two DOI-induced 5-HT(2A) receptor-mediated behaviors in mice. Pharmacol., Biochem. Behav. 68, 311–317. [DOI] [PubMed] [Google Scholar]
- Moreno J. L.; Holloway T.; Albizu L.; Sealfon S. C.; Gonzalez-Maeso J. (2011) Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci. Lett. 493, 76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J.; Ang R. L.; Yuen T.; Chan P.; Weisstaub N. V.; Lopez-Gimenez J. F.; Zhou M.; Okawa Y.; Callado L. F.; Milligan G.; Gingrich J. A.; Filizola M.; Meana J. J.; Sealfon S. C. (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fribourg M.; Moreno J. L.; Holloway T.; Provasi D.; Baki L.; Mahajan R.; Park G.; Adney S. K.; Hatcher C.; Eltit J. M.; Ruta J. D.; Albizu L.; Li Z.; Umali A.; Shim J.; Fabiato A.; Mackerell A. D. Jr.; Brezina V.; Sealfon S. C.; Filizola M.; Gonzalez-Maeso J.; Logothetis D. E. (2011) Decoding the Signaling of a GPCR Heteromeric Complex Reveals a Unifying Mechanism of Action of Antipsychotic Drugs. Cell 147, 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorella D.; Rabin R. A.; Winter J. C. (1995) Role of 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. II: Reassessment of LSD false positives. Psychopharmacology (Berlin, Ger.) 121, 357–363. [DOI] [PubMed] [Google Scholar]
- Fiorella D.; Rabin R. A.; Winter J. C. (1995) The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. I: Antagonist correlation analysis. Psychopharmacology (Berlin, Ger.) 121, 347–356. [DOI] [PubMed] [Google Scholar]
- Winter J. C.; Fiorella D. J.; Timineri D. M.; Filipink R. A.; Helsley S. E.; Rabin R. A. (1999) Serotonergic receptor subtypes and hallucinogen-induced stimulus control. Pharmacol., Biochem. Behav. 64, 283–293. [DOI] [PubMed] [Google Scholar]
- Winter J. C.; Kieres A. K.; Zimmerman M. D.; Reissig C. J.; Eckler J. R.; Ullrich T.; Rice K. C.; Rabin R. A.; Richards J. B. (2005) The stimulus properties of LSD in C57BL/6 mice. Pharmacol., Biochem. Behav. 81, 830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L.; Barrett R. J.; Sanders-Bush E. (2003) Discriminative stimulus properties of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [(±)DOI] in C57BL/6J mice. Psychopharmacology (Berlin, Ger.) 166, 61–68. [DOI] [PubMed] [Google Scholar]
- Doat M. M.; Rabin R. A.; Winter J. C. (2003) Characterization of the discriminative stimulus properties of centrally administered (−)-DOM and LSD. Pharmacol., Biochem. Behav. 74, 713–721. [DOI] [PubMed] [Google Scholar]
- Gresch P. J.; Barrett R. J.; Sanders-Bush E.; Smith R. L. (2007) 5-Hydroxytryptamine (serotonin)2A receptors in rat anterior cingulate cortex mediate the discriminative stimulus properties of d-lysergic acid diethylamide. J. Pharmacol. Exp. Ther. 320, 662–669. [DOI] [PubMed] [Google Scholar]
- Cameron O. G.; Appel J. B. (1973) A behavioral and pharmacological analysis of some discriminable properties of d-LSD in rats. Psychopharmacologia. 33, 117–34. [DOI] [PubMed] [Google Scholar]
- Appel J. B.; West W. B.; Rolandi W. G.; Alici T.; Pechersky K. (1999) Increasing the selectivity of drug discrimination procedures. Pharmacol., Biochem. Behav. 64, 353–358. [DOI] [PubMed] [Google Scholar]
- Killinger B. A.; Peet M. M.; Baker L. E. (2010) Salvinorin A fails to substitute for the discriminative stimulus effects of LSD or ketamine in Sprague-Dawley rats. Pharmacol., Biochem. Behav. 96, 260–265. [DOI] [PubMed] [Google Scholar]
- Winter J. C. (1975) The effects of 2,5-dimethoxy-4-methylamphetamine (DOM), 2,5-dimethoxy-4-ethylamphetamine (DOET), d-amphetamine, and cocaine in rats trained with mescaline as a discriminative stimulus. Psychopharmacologia 44, 29–32. [DOI] [PubMed] [Google Scholar]
- Crawley J. N. (2007) Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 17, 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commissaris R.; Lyness W. H.; Cordon J. J.; Moore K. E.; Rech R. H. (1980) The effects of d-lysergic acid diethylamide (LSD), 2,5-dimethoxy-4-methylamphetamine (DOM) and d-amphetamine on operant responding in control and 6-hydroxydopamine-treated rats. Pharmacol., Biochem. Behav. 13, 621–626. [DOI] [PubMed] [Google Scholar]
- Commissaris R. L.; Lyness W. H.; Moore K. E.; Rech R. H. (1980) Enhancement of the behavioral effects of 2,5-dimethoxy-4-methyl-amphetamine (DOM) by pretreatment with p-chlorophenylalanine. Pharmacol., Biochem. Behav. 13, 605–608. [DOI] [PubMed] [Google Scholar]
- Mokler D. J.; Rech R. H. (1984) Behavioral effects of intracerebroventricular administration of LSD, DOM, mescaline or lisuride. Pharmacol., Biochem. Behav. 21, 281–287. [DOI] [PubMed] [Google Scholar]
- Commissaris R. L.; Lyness W. H.; Moore K. E.; Rech R. H. (1981) Central 5-hydroxytryptamine and the effects of hallucinogens and phenobarbital on operant responding in rats. Pharmacol., Biochem. Behav. 14, 595–601. [DOI] [PubMed] [Google Scholar]
- Thompson D. M.; Winsauer P. J.; Mastropaolo J. (1987) Effects of phencyclidine, ketamine and MDMA on complex operant behavior in monkeys. Pharmacol., Biochem. Behav. 26, 401–405. [DOI] [PubMed] [Google Scholar]
- Mokler D. J.; Stoudt K. W.; Rech R. H. (1985) The 5HT2 antagonist pirenperone reverses disruption of FR-40 by hallucinogenic drugs. Pharmacol., Biochem. Behav. 22, 677–682. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K.; Paulus M. P.; Geyer M. A. (1998) Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology 18, 339–351. [DOI] [PubMed] [Google Scholar]
- Adams L. M.; Geyer M. A. (1985) Patterns of exploration in rats distinguish lisuride from lysergic acid diethylamide. Pharmacol., Biochem. Behav. 23, 461–468. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L.; van der Heijden I.; Ruderman M. A.; Risbrough V. B.; Gingrich J. A.; Geyer M. A.; Powell S. B. (2009) 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology 34, 1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninan I.; Kulkarni S. K. (1998) 5-HT2A receptor antagonists block MK-801-induced stereotypy and hyperlocomotion. Eur. J. Pharmacol. 358, 111–116. [DOI] [PubMed] [Google Scholar]
- O’Neill M. F.; Heron-Maxwell C. L.; Shaw G. (1999) 5-HT2 receptor antagonism reduces hyperactivity induced by amphetamine, cocaine, and MK-801 but not D1 agonist C-APB. Pharmacol., Biochem. Behav. 63, 237–243. [DOI] [PubMed] [Google Scholar]
- Meltzer H.; Massey B. (2011) The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr. Opin. Pharmacol. 11, 59–67. [DOI] [PubMed] [Google Scholar]
- Steed E.; Jones C. A.; McCreary A. C. (2011) Serotonergic involvement in methamphetamine-induced locomotor activity: a detailed pharmacological study. Behav. Brain Res. 220, 9–19. [DOI] [PubMed] [Google Scholar]
- Williams A. A.; Ingram W. M.; Levine S.; Resnik J.; Kamel C. M.; Lish J. R.; Elizalde D. I.; Janowski S. A.; Shoker J.; Kozlenkov A.; Gonzalez-Maeso J.; Gallitano A. L. (XXXX) Reduced Levels of Serotonin 2A Receptors Underlie Resistance of Egr3-Deficient Mice to Locomotor Suppression by Clozapine. Neuropsychopharmacology X, xxx–xxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McOmish C. E.; Lira A.; Hanks J. B.; Gingrich J. A. (XXXX) Clozapine-Induced Locomotor Suppression is Mediated by 5-HT(2A) Receptors in the Forebrain. Neuropsychopharmacology X, xxx–xxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S. B., and Geyer M. A. (2007) Overview of animal models of schizophrenia. Current Protocols in Neuroscience, Chapter 9, Unit 9 24. [DOI] [PubMed] [Google Scholar]
- Vollenweider F. X.; Csomor P. A.; Knappe B.; Geyer M. A.; Quednow B. B. (2007) The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology 32, 1876–1887. [DOI] [PubMed] [Google Scholar]
- Ouagazzal A.; Grottick A. J.; Moreau J.; Higgins G. A. (2001) Effect of LSD on prepulse inhibition and spontaneous behavior in the rat. A pharmacological analysis and comparison between two rat strains. Neuropsychopharmacology 25, 565–575. [DOI] [PubMed] [Google Scholar]
- Wischhof L.; Aho H. E. A.; Koch M. (2012) DOI-induced deficits in prepulse inhibition in Wistar rats are reversed by mGlu2/3 receptor stimulation. Pharmacology, biochemistry, and behavior. 102, 6–12. [DOI] [PubMed] [Google Scholar]
- Farid M.; Martinez Z. A.; Geyer M. A.; Swerdlow N. R. (2000) Regulation of sensorimotor gating of the startle reflex by serotonin 2A receptors. Ontogeny and strain differences. Neuropsychopharmacology 23, 623–632. [DOI] [PubMed] [Google Scholar]
- Brosda J.; Hayn L.; Klein C.; Koch M.; Meyer C.; Schallhorn R.; Wegener N. (2011) Pharmacological and parametrical investigation of prepulse inhibition of startle and prepulse elicited reactions in Wistar rats. Pharmacol., Biochem. Behav. 99, 22–28. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L.; Geyer M. A. (2010) LSD but not lisuride disrupts prepulse inhibition in rats by activating the 5-HT(2A) receptor. Psychopharmacology 208, 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R.; Melon C.; De Vry J. (1998) The role of 5-HT receptor subtypes in the anxiolytic effects of selective serotonin reuptake inhibitors in the rat ultrasonic vocalization test. Psychopharmacology 135, 383–391. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha B. A.; Bourin M.; Hascoët M. (2003) Anxiolytic-like effects of 5-HT2 ligands on three mouse models of anxiety. Behav. Brain Res. 140, 203–214. [DOI] [PubMed] [Google Scholar]
- Winslow J. T.; Insel T. R. (1991) Serotonergic modulation of the rat pup ultrasonic isolation call: studies with 5HT1 and 5HT2 subtype-selective agonists and antagonists. Psychopharmacologia 105, 513–520. [DOI] [PubMed] [Google Scholar]
- Griffiths R. R.; Johnson M. W.; Richards W. A.; Richards B. D.; McCann U.; Jesse R. (2011) Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology 218, 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins M. F.; Uzelac S. M.; Baumeister A. a.; Hearn J. K.; Broussard J. I.; Guillot T. S. (2002) Behavioral responses to stress following central and peripheral injection of the 5-HT(2) agonist DOI. Pharmacol., Biochem. Behav. 73, 537–544. [DOI] [PubMed] [Google Scholar]
- Magalhaes A. C.; Holmes K. D.; Dale L. B.; Comps-Agrar L.; Lee D.; Yadav P. N.; Drysdale L.; Poulter M. O.; Roth B. L.; Pin J.-P.; Anisman H.; Ferguson S. S. G. (2010) CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nature Neurosci. 13, 622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisstaub N. V.; Zhou M.; Lira A.; Lambe E.; Gonzalez-Maeso J.; Hornung J. P.; Sibille E.; Underwood M.; Itohara S.; Dauer W. T.; Ansorge M. S.; Morelli E.; Mann J. J.; Toth M.; Aghajanian G.; Sealfon S. C.; Hen R.; Gingrich J. A. (2006) Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313, 536–540. [DOI] [PubMed] [Google Scholar]
- de Oliveira Sergio T.; de Bortoli V. C.; Zangrossi H. Jr. (2011) Serotonin-2A receptor regulation of panic-like behavior in the rat dorsal periaqueductal gray matter: the role of GABA. Psychopharmacology (Berlin, Ger.) 218, 725–732. [DOI] [PubMed] [Google Scholar]
- Winstanley C. A. (2011) The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br. J. Pharmacol. 164, 1301–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadamitzky M.; Koch M. (2009) Effects of acute intra-cerebral administration of the 5-HT(2A/C) receptor ligands DOI and ketanserin on impulse control in rats. Behav. Brain Res. 204, 88–92. [DOI] [PubMed] [Google Scholar]
- Wischhof L.; Hollensteiner K. J.; Koch M. (2011) Impulsive behaviour in rats induced by intracortical DOI infusions is antagonized by co-administration of an mGlu2/3 receptor agonist. Behav. Pharmacol. 22, 805–813. [DOI] [PubMed] [Google Scholar]
- Koskinen T.; Ruotsalainen S.; Puumala T.; Lappalainen R.; Koivisto E.; Männistö P. T.; Sirviö J. (2000) Activation of 5-HT2A receptors impairs response control of rats in a five-choice serial reaction time task. Neuropharmacology 39, 471–481. [DOI] [PubMed] [Google Scholar]
- Matell M. S.; Meck W. H. (2004) Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Brain research. Cognitive Brain Res. 21, 139–170. [DOI] [PubMed] [Google Scholar]
- Body S.; Cheung T. H. C.; Bezzina G.; Asgari K.; Fone K. C. F.; Glennon J. C.; Bradshaw C. M.; Szabadi E. (2006) Effects of d-amphetamine and DOI (2,5-dimethoxy-4-iodoamphetamine) on timing behavior: interaction between D1 and 5-HT2A receptors. Psychopharmacology 189, 331–343. [DOI] [PubMed] [Google Scholar]
- Hampson C. L.; Body S.; den Boon F. S.; Cheung T. H. C.; Bezzina G.; Langley R. W.; Fone K. C. F.; Bradshaw C. M.; Szabadi E. (2010) Comparison of the effects of 2,5-dimethoxy-4-iodoamphetamine and D-amphetamine on the ability of rats to discriminate the durations and intensities of light stimuli. Behav. Pharmacol. 21, 11–20. [DOI] [PubMed] [Google Scholar]
- MacDonald C. J.; Meck W. H. (2005) Differential effects of clozapine and haloperidol on interval timing in the supraseconds range. Psychopharmacologia 182, 232–244. [DOI] [PubMed] [Google Scholar]
- Miller J. P.; McAuley J. D.; Pang K. C. H. (2006) Effects of the NMDA receptor antagonist MK-801 on short-interval timing in rats. Behav. Neurosci. 120, 162–172. [DOI] [PubMed] [Google Scholar]
- Cheng R.-K.; MacDonald C. J.; Meck W. H. (2006) Differential effects of cocaine and ketamine on time estimation: implications for neurobiological models of interval timing. Pharmacol., Biochem. Behav. 85, 114–122. [DOI] [PubMed] [Google Scholar]
- Wittmann M.; Carter O.; Hasler F.; Cahn B. R.; Grimberg U.; Spring P.; Hell D.; Flohr H.; Vollenweider F. X. (2007) Effects of psilocybin on time perception and temporal control of behaviour in humans. J. Psychopharmacol. (Oxford, U.K.) 21, 50–64. [DOI] [PubMed] [Google Scholar]
- Harvey J. A. (1996) Serotonergic regulation of associative learning. Behav. Brain Res. 73, 47–50. [DOI] [PubMed] [Google Scholar]
- Welsh S. E.; Romano A. G.; Harvey J. A. (1998) Effects of serotonin 5-HT(2A/2C) antagonists on associative learning in the rabbit. Psychopharmacologia 137, 157–163. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Asgeirsdóttir H. N.; Cohen S. J.; Munchow A. H.; Barrera M. P.; Stackman R. W.. Stimulation of serotonin 2A receptors facilitates consolidation and extinction of fear memory in C57BL/6J mice. Neuropharmacology 2012, not supplied. [DOI] [PMC free article] [PubMed]
- McLean T. H.; Parrish J. C.; Braden M. R.; Marona-Lewicka D.; Gallardo-Godoy A.; Nichols D. E. (2006) 1-Aminomethylbenzocycloalkanes: conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists. J. Med. Chem. 49, 5794–5803. [DOI] [PubMed] [Google Scholar]
- Li J.-x.; Rice K. C.; France C. P. (2008) Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane in rhesus monkeys. J. Pharmacol. Exp. Ther. 324, 827–833. [DOI] [PubMed] [Google Scholar]
- White F. J.; Appel J. B. (1982) The role of dopamine and serotonin in the discriminative stimulus effects of lisuride. J. Pharmacol Exp. Ther. 221, 421–427. [PubMed] [Google Scholar]
- Rech R. H.; Commissaris R. L. (1982) Neurotransmitter basis of the behavioral effects of hallucinogens. Neurosci. Biobehav. Rev. 6, 521–527. [DOI] [PubMed] [Google Scholar]
- Leriche L.; Schwartz J.-C.; Sokoloff P. (2003) The dopamine D3 receptor mediates locomotor hyperactivity induced by NMDA receptor blockade. Neuropharmacology 45, 174–181. [DOI] [PubMed] [Google Scholar]
- Ma J.; Leung L. S. (2007) The supramammillo-septal-hippocampal pathway mediates sensorimotor gating impairment and hyperlocomotion induced by MK-801 and ketamine in rats. Psychopharmacology 191, 961–974. [DOI] [PubMed] [Google Scholar]
- Morita T.; Hitomi S.; Saito S.; Fujita T.; Uchihashi Y.; Kuribara H. (1995) Repeated ketamine administration produces up-regulation of muscarinic acetylcholine receptors in the forebrain, and reduces behavioral sensitivity to scopolamine in mice. Psychopharmacologia 117, 396–402. [DOI] [PubMed] [Google Scholar]
- Ukai M.; Kameyama T. (1985) Multi-dimensional analyses of behavior in mice treated with U-50,488H, a purported kappa (non-mu) opioid agonist. Brain Res. 337, 352–356. [DOI] [PubMed] [Google Scholar]
- Sadalge A.; Coughlin L.; Fu H.; Wang B.; Valladares O.; Valentino R.; Blendy J. A. (2003) alpha 1d Adrenoceptor signaling is required for stimulus induced locomotor activity. Mol. Psychiatry 8, 664–672. [DOI] [PubMed] [Google Scholar]
- Gould T. D.; O’Donnell K. C.; Picchini A. M.; Manji H. K. (2007) Strain differences in lithium attenuation of d-amphetamine-induced hyperlocomotion: a mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology 32, 1321–1333. [DOI] [PubMed] [Google Scholar]
- Varty G. B.; Bakshi V. P.; Geyer M. A. (1999) M100907, a serotonin 5-HT2A receptor antagonist and putative antipsychotic, blocks dizocilpine-induced prepulse inhibition deficits in Sprague-Dawley and Wistar rats. Neuropsychopharmacology 20, 311–321. [DOI] [PubMed] [Google Scholar]
- Singer P.; Yee B. K. (2012) Reversal of scopolamine-induced disruption of prepulse inhibition by clozapine in mice. Pharmacol., Biochem. Behav. 101, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M.; Aru G. N.; Frau R.; Orrù M.; Fà M.; Manunta M.; Puddu M.; Mereu G.; Gessa G. L. (2005) Kappa opioid receptor activation disrupts prepulse inhibition of the acoustic startle in rats. Biol. Psychiatry 57, 1550–1558. [DOI] [PubMed] [Google Scholar]
- Tejeda H. a.; Chefer V. I.; Zapata A.; Shippenberg T. S. (2010) The effects of kappa-opioid receptor ligands on prepulse inhibition and CRF-induced prepulse inhibition deficits in the rat. Psychopharmacology 210, 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney G. G.; Burno M.; Campbell U. C.; Hernandez L. M.; Rodriguez D.; Bristow L. J.; Conn P. J. (2003) Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J. Pharmacol. Exp. Ther. 306, 116–123. [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R.; Stephany N.; Wasserman L. C.; Talledo J.; Shoemaker J.; Auerbach P. P. (2003) Amphetamine effects on prepulse inhibition across-species: replication and parametric extension. Neuropsychopharmacology 28, 640–650. [DOI] [PubMed] [Google Scholar]
- Gomes K. S.; Nunes-De-Souza R. L. (2009) Implication of the 5-HT2A and 5-HT2C (but not 5HT1A) receptors located within the periaqueductal gray in the elevated plus-maze test-retest paradigm in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 33, 1261–1269. [DOI] [PubMed] [Google Scholar]
- Zarrindast M. R.; Nasehi M.; Piri M.; Heidari N. (2011) Effects of cholinergic system of dorsal hippocampus of rats on MK-801 induced anxiolytic-like behavior. Neurosci. Lett. 505, 65–70. [DOI] [PubMed] [Google Scholar]
- Rodgers R. J.; Cole J. C. (1995) Effects of scopolamine and its quaternary analogue in the murine elevated plus-maze test of anxiety. Behav. Pharmacol. 6, 283–289. [PubMed] [Google Scholar]
- Braida D.; Capurro V.; Zani A.; Rubino T.; Viganò D.; Parolaro D.; Sala M. (2009) Potential anxiolytic- and antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br. J. Pharmacol. 157, 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biała G.; Kruk M. (2007) Amphetamine-induced anxiety-related behavior in animal models. Pharmacol. Rep. 59, 636–644. [PubMed] [Google Scholar]
- Weiss S. M.; Wadsworth G.; Fletcher A.; Dourish C. T. (1998) Utility of ethological analysis to overcome locomotor confounds in elevated maze models of anxiety. Neurosci. Biobehav. Rev. 23, 265–271. [DOI] [PubMed] [Google Scholar]
- Koskinen T.; Sirviö J. (2001) Studies on the involvement of the dopaminergic system in the 5-HT2 agonist (DOI)-induced premature responding in a five-choice serial reaction time task. Brain Res. Bull. 54, 65–75. [DOI] [PubMed] [Google Scholar]
- Koskinen T.; Ruotsalainen S.; Sirviö J. (2000) The 5-HT(2) receptor activation enhances impulsive responding without increasing motor activity in rats. Pharmacol., Biochem. Behav. 66, 729–738. [DOI] [PubMed] [Google Scholar]
- Higgins G. a.; Enderlin M.; Haman M.; Fletcher P. J. (2003) The 5-HT2A receptor antagonist M100,907 attenuates motor and ’impulsive-type’ behaviours produced by NMDA receptor antagonism. Psychopharmacology 170, 309–319. [DOI] [PubMed] [Google Scholar]
- Fletcher P. J.; Rizos Z.; Noble K.; Higgins G. a. (2011) Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5-HT(2C) receptor stimulation and 5-HT(2A) receptor blockade. Neuropharmacology 61, 468–477. [DOI] [PubMed] [Google Scholar]
- Mendez I. A.; Gilbert R. J.; Bizon J. L.; Setlow B.. Effects of acute administration of nicotinic and muscarinic cholinergic agonists and antagonists on performance in different cost-benefit decision making tasks in rats. Psychopharmacology 2012, not supplied. [DOI] [PMC free article] [PubMed]
- Hayton S. J.; Maracle A. C.; Olmstead M. C. (2012) Opposite effects of amphetamine on impulsive action with fixed and variable delays to respond. Neuropsychopharmacology 37, 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balci F.; Ludvig E. A.; Gibson J. M.; Allen B. D.; Frank K. M.; Kapustinski B. J.; Fedolak T. E.; Brunner D. (2008) Pharmacological manipulations of interval timing using the peak procedure in male C3H mice. Psychopharmacologia 201, 67–80. [DOI] [PubMed] [Google Scholar]
- Bolton M. M.; Heaney C. F.; Sabbagh J. J.; Murtishaw A. S.; Magcalas C. M.; Kinney J. W. (2012) Deficits in emotional learning and memory in an animal model of schizophrenia. Behav. Brain Res. 233, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy J. W. (1996) Scopolamine administered before and after training impairs both contextual and auditory-cue fear conditioning. Neurobiol. Learn. Mem. 65, 73–81. [DOI] [PubMed] [Google Scholar]
- Wood S. C.; Anagnostaras S. G. (2009) Memory and psychostimulants: modulation of Pavlovian fear conditioning by amphetamine in C57BL/6 mice. Psychopharmacology 202, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. Y. M.; McNally G. P. (2009) Conditioned stimulus familiarity determines effects of MK-801 on fear extinction. Behav. Neurosci. 123, 303–314. [DOI] [PubMed] [Google Scholar]
- Santini E.; Sepulveda-Orengo M.; Porter J. T. (2012) Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction. Neuropsychopharmacology 37, 2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmack S. A.; Wood S. C.; Anagnostaras S. G. (2010) Amphetamine and extinction of cued fear. Neurosci. Lett. 468, 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]