Abstract
Degarelix is a gonadotrophin-releasing hormone (GnRH) antagonist for the first-line treatment of androgen-dependent advanced prostate cancer. It has a direct mechanism of action that blocks the action of GnRH on the pituitary with no initial surge in gonadotrophin or testosterone levels. Degarelix is the most extensively studied and widely available GnRH antagonist worldwide. Clinical studies have demonstrated similar efficacy to the GnRH agonist leuprolide in achieving testosterone suppression in patients with prostate cancer. However, degarelix produces a faster suppression of testosterone and prostate-specific antigen (PSA), with no testosterone surge or microsurges, thus preventing the risk of clinical flare in advanced disease. Clinical trials have demonstrated that degarelix can offer improved disease control when compared with a GnRH agonist in terms of superior PSA progression-free survival (suggesting that degarelix likely delays progression to castration-resistant disease), and a more significant impact on bone serum alkaline phosphatase and follicle-stimulating hormone. Degarelix is generally well tolerated, with no reports of systemic allergic reactions in any clinical studies. In conclusion, degarelix offers clinicians a rational first-line hormonal monotherapy option for the management of advanced prostate cancer.
Keywords: degarelix, GnRH agonist, GnRH antagonist, prostate cancer
Introduction
Since the pioneering work of Huggins and Hodges, which demonstrated that surgical castration resulted in significant clinical improvement in men with advanced prostate cancer (PCa) [Huggins and Hodges, 1941], androgen deprivation therapy (ADT) has been the mainstay for management of advanced/metastatic PCa [Heidenreich et al. 2012]. ADT is also recommended in combination with radiotherapy in the management of intermediate and high-risk localized disease [National Comprehensive Cancer Network, 2012].
Surgical castration, the seminal ‘gold standard’ ADT, is irreversible and can have negative psychological effects on patients [Heidenreich et al. 2012; Wirth et al. 2007]. Surgical castration has generally been replaced by medical castration induced by gonadotrophin-releasing hormone (GnRH) agonists [Kelly and Gomella, 2011]. However, GnRH agonists may be associated with mechanism-of-action drawbacks, for example, promoting a counterintuitive initial testosterone surge that might delay the onset of initial testosterone suppression and may also result in potentially detrimental exacerbation of clinical symptoms (clinical flare) in advanced disease [Van Poppel and Nilsson, 2008]. Importantly, testosterone surges can also occur following repeated administration of agonists (microsurges/acute-on-chronic responses) [Tombal, 2005; Klotz et al. 2008]; furthermore, late breakthrough testosterone escapes (>0.5 ng/ml) have been noted as a result of renewed testosterone production due to loss of GnRH receptor sensitivity during long-term treatment [Tombal and Berges, 2008; Morote et al. 2007; Perachino et al. 2010].
The GnRH antagonists offer an alternative ADT that avoids the testosterone surge and microsurges associated with agonists, and thus more closely resembles the original goal of surgical castration. Worldwide, the most extensively studied and widely available antagonist is degarelix. Following its approval in the USA in December 2008 for the treatment of advanced PCa, degarelix is now available in many countries throughout North America, Europe, Australia and South America. This review documents the growing body of clinical evidence that supports the use of degarelix in PCa and examines how its pharmacological profile may impact treatment outcomes in PCa therapy.
Rationale for development of degarelix
The counterintuitive testosterone surge associated with GnRH agonist therapy inspired the development of the GnRH antagonists, whose mechanism of action achieves a more direct effect on testosterone suppression. Thus, GnRH antagonists effect a rapid and competitive receptor binding that blocks the action of GnRH on the pituitary, with no initial increase in gonadotrophin or testosterone. Release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) is prevented and thus testicular testosterone production is rapidly suppressed [Frampton and Lyseng-Williamson, 2009].
However, earlier-generation GnRH antagonists were limited by adverse events (AEs) associated with their histamine-releasing properties [Doehn et al. 2006], which resulted in anaphylactic-like syndrome [Mongiat-Artus and Teillac, 2004]. Therefore, newer GnRH antagonist agents such as degarelix were developed to obviate the histamine-releasing characteristics of the GnRH antagonist drug class.
Preclinical studies
Degarelix has been investigated in rat and rhesus monkey preclinical models, demonstrating that subcutaneous administration produced a rapid, reversible and dose-dependent suppression of the pituitary–gonadal axis, indicated by a reduction in LH and testosterone [Broqua et al. 2002]. Subcutaneous administration of degarelix allows the formation of a gel depot that facilitates sustained drug release [Princivalle et al. 2007]. This is reflected in the prolonged LH and testosterone suppression associated with degarelix which, in comparative studies, displayed a longer duration of action than other GnRH antagonists (abarelix, cetrorelix, ganirelix, azaline B).
With early GnRH antagonists, histamine release from mast cells [Schmidt et al. 1984; Hook et al. 1985] resulted in systemic or local anaphylactoid reactions [Broqua et al. 2002]. Indeed, abarelix, the first antagonist clinically available for PCa, was associated with a risk of systemic allergic reactions [Mongiat-Artus and Teillac, 2004]; this agent was voluntarily withdrawn from the US market for commercial reasons. In animal studies, degarelix displayed only weak histamine-releasing properties, having the lowest propensity for histamine release among the GnRH antagonists tested [Broqua et al. 2002]. Similar findings were revealed in an ex vivo human skin model, with degarelix again displaying the lowest propensity to release histamine versus ganirelix, abarelix and cetrorelix [Koechling et al. 2010].
Pharmacokinetics
Degarelix forms a depot after subcutaneous injection, from which the drug is released in two phases into the circulation [Frampton and Lyseng-Williamson 2009]. This biphasic pattern of disposition comprises a short initial fast-release phase followed by a second slow-release phase in which plasma levels display a half-life of several weeks [Steinberg, 2009; White et al. 2007]. Phase III data from study CS21 showed that in patients with PCa, after a single dose of 240 mg, C max (the maximum plasma level) of degarelix was 66 ng/ml, the area under the concentration–time curve (day 0–28) was 635 ng ⋅day per ml and the mean time to Cmax was 40 h. Based on population pharmacokinetic modeling, estimates of the median terminal half-lives for the starting and maintenance doses were around 43 days and 28 days, respectively. The long half-life after subcutaneous injection of degarelix is a consequence of a very slow release of the drug from the depot that is formed at the injection site. Dose adjustment is not required in older patients or in patients with mild or moderate renal or hepatic impairment. Due to the scarcity or lack of data on patients with severe renal or hepatic dysfunction, caution is warranted in these groups [Frampton and Lyseng-Williamson 2009].
Efficacy
Dose-finding studies
Europe/South Africa and North American studies
To determine the optimal dosing schedule for degarelix, two open-label, randomized, 1-year dose-finding studies of similar overall design were conducted in Europe/South Africa [Van Poppel et al. 2008] and North America [Gittelman et al. 2008]. The European study (n = 189) compared six degarelix treatment groups with starting doses of 200 or 240 mg followed by maintenance doses of 80, 120, or 160 mg. The median age of patients was 72 years and the median testosterone and PSA levels at baseline were 4.13 ng/ml and 27.6 ng/ml, respectively. Disease stage was localized in 22%, locally advanced in 32%, metastatic in 19% and not classifiable in 27%. The North American trial (n = 127) investigated a starting dose of degarelix of 200 mg followed by monthly maintenance doses of 60 or 80 mg. The median age of patients was 76 years and the median testosterone and PSA levels at baseline were 4.13 ng/ml and 13.4 ng/ml, respectively. Disease stage was localized in 43%, locally advanced in 11%, metastatic in 19% and not classifiable in 28%. In both trials, degarelix was well tolerated and degarelix treatment for 1 year was associated with a rapid, profound, and sustained suppression of testosterone to castrate testosterone levels (≤0.5 ng/ml) without an initial testosterone surge. These trials also showed rapid PSA suppression with degarelix and PSA was maintained at low levels throughout both studies. Together, these studies identified a starting dose of 240 mg and maintenance doses of 80 or 160 mg for further phase III investigation.
Japanese study
The efficacy and safety of degarelix 240/80 mg and 240/160 mg was also assessed in a 1-year randomized phase II dose-finding trial in Japanese patients (n = 273) with PCa [Ozono et al. 2012]. In the degarelix 240/80 mg and 240/160 mg groups, the mean age of patients was 75 and 74 years, the median baseline testosterone levels were 4.52 and 4.31 ng/ml and the median baseline PSA levels were 24.65 and 20.20 ng/ml, respectively. Overall, disease stage was localized in 46%, locally advanced in 30%, metastatic in 23% and not classifiable in less than 1%. Both degarelix regimens rapidly and effectively suppressed testosterone to castrate levels, without a testosterone surge. PSA and FSH levels were also rapidly suppressed and low levels maintained during the rest of the study.
The best overall tumor response [complete response (CR) + partial response (PR); according to Response Evaluation Criteria In Solid Tumors (RECIST) guidelines] occurred in 71.4% of patients in the 240/80 mg group and 72.7% in the 240/160 mg group. Tumor response was also evaluated using the Assessment Criteria of Response to Noninvasive Treatment for Prostate Cancer (as defined by the Japanese Urological Association); the overall response rate (CR + PR) showed a rapid and sustained antitumor effect. The risk–benefit balance for efficacy and safety in this trial showed that an appropriate monthly degarelix regimen for Japanese patients with PCa was 240/80 mg.
Phase III trial
A randomized, open-label trial (CS21) in North America/Europe compared the efficacy of degarelix with the GnRH agonist leuprolide for achieving and maintaining testosterone suppression over 1 year [Klotz et al. 2008]. Patients with PCa (n = 610) (localized 31%, locally advanced 29%, metastatic 20%, not classifiable 19%) were randomized to degarelix 240 mg for 1 month followed by monthly maintenance doses of 80 mg (n = 207) or 160 mg (n = 202), or monthly leuprolide 7.5 mg (n = 201). Randomization was stratified by geographical region and body weight. In the leuprolide group, 11% of patients received flare protection via concomitant bicalutamide (given at the investigator’s discretion). The median age of patients was 73 years and the median testosterone and PSA levels at baseline were 3.93 and 19.0 ng/ml, respectively.
Both degarelix regimens were as effective as leuprolide in suppressing testosterone to ≤0.5 ng/ml between days 28 and 364 (primary endpoint, considered a treatment response). Treatment response was achieved by 97.2%, 98.3% and 96.4% of the degarelix 240/80 mg, 240/160 mg and leuprolide groups, respectively (intention-to-treat population). However, degarelix suppressed testosterone significantly faster (Figure 1). At day 3, castrate testosterone (≤0.5 ng/ml) was reached by around 96% of patients in the degarelix 240/160 and 240/80 mg groups; in contrast, no patients on leuprolide had achieved castrate levels [Klotz et al. 2008]. Indeed, with leuprolide, median testosterone increased from baseline by 65% by day 3 (Figure 1), and remained greater than 0.5 ng/ml until measurements on day 28. Moreover, 80% of patients receiving leuprolide experienced a testosterone surge (increase from baseline of ≥15% on any 2 days in the initial 2 weeks of therapy) versus none in the degarelix groups. In addition, testosterone microsurges (increases of >0.25 ng/ml) in the week following the ninth injection occurred in 4% of the leuprolide group (in 2% testosterone increased to >0.5 ng/ml) versus 0% of degarelix recipients [Klotz et al. 2008].
Figure 1.
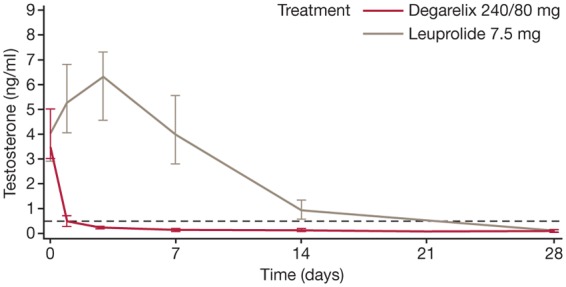
Median serum testosterone levels during the first month of treatment with degarelix versus leuprolide in the phase III CS21 trial. Reprinted from Klotz et al. [2008] with permission from Wiley-Blackwell.
PSA reduction was also significantly faster with degarelix. Median PSA had decreased by 64% and 65% in the 240/80 and 240/160 mg groups, respectively versus 18% with leuprolide by day 14. PSA decreases remained significantly greater with degarelix (85% and 83%, respectively) versus leuprolide (decrease of 68%) by day 28. PSA failure (two consecutive PSA rises of >50% versus nadir and PSA ≥5 ng/ml on two consecutive measurements at least 2 weeks apart) was lowest with degarelix 240/80 mg (the probability of PSA failure during the study was 8.9% with degarelix 240/80 mg versus 14.2% with degarelix 240/160 mg and 14.1% with leuprolide). Degarelix also produced a rapid decrease in median LH and FSH levels, which remained suppressed until the end of the trial. With leuprolide, however, there was an initial increase in LH and FSH, and FSH levels did not fall to the same extent as in the degarelix arms [Klotz et al. 2008].
On the basis of the efficacy and safety findings, the degarelix dosage of 240/80 mg was approved by the US Food and Drug Administration (FDA) in 2008 and the European Medicines Agency in 2009.
Additional analyses from CS21
Prostate-specific antigen
Tombal and colleagues reviewed the CS21 data, and concluded that during the first year of treatment, the degarelix 240/80 mg group displayed a significantly lower risk of PSA failure or death [i.e. improved PSA progression-free survival (PFS)] versus leuprolide (p = 0.05; log rank) [Tombal et al. 2010]. Indeed, after adjusting for baseline PSA and disease stage, the hazard ratio (0.664; 95% confidence interval 0.385–1.146) indicated that the risk of PSA failure or death with degarelix was 34% lower than with leuprolide. In CS21, PSA failure occurred in 26 patients (13%) in the leuprolide group and in 16 patients (8%) in the degarelix 240/80 mg group; death occurred in nine (4%) and five (2%) patients in these groups, respectively.
Around half (49.7%) of the CS21 patient population had advanced disease, and PSA failure mainly occurred in this subgroup. In patients with metastatic disease, PSA failure occurred in 21.6% of degarelix 240/80 mg versus 36.2% of leuprolide patients (p = 0.156). PSA failure was only observed in patients with baseline PSA greater than 20 ng/ml. In this subgroup, the risk of PSA failure was significantly lower for patients receiving degarelix versus leuprolide (p = 0.04). In CS21, PSA failure over time was a preplanned analysis; PSA PFS was a post hoc analysis.
Initial PSA suppression was more rapid with degarelix 240/80 mg than with leuprolide, regardless of baseline disease stage: 59% of degarelix patients achieved PSA less than 4 ng/ml at day 28 versus 34% with leuprolide (p < 0.0001). In patients with metastatic disease, there was an initial increase in PSA with leuprolide but not with degarelix. In this patient cohort, a higher proportion of the degarelix group achieved PSA less than 4 ng/ml over the study period. A large study in patients with metastatic PCa showed that a PSA of ≤4 ng/ml after 7 months of ADT is a strong predictor of survival [Hussain et al. 2006].
Serum alkaline phosphatase
In CS21, baseline levels of the bone marker serum alkaline phosphatase (S-ALP) were high in patients with metastatic disease, indicative of the presence of skeletal metastases [Schröder et al. 2010]. Baseline S-ALP levels were also three to four times higher in patients with baseline PSA >50 versus ≤50 mg/ml. There was an earlier suppression of S-ALP with degarelix 240/80 mg than with leuprolide in patients with baseline metastatic disease or baseline PSA greater than 50 ng/ml. Indeed, at day 364, reduction in S-ALP was significantly greater with degarelix 240/80 mg versus leuprolide in patients with baseline metastatic disease (p = 0.014) and in those with baseline PSA greater than 50 ng/ml (p = 0.007) (Figure 2). There was also a late increase in S-ALP with leuprolide (which might suggest treatment failure) but not with degarelix towards the end of the 1-year trial period in these patient groups (Figure 2). In localized or locally advanced disease, S-ALP levels were maintained around baseline in both treatment groups.
Figure 2.
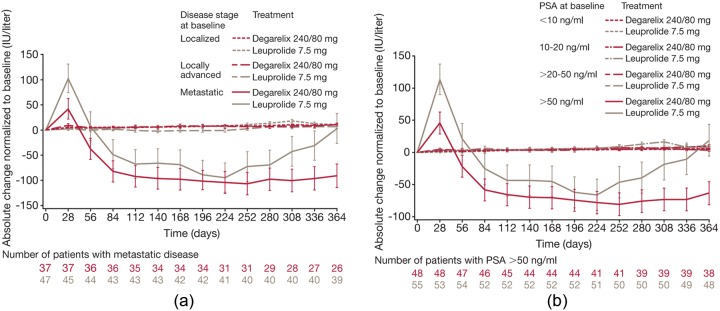
The mean (standard error of the mean) change in serum alkaline phosphatase levels (normalized to baseline) by (a) baseline disease stage and (b) baseline prostate-specific antigen (PSA) level. Reprinted from Schröder et al. [2010] with permission from Wiley-Blackwell.
Health-related quality of life
At the end of CS21, health-related quality of life (HRQoL) [measured using generic Short Form-12 v2 (SF-12) and cancer-specific European Organisation for Research and Treatment of Cancer QLQ-C30 questionnaires] for patients receiving degarelix 240/80 mg versus leuprolide was largely comparable [Gittelman et al. 2011]. However, mean SF-12 scores for the mental component summary and mental health domain were significantly higher (better) with degarelix than with leuprolide. Conversely, leuprolide had a seemingly more favorable effect on insomnia and bodily pain than degarelix; however, the mean increase in insomnia with degarelix may be explained in part by significantly lower baseline insomnia in this group. In patients with metastatic disease, there were significant improvements at month 12 in global health status for degarelix versus leuprolide, as well as role emotional and appetite loss. However, the clinical significance of these findings remains to be determined.
Long-term phase III extension trial (CS21A)
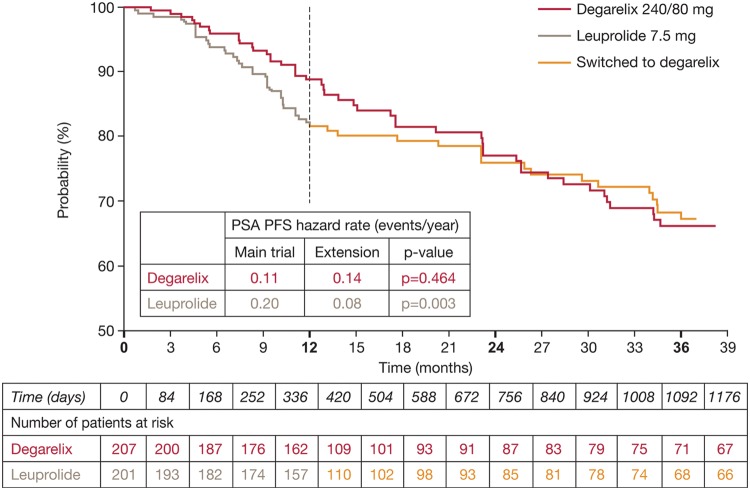
To investigate the long-term efficacy and safety of degarelix, a 5-year extension trial (CS21A) was conducted. After 1 year in CS21, patients treated with degarelix continued with the same monthly maintenance dose (160 or 80 mg), while those treated with leuprolide were randomized to degarelix 240/80 or 240/160 mg. An interim analysis was conducted after a median follow-up of 27.5 months [Crawford et al. 2011]. Data were reported for patients continuing degarelix on the approved 240/80 mg dose; for patients who crossed over from leuprolide to degarelix, the data were pooled. The interim analysis showed an improvement in PSA PFS in patients who crossed over from leuprolide to degarelix (Figure 3) [Crawford et al. 2011]. Thus, after 27.5 months of follow-up, the risk of PSA progression in 1 year was more than halved. PSA PFS hazard rates were reduced from 0.20 events/year in the first year to 0.08 events/year following the crossover from leuprolide to degarelix (p = 0.003). For patients continuing on degarelix 240/80 mg, there was no significant change in PSA PFS hazard rates (0.11 events/year in year 1 in CS21 and 0.14 events/year in CS21A; p = 0.464).
Figure 3.
Prostate-specific antigen (PSA) progression-free survival (PFS) probability in the phase III extension study CS21A (all patients). Reprinted from Crawford et al. [2011] with permission from Elsevier.
The same hazard rate pattern occurred in patients with baseline PSA ≥20 ng/ml. After 27.5 months of follow-up, PSA PFS hazard rates improved from 0.38 events/year in the first year to 0.19 events/year following crossover from leuprolide to degarelix (p = 0.031); corresponding hazard rates for degarelix were 0.23 and 0.23 events/year (p = 0.988). The low levels of testosterone and PSA achieved in year 1 in CS21 were maintained during a further 27.5 months (median) of follow-up in CS21A in patients continuing on degarelix 240/80 mg and those who crossed over from leuprolide to degarelix.
Ongoing clinical development
Intermittent ADT (IAD) with degarelix is under investigation in two phase III trials in PCa, the results of which are eagerly anticipated. CS29 [ClinicalTrials.gov identifier: NCT00801242] is a European noncomparative, two-cycle study of degarelix 240/80 mg in patients requiring ADT. After 7 months of degarelix, patients with PSA less than 4 ng/ml stop therapy until PSA rises to over 4 ng/ml (maximum 24 months). The second trial, CS37 [ClinicalTrials.gov identifier: NCT00928434] is a US randomized comparison of IAD with degarelix versus continuous ADT (CAD) with degarelix or leuprolide in patients with biochemical failure after localized therapy. Patients were randomized to one of three treatment groups: 14 months of CAD with degarelix 240/80 mg or leuprolide (starting dose of 7.5 mg on day 0 followed by maintenance dose of 22.5 mg at day 28 and every 84 days thereafter), or 7 months of degarelix followed by 7 months without treatment (after 7 months, patients were discontinued if PSA levels were >2 ng/ml).
Degarelix is also being studied in the neoadjuvant setting, in which a randomized trial comparing 3 months of degarelix versus goserelin/bicalutamide for prostate size reduction is underway in patients with intermediate- to high-risk PCa who require neoadjuvant hormone therapy prior to radiotherapy (curative intent) (CS30 [ClinicalTrials.gov identifier: NCT00833248]).
Safety
CS21
In CS21, both degarelix and leuprolide were well tolerated, with a similar incidence of treatment-emergent AEs (79%, 83% and 78% of patients in the degarelix 240/80 mg, degarelix 240/160 mg and leuprolide groups, respectively) [Klotz et al. 2008]. Consistent with phase II trials, no systemic allergic reactions occurred with degarelix. Injection-site reactions occurred in 40% of the pooled degarelix groups versus less than 1% of the leuprolide group (p < 0.001). These reactions were mostly mild or moderate and occurred predominantly after the first injection (33% of starting-dose injections versus 4% of maintenance-dose injections). Otherwise, the most frequent AE was flushing in 26% of patients in the pooled degarelix groups and 21% of the leuprolide group. Chills were more common with degarelix (4% versus 0%, p < 0.01), whereas urinary tract infection (9% versus 3%, respectively; p<0.01) and musculoskeletal and connective tissue AEs (26% versus 17%; p < 0.05) were more common with leuprolide.
CS21A
In CS21A, AEs were primarily related to androgen deprivation or the primary disease [Crawford et al. 2011]. The AE frequency in patients who crossed over from leuprolide to degarelix after 1 year and those who continued on degarelix was similar over the 4-year trial period and diminished as the study progressed. While there was an increase in injection-site reactions in year 2 in those patients crossing over from leuprolide to degarelix, in years 3 and 4 the incidence of these effects reduced to a level similar to that of the continuous degarelix group. Patients who crossed from leuprolide to degarelix experienced an improved musculoskeletal AE rate after crossover: first-time musculoskeletal and connective tissue AEs were reported in 17% of the continuous degarelix group versus 20% of the leuprolide/degarelix crossover group (p = 0.532).
Cardiovascular safety
An assessment of cardiovascular (CV) safety data from the CS21 trial showed no significant differences between leuprolide and pooled degarelix groups for mean change in Fridericia’s correction of QT interval [Smith et al. 2010]. A markedly abnormal Fridericia’s correction of QT values occurred in very few patients (≤1%) with either treatment. Ischemic heart disease, the most frequent cardiac disorder, occurred in 4% of degarelix patients versus 10% of leuprolide patients. In this class, the most frequent events were myocardial ischemia and myocardial infarction (each occurring in 2% of leuprolide patients and in less than 1% of those on degarelix). Cardiac failure occurred in less than 1% of degarelix patients versus 2% of leuprolide patients. Supraventricular arrhythmias (occurring in 2% of the pooled degarelix and 4% of the leuprolide group) were the most common type of arrhythmia. Other arrhythmias included bradycardia, atrioventricular conduction disturbances, ventricular arrhythmias, bundle branch block and cardiac arrest (all occurring in ≤1% in both groups).
A pooled analysis of data from 1704 men in nine clinical trials investigated potential relationships between CV disease (CVD) events, baseline patient characteristics and degarelix treatment dose and duration [Smith et al. 2011]. The rate of CVD events was similar before and after degarelix treatment in the overall patient population. Multivariate analysis showed that traditional CV risk factors of age, obesity and baseline CVD were associated with a higher CVD risk, whereas regular alcohol consumption was associated with a lower risk (each p < 0.05). Degarelix dose and treatment duration were not independently associated with CVD events.
It is interesting to note that in 2010 the US FDA required that safety warnings should be included on the labels of GnRH agonists that highlight an increased risk of diabetes and certain CVDs (sudden cardiac death, stroke and heart attack) [US Food and Drug Administration, 2010].
What are the clinical implications of the differential pharmacological profile demonstrated by degarelix?
Effects on testosterone
The rapid testosterone suppression with degarelix allows for a quicker onset of the therapeutic effect of testosterone suppression, avoiding the counterintuitive, supraphysiologic testosterone surge associated with GnRH agonists. Thus, in patients with advanced PCa, rapid relief of PCa-related symptoms may be achieved more efficiently by avoiding the risk of cancer stimulation and worsening of clinical status via a surge-induced clinical flare. Flare symptoms can be serious, and may include bone pain and bladder outlet/ureteral obstruction; as well as potentially rare complications, such as spinal cord compression [Van Poppel and Nilsson, 2008; Thompson, 2001].
Testosterone microsurges (>0.5 ng/ml) have been reported in around 6% of patients with agonists [Tombal, 2005]. However, a study with goserelin reported the occurrence of testosterone surges above a castration threshold of 0.185 ng/ml after at least one repeat injection in 17.7–27% of patients [Zinner et al. 2004]. Reports of the incidence of testosterone breakthroughs (>0.5 ng/ml) with GnRH agonists have varied. While Tombal and Berges reported breakthroughs greater than 0.5 ng/ml in 2–13% of patients [Tombal and Berges, 2005], Morote and colleagues reported breakthroughs greater than 0.5 ng/ml in 24.7% of patients in at least one measurement [Morote et al. 2007]. While the clinical significance of microsurges/breakthroughs has not been fully established, some evidence suggests that maintaining low testosterone may influence outcomes. Thus, in patients with nonmetastatic PCa receiving GnRH agonists (with/without anti-androgens), androgen-independent PFS was related to testosterone breakthrough escape [Morote et al. 2007]. In patients with breakthrough testosterone greater than 0.32 ng/ml, mean PFS was significantly lower (88 months) than in those without such a breakthrough escape (137 months, p < 0.003). Also, in patients with metastatic disease receiving GnRH agonists, high testosterone at 6 months was associated with an increase (1.33-fold) in cancer-specific mortality risk [Perachino et al. 2010]. Analysis of a large, localized PCa population database also showed that, for patients receiving continuous GnRH agonists adjuvant to curative radiotherapy, consistent castrate testosterone levels less than 0.50 ng/ml were associated with a lower PSA nadir before and after radiotherapy and a lower risk of subsequent biochemical relapse [Pickles and Tyldesley, 2011].
Effects on prostate-specific antigen
The improved PSA PFS noted with degarelix versus leuprolide in CS21, and in patients crossing over from leuprolide to degarelix in CS21A, is indicative of delayed progression to castration-resistant disease with degarelix. Disease progression may trigger clinician and patient desire for a change in antineoplastic therapy, with potential attendant physical and psychological morbidities as well as further economic costs. In the subgroup of patients with the highest PSA failure risk (baseline PSA ≥20 ng/ml), the time for 25% of patients to experience PSA failure or death was delayed by around 7 months with degarelix (514 days versus 303 days with leuprolide; p = 0.01) [Boccon-Gibod et al. 2011].
The more rapid PSA suppression associated with degarelix may also be an indicator of prognostic significance. In CS21, the PSA half-life for degarelix was shorter (9–10 days versus 22–23 days with leuprolide) [Van Poppel and Klotz, 2012]. Some studies suggest that a more rapid decrease in PSA, as indicated by a shorter PSA half-life, is associated with improved progression and survival [Hanninen et al. 2009; Lin et al. 2009], although conflicting results have been reported [Park et al. 2009]. In a study of patients with PCa receiving ADT, the median PFS was around 7 months longer (24.6 months) in those with a shorter PSA half-life (≤0.5 months) versus a longer (>0.5 months) PSA half-life (PFS 17.2 months). Overall survival was also greater in patients with a shorter half-life (48 versus 43 months) [Lin et al. 2009]. Notably, in CS21 the PSA half-life for degarelix was less than 0.5 months versus more than 0.5 months for leuprolide.
Effects on follicle-stimulating hormone
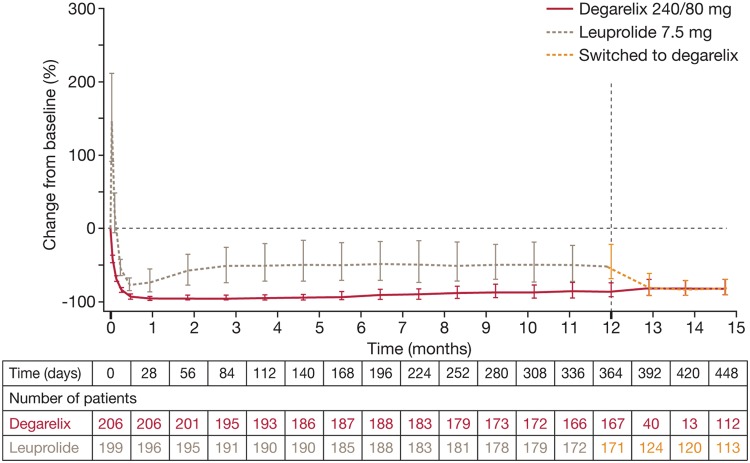
In clinical studies, degarelix produced profound and persistent FSH suppression [Klotz et al. 2008; Van Poppel et al. 2008] compared with only partial FSH suppression with agonists [Klotz et al. 2008]. Leuprolide produced an initial surge in FSH at the start of treatment and levels did not fall to the same extent as with degarelix. Indeed, at the end of study CS21, FSH had fallen by 88.5% with degarelix 240/80 mg versus 54.8% with leuprolide. In patients who crossed over from leuprolide to degarelix in CS21A, FSH levels were further decreased within 1 month to levels similar to those in the continuous degarelix group (Figure 4) [Crawford et al. 2011].
Figure 4.
Median percentage change from baseline and quartiles in follicle-stimulating hormone in patients switched from leuprolide to degarelix and those continuing degarelix in the extension trial. Reprinted from Crawford et al. [2011] with permission from Elsevier.
The implications of persistent FSH suppression with antagonists are not yet fully understood. Nevertheless, several studies suggest a link between FSH and PCa. The prostate can synthesize FSH [Dirnhofer et al. 1998], and prostate cell growth can be stimulated by exogenous FSH in hormone-refractory cell lines [Ben-Josef et al. 1999]. FSH receptors (FSH-R) are generally expressed at higher levels on tumor versus normal tissue [Mariani et al. 2006] and are selectively expressed on the blood vessels of a wide range of tumors, including prostate [Radu et al. 2010]. Also, FSH levels are significantly higher in men with more advanced PCa [Heracek et al. 2007]. Finally, endogenous compounds such as prostatic inhibin peptide (PIP) may inhibit PCa growth by inhibiting FSH [Garde et al. 1993], and PIP expression is reduced in PCa [Zhang et al. 1999]. Indeed, FSH-R may have a potential functional role in angiogenesis and the development of metastatic disease [Gartrell et al. 2012].
Effects on serum alkaline phosphatase
In PCa, increased levels of S-ALP and bone-specific alkaline phosphatase appear to be significant predictors of early mortality [Robinson et al. 2008; Johansen et al. 2007; Ramankulov et al. 2007; Jung et al. 2004] and have been associated with progression of skeletal metastases [Lein et al. 2007; Lorente et al. 1996]. In addition, normalization of bone markers has been associated with improved overall survival [Lipton et al. 2008]. Thus, a decrease in bone turnover marker levels may delay progression of bone metastases and improve survival. In CS21, the initial S-ALP peaks in metastatic patients likely reflect increased osteoblastic activity associated with tumor cell death and rebuilding of bone tissue around skeletal metastases. In general, S-ALP peaks in CS21 were less pronounced with degarelix than with leuprolide, possibly indicating the improved prognosis in terms of PSA PFS previously noted.
Baseline disease stage and pretreatment PSA have been linked to PCa outcome [Stock and Stone, 1997; D’Amico et al. 2007]. In CS21, both patients with baseline metastatic disease and those with PSA greater than 50 ng/ml showed improved S-ALP control with degarelix versus leuprolide (Figure 2). This may suggest prolonged control of skeletal metastases with degarelix compared with an agonist, and might also explain the decreased incidence of musculoskeletal events seen with degarelix in the CS21 trial.
Use of degarelix in the clinic
Degarelix is certainly as effective in achieving testosterone suppression as a GnRH agonist, the most common ADT used in advanced PCa, and has proven long-term efficacy and tolerability, maintaining testosterone and PSA suppression for more than 3 years. Thus, degarelix provides an alternative first-line ADT for advanced disease.
Indeed, the pharmacological profile of degarelix provides several advantages over GnRH agonists. As stated earlier, degarelix offers a more rapid onset of castration and more effective control of testosterone, PSA, S-ALP and FSH. Moreover, the differential pharmacological profile of degarelix versus agonists may also offer clinical advantages.
The fast onset of castration with degarelix is particularly beneficial in symptomatic patients. The lack of surge-induced tumor-promoting effects with degarelix makes it a preferred ADT option in patients with a high tumor burden and risk of acute problems, such as urinary tract symptoms, pain or spinal cord compression. Degarelix also avoids the need for concurrent antiandrogen administration for flare protection, with possible compliance advantages as a result of monotherapy. Concomitant antiandrogens do not completely remove the risk of clinical flare [Heidenreich et al. 2012]. First-line use of degarelix also avoids the risk of anti-androgen toxicity and so offers possible side effect profile advantages and cost savings.
Other therapeutic benefits associated with the differential pharmacological profile of degarelix versus agonists include superior effects on PSA PFS, which delays the onset of castrate-resistant disease (and potentially toxic chemotherapy), while the improved S-ALP profile in patients with metastatic disease may indicate a prolonged control of skeletal metastases in patients with bone metastases.
IAD has been investigated as an alternative to CAD in PCa therapy [Abrahamsson 2010]. IAD alternates active on-treatment periods with off-treatment periods without ADT, which facilitate testosterone recovery. In advanced PCa, IAD aims to minimize ADT-related AEs and improve HRQoL by reducing treatment exposure while providing comparable efficacy. It is also hypothesized that IAD may delay progression to androgen-independent disease. Recent results from a large international trial [S9346 (INT-0162)] comparing IAD and CAD showed that, in hormone-sensitive metastatic PCa, IAD was noninferior to CAD in patients with extensive disease but IAD was statistically inferior in those with minimal disease [Hussain et al. 2012]. The rapid testosterone and PSA suppression observed with degarelix makes it a suitable candidate for IAD; European Association of Urology guidelines consider that GnRH antagonists might provide a valid alternative to agonists for IAD, provided clear results are obtained from randomized studies [Heidenreich et al. 2012]. Results from ongoing trials of IAD with degarelix will clarify the potential role of this agent in IAD. It should be noted that, unlike GnRH agonists, degarelix is currently only available as a 1-month formulation and so cannot offer the potential convenience of longer-term (e.g. 3- to 12-month) depot formulations. However, it remains important for patients to see their physicians on a regular basis, and some men like the support of seeing the physician more often; for some patients, the reassurance gained from frequent physician visits may decrease disease-related anxiety [Sartor, 2006].
In contrast to abarelix, which has been associated with the risk of potentially serious, immediate-onset systemic allergic reactions, degarelix has not been associated with these types of reactions. The hormonal side effect profile of degarelix is comparable with that of agonists. However, in addition to chills, degarelix is associated with a higher incidence of injection-site reactions versus agonists; nevertheless, in the majority of cases no treatment was needed and over-the-counter remedies (e.g. analgesics, cold packs) were effective in 20% of cases. Injection-site reactions with degarelix may be a consequence of a different administration route (subcutaneous degarelix versus intramuscular leuprolide) and a higher injection volume. There have been previous reports of local injection-site reactions with subcutaneously administered GnRH agonists [Oka et al. 2006].
Studies suggest that CV risk with degarelix may be driven by normal aging, with similarities identified between risk factors in studies of the general population and in patients receiving degarelix. In contrast, the US FDA has highlighted an increased risk of diabetes, heart attack, stroke and sudden death with GnRH agonists, with the consequent requirement to add warnings of such risks to GnRH agonist labels [US Food and Drug Administration, 2010]. Interestingly, most but not all studies show no association of orchiectomy with an increased risk of CV events [Levine et al. 2010; Keating et al. 2006, 2010; Alibhai et al. 2009], raising the possibility that CV risk may vary for different forms of ADT [Smith et al. 2011].
Conclusions
Multiple clinical studies have demonstrated that degarelix is an effective and well tolerated treatment for advanced PCa. Degarelix rapidly suppresses testosterone and PSA, without the initial testosterone surge or microsurges associated with GnRH agonists. Degarelix also displays long-term efficacy, maintaining effective testosterone and PSA suppression for over 3 years. Compared with GnRH agonists, degarelix is associated with improved testosterone and PSA control, offering a prolonged time to castration-resistant disease, an improved FSH profile and more favorable effects on S-ALP. Degarelix is generally well tolerated, without systemic allergic reactions; with the exception of injection-site reactions, most AEs reflect androgen suppression or the underlying condition.
The different pharmacological profile of degarelix brings clinical benefits in PCa therapy including: a delay in progression to castration-resistant disease versus agonists, avoidance of the negative clinical effects associated with surge-induced flare, a monotherapy approach to ADT that may improve compliance and avoids the need for antiandrogens (and any attendant AEs), the prolonging of control of skeletal metastases in metastatic disease versus agonists, and possible clinical benefits associated with improved FSH control.
Thus, degarelix, which is now the most comprehensively studied and widely available GnRH antagonist, offers an additional option to GnRH agonists as a first-line ADT for the hormonal management of PCa.
Acknowledgments
Medical writing assistance (funded by Ferring Pharmaceuticals) was provided by Thomas Lavelle of Bioscript Stirling Ltd.
Footnotes
Funding: The degarelix clinical trials included in this review were sponsored by Ferring Pharmaceuticals.
Conflict of interest statement: Neal D. Shore is a consultant for Ferring, Sanofi, Watson, Astellas, Medivation, Dendreon, Janssen and Amgen.
References
- Abrahamsson P. (2010) Potential benefits of intermittent androgen suppression therapy in the treatment of prostate cancer: a systematic review of the literature. Eur Urol 57: 49–59 [DOI] [PubMed] [Google Scholar]
- Alibhai S., Duong-Hua M., Sutradhar R., Fleshner N., Warde P., Cheung A., et al. (2009) Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol 27: 3452–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Josef E., Yang S., Ji T., Bidart J., Garde S., Chopra D., et al. (1999) Hormone-refractory prostate cancer cells express functional follicle-stimulating hormone receptor (FSHR). J Urol 161: 970–976 [PubMed] [Google Scholar]
- Boccon-Gibod L., van der Meulen E., Persson B. (2011) An update on the use of gonadotropin-releasing hormone antagonists in prostate cancer. Ther Adv Urol; 3: 127–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broqua P., Riviere P., Conn P., Rivier J., Aubert M., Junien J. (2002) Pharmacological profile of a new, potent, and longacting gonadotropin-releasing hormone antagonist: degarelix. J Pharmacol Exp Ther 301: 95–102 [DOI] [PubMed] [Google Scholar]
- Crawford E., Tombal B., Miller K., Boccon-Gibod L., Schröder F., Shore N., et al. (2011) A phase III extension trial with a 1-arm crossover from leuprolide to degarelix: comparison of gonadotropin-releasing hormone agonist and antagonist effect on prostate cancer. J Urol 186: 889–897 [DOI] [PubMed] [Google Scholar]
- D’Amico A., Chen M., Catalona W., Sun L., Roehl K., Moul J. (2007) Prostate cancer-specific mortality after radical prostatectomy or external beam radiation therapy in men with 1 or more high-risk factors. Cancer 110: 56–61 [DOI] [PubMed] [Google Scholar]
- Dirnhofer S., Berger C., Hermann M., Steiner G., Madersbacher S., Berger P. (1998) Coexpression of gonadotropic hormones and their corresponding FSH- and LH/CG-receptors in the human prostate. Prostate 35: 212–220 [DOI] [PubMed] [Google Scholar]
- Doehn C., Sommerauer M., Jocham D. (2006) Drug evaluation: degarelix—a potential new therapy for prostate cancer. IDrugs 9: 565–572 [PubMed] [Google Scholar]
- Frampton J., Lyseng-Williamson K. (2009) Degarelix. Drugs 69: 1967–1976 [DOI] [PubMed] [Google Scholar]
- Garde S., Sheth A., Porter A., Pienta K. (1993) Effect of prostatic inhibin peptide (PIP) on prostate cancer cell growth in vitro and in vivo. Prostate 22: 225–233 [DOI] [PubMed] [Google Scholar]
- Gartrell B., Tsao C., Galsky M. (2012) The follicle-stimulating hormone receptor: a novel target in genitourinary malignancies. Urol Oncol 16 April (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittelman M., Brown T., Holm-Larsen T., Persson B. (2011) Comparison of the impact of degarelix and leuprolide on the health-related quality of life of patients with prostate cancer: results of a 12-month phase III clinical trial. UroToday Int J 4(6): art. 81. [Google Scholar]
- Gittelman M., Pommerville P., Persson B., Jensen J., Olesen T. on behalf of the Degarelix Study Group (2008) A 1-year, open-label, randomized phase II dose-finding study of degarelix, a novel gonadotropin-releasing hormone (GnRH) receptor blocker, in the treatment of prostate cancer in North America. J Urol 180: 1986–1992 [DOI] [PubMed] [Google Scholar]
- Hanninen M., Venner P., North S. (2009) A rapid PSA half-life following docetaxel chemotherapy is associated with improved survival in hormone refractory prostate cancer. Can Urol Assoc J 3: 369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich A., Bastian P., Bellmunt J., Bolla M., Joniau S., Masson M., et al. (2012) European Association of Urology. Guidelines on prostate cancer. Available at: http://www.uroweb.org/gls/pdf/08%20Prostate%20Cancer_LR%20March%2013th%202012.pdf (accessed May 2012).
- Heracek J., Urban M., Sachova J., Kuncova J., Eis V., Mandys V., et al. (2007) The endocrine profiles in men with localized and locally advanced prostate cancer treated with radical prostatectomy. Neuro Endocrinol Lett 28: 45–51 [PubMed] [Google Scholar]
- Hook W., Karten M., Siraganian R. (1985) Histamine release by structural analogs of LHRH. Fed Am Soc Exp Biol 44: 1323 [Google Scholar]
- Huggins C., Hodges C. (1941) Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1: 293–297 [DOI] [PubMed] [Google Scholar]
- Hussain M., Tangen C., Higano C., Schelhammer P., Faulkner J., Crawford E., et al. ; Southwest Oncology Group Trial 9346 (INT-0162) (2006) Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol 24: 3984–3990 [DOI] [PubMed] [Google Scholar]
- Hussain M., Tangen C., Higano C., Crawford E., Liu G., Wilding G., et al. (2012) Intermittent (IAD) versus continuous androgen deprivation (CAD) in hormone sensitive metastatic prostate cancer (HSM1PC) patients (pts): Results of S9346 (INT-0162), an international phase III trial. J Clin Oncol 30(Suppl.): abstract 4. Available at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=114&abstractID=92516 (accessed July 2012). [Google Scholar]
- Johansen J., Brasso K., Iversen P., Teisner B., Garnero P., Price P., et al. (2007) Changes of biochemical markers of bone turnover and YKL-40 following hormonal treatment for metastatic prostate cancer are related to survival. Clin Cancer Res 13: 3244–3249 [DOI] [PubMed] [Google Scholar]
- Jung K., Lein M., Stephan C., Von Hösslin K., Semjonow A., Sinha P., et al. (2004) Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. Int J Cancer 111: 783–791 [DOI] [PubMed] [Google Scholar]
- Keating N., O’Malley A., Freedland S., Smith M. (2010) Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst 102: 39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating N., O’Malley J., Smith M. (2006) Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 24: 4448–4456 [DOI] [PubMed] [Google Scholar]
- Kelly W., Gomella L. (2011) Androgen deprivation therapy and competing risks. JAMA 306: 2382–2383 [DOI] [PubMed] [Google Scholar]
- Klotz L., Boccon-Gibod L., Shore N., Andreou C., Persson B., Cantor P., et al. (2008) The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in prostate cancer patients. BJU Int 102: 1531–1538 [DOI] [PubMed] [Google Scholar]
- Koechling W., Hjortkjaer R., Tankó L. (2010) Degarelix, a novel GnRH antagonist, causes minimal histamine release compared with cetrorelix, abarelix and ganirelix in an ex vivo model of human skin samples. Br J Clin Pharmacol 70: 580–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein M., Wirth M., Miller K., Eickenberg H., Weissbach L., Schmidt K., et al. (2007) Serial markers of bone turnover in men with metastatic prostate cancer treated with zoledronic acid for detection of bone metastases progression. Eur Urol 52: 1381–1387 [DOI] [PubMed] [Google Scholar]
- Levine G., D’Amico A., Berger P., Clark P., Eckel R., Keating N., et al. (2010) Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation 121: 833–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G., Yao X., Zhang S., Dai B., Ma C., Zhang H., et al. (2009) Prostate-specific antigen half-life: a new predictor of progression-free survival and overall survival in Chinese prostate cancer patients. Asian J Androl 11: 443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton A., Cook R., Saad F., Major P., Garnero P., Terpos E., et al. (2008) Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer 113: 193–201 [DOI] [PubMed] [Google Scholar]
- Lorente J., Morote J., Raventos C., Encabo G., Valenzuela H. (1996) Clinical efficacy of bone alkaline phosphatase and prostate specific antigen in the diagnosis of bone metastasis in prostate cancer. J Urol 155: 1348–1351 [PubMed] [Google Scholar]
- Mariani S., Salvatori L., Basciani S., Arizzi M., Franco G., Petrangeli E., et al. (2006) Expression and cellular localization of follicle-stimulating hormone receptor in normal human prostate, benign prostatic hyperplasia and prostate cancer. J Urol 175: 2072–2077 [DOI] [PubMed] [Google Scholar]
- Mongiat-Artus P., Teillac P. (2004) Abarelix: the first gonadotrophin-releasing hormone antagonist for the treatment of prostate cancer. Expert Opin Pharmacother 5: 2171–2179 [DOI] [PubMed] [Google Scholar]
- Morote J., Orsola A., Planas J., Trilla E., Raventós C., Cecchini L., et al. (2007) Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol 178: 1290–1295 [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (2012) NCCN Clinical Practice Guidelines in Oncology (NCCN Guideline®). Prostate cancer. Version 3.2012. Available at: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed May 2012).
- Oka D., Shiba M., Arai Y., Nakayama M., Takayama H., Inoue H., et al. (2006) Skin reactions to 3-month depot type of luteinizing hormone-releasing hormone agonist therapy. JMAJ 49: 48–54 [Google Scholar]
- Ozono S., Ueda T., Hoshi S., Yamaguchi A., Maeda H., Fukuyama Y., et al. (2012) The efficacy and safety of degarelix, a GnRH antagonist: a 12-month, multicentre, randomized, maintenance dose-finding phase II study in Japanese patients with prostate cancer. Jpn J Clin Oncol 42: 477–484 [DOI] [PubMed] [Google Scholar]
- Park Y., Hwang I., Jeong C., Kim H., Lee S., Kwak C. (2009) Prostate specific antigen half-time and prostate specific antigen doubling time as predictors of response to androgen deprivation therapy for metastatic prostate cancer. J Urol 181: 2520–2524 [DOI] [PubMed] [Google Scholar]
- Perachino M., Cavalli V., Bravi F. (2010) Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: prognostic significance? BJU Int 105: 648–651 [DOI] [PubMed] [Google Scholar]
- Pickles T., Tyldesley S. (2011) Testosterone breakthrough during LHRH agonist androgen deprivation with curative radiation: Impact on PSA kinetics and subsequent biochemical outcomes. Eur Urol Suppl 10: 239 (abstract 752). [Google Scholar]
- Princivalle M., Broqua P., White R., Meyer J., Mayer G., Elliott L., et al. (2007) Rapid suppression of plasma testosterone levels and tumor growth in the dunning rat model treated with degarelix, a new gonadotropin-releasing hormone antagonist. J Pharmacol Exp Ther 320: 1113–1118 [DOI] [PubMed] [Google Scholar]
- Radu A., Pichon C., Camparo P., Antoine M., Allory Y., Couvelard A., et al. (2010) Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med 363: 1621–1630 [DOI] [PubMed] [Google Scholar]
- Ramankulov A., Lein M., Kristiansen G., Loening S., Jung K. (2007) Plasma osteopontin in comparison with bone markers as indicator of bone metastasis and survival outcome in patients with prostate cancer. Prostate 67: 330–340 [DOI] [PubMed] [Google Scholar]
- Robinson D., Sandblom G., Johansson R., Garmo H., Stattin P., Mommsen S., et al. (2008) Prediction of survival of metastatic prostate cancer based on early serial measurements of prostate specific antigen and alkaline phosphatase. J Urol 179: 117–122 [DOI] [PubMed] [Google Scholar]
- Sartor O. (2006) Eligard® 6: a new form of treatment for prostate cancer. Eur Urol Suppl 5: 905–910 [Google Scholar]
- Schmidt F., Sundaram K., Thau R., Bardin C. (1984) (Ac-D-Dal (2), 4FD-Phe, D-Trp, D-Arg)-LHRH, a potent antagonist of LHRH, produces transient edema and behavioral changes in rats. Contraception 29: 283–289 [DOI] [PubMed] [Google Scholar]
- Schröder F., Tombal B., Miller K., Boccon-Gibod L., Shore N., Crawford E., et al. (2010) Alkaline phosphatase changes in prostate cancer patients receiving degarelix or leuprolide: results from a 12-month, comparative, phase III study (CS21). BJU Int 106: 182–187 [DOI] [PubMed] [Google Scholar]
- Smith M., Klotz L., Persson B., Olesen T., Wilde A. (2010) Cardiovascular safety of degarelix: results from a 12-month, comparative, randomized, open label, parallel-group phase III trial in patients with prostate cancer. J Urol 184: 2313–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Klotz L., van der Meulen E., Colli E., Tankó L. (2011) Gonadotropin-releasing hormone blockers and cardiovascular disease risk: analyses of prospective clinical trials of degarelix. J Urol 186: 1835–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. (2009) Degarelix: a gonadotropin releasing hormone antagonist for the management of prostate cancer. Clin Ther 31: 2312–2331 [DOI] [PubMed] [Google Scholar]
- Stock R., Stone N. (1997) The effect of prognostic factors on therapeutic outcome following transperineal prostate brachytherapy. Semin Surg Oncol 13: 454–460 [DOI] [PubMed] [Google Scholar]
- Thompson I. (2001) Flare associated with LHRH-agonist therapy. Rev Urol 3(Suppl. 3): S10–S14 [PMC free article] [PubMed] [Google Scholar]
- Tombal B. (2005) Appropriate castration with luteinising hormone releasing hormone (LHRH) agonists: what is the optimal level of testosterone? Eur Urol Suppl 4: 14–19 [Google Scholar]
- Tombal B., Berges R. (2005) How good do current LHRH agonists control testosterone? Can this be improved with Eligard®? Eur Urol Suppl 4: 30–36 [Google Scholar]
- Tombal B., Berges R. (2008) Optimal control of testosterone: a clinical case-based approach of modern androgen-deprivation therapy. Eur Urol Suppl 7: 15–21 [Google Scholar]
- Tombal B., Miller K., Boccon-Gibod L., Schröder F., Shore N., Crawford E., et al. (2010) Additional analysis of the secondary end point of biochemical recurrence rate in a phase 3 trial (CS21) comparing degarelix 80 mg versus leuprolide in prostate cancer patients segmented by baseline characteristics. Eur Urol 57: 836–842 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration (FDA) (2010) FDA Drug Safety Communication, 20 October Available from: http://www.fda.gov/Drugs/DrugSafety/ucm229986.htm (accessed July 2012).
- Van Poppel H., Klotz L. (2012) Gonadotropin-releasing hormone: an update review of the antagonists versus agonists. Int J Urol 19: 594–601 [DOI] [PubMed] [Google Scholar]
- Van Poppel H., Nilsson S. (2008) Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology 71: 1001–1006 [DOI] [PubMed] [Google Scholar]
- Van Poppel H., Tombal B., de la Rosette J., Persson B., Jensen J., Olesen T. on behalf of the Degarelix Study Group (2008) Degarelix: a novel gonadotropin-releasing hormone (GnRH) receptor blocker – results from a 1-yr, multicentre, randomised, phase II dose-finding study in the treatment of prostate cancer. Eur Urol 54: 805–815 [DOI] [PubMed] [Google Scholar]
- White R., Schwach G., Schteingart C. (2007) Degarelix, a unique, sustained-release depot GnRH blocker with a long duration of action. 1st European Multidisciplinary Meeting on Urological Cancers, Barcelona, 24 November 2007. Abstract P92. Available at: http://www.emucbarcelona2007.org/fileadmin/user_upload/downloads/EMUC_Binnen.pdf (accessed 31 July 2012). [Google Scholar]
- Wirth M., Hakenberg O., Froehner M. (2007) Antiandrogens in the treatment of prostate cancer. Eur Urol 51: 306–313 [DOI] [PubMed] [Google Scholar]
- Zhang P., Driscoll D., Lee H., Nolan C., Velagapudi S. (1999) Decreased immunoexpression of prostate inhibin peptide in prostatic carcinoma: a study with monoclonal antibody. Hum Pathol 30: 168–172 [DOI] [PubMed] [Google Scholar]
- Zinner N., Bidair M., Centeno A., Tomera K. (2004) Similar frequency of testosterone surge after repeat injections of goserelin (Zoladex) 3.6mg and 10.8mg: results of a randomized open-label trial. Urology 64: 1177–1181 [DOI] [PubMed] [Google Scholar]