Abstract
Neuropsychiatric disorders have long been linked to both immune system activation and alterations in serotonin (5-HT) signaling. In the CNS, the contributions of 5-HT modulate a broad range of targets, most notably, hypothalamic, limbic and cortical circuits linked to the control of mood and mood disorders. In the periphery, many are aware of the production and actions of 5-HT in the gut but are unaware that the molecule and its receptors are also present in the immune system where evidence suggests they contribute to the both innate and adaptive responses. In addition, there is clear evidence that the immune system communicates to the brain via both humoral and neuronal mechanisms, and that CNS 5-HT neurons are a direct or indirect target for these actions. Following a brief primer on the immune system, we describe our current understanding of the synthesis, release, and actions of 5-HT in modulating immune function, including the expression of 5-HT biosynthetic enzymes, receptors, and transporters that are typically studied with respect to the roles in the CNS. We then orient our presentation to recent findings that pro-inflammatory cytokines can modulate CNS 5-HT signaling, leading to a conceptualization that among the many roles of 5-HT in the body is an integrated physiological and behavioral response to inflammatory events and pathogens. From this perspective, altered 5-HT/immune conversations are likely to contribute to risk for neurobehavioral disorders historically linked to compromised 5-HT function or ameliorated by 5-HT targeted medications, including depression and anxiety disorders, obsessive-compulsive disorder (OCD), and autism. Our review raises the question as to whether genetic variation impacting 5-HT signaling genes may contribute to maladaptive behavior as much through perturbed immune system modulation as through altered brain mechanisms. Conversely, targeting the immune system for therapeutic development may provide an important opportunity to treat mental illness.
Keywords: Serotonin, serotonin transporter, immune system, P38 MAPK, interleukin-1 beta, depression
The monoamine neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) regulates many fundamental aspects of physiology and behavior including mood,1 aggression,2,3 sleep,4 appetite,5 pain sensation,6 bone mass,7,8 tissue regeneration,9 platelet coagulation,10,11 and gastrointestinal function.12 In the central nervous system, serotonergic cell bodies are clustered in midbrain and brainstem raphe nuclei from which they project locally, as well as to spinal cord and a large number of forebrain targets.13,14 Not surprisingly, dysregulation of 5-HT signaling has been implicated in multiple neurobehavioral disorders,15 including anxiety, depression, obsessive-compulsive disorder (OCD), and addiction. Understanding the mechanisms by which 5-HT neurotransmission is regulated remains an active area of investigation, particularly with respect to how genetic perturbations interact with the environment to modify complex behaviors and drive disease risk.16,17 Among the genes receiving significant attention in this context, the 5-HT transporter (SERT) gene (SLC6A4) arguably tops the list, driven by the transporter’s key role in the termination of 5-HT signaling.18,19 SERT is antagonized by the 5-HT selective reuptake inhibitor (SSRI) class of antidepressant medications that continue to represent the most common pharmacological treatment for affective disorders.20 SERT also interacts with multiple drugs of abuse, including cocaine21 and 3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”).22 Alterations in SERT gene expression or transport activity have been associated with multiple neuropsychiatric disorders including depression,23,24 anxiety,25 OCD,26,27 alcoholism,28 and autism.29 A more detailed understanding of SERT regulation, particularly in native preparations, is needed to elucidate the contributions of this key regulatory molecule in 5-HT signaling and how its compromised actions contribute to 5-HT linked brain disorders.
Many are unaware that SERT and 5-HT receptors are expressed in the immune system,30,31 likely due to the fact that the function of these proteins in immune signaling remains ill-defined and not yet consistently linked to physiological and behavioral disorders. Nonetheless, biomarkers of immune system dysregulation (e.g., inflammatory cytokine levels) have been found in many subjects with mood disorders and other affective pathologies32 as well as autism,33 and 5-HT can be reliably detected in lymphatic tissue,31 including spleen,34 thymus,35 lymph nodes, and lymphatic fluid.36 These findings dictate that we consider the degree to which genetic, environmental, and pharmacological influences thought to impact brain development, function, and behavior through brain 5-HT signaling actually arise from a perturbation of immune system 5-HT signaling. Additionally, we might ask whether changes in CNS 5-HT signaling can be brought about through primary alterations of immune function. Below we discuss the function of 5-HT in the immune system, and how immune system activation can impact CNS 5-HT signaling, a topic we have been drawn to through our recent findings that CNS SERT can be modulated by the peripheral immune system activation.37
A Brief Primer on the Structure and Function of the Immune System
The complexity of immune system structure and signaling can only be superficially summarized in a brief review. To familiarize the reader with the key components of immune system structure and function that we will discuss in later parts of this review, we present a brief overview of key features. (see ref (38) for a more detailed presentation). Table 1 lists the cells involved in immune responses and summarizes their functions.
Table 1. Cells Involved in Mediating Innate and Adaptive Immunitya.
| cell | immune function | serotonergic phenotype |
|---|---|---|
| innate immunity | ||
| macrophage | phagocytosis; APCs; secrete cytokines, GCSF, MCSF, interferons, TNFα and β, TGFβ | SERT; 5-HT receptors 5-HT1A, 5-HT2, 5-HT2A, 5-HT3, 5-HT4, 5-HT7 |
| mast cell | release heparin, histamine and serotonin; produce leukotrienes, prostaglandins, and PAF | SERT; 5-HT receptors 5-HT1A |
| dendritic cell | express MHC II (APCs) | SERT; 5-HT receptors 5-HT1B, 5-HT1E, 5-HT2A, 5-HT2B, 5-HT3, 5-HT4, 5-HT7 |
| basophil | release heparin, histamine and serotonin | SERT? |
| neutrophil | phagocytosis | SERT?; 5-HT receptors 5-HT7 |
| eosinophil | phagocytosis; release histaminase and other enzymes involved in allergic and parasitic immune responses | SERT?; 5-HT receptors 5-HT2A |
| natural killer | express MHC I; Apoptosis of “non-self” cells | SERT?; 5-HT receptors 5-HT1A |
| adaptive immunity | ||
| T cells | activation of macrophages; cytotoxicity; memory | 5-HT receptors 5-HT1A, 5-HT1B, 5-HT2, 5-HT3, 5-HT7 |
| B cells | memory; antibody production | SERT; 5-HT receptors 5-HT1A, 5-HT2, 5-HT3, 5-HT7 |
Key: antigen-presenting cells (APCs); granulocyte colony stimulating factor (GCSF); monocyte colony stimulating factor (MCSF); tumor necrosis factor (TNF); transforming growth factor (TGF); platelet activating factor (PAF); major histocompatibility complex (MHC).
Defense against pathogens is mediated by innate and adaptive immune mechanisms that act in the periphery and the CNS.39 Within minutes of injury or pathogen breach of the skin or mucosa, an acute, systemic inflammatory response occurs, mediated by cells of the innate immune system. This response is marked by changes in vascular permeability, migration of immune cells and the elevation of pro- and anti-inflammatory molecules at the site of infection or injury. This “first wave” of innate immune system function involves macrophages, mast cells, and dendritic cells (DCs), that act in the area of infection or injury, leading to the identification and destruction of pathogens. Innate immunity is primarily responsible for recognizing and eradicating “nonself” molecules presented by the pathogen, and is therefore confined to recognizing extracellular pathogens (bacteria vs viruses). This response is nonspecific with respect to particular invaders, and host tissue can be mistakenly destroyed along with pathogens. Innate immunity provides immediate host defense against pathogens via pattern recognition by toll-like receptors (TLRs). Pathogen-associated molecular patterns (PAMPs) on pathogens (e.g., peptidoglycans, bacterial lipopolysaccharides) bind TLRs on antigen-presenting cells (APCs), namely, DCs34 and macrophages. APCs then phagocytize pathogens and display pathogen-derived peptides via the major histocompatibility complex (MHC) on their cell surface for recognition by leukocytes of the “adaptive” immune system (see below). APCs also secrete pro-inflammatory cytokines (e.g., IL1β, IL-6, TNFα), prostaglandins, and histamine, which further activate physiological responses, alerting the body to infection/invasion.38 In addition to cellular protective mechanisms, innate immunity also includes the complement system, which consists of more than 20 glycoproteins, activated in a cascade by foreign substances, antigen–antibody complexes (classical pathway), and Gram-negative bacteria (alternative pathway). This system leads to lysis of cells, increased vascular permeability (allowing antibodies, innate immune cells, and fluid to enter tissue more readily), and chemotaxis. The complement system also helps to activate APCs, namely, follicular DCs and B cells in the specific immune responses. Innate immunity therefore also functions to communicate pathogen presence to cells involved in adaptive immune responses.
The response of a second immune system division, termed the adaptive, or specific, immune system, occurs within hours to days of an infection and involves antigen-specific recognition and destruction of pathogens by T and B lymphocytes. The two components of the adaptive immune system involve cell-mediated and humoral immunity. Cell-mediated immunity is carried out by T cells located in the thymus, lymph nodes, and circulation. APCs that migrate to lymph nodes prime and educate T cells as to the nature of the pathogen. T cells then proliferate and differentiate into either CD4+ T helper inflammatory cells (Th1) that activate macrophages, CD4+ Th2 cells that aid antibody responses, or CD8+ cytotoxic cells that target cells infected with intracellular microbes. It is beyond the scope of the present review to note all of the other various surface antigens (e.g., CD20+, CD3+CD4+, CD3+CD8+, etc.) that T and B cells exhibit as they differentiate and carry out specific immune functions. The reader is referred to excellent texts that provide more in depth descriptions of immune cell types.31,38 The second component of adaptive immunity involves the contributions of B cells, located in lymph tissue, spleen, and in the circulation. Upon stimulation, B cells become plasma cells (with or without the help of Th2),40 that produce and secrete antibodies (immunoglobulins). Memory T and B cells recognize specific antigens and respond quickly if a specific antigen is encountered again. Thus, the adaptive immune is distinguished from the innate immune system by its ability to identify, remember, and eliminate pathogens that have been designated as nonself. Together, the innate and adaptive immune responses work together, but over different time scales, to provide cellular, molecular, and chemical defenses against potentially harmful substances, microbes, and viruses.
The Presence and Role of 5-HT and Its Targets in the Peripheral Immune System
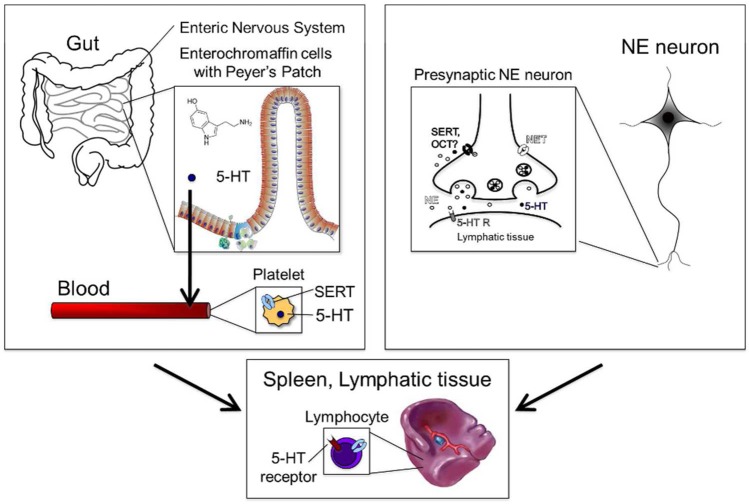
Only about 5% of the body’s 5-HT is produced in the brain,41 and therefore, 5-HT in lymphatic tissue is supplied primarily from peripheral sources (Figure 1). The majority of peripheral 5-HT is found in the gut, where approximately 90% is synthesized by enterochromaffin (EC) cells of the gastrointestinal (GI) tract and 5% by myenteric neurons.42 5-HT released from EC cells into the bloodstream is transported into blood platelets via SERT. Because 5-HT does not cross the BBB, almost all of the 5-HT in the blood is from this GI pathway.43 Platelets carry 5-HT to various tissues and represent the major source of 5-HT for immune cells and lymphatic vessels which express MAOA,44 but not MAOB, for degradation of 5-HT. The blood therefore represents the primary source of 5-HT for lymph tissue, although monocytes,45 mast cells,46 and T cells also appear able to synthesize small amounts of 5-HT.47 In addition to the circulation and immune cells, sympathetic nerves represents another source of 5-HT in lymphatic tissue, which is heavily innervated by sympathetic neurons that appear capable of accumulating 5-HT48,49 and co-releasing norepinephrine (NE) and 5-HT.50−53 Whether accumulation of 5-HT by adrenergic nerve terminals is mediated by SERT remains to be determined. Interestingly though, SERT54 and 5-HT55,56 have been shown to reside with NE in adrenal chromaffin cells, but again the role of these sites of expression has only been superficially explored. Lymphoid tissue levels of 5-HT can reach up to 1 μM, and therefore, clearance of 5-HT in lymphatic tissue by noradrenergic terminals could be mediated by other low-affinity 5-HT transporters, such as the norepinephrine transporter (NET), one or more organic cation transporters (OCT), or the peripheral monoamine transporter (PMAT). Together these findings provide strong evidence for the presence of 5-HT in immune tissues, though certain aspects related to cellular subtypes, releasable pools, and stimuli that evoke release deserve considerably more attention.
Figure 1.
Enterochromaffin cells of the gut produce serotonin (5-HT) and release it into the blood. 5-HT is then taken up by the serotonin transporter (SERT) into platelets. 5-HT can then interact with immune cells in the blood or in the tissue. In lymph tissue, B lymphocytes take up and release 5-HT. 5-HT is also released in lymph tissue by noradrenergic neurons.
Innate (Nonspecific) Immune System
In Table 1, we provide a summary of the reported 5-HT related characteristics, including expression of SERT and/or 5-HT receptors, of immune cells. Caution should be noted, however, with respect to such tabulations, as for some 5-HT receptors, studies suggest that some are expressed by either rodent or human cells, but not both. With respect to 5-HT transport, DCs,34 macrophages,57,58 monocytes,45 and mast cells59 have been reported to take up 5-HT. Mast cells also store 5-HT;60 however, the abundance of 5-HT in mast cells is species-specific. Although rodent mast cells contain 5-HT, human mast cells, which express TPH-1,46 appear to sequester only small amounts of 5-HT with larger amounts of histamine. The presence of 5-HT receptors and the ability of these cells to take up 5-HT suggests that 5-HT influences the activity of the innate immune cells.31 Following injury or release of inflammatory substances, such as C3a, C5a, and IgE complexes, and PAF,61 5-HT is rapidly released from mast cells, basophils, and platelets62,63 and functions in several ways to coordinate the movement and secretion of several cellular cascades involved in carrying out immune responses,30 including inflammation, chemotaxis, and phagocytosis. 5-HT is chemotactic for eosinophils,64 dendritic cells,65 and mast cells,66 attracting cells to sites of inflammation. In mouse and human mast cells in particular, the chemotaxis-promoting effect of 5-HT was lost in 5-HT1A receptor knockout mice, suggesting this effect is mediated by a 5-HT1A receptor pathway.66 The chemotactic properties of human eosinophils and mouse DCs have also been reported to be mediated through 5-HT2A64 and 5-HT1/5-HT265 receptor pathways, respectively. Although 5-HT has not been reported to be chemotactic for macrophages or NK cells, 5-HT regulates several functions of macrophages and NK cells that relate to cell death. For example in macrophages, 5-HT stimulates phagocytosis67 of cells that contained zymosan, a component of yeast cell walls that is used to induced sterile inflammation.68 5-HT also plays a role in enhancing the cytotoxicity of NK cells67,69 cells in blood and lymph that are not phagocytic but promote cell lysis when stimulated. Together, these findings indicate that multiple cells of the native immune system possess the machinery to produce, store, release, and respond to 5-HT, with the overall picture being one to mobilize cells to target to, and destroy, pathogens.
DCs, macrophages, and endo/epithelial cells produce cytokines, which are small molecules that are released in response to immune system activation to orchestrate chemotactic and inflammatory processes. Cytokines are key markers of immune system activation, and there is evidence that multiple cytokines are elevated in patients with mental illness, including major depressive disorder (MDD).70 The bulk of investigations regarding 5-HT and the modulation of cytokine production/release suggest that 5-HT mediates pro-inflammatory responses. For example, 5-HT has been reported to enhance human NK cell production of interferon-gamma (IFN-γ)71 and alter the types of cytokines released by DCs,72 enhancing IL-1β and IL-8 and decreasing IL-12 and TNFα.72 This “cytokine switch” has been shown to alter whether the immune system is stimulated or suppressed.73 5-HT also has been shown to enhance the levels of IL-1α and IL-1β in smooth muscle cells,74 as well as TNFα and IL-6 mRNA expression in hippocampal astrocytes.75 Pro-inflammatory cytokines, including IFN-β1b and IFN-γ (with a synergistic effect of TNF-α76), have been shown to reduce the availability of tryptophan, which is required for 5-HT synthesis, through activation of indoleamine-2,3-dioxygenase (IDO),77−79 a tryptophan-degrading enzyme, and elevate kynurenine metabolites, that can be neurotoxic.80 Changes in tryptophan, through manipulation of IDO, influence brain 5-HT. Upregulation of IDO by IL-6 has been shown to reduce 5-HT/tryptophan ratios, increase kynurenine/tryptophan ratios in hippocampus, and produce a depressive-like effect on behavior that is lost in IDO knockout mice.81
Adaptive (Specific) Immune System
As noted in Table 1, some lymphocytes express SERT61,82 as well as 5-HT receptors. Whereas B lymphocytes express SERT,61,83,84 T lymphocytes do not.34 Possibly, 5-HT uptake in T cells occurs through the dopamine transporter (DAT),34 as this molecule has been reported to be expressed by blood lymphocytes85−87 though the studies cited did not differentiate B and T cells, and DAT is a low-affinity 5-HT uptake mechanism.88,89 Interestingly, T cells have been reported to produce 5-HT.47 5-HT regulates murine T cell function,90 and although there are reports that 5-HT both promotes and inhibits T cell proliferation, depending on the receptor signaling pathway activated, the strongest evidence points to a stimulatory effect of 5-HT on T cell proliferation. Either through an autocrine or paracrine mechanism, 5-HT can stimulate T cells via 5-HT7 receptors, ERK phosphorylation, and NFkB activation. Alternately, 5-HT has been shown to reverse forskolin inhibition of T cell proliferation by decreasing cAMP levels via 5-HT1A receptor activation.91,92 Further complicating this picture, T cell activation leads to expression of 5-HT2A and 5-HT1B receptors.47 Similar to cells of the innate immune system, T helper (Th) cells also secrete cytokines, and one role of 5-HT in the immune response appears to be in modulating the balance of cells promoting the release of pro-inflammatory (Th1) versus anti-inflammatory (Th2) cytokines.93 The actions of 5-HT in the adaptive immune system are not limited to T-cells, as 5-HT, together with platelet-activating factor (PAF), activates B cells94 that both express SERT61,95 and can cross the BBB,96 albeit in very low numbers without damage to this barrier. It is known that activated T cells also cross the BBB,97,98 and therefore, the finding that B cells may cross the BBB suggests that humoral, as well cellular, immune mechanisms may be important in the CNS, which has long been considered to exclude most peripheral immune cells.
In studies of platelets, Walther et al. described a SERT-mediated signaling pathway where transported, intracellular 5-HT is used to “serotonylate” small intracellular GTPases (RhoA and Rab4) via the action of intracellular transglutaminase.99 Because these GTPases are also expressed in lymphocytes and other immune cells, transport of 5-HT and a serotonylation pathway may act intracellularly to affect the secretory functions of lymphocytes.95,100 Schneider and co-workers have also described an intracellular action of 5-HT in reducing IL-4 production mediated by both SERT and the organic cation transporter 3 (OCT3).101 How such mechanisms complement the actions of 5-HT on immune cells via cell-surface receptors is unclear, though the same can be said for platelets. What is clear is that 5-HT exerts significant effects on numerous types of immune cells and processes involved in adaptive immunity. Future studies with methods that allow for the isolation of components of the immune system, for example, using flow cytometry to dissect T- and B-cell subsets, methods that target surface versus intracellular actions of 5-HT, and conditional transgenic methods that could manipulate 5-HT targets in these cells, are needed to take us further toward a better understanding of these mechanisms. Nonetheless, enough evidence is present to consider that we are beyond the point of hypothesis related to the possibility of a role for 5-HT in both innate and adaptive immunity. More so, we now need to dig deeper with better cellular and time resolution on where and when 5-HT contributes to pathogen responses.
Although we have discussed the innate and adaptive immune systems in isolation, a role for 5-HT in communication between these two systems has been described.30 As examples, 5-HT has been reported to contribute to interactions between monocytes and NK cells,102 5-HT may promote macrophage-stimulation of T cell activity, and depletion of 5-HT via treatment with the irreversible tryptophan hydroxylase (TPH) inhibitor parachlorophenylalanine (PCPA) appears to impair the ability of macrophages to activate T cells.90,103,104 DCs in blood function as APCs, which communicate between innate and adaptive immune systems, and 5-HT has been shown to modulate the differentiation of DCs from human monocytes,105 the blood-borne phagocytic precursors of macrophages.
Serotonin-Immune Cell Link to Neurobehavioral Disorders
The role of 5-HT in regulating cells involved in neuroinflammation is interesting given evidence for altered immune function in several psychiatric disorders where compromised 5-HT signaling has also been illustrated. For example, one group found that activated microglia and astrocytes are located near senile plaques in inflamed tissue of Alzheimer’s patients106 and that 5-HT2C receptors are pathologically expressed in NK cells in Alzheimer’s patients.107 Furthermore, alterations in immune function have been reported in subjects with autism;33 autism is also associated with elevated blood levels of serotonin.108 A human, gain-of-function SERT coding variant, Gly56Ala, was identified among four other similar hyperactive variants in a study of rare gene variation in subjects with autism.109 The Gly56 to Ala56 change leads to hyperphosphorylation of SERT, and mice carrying the Ala56 variant display hyperserotonemia, 5-HT receptor hypersensitivity, social impairment, and repetitive behavior,110 characteristics displayed by subjects with autism. Since autism is often associated with gastrointestinal disorder (GID)111 and immune disturbances,112 these findings have elevated our interest in 5-HT actions in the immune system. In preliminary studies, our group has found changes in gene expression linked to activation of p38 MAPK in lymphoblastoid cells from autism-associated SERT gene variant carriers,113 with pathway analysis pointing to activation of p38 MAPK signaling, as well as molecular circuits responding to oxidative stress. As we note below, p38 MAPK pathways also regulate SERT, suggesting the opportunity for a positive feedback regulation of 5-HT accumulation in immune cells should the balance between uptake, metabolism, and release of 5-HT be altered. Lymphoblastoid cells are derived from B lymphocytes following immortalization with Epstein–Barr virus (EBV). Since these cells are typically the source of patient DNA samples, they are a convenient resource to connect genotype with phenotype, when the molecule of choice is expressed in B cells (e.g., SERT). However, it must be recognized that as they are transformed, these cells may exhibit characteristics distinct from native B cells. For example, we find that SERT protein levels and 5-HT uptake activity are much higher in native B cells than in EBV-transformed cells (Han and Blakely, unpublished findings).
Since the discussion above notes a connection between inflammatory cytokine signaling, SERT, and neurobehavioral disorders, it is interesting to note that functional coding variation exists in the transporter across inbred strains of mice. Many inbred mouse strains express Glu-39 and Arg-152 coding sequences (ER isoform) whereas a few others, and notably C57Bl/6 express Gly-39 and Lys-152 (GK isoform).114 Since these two haplotypes are present in one each of the progenitors of BXD recombinant inbred lines, Carneiro and colleagues were able to demonstrate that not only does the ER SERT variant exhibit elevated 5-HT uptake capacity compared to the GK isoform, but also that the ER variant is associated with reduced T cell proliferation and T cell receptor expression.114 Although it is not possible to know if this association is driven by a direct effect of SERT activity in the immune system proper, this seems a parsimonious hypothesis. Possibly, elevated 5-HT clearance in the immune system diminishes extracellular 5-HT availability that in turn limits T cell proliferation. As both the mouse ER/GK variation and the human Ala56Gly SERT variant associate with behavioral changes, studies are now needed that distinguish their immune system impact from other sites of action of the transporter and 5-HT.
Depression has long been linked to aberrations in both the immune and serotonergic systems. Patients diagnosed with major depressive disorder often display elevated levels of immune markers, as well as associations with single nucleotide polymorphisms (SNPs) in pro-inflammatory cytokine genes,115 such as IL-1β,116,117 TNFα,118,119 and cyclooxygenase-2.120 The observation that pro-inflammatory cytokine levels are elevated in depressed patients121 led Smith to propose a “macrophage theory of depression”.122 Evidence that cytokine elevations may be causally related to mental illness derives from the depressive symptoms that occur in patients treated with cytokines (IFN-α) as part of cancer therapy.123,124 SSRIs, such as Prozac and Zoloft, elevate 5-HT levels in the brain and periphery, and effects of SSRIs on adaptive immune cells have been described. In turn, SSRIs enhance the cytolytic function of NK cells,125 whereas fluoxetine and paroxetine treatment can reverse the blunted NK cell activity reported in depressed patients.126 Long-term SSRI treatment has also been shown to enhance NK and B cell proliferation.127 Zimelidine increases MHC class II expression on macrophages in vitro.128 Finally, paroxetine and sertraline inhibit CNS microglial activation via inhibition of IFN-γ-induced increases in intracellular Ca2+.129 Finally, SSRI treatment has been shown to normalize elevated cytokine levels in depressed patients.130 Another link between immune function and SSRI action arises from findings that reduced lymphocyte SERT expression in patients with MDD is normalized by administration of SSRIs.131 A caveat to a mechanistic interpretation of these studies is the possibility that the actions of SSRIs in immune system function may be mediated by proteins other than SERT, particularly when the evidence derives from in vitro studies of cell suspensions where blockade of the transporter is unlikely to elevate extracellular 5-HT to an extent that would impact cell-surface 5-HT receptors in the way we expect in intact systems. We recently developed a knock-in mouse strain (SERT I172M) that results in significantly reduced sensitivity to multiple SSRIs.132 The SERT I172 M model may be useful in pursuing this question.
Several lines of evidence suggest that treatment with antidepressant medications can have anti-inflammatory properties.133 Sacre et al.134 reported that fluoxetine and citalopram reduced inflammation by inhibiting TLR function. Elevated cytokine levels in depressed patients have also been shown to normalize following SSRI treatment;135,136 however, normalization of cytokine levels appears not to occur in patients that did not respond to SSRIs,137 raising the possibility that immune system status could be a useful biomarker in structuring personalized depression therapies. Since T cells appear not to express SERT47 (though note the caveat mentioned regarding T cell subtypes above), they do express 5-HT receptors47 and thus the effects of SSRIs in vivo may also be indirect. In the CNS, SSRIs have been reported to potently inhibit microglial TNFα and NO production after stimulation by LPS.138 Contrary to these findings, others have reported that SSRIs and other antidepressants elevate levels of cytokines in the brain, including TNFα and IFNγ, but this effect is lost when animals are cotreated with a nonsteroidal anti-inflammatory (NSAID) drug.139 Warner-Schmidt et al.139 recently reported that NSAIDs attenuate the antidepressant effects of SSRIs in both mice and humans. The discrepancies in the ability of SSRIs to increase or reduce cytokine levels could be a result of varying concentrations of these agents across studies and the duration of exposure to these drugs, as well as brain region specificity, and thus will require further study. Interestingly, macrophage migration inhibitory factor (MMIF) has been reported to be required for the antidepressant effects of voluntary exercise,140 as these effects of exercise are lost in MIF knockout mice, extending the immune system connection to treatments for depression beyond those of antidepressant medications.
Polymorphisms in interleukin genes linked to inflammation and depression have been identified, raising the possibility that a genetic predisposition to mood disorders (or resiliency) could arise not from the brain but from an immune system action. Individuals homozygous for the −511T allele of the IL-1β gene have fewer symptoms associated with depression and respond better to fluoxetine141 and paroxetine142 treatment than those143 carrying the −511C isoform, but not in patients carrying a variable number of tandom repeats (VNTR) polymorphism of IL-1Ra, an IL-1R antagonist.142 Another study showed a positive association between a polymorphism in the IL-11 gene (rs1126757) and response to escitalopram.144 Finally, two SNPs (rs2929115 and rs2929116) in the IDO2 gene, which has been linked to both 5-HT homeostasis (via tryptophan metabolism) and immune function, have been associated with SSRI efficacy.145 Since these genes are expressed in the brain, as well as the periphery, work remains to understand where these polymorphisms may impact behavior and/or antidepressant action.
Loss of SERT in knockout mice has been reported to trigger changes in levels of pulmonary immune molecule transcripts,146 to exacerbate intestinal inflammatory/immune responses to 2,4,6-trinitrobenzene sulfonic acid (TNBS) exposure,147 and to reduce severity of immune responses in an experimental autoimmune encephalomyelitis paradigm.148 TPH1 knockout mice have been shown to have reduced levels of 5-HT and production of cytokines in the gut, as well as reduced severity of colitis.149 In humans80,140 and rhesus monkeys,150,151 a commonly studied SERT promoter polymorphism, the 5-HTTLPR, has been reported to impact SERT mRNA levels, protein expression, and uptake function.83,143 Carvalho and co-workers have reported that patients diagnosed with fibromyalgia expressed different T-cell surface antigens, depending on whether they were carriers of the long “l” or short “s” variants of the 5-HTTLPR. In addition, NK cells were also reduced in s carriers.152 Additionally, Lima and colleagues described effects of 5-HTTLPR status on lymphocyte SERT expression and found that SERT mRNA expression is reduced in lymphocytes of depressed patients carrying the l allele.153 The sensitivity of SERT gene expression to IL-4 stimulation in EBV-transformed lymphoblasts has also been reported to depend on 5-HTTLPR status.154 Finally, multiple studies indicate that polymorphism status reveals itself with respect to mental illness in the context of a significant life history of stress.16 In this regard, Matsunaga and colleagues reported that amygdala activity occasioned by seeing a “favorite person” is correlated with levels of NK cells.155 Thus, further work is needed to determine whether environment/5-HT gene interactions might drive changes in immune function that can ultimately alter behavior.
Pathways Mediating Immune–Brain Communication
The impact of immune system activation on behavior and the intersections noted above between antidepressant action and immune signaling raise the critical question as to the path(s) by which peripheral immune system activation could initiate or stabilize changes in behavior. Activation of the immune system, whether ectopic or via peripheral injection of lipopolysaccharide (LPS), a constituent of the cell walls of gram-negative bacteria, leads to elevation of levels of pro-inflammatory cytokines, such as IL-1β,156 both systemically and centrally.157−159 In the periphery (Figure 2), LPS binds to pathogen-associated molecular pattern (PAMP)-recognizing receptors, toll-like receptor-4 (TLR4) and CD14 expressed on macrophages, dendritic cells, and neutrophils. LPS stimulates monocytes and macrophages to synthesize IL-1β, which then enters the blood. IL-1β generated in the periphery does not readily cross the BBB because the peptide is fairly large (∼15 kDa) and hydrophilic. Cytokines such as IL-1β, however, can cross into the brain at the circumventricular organs (CVOs),160 such as the organum vasculosum lamina terminalis, where the BBB is weak or absent. Products of both the innate (i.e., monocytes,161 cytokines162) and adaptive (T cells/B cells) immune systems that do enter the brain activate central immune cells, though it is important to note that many peripherally activated components of the immune system are unable to cross the tightly packed cells of the BBB, including many antibodies. Furthermore, under physiological conditions, only T cells that have been activated have been shown to cross the BBB easily and to accumulate in CNS tissue.98 Several immune components, including cytokines,163 influence the permeability of the BBB. Astrocytes assist in BBB function through influencing the permeability of brain microvascular endothelial cells that comprise the BBB,164 and activated microglia damage the BBB via NFκB, JAK-STAT, and JNK stress kinase signaling pathways.165 The BBB, in turn, also has been shown to release cytokines in response to peripheral administration of LPS166 or IL-1β.167 A compromised BBB would allow peripheral cytokines to access enter the brain more easily and thereby influence central neural pathways, likely compounding other problems. Another route by which cytokines could gain access to the brain is through active transport mechanisms. Indeed, active transport mechanisms have been reported which carry cytokines across the BBB,168 including IL-1α,169 IL-1β,162,170−172 TNFα,173 and IL-1RA.174 Multiple other mechanisms for transport of cytokines across the BBB have been described,162 although the specific details of which proteins mediate these transport activities remain an area of investigation.
Figure 2.
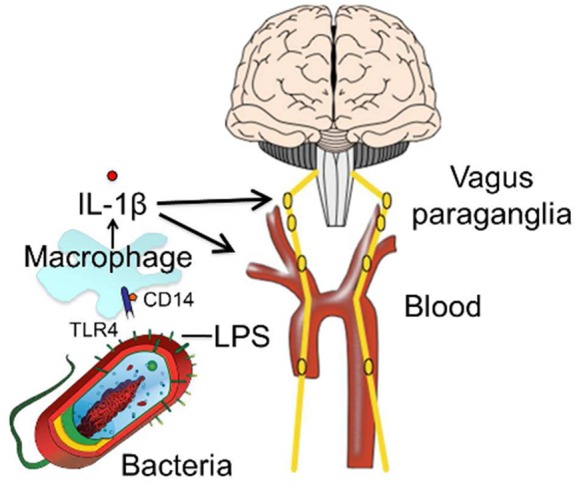
Peripheral lipopolysaccharide (LPS) binds to toll-like receptor-4 (TLR4) on macrophages and stimulates release of interleukin 1β (IL-1β). IL-1β in the blood can enter the brain through circumventricular organs (CVOs) or active transport mechanisms. IL-1β also can stimulate the vagus paraganglia.
Other lines of evidence point to, in addition to a BBB-mediated route, a neural mechanism of immune–brain communication. One study reported that peripheral LPS increased CSF IL-1β within 15 min.175 In another study, Fos protein expression was measured 1 h following immune activation with IL-1β, suggesting that the cfos gene must have been activated within minutes,176 since blood-borne mechanisms would have a more delayed action. Together, these findings that initial responses to peripheral LPS and IL-1β are rapid suggest a role for activation of neural inputs to the CNS following peripheral immune activation. The vagus nerve is one neural mechanism that mediates neural communication between the brain and peripheral immune system. The vagus is important in controlling autonomic responses and innervates lymphatic tissue, including spleen,177 thymus,178 and lymph glands,179 as well as sites where pathogens enter the body (lungs, peritoneum, and organs important for innate immunity such as the liver). The vagus nerve contains both efferent and afferent fibers both from and to the CNS, respectively, linking brain and lymphatic tissue.180 The efferent, cholinergic fibers of the vagus have been shown to suppress microglia activation. In addition, IL-1β increases activity of vagal efferent fibers.181 Peripheral immune activation (via i.p. injection of LPS182 or IL-1β183) also activates afferent vagal fibers182 and induces perivagal immune cells to rapidly express IL-1β immunoreactivity.184 Activation of afferent vagal fibers has been shown to produce neural changes characteristic of sickness.185 Severing the vagus reduces or eliminates the ability of LPS to produce sickness behavior186,187 but not to produce fever. This suggests different effects of LPS on vagal circuitry controlling behavioral and hypothalamic processes. Together, these lines of evidence point to a key role of the vagus nerve in relaying information between the brain and immune system via afferent and efferent pathways. It is important to note, however, that the vagus is not the only nerve that can signal occurrence of immune activation. The skin and oral cavity also respond to infection/injury but are not innervated by the vagus nerve, and therefore, other nerves in those regions may function to communicate immune activation to the CNS.188 Taken together, these lines of evidence suggest that peripheral cytokines such as IL-1β employ several routes of communication (through the BBB or peripheral nerves) to the brain to potentially influence behavior and suggest that blood-bourne and neural inputs collaborate in communicating to the brain in the presence of peripheral inflammation.
Immune System Activation and CNS 5-HT Neurotransmission
There are several mechanisms by which inflammatory cytokines acting within the CNS can modulate the functions of key neural circuits, including 5-HT, that drive various physiological and behavioral responses. Although there is evidence that IL-1β can inhibit the firing of 5-HT neurons in the dorsal raphe,189 there is overarching consensus that acute activation of the immune system and IL-1β increase extracellular 5-HT levels in the brain. Microdialysis studies show that administration of IL-1β either i.p.190 or centrally191 elevates extracellular brain 5-HT, and systemic LPS increases 5-HT in hippocampus.192 Increased 5-HT synthesis in nerve terminals could explain elevations in central 5-HT following immune activation; however, systemic administration of IL-1β elevates levels of both the 5-HT precursor, tryptophan, and its metabolite, 5-HIAA,193−195 an effect that appears to depend on activation of the sympathetic nervous system.196 This finding is interesting in light of clinical evidence that serum tryptophan levels are reduced in patients with treatment-resistant depression.197 Levels of 5-HT are also influenced by cytokines other than IL-1β, as peripheral IL-6 increases 5-HT levels in rat striatum.198
Cytokines have been shown to control proteins that regulate 5-HT levels. Cytokine regulation of SERT transcription and activity has been previously demonstrated by several groups (for review, see ref (199)). The pro-inflammatory cytokines TNFα200 and IL-1β201 have been reported to elevate SERT activity via increased production of SERT mRNA levels in human JAR cells, a choriocarcinoma cell line derived from the placenta that constitutively expresses SERT. Similarly, interferon (IFN)-α and IFN-γ, also pro-inflammatory, have been found to increase SERT activity in BeWo cells, another human choriocarcinoma cell line.202 An anti-inflammatory cytokine, IL-4, was shown to reduce SERT activity in B lymphoblastoid cell lines, which express SERT.203 In general, chronic stimulation by inflammatory cytokines appears to stimulate SERT transcription, whereas anti-inflammatory cytokines reduce SERT activity. Altered SERT expression and activity has long been linked to neuropsychiatric disorders, such as depression. Clinically, carriers of the s allele of the SERT-linked polymorphic region (5-HTTLPR), which several groups report as producing reduced SERT expression, showed elevated risk for depression and circulating levels of IL-6, another anti-inflammatory cytokine.204 Other clinical studies have found a correlation between cytokine levels and depression, reporting higher levels of IL-6 in men with depression,205 and two other meta-analyses indicate that levels of IL-6 and TNFα were elevated in depressed subjects.206,207 It may seem odd to have a pro-inflammatory cytokine elevate raphe firing and extracellular levels of 5-HT yet associate with depression or elevated SERT expression (and activity, as noted below). One way to reconcile these findings, and our working model, is that changes in 5-HT transport capacity reflect a homeostatic mechanism, scaled with elevated serotonergic signaling. The more 5-HT is secreted, the greater the need for uptake activity via SERT to ensure that the elevated amplitude of 5-HT signals can scale in magnitude, without also broadening the temporal or spatial impact of these effects. Seen in this light, disease risk can arise is when these homeostatic mechanisms are activated independent of the normal stimulus (such that 5-HT modulation occurs in a context devoid of the actions of cytokines elsewhere in the brain) or when inflammatory stimuli that should drive a coordinated serotonergic response, produce one involving an incomplete constellation of contributors (e.g., uptake modulation without a change in raphe firing). In this regard, our group recently reported that a SERT coding variant that lacks a capacity for regulation by cytokine-linked signaling pathways109,208 produces behaviors reminiscent of autism when expressed in transgenic mice.110
Since SERTs are critical modulators of 5-HT signaling and are the primary target for many antidepressant medications, our lab and others have investigated how this transporter can be regulated by multiple signaling pathways and interacting proteins.209 In relation to this review, our lab discovered that pathways activated in the CNS by the pro-inflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor-α (TNFα) regulate SERT via rapid effects mediated by p38 mitogen activated protein kinase (MAPK)-linked pathways.210 The ability of cytokines to regulate SERT activity derives from studies initially exploring how SERT is regulated by G-protein coupled receptors. SERT proteins do not reside constitutively at the plasma membrane, but rather, like many receptors and transporters, are dynamically trafficked as a consequence of protein–protein associations and post-translational modifications, including those mediated by PKC211,212 glycosylation,213 PKG/p38 MAPK,214 and cGMP.215 SERT is a phosphoprotein,216 and alterations in transporter phosphorylation also appear to modulate both trafficking and SERT catalytic activity.213,214,210 Several receptor-signaling pathways are now known to regulate SERT. Thus, activation of the A3 adenosine receptor (A3AR),214 α2-adrenoreceptor,217 5-HT1B autoreceptor,218 and interleukin-1 receptor (IL-1R)37 have been reported to rapidly modulate the intrinsic activity or membrane density of SERT. Our group found that A3AR activation elevates SERT plasma membrane density in cultured cells and synaptosomes via PKG1α signaling and catalytic activation of SERT in parallel via PKG-dependent regulation of p38 MAPK.209 Activation of both PKG1α and p38 MAPK isoforms (not yet well-defined, though antagonist SB203580 targets α and β subtypes) leads to SERT phosphorylation. Ramamoorthy and co-workers have identified Thr276 as the essential phosphorylation site on SERT that supports PKG1-dependent SERT regulation.219 Although PKG1α activation can modulate SERT trafficking, we have argued209 that the T276A site may be phosphorylated following PKG1-dependent p38 MAPK activation and, therefore, is of greatest relevance to catalytic activation. Recent studies by Rudnick and colleagues have found that, in transfected cells, PKG does not directly phosphorylate SERT, nor does p38 MAPK appear to be the critical intermediate, necessitating further studies.220 Finally, we have recently demonstrated using cultured raphe cell line (RN46A cells) that the IL-1R/p38 MAPK pathway mobilizes cell surface SERT in lipid-raft microdomains through detatchment of the transporter from cytoskeletal anchors that normally stabilize the transporter in less effective conformations.221 Clearly, a sophisticated network of controls is in place for altered immune function to modulate at least one critical component of 5-HT signaling regulation.
Finding that IL-1β and TNFα stimulated SERT activity in RN46A cells, as well as in mouse brain synaptosomes,222 we asked whether IL-1β effects could be blocked by the IL-1R antagonist IL-1Ra, as well as a p38 MAPK inhibitor, SB203580,210 and in both cases the answer was yes. We next wanted to determine whether peripheral induction of IL-1R secretion by stimulation of the native immune system could modulate CNS SERT activity. We recently reported that peripheral administration of LPS rapidly stimulates SERT activity (as much as a 100% increase) in mouse midbrain and hippocampal synaptosomes, as well increases the clearance rate of 5-HT in the CNS.37 These effects occur within 1 h of peripheral LPS injection, suggesting that they arise from a post-translational modification of SERT, one that our report indicates results in a shift of SERT to a high affinity state for 5-HT.37 Use of an IL-R KO or systemic administration of SB203580 abolished this effect, suggesting that the activation of IL-1R affects SERT via an IL-1R and p38 MAPK-dependent pathway (Figure 3). In chronoamperometric studies, peripheral administration of LPS also stimulated 5-HT uptake in hippocampus,37 further supporting the notion that CNS SERT activity in vivo is under the control of the peripheral immune system. Behaviorally, mice treated with LPS spent more time immobile in the tail suspension test (TST), a screen for antidepressant efficacy. The depressive-like effect of LPS on behavior is lost in mice treated with SB203580, as well as in IL-1R KO and SSRI-treated mice. Together, these biochemical and behavioral studies provide strong evidence that LPS stimulates SERT activity through a p38 MAPK/IL-1R pathway, and may explain components of the depressive-like phenotype that follows immune activation in humans.223,224 However, there are many steps between LPS activation of the peripheral immune system and CNS SERT regulation at which loss of IL-1Rs or pharmacological antagonism of p38 MAPK could attenuate SERT upregulation. For example, these manipulations may simply depress peripheral immune system reactivity that leads to IL-1β stimulation. We have reported that if synaptosomes prepared from LPS-injected mice are treated with SB203580, stimulation of SERT is lost. These data indicate that ongoing CNS p38 MAPK activity in serotonergic terminals is required at least to sustain rapid upregulation of neuronal SERT initiated by peripheral immune system activation.
Figure 3.
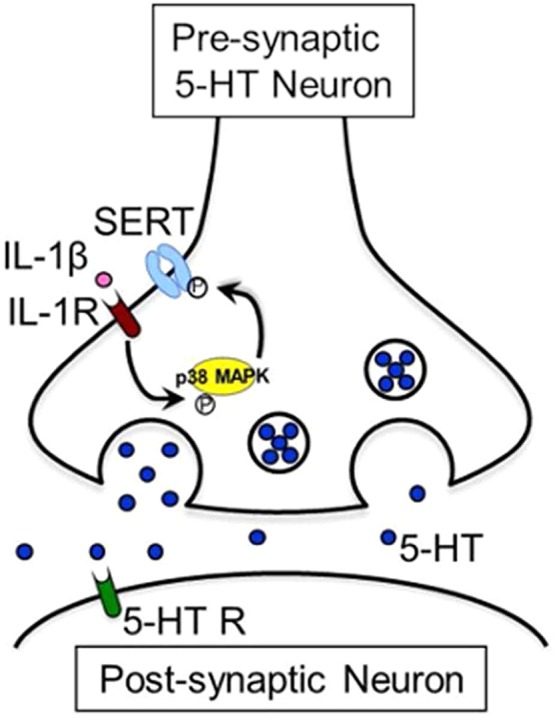
Interleukin-1β (IL-1β) stimulates SERT activity through an interleukin-1 receptor (IL-1R)- and p38 MAPK-dependent signaling pathway.
In addition to SERT, pro-inflammatory cytokines have been shown to influence the function of other proteins, such as p11 (S100A10), that interact with 5-HT receptors.225,226 The level of p11 expression has been negatively correlated with depressive behavior, with p11 knockout mice displaying increased immobility time in both the forced swim (FST) and tail suspension (TST) tests.225 Interestingly, SSRIs, tricyclic antidepressants (TCAs), and electroconvulsive therapy (ECT) all appear to increase both cytokine (specifically IL-1β, IL-3, IL-6, IFNγ, TNFα, and IL-12)139 and p11 levels in some brain regions of mice.225,227 The SSRI-induced elevation of p11 was abolished in mice with genetic depletion of cytokines, suggesting that SSRIs could elevate cytokine levels via a p11-mediated pathway. These findings suggest that SSRI-stimulation of cytokine production leads to elevated p11 and has an antidepressant effect on behavior. How elevated p11 elicits an effect on mood, however, remains unknown. In this regard, there is evidence that p11 binds to and upregulates surface expression of both 5-HT1B225 and 5-HT4226 receptors. p11 could therefore play a role in mediating immune-brain communication and the risk for depression. Again, one would presume that regulation of p11 has likely not evolved to establish a mood disorder, but in concert with other immune targets such as those expressed presynaptically by 5-HT neurons, to provide for highly regulated brain signaling and behaviors responsive to inflammatory insults.
Immune Modulation of Serotonin-Related Behaviors
As noted earlier, peripheral LPS228 and pro-inflammatory cytokines trigger “sickness behavior”70,229 that is characterized by fatigue, loss of appetite, inactivity, malaise, and lack of sociability. This “sickness syndrome” is strikingly similar to symptoms presented by depressed patients.70 Importantly, vaccination and LPS administration can induce sickness behavior and depressive behavior in both humans and rodents.230 Our lab has previously shown that systemic treatments with LPS altered behavior in behavioral assays that are commonly used to screen for antidepressant drugs, namely, the FST and TST.37 This effect of LPS on behavior was lost in mice treated with SB203580 or with a constitutive knockout of the IL-1R, supporting the hypothesis that the despair-like effect of LPS is p38 MAPK- and IL-1R-dependent. As described above, cytokines can alter levels of 5-HT in the brain,70 and IL-1β upregulates SERT density and function and enhances SERT activity. As p38 MAPK-dependent activation of SERT could involve other cytokines that signal through p38 MAPK besides IL-1β, we have initiated studies of the constitutive elimination of p38 MAPK in raphe neurons (p38MAPKflox/flox:ePet-1::Cre). Our studies231,232 indicate that animals positive for both ePet-1:Cre and floxed p38 MAPKα are viable with no gross evidence of developmental perturbations as expected from studies using virus-based raphe excision of p38 MAPKα by Chavkin and co-workers.233 Our preliminary biochemical and behavioral studies231,232 with these mice are consistent with a requirement for p38 MAPKα expression by raphe neurons in producing both a rise in synaptosomal 5-HT uptake, as well as alterations in 5-HT and SSRI-linked behaviors. To determine if LPS effects arise from IL-1R stimulated MAPK pathways, we have also engineered mice that express a floxed allele of the IL-1R gene (IL-1Rflox/flox), and these mice recently achieved germ-line transmission. As with constitutive IL-1R KO mice, these animals are viable, of normal size and fecundity, and appear to have no gross morphological alterations of the CNS or perturbation of 5-HT levels. Following initial characterization to ensure that the floxed construct does not perturb IL-1R expression, we can achieve serotonin neuron-specific elimination of IL-1R signaling potential using raphe-specific Cre expression, further defining a signaling path by which peripheral inflammation can modulate behaviors linked to depression and antidepressant action.
Although we have much work to do before we can say that we have defined the molecular and circuit-level underpinnings of mental illness, the studies reviewed above make a strong case that we cannot remain brain-centric in our thinking as to mechanism, both in terms of physiological processes involved, in general, and the contributions of compromised 5-HT signaling, in particular. It seems likely that the behavioral changes arising as a consequence of immune system activation have evolved to provide survival benefit following pathogen invasion. A reduction in eating, a conservation of energy resources, and social withdrawal are things an organism should do to prevent exacerbation of infections and the potential for spread of potentially fatal agents to other members of the community. In this view, risk for mood disorders in some subjects may arise from a propensity for an enhanced or ectopic activation of what normally is a beneficial immune–brain dialogue, one that often speaks the language of 5-HT. Although this evolution-centered hypothesis is straightforward in description, specific mechanisms remained to be defined, as receptors and signaling pathways for immune molecules are widely expressed, as are determinants of 5-HT signaling. We must also remember that early actions that perturb maternal, embryonic, and neonatal immune and 5-HT signaling likely have enduring consequences on behavior.234−237 Regardless of complexity, the inclusion of the immune system in our concepts of the determinants of both normal behavior and risk for mental illness has the opportunity to provide new opportunities for improved diagnosis and treatment of disorders long linked to changes in 5-HT signaling.
Author Contributions
N.B. and R.B. researched and wrote the article.
Funding MH094527 and MH078668 (to R.D.B.) and N5007491 (to N.L.B.) supported our studies.
The authors declare no competing financial interest.
References
- Canli T.; Lesch K. P. (2007) Long story short: the serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 10, 1103–1109. [DOI] [PubMed] [Google Scholar]
- Passamonti L.; Crockett M. J.; Apergis-Schoute A. M.; Clark L.; Rowe J. B.; Calder A. J.; Robbins T. W. (2012) Effects of acute tryptophan depletion on prefrontal-amygdala connectivity while viewing facial signals of aggression. Biol. Psychiatry 71, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp A. M.; Miczek K. A. (2000) Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J. Neurosci. 20, 9320–9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti J. M. (2011) Serotonin control of sleep-wake behavior. Sleep Med. Rev. 15, 269–281. [DOI] [PubMed] [Google Scholar]
- Blundell J. E. (1984) Serotonin and appetite. Neuropharmacology 23, 1537–1551. [DOI] [PubMed] [Google Scholar]
- Bardin L. (2011) The complex role of serotonin and 5-HT receptors in chronic pain. Behav. Pharmacol. 22, 390–404. [DOI] [PubMed] [Google Scholar]
- Ducy P. (2011) 5-HT and bone biology. Curr. Opin. Pharmacol. 11, 34–38. [DOI] [PubMed] [Google Scholar]
- Karsenty G.; Yadav V. K. (2011) Regulation of bone mass by serotonin: molecular biology and therapeutic implications. Annu. Rev. Med. 62, 323–331. [DOI] [PubMed] [Google Scholar]
- Lesurtel M.; Graf R.; Aleil B.; Walther D. J.; Tian Y.; Jochum W.; Gachet C.; Bader M.; Clavien P. A. (2006) Platelet-derived serotonin mediates liver regeneration. Science 312, 104–107. [DOI] [PubMed] [Google Scholar]
- White J. G. (1970) A biphasic response of platelets to serotonin. Scand. J. Haematol. 7, 145–151. [DOI] [PubMed] [Google Scholar]
- Li N.; Wallen N. H.; Ladjevardi M.; Hjemdahl P. (1997) Effects of serotonin on platelet activation in whole blood. Blood Coagulation Fibrinolysis 8, 517–523. [DOI] [PubMed] [Google Scholar]
- Lesurtel M.; Soll C.; Graf R.; Clavien P. A. (2008) Role of serotonin in the hepato-gastroIntestinal tract: an old molecule for new perspectives. Cell. Mol. Life Sci. 65, 940–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. Y.; Halaris A. E.; Jones B. E. (1978) Serotonin neurons of the midbrain raphe: ascending projections. J. Comp. Neurol. 180, 417–438. [DOI] [PubMed] [Google Scholar]
- Bang S. J.; Jensen P.; Dymecki S. M.; Commons K. G. (2012) Projections and interconnections of genetically defined serotonin neurons in mice. Eur. J. Neurosci. 35, 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. (1998) The spectrum of behaviors influenced by serotonin. Biol. Psychiatry 44, 151–162. [DOI] [PubMed] [Google Scholar]
- Caspi A.; Sugden K.; Moffitt T. E.; Taylor A.; Craig I. W.; Harrington H.; McClay J.; Mill J.; Martin J.; Braithwaite A.; Poulton R. (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301, 386–389. [DOI] [PubMed] [Google Scholar]
- Uher R.; McGuffin P. (2008) The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol. Psychiatry 13, 131–146. [DOI] [PubMed] [Google Scholar]
- Blakely R. D.; Berson H. E.; Fremeau R. T. Jr.; Caron M. G.; Peek M. M.; Prince H. K.; Bradley C. C. (1991) Cloning and expression of a functional serotonin transporter from rat brain. Nature 354, 66–70. [DOI] [PubMed] [Google Scholar]
- Fuller R. W.; Wong D. T. (1990) Serotonin uptake and serotonin uptake inhibition. Ann. N.Y. Acad. Sci. 600, 68–78. [DOI] [PubMed] [Google Scholar]
- Blakely R. D.; Ramamoorthy S.; Schroeter S.; Qian Y.; Apparsundaram S.; Galli A.; DeFelice L. J. (1998) Regulated phosphorylation and trafficking of antidepressant-sensitive serotonin transporter proteins. Biol. Psychiatry 44, 169–178. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S.; Bauman A. L.; Moore K. R.; Han H.; Yang-Feng T.; Chang A. S.; Ganapathy V.; Blakely R. D. (1993) Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc. Natl. Acad. Sci. U.S.A. 90, 2542–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S.; Blakely R. D. (1999) Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science 285, 763–766. [DOI] [PubMed] [Google Scholar]
- Lotrich F. E.; Pollock B. G. (2004) Meta-analysis of serotonin transporter polymorphisms and affective disorders. Psychiatr. Genet. 14, 121–129. [DOI] [PubMed] [Google Scholar]
- Lasky-Su J. A.; Faraone S. V.; Glatt S. J.; Tsuang M. T. (2005) Meta-analysis of the association between two polymorphisms in the serotonin transporter gene and affective disorders. Am. J. Med. Genet., Part B 133, 110–115. [DOI] [PubMed] [Google Scholar]
- Schinka J. A.; Busch R. M.; Robichaux-Keene N. (2004) A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry 9, 197–202. [DOI] [PubMed] [Google Scholar]
- McDougle C. J.; Epperson C. N.; Price L. H.; Gelernter J. (1998) Evidence for linkage disequilibrium between serotonin transporter protein gene (SLC6A4) and obsessive compulsive disorder. Mol. Psychiatry 3, 270–273. [DOI] [PubMed] [Google Scholar]
- Lin P. Y. (2007) Meta-analysis of the association of serotonin transporter gene polymorphism with obsessive-compulsive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 31, 683–689. [DOI] [PubMed] [Google Scholar]
- Feinn R.; Nellissery M.; Kranzler H. R. (2005) Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. Am. J. Med. Genet., Part B 133B, 79–84. [DOI] [PubMed] [Google Scholar]
- Cook E. H. Jr.; Courchesne R.; Lord C.; Cox N. J.; Yan S.; Lincoln A.; Haas R.; Courchesne E.; Leventhal B. L. (1997) Evidence of linkage between the serotonin transporter and autistic disorder. Mol Psychiatry 2, 247–250. [DOI] [PubMed] [Google Scholar]
- Mossner R.; Lesch K. P. (1998) Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav Immun 12, 249–271. [DOI] [PubMed] [Google Scholar]
- Ahern G. P. (2011) 5-HT and the immune system. Curr Opin Pharmacol 11, 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M. R.; Miller A. H. (2007) Depressive disorders and immunity: 20 years of progress and discovery. Brain, Behav., Immun. 21, 374–383. [DOI] [PubMed] [Google Scholar]
- Onore C.; Careaga M.; Ashwood P. (2012) The role of immune dysfunction in the pathophysiology of autism. Brain, Behav., Immun. 26, 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell P. J.; Wang X.; Leon-Ponte M.; Griffiths C.; Pingle S. C.; Ahern G. P. (2006) A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood 107, 1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csaba G.; Kovacs P. (2006) Perinuclear localization of biogenic amines (serotonin and histamine) in rat immune cells. Cell Biol. Int. 30, 861–865. [DOI] [PubMed] [Google Scholar]
- Reddy N. P. (1986) Lymph circulation: physiology, pharmacology, and biomechanics. Crit. Rev. Biomed. Eng 14, 45–91. [PubMed] [Google Scholar]
- Zhu C. B.; Lindler K. M.; Owens A. W.; Daws L. C.; Blakely R. D.; Hewlett W. A. (2010) Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 35, 2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin J.; Cohen B. (2001) An overview of the immune system. Lancet 357, 1777–1789. [DOI] [PubMed] [Google Scholar]
- Ransohoff R. M.; Brown M. A. (2012) Innate immunity in the central nervous system. J. Clin. Invest. 122, 1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Shelef M.; Calame K. (2005) Regulation of plasma-cell development. Nat. Rev. Immunol. 5, 230–242. [DOI] [PubMed] [Google Scholar]
- Gershon M. D.; Tack J. (2007) The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414. [DOI] [PubMed] [Google Scholar]
- Gershon M. D.; Drakontides A. B.; Ross L. L. (1965) Serotonin: Synthesis and Release from the Myenteric Plexus of the Mouse Intestine. Science 149, 197–199. [DOI] [PubMed] [Google Scholar]
- Kim D. Y.; Camilleri M. (2000) Serotonin: a mediator of the brain-gut connection. Am. J. Gastroenterol. 95, 2698–2709. [DOI] [PubMed] [Google Scholar]
- Rodriguez M. J.; Saura J.; Billett E. E.; Finch C. C.; Mahy N. (2001) Cellular localization of monoamine oxidase A and B in human tissues outside of the central nervous system. Cell Tissue Res. 304, 215–220. [DOI] [PubMed] [Google Scholar]
- Finocchiaro L. M.; Arzt E. S.; Fernandez-Castelo S.; Criscuolo M.; Finkielman S.; Nahmod V. E. (1988) Serotonin and melatonin synthesis in peripheral blood mononuclear cells: stimulation by interferon-gamma as part of an immunomodulatory pathway. J. Interferon Res. 8, 705–716. [DOI] [PubMed] [Google Scholar]
- Kushnir-Sukhov N. M.; Brown J. M.; Wu Y.; Kirshenbaum A.; Metcalfe D. D. (2007) Human mast cells are capable of serotonin synthesis and release. J. Allergy Clin. Immunol. 119, 498–499. [DOI] [PubMed] [Google Scholar]
- Leon-Ponte M.; Ahern G. P.; O’Connell P. J. (2007) Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood 109, 3139–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. A. (1985) Platelet-induced neurogenic coronary contractions due to accumulation of the false neurotransmitter, 5-hydroxytryptamine. J. Clin. Invest. 75, 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. A.; Zitnay K. M.; Weisbrod R. M. (1987) Accumulation of 5-hydroxytryptamine leads to dysfunction of adrenergic nerves in canine coronary artery following intimal damage in vivo. Circ. Res. 61, 829–833. [DOI] [PubMed] [Google Scholar]
- Thoa N. B.; Eccleston D.; Axelrod J. (1969) The accumulation of C14-serotonin in the guinea-pig vas deferens. J. Pharmacol. Exp. Ther. 169, 68–73. [PubMed] [Google Scholar]
- Verbeuren T. J.; Jordaens F. H.; Herman A. G. (1983) Accumulation and release of [3H]-5-hydroxytryptamine in saphenous veins and cerebral arteries of the dog. J. Pharmacol. Exp. Ther. 226, 579–588. [PubMed] [Google Scholar]
- Kawasaki H.; Takasaki K. (1984) Vasoconstrictor response induced by 5-hydroxytryptamine released from vascular adrenergic nerves by periarterial nerve stimulation. J. Pharmacol. Exp. Ther. 229, 816–822. [PubMed] [Google Scholar]
- Saito A.; Lee T. J. (1987) Serotonin as an alternative transmitter in sympathetic nerves of large cerebral arteries of the rabbit. Circ. Res. 60, 220–228. [DOI] [PubMed] [Google Scholar]
- Schroeter S.; Levey A. I.; Blakely R. D. (1997) Polarized expression of the antidepressant-sensitive serotonin transporter in epinephrine-synthesizing chromaffin cells of the rat adrenal gland. Mol. Cell. Neurosci. 9, 170–184. [DOI] [PubMed] [Google Scholar]
- Holzwarth M. A.; Brownfield M. S. (1985) Serotonin coexists with epinephrine in rat adrenal medullary cells. Neuroendocrinology 41, 230–236. [DOI] [PubMed] [Google Scholar]
- Verhofstad A. A. J.; Jonsson G. (1983) Immunohistochemical and neurochemical evidence for the presence of serotonin in the adrenal medulla of the rat. Neuroscience 10(4), 1443–1453. [DOI] [PubMed] [Google Scholar]
- Jackson J. C.; Walker R. F.; Brooks W. H.; Roszman T. L. (1988) Specific uptake of serotonin by murine macrophages. Life Sci. 42, 1641–1650. [DOI] [PubMed] [Google Scholar]
- Rudd M. L.; Nicolas A. N.; Brown B. L.; Fischer-Stenger K.; Stewart J. K. (2005) Peritoneal macrophages express the serotonin transporter. J. Neuroimmunol. 159, 113–118. [DOI] [PubMed] [Google Scholar]
- Hoffman B. J.; Mezey E.; Brownstein M. J. (1991) Cloning of a serotonin transporter affected by antidepressants. Science 254, 579–580. [DOI] [PubMed] [Google Scholar]
- Tamir H.; Theoharides T. C.; Gershon M. D.; Askenase P. W. (1982) Serotonin storage pools in basophil leukemia and mast cells: characterization of two types of serotonin binding protein and radioautographic analysis of the intracellular distribution of [3H]serotonin. J. Cell Biol. 93, 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J.; Barnes N. M. (2003) Lymphocytes transport serotonin and dopamine: agony or ecstasy?. Trends Immunol. 24, 438–443. [DOI] [PubMed] [Google Scholar]
- Pfeiffer J. R.; Seagrave J. C.; Davis B. H.; Deanin G. G.; Oliver J. M. (1985) Membrane and cytoskeletal changes associated with IgE-mediated serotonin release from rat basophilic leukemia cells. J. Cell Biol. 101, 2145–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig B.; Bergmann U.; Konig W. (1992) Induction of inflammatory mediator release (serotonin and 12-hydroxyeicosatetraenoic acid) from human platelets by Pseudomonas aeruginosa glycolipid. Infect. Immun. 60, 3150–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme S. A.; Lio F. M.; Sikora L.; Pandit T. S.; Lavrador K.; Rao S. P.; Sriramarao P. (2004) Cutting edge: serotonin is a chemotactic factor for eosinophils and functions additively with eotaxin. J. Immunol. 173, 3599–3603. [DOI] [PubMed] [Google Scholar]
- Muller T.; Durk T.; Blumenthal B.; Grimm M.; Cicko S.; Panther E.; Sorichter S.; Herouy Y.; Di Virgilio F.; Ferrari D.; Norgauer J.; Idzko M. (2009) 5-hydroxytryptamine modulates migration, cytokine and chemokine release and T-cell priming capacity of dendritic cells in vitro and in vivo. PLoS One 4, e6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir-Sukhov N. M.; Gilfillan A. M.; Coleman J. W.; Brown J. M.; Bruening S.; Toth M.; Metcalfe D. D. (2006) 5-hydroxytryptamine induces mast cell adhesion and migration. J. Immunol. 177, 6422–6432. [DOI] [PubMed] [Google Scholar]
- Hellstrand K.; Hermodsson S. (1990) Enhancement of human natural killer cell cytotoxicity by serotonin: role of non-T/CD16+ NK cells, accessory monocytes, and 5-HT1A receptors. Cell. Immunol. 127, 199–214. [DOI] [PubMed] [Google Scholar]
- Freire-Garabal M.; Nunez M. J.; Balboa J.; Lopez-Delgado P.; Gallego R.; Garcia-Caballero T.; Fernandez-Roel M. D.; Brenlla J.; Rey-Mendez M. (2003) Serotonin upregulates the activity of phagocytosis through 5-HT1A receptors. Br. J. Pharmacol. 139, 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrand K.; Hermodsson S. (1987) Role of serotonin in the regulation of human natural killer cell cytotoxicity. J. Immunol. 139, 869–875. [PubMed] [Google Scholar]
- Dantzer R.; O’Connor J. C.; Freund G. G.; Johnson R. W.; Kelley K. W. (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrand K.; Czerkinsky C.; Ricksten A.; Jansson B.; Asea A.; Kylefjord H.; Hermodsson S. (1993) Role of serotonin in the regulation of interferon-gamma production by human natural killer cells. J. Interferon Res. 13, 33–38. [DOI] [PubMed] [Google Scholar]
- Idzko M.; Panther E.; Stratz C.; Muller T.; Bayer H.; Zissel G.; Durk T.; Sorichter S.; Di Virgilio F.; Geissler M.; Fiebich B.; Herouy Y.; Elsner P.; Norgauer J.; Ferrari D. (2004) The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J. Immunol. 172, 6011–6019. [DOI] [PubMed] [Google Scholar]
- Kelly R. W.; Carr G. G.; Critchley H. O. (1997) A cytokine switch induced by human seminal plasma: an immune modulation with implications for sexually transmitted disease. Hum. Reprod. 12, 677–681. [DOI] [PubMed] [Google Scholar]
- Wilcox B. D.; Dumin J. A.; Jeffrey J. J. (1994) Serotonin regulation of interleukin-1 messenger RNA in rat uterine smooth muscle cells. Relationship to the production of interstitial collagenase. J. Biol. Chem. 269, 29658–29664. [PubMed] [Google Scholar]
- Pousset F.; Fournier J.; Legoux P.; Keane P.; Shire D.; Soubrie P. (1996) Effect of serotonin on cytokine mRNA expression in rat hippocampal astrocytes. Brain Res. 38, 54–62. [DOI] [PubMed] [Google Scholar]
- Babcock T. A.; Carlin J. M. (2000) Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon-treated epithelial cells. Cytokine 12, 588–594. [DOI] [PubMed] [Google Scholar]
- Guillemin G. J.; Kerr S. J.; Pemberton L. A.; Smith D. G.; Smythe G. A.; Armati P. J.; Brew B. J. (2001) IFN-beta1b induces kynurenine pathway metabolism in human macrophages: potential implications for multiple sclerosis treatment. J. Interferon Cytokine Res. 21, 1097–1101. [DOI] [PubMed] [Google Scholar]
- Sakash J. B.; Byrne G. I.; Lichtman A.; Libby P. (2002) Cytokines induce indoleamine 2,3-dioxygenase expression in human atheroma-asociated cells: implications for persistent Chlamydophila pneumoniae infection. Infect. Immun. 70, 3959–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y.; Dale W. E.; Brown O. R. (2000) Comparative effects of oxygen on indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase of the kynurenine pathway. Free. Radical Biol. Med. 28, 615–624. [DOI] [PubMed] [Google Scholar]
- Dantzer R.; O’Connor J. C.; Lawson M. A.; Kelley K. W. (2011) Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology 36, 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.; Chen L.; Lim G.; Sung B.; Wang S.; McCabe M. F.; Rusanescu G.; Yang L.; Tian Y.; Mao J. (2012) Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J. Clin. Invest. 122, 2940–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D.; Consoli G.; Masala I.; Catena Dell’Osso M.; Baroni S. (2010) Latest advancements on serotonin and dopamine transporters in lymphocytes. Mini Rev. Med. Chem. 10, 32–40. [DOI] [PubMed] [Google Scholar]
- Lesch K. P.; Bengel D.; Heils A.; Sabol S. Z.; Greenberg B. D.; Petri S.; Benjamin J.; Muller C. R.; Hamer D. H.; Murphy D. L. (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531. [DOI] [PubMed] [Google Scholar]
- Meredith E. J.; Chamba A.; Holder M. J.; Barnes N. M.; Gordon J. (2005) Close encounters of the monoamine kind: immune cells betray their nervous disposition. Immunology 115, 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttarelli F. R.; Fanciulli A.; Pellicano C.; Pontieri F. E. (2011) The dopaminergic system in peripheral blood lymphocytes: from physiology to pharmacology and potential applications to neuropsychiatric disorders. Curr. Neuropharmacol. 9, 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenta F.; Bronzetti E.; Cantalamessa F.; El-Assouad D.; Felici L.; Ricci A.; Tayebati S. K. (2001) Identification of dopamine plasma membrane and vesicular transporters in human peripheral blood lymphocytes. J. Neuroimmunol. 117, 133–142. [DOI] [PubMed] [Google Scholar]
- Marazziti D.; Baroni S.; Catena Dell’Osso M.; Masala I.; Fabbrini L.; Betti L.; Giannaccini G.; Dell’osso B.; Lucacchini A. (2008) Presence and characterization of the dopamine transporter in human resting lymphocytes. Neurochem. Res. 33, 1011–1016. [DOI] [PubMed] [Google Scholar]
- Stamford J. A.; Kruk Z. L.; Millar J. (1990) Striatal dopamine terminals release serotonin after 5-HTP pretreatment: in vivo voltammetric data. Brain Res. 515, 173–180. [DOI] [PubMed] [Google Scholar]
- Jackson B. P.; Wightman R. M. (1995) Dynamics of 5-hydroxytryptamine released from dopamine neurons in the caudate putamen of the rat. Brain Res. 674, 163–166. [DOI] [PubMed] [Google Scholar]
- Kut J. L.; Young M. R.; Crayton J. W.; Wright M. A.; Young M. E. (1992) Regulation of murine T-lymphocyte function by spleen cell-derived and exogenous serotonin. Immunopharmacol. Immunotoxicol. 14, 783–796. [DOI] [PubMed] [Google Scholar]
- Aune T. M.; Kelley K. A.; Ranges G. E.; Bombara M. P. (1990) Serotonin-activated signal transduction via serotonin receptors on Jurkat cells. J. Immunol. 145, 1826–1831. [PubMed] [Google Scholar]
- Aune T. M.; Golden H. W.; McGrath K. M. (1994) Inhibitors of serotonin synthesis and antagonists of serotonin 1A receptors inhibit T lymphocyte function in vitro and cell-mediated immunity in vivo. J. Immunol. 153, 489–498. [PubMed] [Google Scholar]
- Crews F. T.; Bechara R.; Brown L. A.; Guidot D. M.; Mandrekar P.; Oak S.; Qin L.; Szabo G.; Wheeler M.; Zou J. (2006) Cytokines and alcohol. Alcohol.: Clin. Exp. Res. 30, 720–730. [DOI] [PubMed] [Google Scholar]
- Matsumura Y.; Byrne S. N.; Nghiem D. X.; Miyahara Y.; Ullrich S. E. (2006) A role for inflammatory mediators in the induction of immunoregulatory B cells. J. Immunol. 177, 4810–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith E. J.; Holder M. J.; Chamba A.; Challa A.; Drake Lee A.; Bunce C. M.; Drayson M. T.; Pilkington G.; Blakely R. D.; Dyer M. J.; Barnes N. M.; Gordon J. (2005) The serotonin transporter (SLC6A4) is present in B-cell clones of diverse malignant origin: probing a potential antitumor target for psychotropics. FASEB J. 19, 1187–1189. [DOI] [PubMed] [Google Scholar]
- Anthony I. C.; Crawford D. H.; Bell J. E. (2003) B lymphocytes in the normal brain: contrasts with HIV-associated lymphoid infiltrates and lymphomas. Brain 126, 1058–1067. [DOI] [PubMed] [Google Scholar]
- Pryce G.; Santos W.; Male D. (1994) An assay for the analysis of lymphocyte migration across cerebral endothelium in vitro. J. Immunol. Methods 167, 55–63. [DOI] [PubMed] [Google Scholar]
- Hickey W. F.; Hsu B. L.; Kimura H. (1991) T-lymphocyte entry into the central nervous system. J. Neurosci. Res. 28, 254–260. [DOI] [PubMed] [Google Scholar]
- Walther D. J.; Peter J. U.; Winter S.; Holtje M.; Paulmann N.; Grohmann M.; Vowinckel J.; Alamo-Bethencourt V.; Wilhelm C. S.; Ahnert-Hilger G.; Bader M. (2003) Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell 115, 851–862. [DOI] [PubMed] [Google Scholar]
- Serafeim A.; Grafton G.; Chamba A.; Gregory C. D.; Blakely R. D.; Bowery N. G.; Barnes N. M.; Gordon J. (2002) 5-Hydroxytryptamine drives apoptosis in biopsylike Burkitt lymphoma cells: reversal by selective serotonin reuptake inhibitors. Blood 99, 2545–2553. [DOI] [PubMed] [Google Scholar]
- Schneider E.; Machavoine F.; Bricard-Rignault R.; Levasseur M.; Petit-Bertron A. F.; Gautron S.; Ribeil J. A.; Launay J. M.; Mecheri S.; Cote F.; Dy M. (2011) Downregulation of basophil-derived IL-4 and in vivo T(H)2 IgE responses by serotonin and other organic cation transporter 3 ligands. J. Allergy Clin. Immunol. 128, 864–871; e862.. [DOI] [PubMed] [Google Scholar]
- Hellstrand K.; Hermodsson S. (1993) Serotonergic 5-HT1A receptors regulate a cell contact-mediated interaction between natural killer cells and monocytes. Scand. J. Immunol. 37, 7–18. [DOI] [PubMed] [Google Scholar]
- Young M. R.; Kut J. L.; Coogan M. P.; Wright M. A.; Young M. E.; Matthews J. (1993) Stimulation of splenic T-lymphocyte function by endogenous serotonin and by low-dose exogenous serotonin. Immunology 80, 395–400. [PMC free article] [PubMed] [Google Scholar]
- Young M. R.; Matthews J. P. (1995) Serotonin regulation of T-cell subpopulations and of macrophage accessory function. Immunology 84, 148–152. [PMC free article] [PubMed] [Google Scholar]
- Katoh N.; Soga F.; Nara T.; Tamagawa-Mineoka R.; Nin M.; Kotani H.; Masuda K.; Kishimoto S. (2006) Effect of serotonin on the differentiation of human monocytes into dendritic cells. Clin. Exp. Immunol. 146, 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumery L.; Bourdel F.; Soussan Y.; Fialkowsky A.; Viale S.; Nicolas P.; Reboud-Ravaux M. (2001) beta-Amyloid protein aggregation: its implication in the physiopathology of Alzheimer’s disease. Pathol. Biol. 49, 72–85. [DOI] [PubMed] [Google Scholar]
- Martins L. C.; Rocha N. P.; Torres K. C.; Dos Santos R. R.; Franca G. S.; de Moraes E. N.; Mukhamedyarov M. A.; Zefirov A. L.; Rizvanov A. A.; Kiyasov A. P.; Vieira L. B.; Guimaraes M. M.; Yalvac M. E.; Teixeira A. L.; Bicalho M. A.; Janka Z.; Romano-Silva M. A.; Palotas A.; Reis H. J. (2012) Disease-specific expression of the serotonin-receptor 5-HT(2C) in natural killer cells in Alzheimer’s dementia. J. Neuroimmunol. 251, 73–79. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J.; Blakely R. D. (2012) Networking in autism: leveraging genetic, biomarker and model system findings in the search for new treatments. Neuropsychopharmacology 37, 196–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. S.; Delahanty R. J.; Prasad H. C.; McCauley J. L.; Han Q.; Jiang L.; Li C.; Folstein S. E.; Blakely R. D. (2005) Allelic Heterogeneity at the Serotonin Transporter Locus (SLC6A4) Confers Susceptibility to Autism and Rigid-Compulsive Behaviors. Am. J. Hum. Genet. 77, 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J.; Muller C. L.; Iwamoto H.; Sauer J. E.; Owens W. A.; Shah C. R.; Cohen J.; Mannangatti P.; Jessen T.; Thompson B. J.; Ye R.; Kerr T. M.; Carneiro A. M.; Crawley J. N.; Sanders-Bush E.; McMahon D. G.; Ramamoorthy S.; Daws L. C.; Sutcliffe J. S.; Blakely R. D. (2012) Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc. Natl. Acad. Sci. U.S.A. 109, 5469–5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind D. H. (2009) Advances in autism. Annu. Rev. Med. 60, 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao E. Y.; McBride S. W.; Chow J.; Mazmanian S. K.; Patterson P. H. (2012) Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc. Natl. Acad. Sci. U.S.A. 109, 12776–12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad H. C., Hewlett W. A., Sutcliffe J. S., and Blakely R. D. (2012) Immune System Dysregulation in Autism Linked to Hypperactivity of the Serotonin Transporter. Presented at the Biological Psychiatry Conference, Philadelphia, PA, May 3, 2012.
- Carneiro A. M.; Airey D. C.; Thompson B.; Zhu C. B.; Lu L.; Chesler E. J.; Erikson K. M.; Blakely R. D. (2009) Functional coding variation in recombinant inbred mouse lines reveals multiple serotonin transporter-associated phenotypes. Proc. Natl. Acad. Sci. U.S.A. 106, 2047–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. L.; Dong C.; Maestre-Mesa J.; Licinio J. (2008) Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol. Psychiatry 13, 800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune B. T.; Dannlowski U.; Domschke K.; Janssen D. G.; Jordan M. A.; Ohrmann P.; Bauer J.; Biros E.; Arolt V.; Kugel H.; Baxter A. G.; Suslow T. (2010) The interleukin 1 beta (IL1B) gene is associated with failure to achieve remission and impaired emotion processing in major depression. Biol. Psychiatry 67, 543–549. [DOI] [PubMed] [Google Scholar]
- Hwang J. P.; Tsai S. J.; Hong C. J.; Yang C. H.; Hsu C. D.; Liou Y. J. (2009) Interleukin-1 beta −511C/T genetic polymorphism is associated with age of onset of geriatric depression. Neuromol. Med. 11, 322–327. [DOI] [PubMed] [Google Scholar]
- Cerri A. P.; Arosio B.; Viazzoli C.; Confalonieri R.; Teruzzi F.; Annoni G. (2009) -308(G/A) TNF-alpha gene polymorphism and risk of depression late in the life. Arch. Gerontol. Geriatr. 49(Suppl 1), 29–34. [DOI] [PubMed] [Google Scholar]
- Cerri A. P.; Arosio B.; Viazzoli C.; Confalonieri R.; Vergani C.; Annoni G. (2010) The −308 (G/A) single nucleotide polymorphism in the TNF-alpha gene and the risk of major depression in the elderly. Int. J. Geriatr. Psychiatry 25, 219–223. [DOI] [PubMed] [Google Scholar]
- Galecki P.; Florkowski A.; Bienkiewicz M.; Szemraj J. (2010) Functional polymorphism of cyclooxygenase-2 gene (G-765C) in depressive patients. Neuropsychobiology 62, 116–120. [DOI] [PubMed] [Google Scholar]
- Schiepers O. J.; Wichers M. C.; Maes M. (2005) Cytokines and major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 201–217. [DOI] [PubMed] [Google Scholar]
- Smith R. S. (1991) The macrophage theory of depression. Med. Hypotheses 35, 298–306. [DOI] [PubMed] [Google Scholar]
- Capuron L.; Ravaud A. (1999) Prediction of the depressive effects of interferon alfa therapy by the patient’s initial affective state. N. Engl. J. Med. 340, 1370. [DOI] [PubMed] [Google Scholar]
- Capuron L.; Ravaud A.; Dantzer R. (2000) Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J. Clin. Oncol. 18, 2143–2151. [DOI] [PubMed] [Google Scholar]
- Evans D. L.; Lynch K. G.; Benton T.; Dube B.; Gettes D. R.; Tustin N. B.; Lai J. P.; Metzger D.; Douglas S. D. (2008) Selective serotonin reuptake inhibitor and substance P antagonist enhancement of natural killer cell innate immunity in human immunodeficiency virus/acquired immunodeficiency syndrome. Biol. Psychiatry 63, 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M. G.; Hendricks S. E.; Johnson D. R.; Wieseler J. L.; Burke W. J. (1999) Antidepressants augment natural killer cell activity: in vivo and in vitro. Neuropsychobiology 39, 18–24. [DOI] [PubMed] [Google Scholar]
- Hernandez M. E.; Martinez-Fong D.; Perez-Tapia M.; Estrada-Garcia I.; Estrada-Parra S.; Pavon L. (2010) Evaluation of the effect of selective serotonin-reuptake inhibitors on lymphocyte subsets in patients with a major depressive disorder. Eur Neuropsychopharmacol. 20, 88–95. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Mix E.; Olsson T.; Link H. (1995) Influence of ion channel modulation on in vitro interferon-gamma induced MHC class I and II expression on macrophages. Immunopharmacol. Immunotoxicol. 17, 109–136. [DOI] [PubMed] [Google Scholar]
- Horikawa H.; Kato T. A.; Mizoguchi Y.; Monji A.; Seki Y.; Ohkuri T.; Gotoh L.; Yonaha M.; Ueda T.; Hashioka S.; Kanba S. (2010) Inhibitory effects of SSRIs on IFN-gamma induced microglial activation through the regulation of intracellular calcium. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 1306–1316. [DOI] [PubMed] [Google Scholar]
- Hannestad J.; DellaGioia N.; Bloch M. (2011) The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology 36, 2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima L.; Urbina M. (2002) Serotonin transporter modulation in blood lymphocytes from patients with major depression. Cell. Mol. Neurobiol. 22, 797–804. [DOI] [PubMed] [Google Scholar]
- Thompson B. J.; Jessen T.; Henry L. K.; Field J. R.; Gamble K. L.; Gresch P. J.; Carneiro A. M.; Horton R. E.; Chisnell P. J.; Belova Y.; McMahon D. G.; Daws L. C.; Blakely R. D. (2011) Transgenic elimination of high-affinity antidepressant and cocaine sensitivity in the presynaptic serotonin transporter. Proc. Natl. Acad. Sci. U.S.A. 108, 3785–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M. (2001) The immunoregulatory effects of antidepressants. Hum. Psychopharmacol. 16, 95–103. [DOI] [PubMed] [Google Scholar]
- Sacre S.; Medghalchi M.; Gregory B.; Brennan F.; Williams R. (2010) Fluoxetine and citalopram exhibit potent antiinflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum 62, 683–693. [DOI] [PubMed] [Google Scholar]
- Tsao C. W.; Lin Y. S.; Chen C. C.; Bai C. H.; Wu S. R. (2006) Cytokines and serotonin transporter in patients with major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 899–905. [DOI] [PubMed] [Google Scholar]
- Basterzi A. D.; Aydemir C.; Kisa C.; Aksaray S.; Tuzer V.; Yazici K.; Goka E. (2005) IL-6 levels decrease with SSRI treatment in patients with major depression. Hum. Psychopharmacol. 20, 473–476. [DOI] [PubMed] [Google Scholar]
- O’Brien S. M.; Scully P.; Fitzgerald P.; Scott L. V.; Dinan T. G. (2007) Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J. Psychiatr. Res. 41, 326–331. [DOI] [PubMed] [Google Scholar]
- Tynan R. J.; Weidenhofer J.; Hinwood M.; Cairns M. J.; Day T. A.; Walker F. R. (2012) A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav. Immun 26, 469–479. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt J. L.; Vanover K. E.; Chen E. Y.; Marshall J. J.; Greengard P. (2011) Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc. Natl. Acad. Sci. U.S.A. 108, 9262–9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. Y.; Kim S. H.; Yang Y. R.; Song P.; Yu H. S.; Park H. G.; Hwang O.; Lee-Kwon W.; Seo J. K.; Hwang D.; Choi J. H.; Bucala R.; Ryu S. H.; Kim Y. S.; Suh P. G. (2012) Macrophage migration inhibitory factor mediates the antidepressant actions of voluntary exercise. Proc. Natl. Acad. Sci. U.S.A. 109, 13094–13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. W.; Chen T. J.; Hong C. J.; Chen H. M.; Tsai S. J. (2003) Association study of the interleukin-1 beta (C-511T) genetic polymorphism with major depressive disorder, associated symptomatology, and antidepressant response. Neuropsychopharmacology 28, 1182–1185. [DOI] [PubMed] [Google Scholar]
- Tadic A.; Rujescu D.; Muller M. J.; Kohnen R.; Stassen H. H.; Szegedi A.; Dahmen N. (2008) Association analysis between variants of the interleukin-1beta and the interleukin-1 receptor antagonist gene and antidepressant treatment response in major depression. Neuropsychiatr. Dis. Treat. 4, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. L.; Lesch K. P. (2008) Targeting the murine serotonin transporter: insights into human neurobiology. Nat. Rev. Neurosci. 9, 85–96. [DOI] [PubMed] [Google Scholar]
- Uher R.; Perroud N.; Ng M. Y.; Hauser J.; Henigsberg N.; Maier W.; Mors O.; Placentino A.; Rietschel M.; Souery D.; Zagar T.; Czerski P. M.; Jerman B.; Larsen E. R.; Schulze T. G.; Zobel A.; Cohen-Woods S.; Pirlo K.; Butler A. W.; Muglia P.; Barnes M. R.; Lathrop M.; Farmer A.; Breen G.; Aitchison K. J.; Craig I.; Lewis C. M.; McGuffin P. (2010) Genome-wide pharmacogenetics of antidepressant response in the GENDEP project. Am. J. Psychiatry 167, 555–564. [DOI] [PubMed] [Google Scholar]
- Cutler J. A.; Rush A. J.; McMahon F. J.; Laje G. (2012) Common genetic variation in the indoleamine-2,3-dioxygenase genes and antidepressant treatment outcome in major depressive disorder. J. Psychopharmacol. 26, 360–367. [DOI] [PubMed] [Google Scholar]
- Crona D.; Harral J.; Adnot S.; Eddahibi S.; West J. (2009) Gene expression in lungs of mice lacking the 5-hydroxytryptamine transporter gene. BMC Pulm. Med. 9, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff S. C.; Mailer R.; Pabst O.; Weier G.; Sedlik W.; Li Z.; Chen J. J.; Murphy D. L.; Gershon M. D. (2009) Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am. J. Physiol.: Gastrointest. Liver Physiol. 296, G685–695. [DOI] [PubMed] [Google Scholar]
- Hofstetter H.; Gold R.; Hartung H. P. (2009) Th17 Cells in MS and Experimental Autoimmune Encephalomyelitis. Int. MS J. 16, 12–18. [PubMed] [Google Scholar]
- Li N.; Ghia J. E.; Wang H.; McClemens J.; Cote F.; Suehiro Y.; Mallet J.; Khan W. I. (2011) Serotonin activates dendritic cell function in the context of gut inflammation. Am. J. Pathol. 178, 662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Y. S.; Sawarynski L. E.; Michael H. M.; Ferrell R. E.; Murphey-Corb M. A.; Swain G. M.; Patel B. A.; Andrews A. M. (2010) Boron-Doped Diamond Microelectrodes Reveal Reduced Serotonin Uptake Rates in Lymphocytes from Adult Rhesus Monkeys Carrying the Short Allele of the 5-HTTLPR. ACS Chem. Neurosci. 1, 49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Y. S.; Altieri S. C.; Gilman T. L.; Michael H. M.; Tomlinson I. D.; Rosenthal S. J.; Swain G. M.; Murphey-Corb M. A.; Ferrell R. E.; Andrews A. M. (2012) Differential serotonin transport is linked to the rh5-HTTLPR in peripheral blood cells. Transl. Psychiatry 2, e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho L. S.; Correa H.; Silva G. C.; Campos F. S.; Baiao F. R.; Ribeiro L. S.; Faria A. M.; d’Avila Reis D. (2008) May genetic factors in fibromyalgia help to identify patients with differentially altered frequencies of immune cells?. Clin. Exp. Immunol. 154, 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima L.; Mata S.; Urbina M. (2005) Allelic isoforms and decrease in serotonin transporter mRNA in lymphocytes of patients with major depression. Neuroimmunomodulation 12, 299–306. [DOI] [PubMed] [Google Scholar]
- Mossner R.; Daniel S.; Schmitt A.; Albert D.; Lesch K. P. (2001) Modulation of serotonin transporter function by interleukin-4. Life Sci. 68, 873–880. [DOI] [PubMed] [Google Scholar]
- Matsunaga M.; Murakami H.; Yamakawa K.; Isowa T.; Kasugai K.; Yoneda M.; Kaneko H.; Fukuyama S.; Shinoda J.; Yamada J.; Ohira H. (2010) Genetic variations in the serotonin transporter gene-linked polymorphic region influence attraction for a favorite person and the associated interactions between the central nervous and immune systems. Neurosci. Lett. 468, 211–215. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. (1984) Interleukin-1. Rev. Infect. Dis. 6, 51–95. [DOI] [PubMed] [Google Scholar]
- Loppnow H.; Libby P.; Freudenberg M.; Krauss J. H.; Weckesser J.; Mayer H. (1990) Cytokine induction by lipopolysaccharide (LPS) corresponds to lethal toxicity and is inhibited by nontoxic Rhodobacter capsulatus LPS. Infect. Immun. 58, 3743–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn M. J.; Killcross S. (2006) Differential attenuation of d-amphetamine-induced disruption of conditional discrimination performance by dopamine and serotonin antagonists. Psychopharmacology (Berlin, Ger.) 188, 183–192. [DOI] [PubMed] [Google Scholar]
- Qin L.; He J.; Hanes R. N.; Pluzarev O.; Hong J. S.; Crews F. T. (2008) Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflammation 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatteis C. M. (1990) Neuromodulative actions of cytokines. Yale J. Biol. Med. 63, 133–146. [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y.; Stins M.; Way D.; Witte M. H.; Weinand M.; Kim K. S.; Bock P.; Gendelman H. E.; Fiala M. (1997) A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J. Immunol. 158, 3499–3510. [PubMed] [Google Scholar]
- Banks W. A.; Kastin A. J.; Broadwell R. D. (1995) Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 2, 241–248. [DOI] [PubMed] [Google Scholar]
- Mark K. S.; Miller D. W. (1999) Increased permeability of primary cultured brain microvessel endothelial cell monolayers following TNF-alpha exposure. Life Sci. 64, 1941–1953. [DOI] [PubMed] [Google Scholar]
- Raub T. J.; Kuentzel S. L.; Sawada G. A. (1992) Permeability of bovine brain microvessel endothelial cells in vitro: barrier tightening by a factor released from astroglioma cells. Exp. Cell Res. 199, 330–340. [DOI] [PubMed] [Google Scholar]
- Kacimi R.; Giffard R. G.; Yenari M. A. (2011) Endotoxin-activated microglia injure brain derived endothelial cells via NF-kappaB, JAK-STAT and JNK stress kinase pathways. J. Inflammation (London, U.K.) 8, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N.; Whiteside M.; Kim L.; Herkenham M. (1997) Induction of inhibitory factor kappaBalpha mRNA in the central nervous system after peripheral lipopolysaccharide administration: an in situ hybridization histochemistry study in the rat. Proc. Natl. Acad. Sci. U.S.A. 94, 10985–10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M.; Lee H. Y.; Baker R. A. (1998) Temporal and spatial patterns of c-fos mRNA induced by intravenous interleukin-1: a cascade of non-neuronal cellular activation at the blood-brain barrier. J. Comp. Neurol. 400, 175–196. [DOI] [PubMed] [Google Scholar]
- Banks W. A. (2005) Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr. Pharm. Des. 11, 973–984. [DOI] [PubMed] [Google Scholar]
- Banks W. A.; Kastin A. J.; Gutierrez E. G. (1993) Interleukin-1 alpha in blood has direct access to cortical brain cells. Neurosci. Lett. 163, 41–44. [DOI] [PubMed] [Google Scholar]
- McLay R. N.; Kastin A. J.; Zadina J. E. (2000) Passage of interleukin-1-beta across the blood-brain barrier is reduced in aged mice: a possible mechanism for diminished fever in aging. Neuroimmunomodulation 8, 148–153. [DOI] [PubMed] [Google Scholar]
- Magni F.; Bruschi F.; Kasti M. (1987) The afferent innervation of the thymus gland in the rat. Brain Res. 424, 379–385. [DOI] [PubMed] [Google Scholar]
- Dunn A. J. (1992) Endotoxin-induced activation of cerebral catecholamine and serotonin metabolism: comparison with interleukin-1. J. Pharmacol. Exp. Ther. 261, 964–969. [PubMed] [Google Scholar]
- Gutierrez E. G.; Banks W. A.; Kastin A. J. (1993) Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J. Neuroimmunol. 47, 169–176. [DOI] [PubMed] [Google Scholar]
- Gutierrez E. G.; Banks W. A.; Kastin A. J. (1994) Blood-borne interleukin-1 receptor antagonist crosses the blood-brain barrier. J. Neuroimmunol. 55, 153–160. [DOI] [PubMed] [Google Scholar]
- Ma X. C.; Chen L. T.; Oliver J.; Horvath E.; Phelps C. P. (2000) Cytokine and adrenal axis responses to endotoxin. Brain Res. 861, 135–142. [DOI] [PubMed] [Google Scholar]
- Ericsson A.; Kovacs K. J.; Sawchenko P. E. (1994) A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J. Neurosci. 14, 897–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M.; Ochani M.; Parrish W. R.; Ochani K.; Harris Y. T.; Huston J. M.; Chavan S.; Tracey K. J. (2008) Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. U.S.A. 105, 11008–11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulloch K.; Pomerantz W. (1984) Autonomic nervous system innervation of thymic-related lymphoid tissue in wildtype and nude mice. J. Comp. Neurol. 228, 57–68. [DOI] [PubMed] [Google Scholar]
- Pospieszny N. (1979) Distribution of the vagus nerve of the stomach and certain lymph nodes of the sheep in the prenatal period (author’s transl). Anat. Anz. 146, 47–59. [PubMed] [Google Scholar]
- Olofsson P. S.; Rosas-Ballina M.; Levine Y. A.; Tracey K. J. (2012) Rethinking inflammation: neural circuits in the regulation of immunity. Immunol. Rev. 248, 188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niijima A.; Hori T.; Katafuchi T.; Ichijo T. (1995) The effect of interleukin-1 beta on the efferent activity of the vagus nerve to the thymus. J. Auton. Nerv. Syst. 54, 137–144. [DOI] [PubMed] [Google Scholar]
- Goehler L. E.; Gaykema R. P.; Hammack S. E.; Maier S. F.; Watkins L. R. (1998) Interleukin-1 induces c-Fos immunoreactivity in primary afferent neurons of the vagus nerve. Brain Res. 804, 306–310. [DOI] [PubMed] [Google Scholar]
- Ek M.; Kurosawa M.; Lundeberg T.; Ericsson A. (1998) Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins. J. Neurosci. 18, 9471–9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler L. E.; Gaykema R. P.; Nguyen K. T.; Lee J. E.; Tilders F. J.; Maier S. F.; Watkins L. R. (1999) Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems?. J. Neurosci. 19, 2799–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosevelt R. W.; Smith D. C.; Clough R. W.; Jensen R. A.; Browning R. A. (2006) Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 1119, 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan W.; Wetmore L.; Sorensen C. M.; Greenberg A. H.; Nance D. M. (1994) Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res. Bull. 34, 7–14. [DOI] [PubMed] [Google Scholar]
- Konsman J. P.; Luheshi G. N.; Bluthe R. M.; Dantzer R. (2000) The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur. J. Neurosci. 12, 4434–4446. [DOI] [PubMed] [Google Scholar]
- Romeo H. E.; Tio D. L.; Taylor A. N. (2003) Effects of glossopharyngeal nerve transection on central and peripheral cytokines and serum corticosterone induced by localized inflammation. J. Neuroimmunol. 136, 104–111. [DOI] [PubMed] [Google Scholar]
- Brambilla D.; Francoisi S.; Opp M. R.; Imeri L. (2007) Interleukin-1 inhibits firing of serotonergic neurons in the dorsal raphe nucleus and enhances GABAergic inhibitory post-synaptic potentials. Eur. J. Neurosci. 26, 1862–1869. [DOI] [PubMed] [Google Scholar]
- Merali Z.; Lacosta S.; Anisman H. (1997) Effects of interleukin-1beta and mild stress on alterations of norepinephrine, dopamine and serotonin neurotransmission: a regional microdialysis study. Brain Res. 761, 225–235. [DOI] [PubMed] [Google Scholar]
- Shintani F.; Kanba S.; Nakaki T.; Nibuya M.; Kinoshita N.; Suzuki E.; Yagi G.; Kato R.; Asai M. (1993) Interleukin-1b a augments release of norepinephrine, dopamine, and serotonin in the rat anterior hypothalamus. J. Neurosci. 13(8), 3574–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst A. C.; Flachskamm C.; Holsboer F.; Reul J. M. (1996) Activation of serotonergic and noradrenergic neurotransmission in the rat hippocampus after peripheral administration of bacterial endotoxin: involvement of the cyclo-oxygenase pathway. Neuroscience 72, 989–997. [DOI] [PubMed] [Google Scholar]
- Dunn A. J. (1988) Systemic interleukin-1 administration stimulates hypothalamic norepinephrine metabolism parallelling the increased plasma corticosterone. Life Sci. 43, 429–435. [DOI] [PubMed] [Google Scholar]
- Kabiersch A.; del Rey A.; Honegger C. G.; Besedovsky H. O. (1988) Interleukin-1 induces changes in norepinephrine metabolism in the rat brain. Brain, Behav., Immun. 2, 267–274. [DOI] [PubMed] [Google Scholar]
- Mohankumar P. S.; Thyagarajan S.; Quadri S. K. (1993) Interleukin-1 beta increases 5-hydroxyindoleacetic acid release in the hypothalamus in vivo. Brain Res. Bull. 31, 745–748. [DOI] [PubMed] [Google Scholar]
- Dunn A. J.; Welch J. (1991) Stress- and endotoxin-induced increases in brain tryptophan and serotonin metabolism depend on sympathetic nervous system activity. J. Neurochem. 57, 1615–1622. [DOI] [PubMed] [Google Scholar]
- Maes M.; Verkerk R.; Vandoolaeghe E.; Van Hunsel F.; Neels H.; Wauters A.; Demedts P.; Scharpe S. (1997) Serotonin-immune interactions in major depression: lower serum tryptophan as a marker of an immune-inflammatory response. Eur. Arch. Psychiatry Clin. Neurosci. 247, 154–161. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Terreni L.; De Simoni M. G.; Dunn A. J. (2001) Peripheral interleukin-6 administration increases extracellular concentrations of serotonin and the evoked release of serotonin in the rat striatum. Neurochem. Int. 38, 303–308. [DOI] [PubMed] [Google Scholar]
- Masson J.; Hamon M. (2009) Monaomine Transporters: Focus on the Regulation of Serotonin Transpoter by Cytokines. Encycl. Neurosci. 921–929. [Google Scholar]
- Mossner R.; Heils A.; Stober G.; Okladnova O.; Daniel S.; Lesch K. P. (1998) Enhancement of serotonin transporter function by tumor necrosis factor alpha but not by interleukin-6. Neurochem. Int. 33, 251–254. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S.; Ramamoorthy J. D.; Prasad P.; Bhat G. K.; Mahesh V. B.; Leibach F. H.; Ganapathy V. (1995) Regulation of the human serotonin transporter by interleukin-1β. Biochem. Biophys. Res. Commun. 216, 560–567. [DOI] [PubMed] [Google Scholar]
- Morikawa O.; Sakai N.; Obara H.; Saito N. (1998) Effects of interferon-alpha, interferon-gamma and cAMP on the transcriptional regulation of the serotonin transporter. Eur. J. Pharmacol. 349, 317–324. [DOI] [PubMed] [Google Scholar]
- Mössner R.; Daniel S.; Schmitt A.; Albert D.; Lesch K. P. (2001) Modulation of serotonin transporter function by interleukin-4. Life Sci. 68, 873–880. [DOI] [PubMed] [Google Scholar]
- Su S.; Zhao J.; Bremner J. D.; Miller A. H.; Tang W.; Bouzyk M.; Snieder H.; Novik O.; Afzal N.; Goldberg J.; Vaccarino V. (2009) Serotonin transporter gene, depressive symptoms, and interleukin-6. Circ Cardiovasc Genet 2, 614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N.; Duivis H. E.; Beekman A. T.; Kluft C.; Neuteboom J.; Hoogendijk W.; Smit J. H.; de Jonge P.; Penninx B. W. (2012) Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl Psychiatry 2, e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y.; Herrmann N.; Swardfager W.; Liu H.; Sham L.; Reim E. K.; Lanctot K. L. (2010) A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457. [DOI] [PubMed] [Google Scholar]
- Howren M. B.; Lamkin D. M.; Suls J. (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 71, 171–186. [DOI] [PubMed] [Google Scholar]
- Prasad H. C.; Zhu C. B.; McCauley J. L.; Samuvel D. J.; Ramamoorthy S.; Shelton R. C.; Hewlett W. A.; Sutcliffe J. S.; Blakely R. D. (2005) Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc. Natl. Acad. Sci. U.S.A. 102, 11545–11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J. A.; Carneiro A. M.; Blakely R. D. (2008) Going with the flow: trafficking-dependent and -independent regulation of serotonin transport. Traffic 9, 1393–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C. B.; Carneiro A. M.; Dostmann W. R.; Hewlett W. A.; Blakely R. D. (2005) p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J. Biol. Chem. 280, 15649–15658. [DOI] [PubMed] [Google Scholar]
- Qian Y.; Galli A.; Ramamoorthy S.; Risso S.; DeFelice L. J.; Blakely R. D. (1997) Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J. Neurosci. 17, 45–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S., and Blakely R. D. (1998) 5HT-modulates protein kinase C-dependent phosphorylation and trafficking of human serotonin transporter. Presented at the Society for Neuroscience Conference, Los Angeles, CA, 1998.
- Ramamoorthy S.; Melikian H. E.; Qian Y.; Blakely R. D. (1998) Biosynthesis, N-glycosylation, and surface trafficking of biogenic amine transporter proteins. Methods Enzymol. 296, 347–370. [DOI] [PubMed] [Google Scholar]
- Zhu C. B.; Hewlett W. A.; Feoktistov I.; Biaggioni I.; Blakely R. D. (2004) Adenosine Receptor, Protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol. Pharmacol. 65, 1462–1474. [DOI] [PubMed] [Google Scholar]
- Zhu C. B.; Hewlett W. A.; Francis S. H.; Corbin J. D.; Blakely R. D. (2004) Stimulation of serotonin transport by the cyclic GMP phosphodiesterase inhibitor sildenafil. Eur. J. Pharmacol. 504, 1–6. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S.; Giovanetti E.; Qian Y.; Blakely R. D. (1998) Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J. Biol. Chem. 273, 2458–2466. [DOI] [PubMed] [Google Scholar]
- Ansah T. A. R., S.; Montanez S.; Daws L. C.; Blakely R. D. (2003) Calcium-dependent inhibition of synaptosomal serotonin transport by the alpha2-adrenergic receptor agonist UK14304. J. Pharmacol. Exp. Ther. 305, 956–965. [DOI] [PubMed] [Google Scholar]
- Daws L. C.; Gould G. G.; Teicher S. D.; Gerhardt G. A.; Frazer A. (2000) 5-HT(1B) receptor-mediated regulation of serotonin clearance in rat hippocampus in vivo. J. Neurochem. 75, 2113–2122. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S.; Samuvel D. J.; Buck E. R.; Rudnick G.; Jayanthi L. D. (2007) Phosphorylation of threonine residue 276 is required for acute regulation of serotonin transporter by cyclic GMP. J. Biol. Chem. 282, 11639–11647. [DOI] [PubMed] [Google Scholar]
- Wong A.; Zhang Y. W.; Jeschke G. R.; Turk B. E.; Rudnick G. (2012) Cyclic-GMP-dependent stimulation of serotonin transport does not involve direct transporter phosphorylation by cGMP-dependent protein kinase. J. Biol. Chem. 287, 36051–36058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. C.; Tomlinson I. D.; Warnement M. R.; Ustione A.; Carneiro A. M.; Piston D. W.; Blakely R. D.; Rosenthal S. J. (2012) Single molecule analysis of serotonin transporter regulation using antagonist-conjugated quantum dots reveals restricted, p38 MAPK-dependent mobilization underlying uptake activation. J. Neurosci. 32, 8919–8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C. B.; Blakely R. D.; Hewlett W. A. (2006) The Proinflammatory Cytokines Interleukin-1beta and Tumor Necrosis Factor-Alpha Activate Serotonin Transporters. Neuropsychopharmacology 31, 2121–2131. [DOI] [PubMed] [Google Scholar]
- Eisenberger N. I.; Berkman E. T.; Inagaki T. K.; Rameson L. T.; Mashal N. M.; Irwin M. R. (2010) Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry 68, 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N. I.; Inagaki T. K.; Mashal N. M.; Irwin M. R. (2010) Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, Behav., Immun. 24, 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P.; Chergui K.; Rachleff I.; Flajolet M.; Zhang X.; El Yacoubi M.; Vaugeois J. M.; Nomikos G. G.; Greengard P. (2006) Alterations in 5-HT1B receptor function by p11 in depression-like states. Science 311, 77–80. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt J. L.; Flajolet M.; Maller A.; Chen E. Y.; Qi H.; Svenningsson P.; Greengard P. (2009) Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J. Neurosci. 29, 1937–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt J. L.; Chen E. Y.; Zhang X.; Marshall J. J.; Morozov A.; Svenningsson P.; Greengard P. (2010) A role for p11 in the antidepressant action of brain-derived neurotrophic factor. Biol. Psychiatry 68, 528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. (2001) Cytokine-induced sickness behavior: mechanisms and implications. Ann. N.Y. Acad. Sci. 933, 222–234. [DOI] [PubMed] [Google Scholar]
- Kent S.; Bluthe R. M.; Kelley K. W.; Dantzer R. (1992) Sickness behavior as a new target for drug development. Trends Pharmacol. Sci. 13, 24–28. [DOI] [PubMed] [Google Scholar]
- Yirmiya R.; Pollak Y.; Morag M.; Reichenberg A.; Barak O.; Avitsur R.; Shavit Y.; Ovadia H.; Weidenfeld J.; Morag A.; Newman M. E.; Pollmacher T. (2000) Illness, cytokines, and depression. Ann. N.Y. Acad. Sci. 917, 478–487. [DOI] [PubMed] [Google Scholar]
- Lindler K. M., Baganz N. L., Zhu C. B., Deneris E., Hewlett W. A., and Blakely R. D. (2012) Conditional Elimination of p38 MAPK and IL-1 Receptors in Raphe Serotonergic Neurons: Impact on Inflammatory Cytokine Regulation of the Serotonin Transporter and Cytokine-Modulated Behavior. In Society for Neuroscience, New Orleans, LA. [Google Scholar]
- Baganz N. L., Harbom L. J., Lindler K. M., Zhu C. B., and Blakely R. D. (2012) The role of inflammatory cytokine-linked pathways in anxiety and depressive behavior following maternal separation. In Society for Neuroscience, New Orleans, LA. [Google Scholar]
- Bruchas M. R.; Schindler A. G.; Shankar H.; Messinger D. I.; Miyatake M.; Land B. B.; Lemos J. C.; Hagan C. E.; Neumaier J. F.; Quintana A.; Palmiter R. D.; Chavkin C. (2011) Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron 71, 498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H. (2011) Maternal infection and immune involvement in autism. Trends Mol. Med. 17, 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge M. S.; Zhou M.; Lira A.; Hen R.; Gingrich J. A. (2004) Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306, 879–881. [DOI] [PubMed] [Google Scholar]
- Gaspar P.; Cases O.; Maroteaux L. (2003) The developmental role of serotonin: news from mouse molecular genetics. Nat. Rev. Neurosci. 4, 1002–1012. [DOI] [PubMed] [Google Scholar]
- Bonnin A.; Levitt P. (2011) Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 197, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]