Abstract
Orally active, selective inhibitors of phosphodiesterase type 5 (PDE 5, cyclic GMP PDE), such as sildenafil, tadalafil and vardenafil, are currently the first-choice treatment options for the clinical management of erectile dysfunction (ED) of various etiologies and severities. However, a significant number of patients remain dissatisfied with the available therapies due a lack of efficacy or discomfort arising from adverse events. Several new PDE5 inhibitors, among which are avanafil (TA-1790), lodenafil, mirodenafil, udenafil, SLX-2101, JNJ-10280205 and JNJ-10287069, have recently been approved and introduced into the market or are in the final stages of their clinical development. Avanafil (marketed in the US under the brand name STENDRA™) has been developed by VIVUS Inc. (Mountain View, CA, USA) and has recently received approval from the US Food and Drug Administration (FDA) for use in the treatment of male ED. The drug has demonstrated improved selectivity for PDE5, is rapidly absorbed after oral administration with a fast onset of action and a plasma half-life that is comparable to sildenfil and vardenafil. In phase II and phase III clinical trials that included a large number of patients, avanafil has been shown to be effective and well tolerated. Owing to its favorable pharmacodynamic and pharmacokinetic profile, avanafil is considered as a promising new option in the treatment of ED. The present article summarizes the initial data and clinical key properties of avanafil.
Keywords: avanafil, erectile dysfunction, oral pharmacotherapy, PDE5 inhibitors
Introduction
Erectile dysfunction (ED), one of the most common sexual disturbances in the adult male, is defined as a man’s consistent or recurrent inability to attain and/or maintain penile erection sufficient for satisfactory sexual performance [Montorsi et al. 2010]. According to epidemiologic studies in this field, such as the Massachusetts Male Aging Study and the Cologne Male Survey, which surveyed several thousands of males aged between 30 and 80 years, the overall prevalence of ED is approximately 20–40% at 40 years of age and 70% at 70 years [Feldman et al. 1994; Braun et al. 2000]. As the proportion of the elderly population is continuously increasing, it has been estimated that in 2025 an estimated number of 320 million men will be affected by ED, many of them are expected to seek medical therapy to treat this condition [Aytac et al. 1999]. The discovery of nitric oxide (NO) and cyclic GMP as the pivotal signaling molecules in the process of penile smooth muscle relaxation was a scientific landmark and has led to the identification of drugs that are able to elevate intracellular levels of cyclic GMP, such as the NO donor drugs sodium nitroprusside (SNP), nitroglycerine and linsidomine (SIN-1). To date, the use of selective inhibitors of phosphodiesterase type 5 (PDE5, cyclic GMP PDE), for example, sildenafil, vardenafil and tadalafil, in the treatment of ED has gained widespread acceptance in the field of urology and is considered the first-line strategy to target ED [Kalsi and Kell, 2004]. PDE5 inhibitors are characterized as nonhydrolyzable analogs to cyclic GMP that counteract the degradation of this cyclic nucleotide by competitive binding to the catalytic site of the PDE5, thereby enhancing the relaxation brought about by NO and cyclic GMP of penile erectile tissue. The efficacy of sildenafil, vardenafil and tadalafil has been evaluated in various clinical trials involving a broad spectrum of male subjects with different causes of ED, including psychogenic ED, as well as patients with concomitant diabetes, cardiovascular diseases and histories of spinal cord injury or pelvic surgery (radical cystectomy/prostatectomy). In all trials, men receiving a PDE5 inhibitor reported erections sufficient for sexual intercourse more often than did those who received placebo; the overall response rates have been reported at 60–80% [Kalsi and Kell, 2004]. Common adverse events include headache (10–16%), flushing (5–12%), dyspepsia (4–12%), nasal congestion (1–10%) and dizziness (2–3%). In view of the significant number of patients who remain dissatisfied with the available therapies due to a lack of efficacy or discomfort arising from adverse events, the development of new PDE5 inhibitors with an enhanced selectivity for PDE5, a faster onset of action, improved oral bioavailability and an extended duration of drug action persists as an important topic and is being pursued vigorously. Currently, avanafil (originally developed by Mitsubishi Tanabe Pharma Corp., Yokohama, Japan, under the substance code TA-1790), JNJ-10280205, JNJ-10287069, lodenafil, mirodenafil, SLX-2101 and udenafil are under development for future use in the treatment of ED [Qiu et al. 2006; Hatzimouratidis and Hatzichristou, 2008; Eardley et al. 2010]. Some of these drugs have already entered into the clinical stage of testing (avanafil, lodenafil and mirodenafil) or have already been approved in some countries by the drug authorities in charge and are now being offered to patients (udenafil). This review focuses on avanafil, a new, highly selective, an orally active PDE5 inhibitor that has recently completed the final phase of clinical development by VIVUS Inc. (Mountain View, CA, USA). Avanafil has been characterized as a pyrimidine derivative (chemical name: 4-[(3-chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl]-N-(2-pyrimidinylmethyl)-5 -pyrimidinecarboxamide;(S)-2-(2-hydroxymethyl-1-pyrrolidinyl)-4-(3-chloro-4-methoxybenzylamino)-5-[(2-pyrimidinylmethyl)carbamoyl] pyrimidine, molecular weight = 483.95 D) that exists as a single enantiomer (= one stereoisomer only with no nonsuperposable mirror image of itself) with the ability to rotate plane-polarized light to the left (known as S-stereochemistry). The chemical structure of avanafil is different from the standard (nucleo)base/sugar/phosphate diester model of sildenafil, vardenafil and tadalafil. Therefore, avanafil can bind to the catalytic site of PDE5 independent of the spatial orientation of the molecule. This unique property may significantly increase the affinity towards the target enzyme PDE5 and, thus, the clinical efficacy of the drug.
Figure 1.
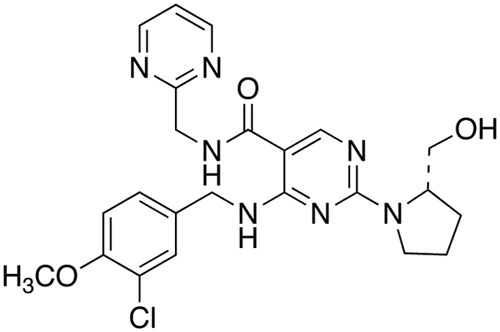
Structural formula of avanafil. In order to bind effectively to the catalytic site of the PDE5, a selective inhibitor has to comprise three major structural principles: a guanine-like base, a ribose- or desoxyribose-like system and, finally, a phosphate diester-like bond. In the avanafil molecule, the central structure is formed by a nitrogen derivative of a pyrimidine carboxamide where the nitrogen atom of the amide is bound to a pyrimidinylmethyl group. It seems likely that the ribose (sugar)-phosphate component is represented by a cyclic chloromethoxybenzylamino structure; in this case, the Cl-atom and the methoxy ligand resemble the phosphate group seen in, for example, sildenafil and vardenafil.
Basic research findings
The inhibitory activities of avanafil on various PDE isoenzymes were tested in preclinical studies and compared with those of sildenafil, vardenafil and tadalafil. Avanafil strongly inhibits PDE5 in a competitive manner; with a half-maximal concentration (IC50) of 5.2 nM, the drug is a 100-fold more potent for PDE5 than for PDE6, the PDE isoenzyme that is found exclusively in the retina where it is responsible for the mechanism of phototransduction. Avanafil shows higher selectivity for PDE5 versus PDE6 (120-fold) than sildenafil (16-fold) and vardenafil (21-fold), its selectivity for PDE5 versus PDE1 (cyclic AMP/cyclic GMP PDE, dependent on calcium and calmodulin) is greater than 10,000-fold (sildenafil: 380-fold; vardenafil: 1000-fold). In contrast to tadalafil, considerable inhibition by avanafil of PDE11 (cyclic AMP/cyclic GMP PDE, predominantly found in testis, prostate and striated muscle) was not registered (selectivity for PDE5 versus PDE11: >19,000-fold; tadalafil: 25-fold). These findings imply that avanafil has little tendency to cause visual disturbances (blue vision) and myalgia, adverse effects reported by patients who are on sildenafil or tadalafil, respectively. In in vivo animal studies, the effects of avanafil and sildenafil on retinal function were examined by means of electroretinogram recordings. At pharmacologically relevant doses, the drug was less likely to affect retinal function than sildenafil, thereby providing further evidence for a significant reduction of the nonspecific inhibition of PDE6 by avanafil [Mochida et al. 2006]. In the anesthetized dog model, avanafil, administered either intravenously (i.v.; 3–300 µg/kg) or intraduodenally (i.d.; 0.1–1 mg/kg), has been shown to potentiate in a dose-dependent manner penile tumescence (as measured by means of the increase in intracavernous pressure) brought about by electrical stimulation of the pelvic nerves of the animals [Kotera et al. 2012]. The efficacy of the drug was equivalent to that of sildenafil: the percentages of enhancement by avanafil and sildenafil at maximum drug concentrations were 325% and 304% (i.v.), and 415% and 358% (i.d.), respectively. After i.v. administration, the 200% effective dose of avanafil was 37.5 μg/kg (sildenafil: 34.6 μg/kg), the respective dose given i.d. amounted to 151.7 μg/kg (sildenafil: 79.0 μg/kg) at peak time (= time to peak response). The time to peak response was 10 min with avanafil and 30 min with sildenafil, indicating a more rapid onset of the avanafil action. The hypotensive response to the intravenous administration of avanafil (300 µg/kg/min) was weaker than the drop in blood pressure registered after the application of the same dose of sildenafil (–12 mmHg versus –22 mmHg, respectively). After intraduodenal administration (0.1–1 mg/kg), the potentiation by avanafil of the hypotension induced by nitroglycerin was significantly less pronounced than the effect exerted by sildenafil (peak area under curve at maximum concentration: 146% for avanafil, 207% for sildenafil) [Mochida et al. 2006].
Clinical key data
Avanafil, administered in doses of 50 to 200 mg (maximum dose), is rapidly absorbed, the median time to maximum plasma concentration (T max) is 30–45 min in the fasting state and 1 h 20 min when the drug is taken together with a high fat meal. Owing to clearance by the hepatic metabolism, avanafil is degraded into two major metabolites, designated as M4 and M16. The M4 metabolite accounts for approximately 23% of the parent compound and 4% of its pharmacologic activity, with an in vitro inhibitory potency for PDE5 of 18% of that of avanafil. The M16 metabolite, accounting for approximately 29% of the parent compound, is inactive against PDE5. In phase I settings to investigate the tolerability and pharmacokinetic properties of the drug, avanafil was shown to be rapidly absorbed after oral administration, with a T max of approximately 35–45 min. With regard to the elimination time from the plasma, discordant data have been presented: the apparent T 1/2 was estimated within a range from 1 h 10 min to 1 h 20 min to a maximum of 5 h, as well as even 11 h (sildenafil: 3–4 h; vardenafil: 4–5 h; tadalafil: 17.5 h). Although these values were similar among the different dose units administered to the volunteers in the respective studies (50 mg, 100 mg and 200 mg), it remains to be elucidated whether or not the T 1/2 can be considered a pharmacokinetic property that distinguishes avanafil from, for example, sildenafil and vardenafil [Allison et al. 2011; Jung et al. 2010; Peterson and Swearingen, 2006]. The drug was well tolerated, with headache and facial flushing being the most common adverse events demonstrated (4–11%). It was shown that the daily administration of 200 mg avanafil for 14 days did not result in a significant accumulation in the plasma. This observation was regardless of the dosage regimen, namely once or twice daily.
The safety and clinical efficacy of avanafil were evaluated in several phase II and III trials involving a large number of male subjects with various causes of ED. In a double-blind, randomized, crossover, at-home phase II trial in a cohort of 83 patients (mean age 52 years) with mild to moderate ED of a mean duration of 58 months, avanafil at doses of 50 mg, 100 mg or 200 mg induced significantly greater penile rigidity, as measured by means of RigiScan®, than did placebo. Responses to avanafil were similar to or greater than those to 50 mg sildenafil citrate. Both drugs induced erections sufficient to achieve vaginal penetration in approximately 80% of the attempts within an average time of 20 min; however, the peak response to avanafil occurred within 20–40 min after administration, while the peak response to sildenafil occurred in the median (60–80 min) or late (100–120 min) time window [Hellstrom et al. 2012]. This provides further evidence that avanafil is rapidly absorbed, with a fast onset of drug action.
A multicenter, double-blind, randomized, parallel-design phase II trial assessed the efficacy and safety of different doses of avanafil. After a 4-week nontreatment run-in period, 284 men (aged 32–70 years) with mild to moderate ED were randomly assigned to treatment for 12 weeks with placebo or avanafil at doses of 50 mg, 100 mg, 200 mg or 300 mg. The mean duration of ED was 66.7 months, 87% of the subjects had used oral ED medications prior to their enrollment in the study. Subjects were instructed to take the drug 30 min before the initiation of sexual activities. Primary efficacy outcomes included questions 2 and 3 of the Sexual Encounter Profile (SEP) (SEP 2 = the percentage of sexual attempts in which subjects were able to achieve vaginal penetration; SEP 3 = the percentage of sexual attempts in which subjects were able to maintain erections long enough for successful completion of intercourse) and the erectile function (EF) domain score of the International Index of Erectile Function (IIEF) questionnaire. All primary outcome parameters were significantly improved at all doses. Avanafil induced erections sufficient for vaginal penetration on 76%, 79%, 80% and 84% of the attempts for the 50 mg, 100 mg, 200 mg and 300 mg doses, respectively (placebo 60%). SEP 3 rates were 54%, 59%, 62% and 64% for the 50 mg, 100 mg, 200 mg and 300 mg doses, respectively (versus 28% of attempts in the placebo group) [Kaufman and Dietrich, 2006].
Table 1.
Randomized, placebo-controlled phase II and III clinical trials investigating the efficacy of the phosphodiesterase type 5 (PDE5) inhibitor avanafil in patients with mild to severe erectile dysfunction. Treatment period in all trials listed was 12 weeks (after a 4-week nontreatment run-in period). For the clinical key data presented by Zhao et al. [2012], see the text.
| Study | Drug doses evaluated | Number of patients | Improvement in SEP 2 (%) (versus placebo) | Improvement in SEP 3 (%) (versus placebo) | Successful sexual attempts (SEP 3) (%) at ≤ 15 min (versus placebo) | Improvement in IIEF-EF score (versus placebo) | Shift to normal erectile function (EF score ≥ 26) (%) (versus placebo) |
|---|---|---|---|---|---|---|---|
| Kaufmann and Dietrich [2006] | 50 mg 100 mg 200 mg 300 mg |
284 | 16.1a 19.1b 19.7b 23.6c |
25.9c 30.6c 34.1c 36.3c |
NE | + 3.9b + 5.3c + 6.0c + 6.7c |
NE |
| Zhao et al. [2012] | 100 mg 200mg |
200 | 11.3c 13.5c |
28.5c 29.5c |
NE | + 5.0c + 5.3c |
28.9c 22.7c |
| Goldstein et al. [2012b] | 50 mg 100 mg 200 mg |
646 | 10.0d 20.0e 23.0e |
14.0d 30.0e 30.0e |
37 40 44 |
+ 2.8d + 5.6e + 6.9e |
9.1 28.7 32.1 |
| Goldstein et al. [2012a] REVIVE-D Study (#TA-302) | 100 mg 200 mg |
390 | 12.0d 21.0d |
14.0d 20.0d |
See Goldstein et al. [2012a] | + 2.7d + 4.1d |
NE |
| Mulhall et al. [2012] | 100 mg 200 mg |
298 | 12.0c 21.0c |
14.0c 17.0c |
45 28 |
+ 3.3c + 5.4c |
NE |
p < 0.05; bp < 0.01; cp < 0.001; dp < 0.005; ep < 0.0001.
EF, erectile function; IIEF, International Index of Erectile Function; NE, not evaluated; SEP, Sexual Encounter Profile.
A multicenter, randomized, double-blind, placebo-controlled phase III study assessed the efficacy and safety of 100 mg and 200 mg of avanafil in 200 patients (mean age 56 years, mean duration of ED ≥ 6 months) with so-called generalized ED of varying etiologies. IIEF-EF domain scores at the time of enrollment were: ≤10 = severe: 20% of patients; 11–16 = moderate: 48% of patients; 17–25 = mild: 32% of patients. 40% of the subjects had used oral ED medications prior to the study. After a 4-week run-in period, subjects were assigned to treatment for 12 weeks with placebo or avanafil (taken 30 min before the initiation of sexual activities). There was no restriction on alcohol or food intake. The primary efficacy endpoint was the change in the IIEF-EF domain score; secondary efficacy parameters were SEP 2 and SEP 3, potential shift to normal EF domain score (≥26), and the response to the Global Assessment Questionnaire (GAQ, Has the treatment you have been taking improved your erections?). Avanafil was significantly superior to placebo for all primary and secondary outcome parameters. No significant differences were observed between the two dose units. After 12 weeks of treatment, IIEF-EF domain scores were shifted from 15.2 to 23.7 points with 100 mg avanafil and from 14.1 to 22.9 points with 200 mg (placebo: 14.5 to 18 points) [Zhao et al. 2012]. Surprisingly, the authors present data from their study indicating that, in the group of patients who were on avanafil, the response rates indicating successful vaginal penetration (SEP 2) were lower (27% and 29%, respectively, versus 15.7% in the placebo group) than the SEP 3 rates (increase to 54.3% after 12 weeks at 100 mg and 55.3% at 200 mg versus 25.8% for placebo). Unfortunately, the manuscript does not provide any guidance to help the reader to understand these data. One possible explanation might be that the authors (i) took, by mistake, SEP 3 for SEP 2 and vice versa or (ii) the SEP2 data given are likely to indicate negative attempts (failure) than positive response rates. However, the improvements in the orgasmic function, sexual desire, intercourse satisfaction and overall satisfaction scores also favored avanafil over placebo. With regard to the efficacy of the drug, no significant differences were observed between patients who had previous PDE5 inhibitor experiences and those who did not (PDE5 inhibitor-naive). The adverse events seen were in general mild to moderate in severity and attenuated after termination of treatment.
In another multicenter, double-blind, phase III trial, 646 men with mild to severe ED were randomized to either avanafil (50 mg, 100 mg or 200 mg) or placebo for 12 weeks. Subjects had an average age of 56 years, with an average duration of their ED of 79.3 months and an average IIEF domain score of 12.7 points. A total of 73% of the subjects surveyed had a history of previous oral ED treatment. Primary efficacy outcomes were the improvement in erectile function as measured by SEP 2 and SEP 3 and improvements in the IIEF-EF domain score. Secondary outcome parameters were changes in the responses regarding the other IIEF domains (orgasmic function, sexual desire, intercourse satisfaction and overall satisfaction), determination of the proportion of subjects with a normalized IIEF-EF domain score, as well as an analysis of the number of successful intercourse attempts at various times post-dosage. The 50 mg dose was inferior to the 100 and 200 mg doses, while the efficacy of 100 and 200 mg of avanafil were similar. At the end of the treatment period, SEP 2 rates had increased from 45% to 64% (50 mg), 47% to 74% (100 mg) and 48% to 77% (200 mg), while success rates in the placebo group had improved from 47% to 54%. The SEP 3 rates increased from 13% to 41% (50 mg), 14% to 57% (100 mg) and 12% to 57% (200 mg) versus 13% to 27% in the placebo group. The IIEF-EF domain score demonstrated significant improvements from 12.6 to 18.1 (50 mg), 12.6 to 20.9 (100 mg) and 12.8 to 22.2 (200 mg). Changes in other domains of the IIEF (orgasmic function, sexual desire, intercourse satisfaction and overall satisfaction) also significantly favored avanafil over placebo. Avanafil in doses of 50, 100 and 200 mg normalized the IIEF-EF domain score in 12% (50 mg), 29% (100 mg) and 31% (200 mg) of subjects with severe ED, 19%, 43% and 46% with moderate ED, and in 35%, 52% and 57% of those with mild ED, respectively. Out of the total of 300 sexual attempts for sexual intercourse that were made within 15 min after dosage, 64–71% were successful in the avanafil group in comparison with 27% in the placebo group. The number of successful attempts occurring at 15–30 min, 30–45 min, 2–4 hours and 4–6 hours post-dosing were also significantly higher in comparison with placebo. Interestingly, of the 80 sexual attempts occurring >6 hours post-dosage, 59–83% were successful with avanafil (placebo: 25%). Rates of discontinuation from the avanafil dose groups due to adverse events were between only 1.9% and 3.7% [Goldstein et al. 2012b].
The efficacy of avanafil was also evaluated in so-called difficult-to-treat cohorts, including ED patients with comorbid diabetes, as well as individuals following radical prostatectomy due to localized cancer. In 390 men with ED and comorbid diabetes, avanafil, administered in doses of 100 or 200 mg, significantly improved all primary endpoints: SEP 2, SEP 3 and the IIEF-EF domain score. SEP 2 rates increased from 32% to 54% and 42% to 63% for the 100 and 200 mg doses, respectively. In the placebo group, success rates increased from 36% to 42%. SEP 3 rates were enhanced from 8% to 34% (with the 100 mg dose) and 40% (with the 200 mg dose) versus 10 % up to 20% with placebo. Mean changes in the IIEF-EF domain were 4.6 and 5.4 points for 100 and 200 mg doses, respectively (placebo: 1.9 points). Successful intercourses were also reported from subjects who conducted attempts at <15 min and >6 hours post-dosing [Goldstein et al. 2012a].
In another study, 298 men with mild to severe ED, who had undergone bilateral nerve-sparing radical prostatectomy, were randomized to either avanafil 100 or 200 mg or placebo for 12 weeks. The average duration of ED prior to the inclusion in the study was 19 months post-surgery. A total of 72% of the subjects had severe ED at baseline. Primary endpoints included improvements in SEP 2, SEP 3 and the IIEF-EF domain score. A total of 252 subjects completed the study. In comparison with placebo, both doses of avanafil significantly improved all primary endpoints. At the end of treatment, SEP 2 rates had increased from 17% to 32% and 20% to 41% with the 100 and 200 mg doses, respectively (placebo: 0%). SEP 3 rates were enhanced from 5% to 23% (with 100 mg of avanafil) and 26% (with 200 mg) (placebo group: from 4% to 9%). Mean changes in the IIEF-EF domain were 3.5 points and 5.2 points with 100 and 200 mg doses, respectively (placebo: 0.1 points). Among the avanafil treatment groups, one-third to one-half of all intercourse attempts conducted at ≤15 min and ≤30 min, respectively, after administration of the drug were successful. The observation that efficacy rates were relatively low in the difficult-to-treat populations is well in agreement with the hypothesis that the action of all PDE5 inhibitors requires unimpaired neuronal input into the corpus cavernosum as well as intact vascular/cavernous endothelial structures [Mulhall et al. 2012].
An open-label extension study of the two multicenter, randomized, double-blind, placebo-controlled phase III trials, as mentioned above, has evaluated the safety and efficacy of avanafil over a period of up to 52 weeks. A total of 712 subjects with mild to severe ED with or without comorbid diabetes were enrolled and subjected to 100 mg of avanafil. There was no restriction with regard to the intake of the drug together with food or alcohol; patients who were on alpha-blocker medication were not excluded from the study. Increase to 200 mg or decrease to 50 mg was permitted if necessary to increase efficacy or to improve tolerability. The mean duration of ED was 76 months, 32% of the subjects had diabetes mellitus type 1 or 2, and 38% of the subjects had severe ED at baseline. A total of 493 subjects completed at least 26 weeks on active treatment and 153 subjects completed ≥52 weeks; 75% of patients increased their dose to 200 mg. Primary endpoints were improvements in SEP 2, SEP 3 and the IIEF-EF domain score. During treatment, the SEP 2 rate increased from 44% to 83.3% with 100 mg only (147 subjects) and to 79.4% in those who escalated the dose from 100 to 200 mg (535 subjects). Corresponding results for SEP 3 rates were 67.7% (baseline: 13.3%) in the 100 mg group and 66.3% (baseline: 11.4%) in the dose escalation group. Mean changes in the IIEF-EF domain were 8.6 and 10.8 points with 100 mg and 100/200 mg, respectively. A total of 84% of sexual attempts were made within 60 min after dosing, with 79.6% of the successful intercourses reported as early as 15 min. In some subjects, the erectogenic effect of the drug was sustained beyond 6 h [Belkoff et al. 2011]. There were no serious drug-related adverse events. Not surprisingly, the drop-out rate due to adverse events was <3%.
Protocols to assess the hemodynamic effects of the co-administration of avanafil with glyceryl trinitrate (GTN) were also carried out using a double-blind, cross-over design. A total of 106 healthy males, aged 30–60 years, were randomly given at separate visits placebo, 200 mg avanafil and 100 mg sildenafil, and the systolic blood pressure (SBP) and heart rate (HR) measured. When administered 12 h prior to nitrate, avanafil had no considerable effect on SBP or HR. Clinically significant drops in SBP (30 mmHg or more) were seen in 11 subjects following placebo, 14 subjects following avanafil and 28 subjects following sildenafil. In comparison with sildenafil, the co-administration of avanafil and GTN resulted in smaller changes in SBP and HR, a shorter duration of interaction, and fewer subjects with significant hypotension events. Thus, avanafil might be a preferable ED medication for those patients who are using nitrates [Nehra et al. 2006].
Conclusion
When compared with the other PDE5 inhibitors that have been made available to the international markets, avanafil has a unique selectivity profile, resulting in a favorable in vitro and in vivo potency and fast onset of action. Penile erection occurs between 20 and 40 min after dosage, and successful intercourse has been observed even as early as 15 min after administration of the drug. Moreover, avanafil is associated with low rates of hemodynamic side effects and a shorter duration of interaction in combination with NO-releasing drugs, such as glyceryl trinitrate. The faster onset of action and sustained duration of the erectogenic effect of the drug could be associated with more spontaneous sexual activity for ED patients and their female partners. The cumulative data also suggest that avanafil might be a particularly suitable medication for patients with ED and comorbid cardiovascular diseases who are on nitrates.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
George T. Kedia, Department of Urology and Urological Oncology, Hannover Medical School, Carl-Neuberg-Strasse 1, Hannover 30625, Germany
Stefan Ückert, Department of Urology and Urological Oncology, Hannover Medical School, Hannover, Germany.
Farhang Assadi-Pour, Department of Urology and Urological Oncology, Hannover Medical School and FAPO GmbH, Medical Park Business Area, Hannover, Germany.
Markus A. Kuczyk, Department of Urology and Urological Oncology, Hannover Medical School, Hannover, Germany
Knut Albrecht, Departments of Urology and Urological Oncology and Forensic (Legal) Medicine, Hannover Medical School, Hannover, Germany.
References
- Allison M., Grant T., Obaidi M., Marenco T., Yee S., Day W. (2011) Pharmacokinetics of avanafil; a novel, rapidly-absorbed, selective PDE5 inhibitor for the treatment of mild to severe erectile dysfunction. J Sex Med 8(Suppl. 5): 466–467 [Google Scholar]
- Aytac I., McKinlay J., Krane R. (1999) The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int 84: 50–56 [DOI] [PubMed] [Google Scholar]
- Belkoff L., McCullough A., Goldstein I., Di Donato K., Trask B., Bowden C. (2011) An open-label, long-term evaluation of the safety and tolerability of avanafil in men with erectile dysfunction. J Sex Med 8(Suppl. 5): 396 (abstract). [Google Scholar]
- Braun M., Wassmer G., Klotz T., Reifenrath B., Mathers M., Engelmann U. (2000) Epidemiology of erectile dysfunction: results of the Cologne Male Survey. Int J Impot Res (IJIR) 12: 305–311 [DOI] [PubMed] [Google Scholar]
- Eardley I., Donatucci C., Corbin J., El-Meliegy A., Hatzimouratidis K., McVary K., et al. (2010) Pharmacotherapy for erectile dysfunction. J Sex Med 7: 524–540 [DOI] [PubMed] [Google Scholar]
- Feldman H., Goldstein I., Hatzichristou D., Krane R., McKinlay J. (1994) Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 151: 54–61 [DOI] [PubMed] [Google Scholar]
- Goldstein I., Jones L., Belkoff L., Karlin G., Bowden C., Peterson C., et al. (2012a) Avanafil for the treatment of erectile dysfunction: a multicenter, randomized, double-blind study in men with diabetes. Mayo Clin Proc 87: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I., McCullough A., Jones L., Hellstrom W., Bowden C., Di Donato K., et al. (2012b) A randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of avanafil in subjects with erectile dysfunction. J Sex Med 9: 1122–1133 [DOI] [PubMed] [Google Scholar]
- Hatzimouratidis K., Hatzichristou D. (2008) Looking to the future for erectile dysfunction therapies. Drugs 68: 231–250 [DOI] [PubMed] [Google Scholar]
- Hellstrom W., Freier M., Serefoglu E., Lewis R., Didonato K., Peterso C. (2012) A phase II, single-blind, randomized, cross-over evaluation of the safety and efficacy of avanafil using visual sexual stimulation in patients with mild to moderate erectile dysfunction. BJU Int, DOI: 10.1111/j.1464-410X.2012.11267 [DOI] [PubMed] [Google Scholar]
- Jung J., Choi S., Cho S., Ghim J., Hwang A., Kim U., et al. (2010) Tolerability and pharmacokinetics of avanafil, a phosphodiesterase type 5 inhibitor: a single- and multiple-dose, double-blind, randomized, placebo-controlled, dose-escalation study in healthy Korean male volunteers. Clin Ther 32: 1178–1187 [DOI] [PubMed] [Google Scholar]
- Kalsi J., Kell P. (2004) Update on the oral treatments for male erectile dysfunction. Eur Acad Dermatol Venereol 18: 267–274 [DOI] [PubMed] [Google Scholar]
- Kaufman J., Dietrich J. (2006) Safety and efficacy of avanafil, a new PDE5 inhibitor for treating erectile dysfunction. J Sex Med 3(Suppl. 3): 180 (abstract). [Google Scholar]
- Kotera J., Mochida H., Inoue H., Noto T., Fujishige K., Sasaki T., et al. (2012) Avanafil, a potent and highly selective phosphodiesterase 5 inhibitor for erectile dysfunction. J Urol 188: 668–674 [DOI] [PubMed] [Google Scholar]
- Mochida H., Noto T., Inoue H., Yano K., Kikkawa K. (2006) Avanafil, a highly selective phosphodiesterase 5 inhibitor for erectile dysfunction, has pharmacological properties different from sildenafil on hemodynamics and electroretinogram in anesthetized dogs. J Sex Med 3(Suppl. 1): 45 (abstract). [Google Scholar]
- Montorsi F., Adaikan G., Becher E., Giuliano F., Khoury S., Lue T., et al. (2010) Summary of the recommendations on sexual dysfunctions in men. J Sex Med 7: 3572–3588 [DOI] [PubMed] [Google Scholar]
- Mulhall J., Moul J., Wang R., Shin D., Engel J., Day W., et al. (2012) A phase III, placebo-controlled study of the safety and efficacy of avanafil in the treatment of erectile dysfunction following bilateral, nerve-sparing radical prostatectomy. J Sex Med 9(Suppl. 1): 42–43 (abstract). [Google Scholar]
- Nehra A., Swearingen D., Dietrich J., Peterson C. (2006) Hemodynamic effects of co-administration of avanafil and glyceryl trinitrate. J Sex Med 3(Suppl. 1): 49 (abstract). [Google Scholar]
- Peterson C., Swearingen D. (2006) Pharmacokinetics of avanafil, a new PDE5 inhibitor being developed for treating erectile dysfunction. J Sex Med 3(Suppl. 3): 253–254 (abstract).16490018 [Google Scholar]
- Qiu Y., Bhattacharjee S., Kraft P., Mathew J., Haynes-Johnson D., Jiang W., et al. (2006) JNJ-10280205 and JNJ-10287069: novel PDE5 inhibitors as clinical candidates for erectile dysfunction. Int J Impot Res (IJIR) 18: 477–483 [DOI] [PubMed] [Google Scholar]
- Zhao C., Kim S., Yang D., Kim J., Park N., Lee S., et al. (2012) Efficacy and safety of avanafil for treating erectile dysfunction: results of a multi-centre, randomized, double-blind, placebo-controlled trial. BJU Int, DOI: 10.1111/j.1464-410X.2012.11095 [DOI] [PubMed] [Google Scholar]


