Abstract
Introduction
Left ventricular noncompaction (LVNC) is classified as a genetic cardiomyopathy characterized by a progressive systolic dysfunction. It may occur alone or in association with congenital cardiac anomalies. The combination of left ventricular noncompaction with partial atrioventricular canal defect is rare and has not, to our knowledge, been described previously.
Case presentation
A 21-year-old male who traveled to our center from a neighboring country presented with signs of heart failure. Transthorarcic echocardiography showed prominent trabeculations in the left ventricle predominantly in the left ventricle involving the apical lateral and mid anterolateral segments associated with a partial atrioventricular canal defect. There was a biventricular systolic dysfunction. There was good response to medical treatment.
Conclusion
This case stresses the importance of maintaining a high degree of suspicion for this rare cardiomyopathy and the need to systematically look for other associated anomalies in order to institute proper short- and long-term managements.
Keywords: left ventricular, noncompaction, atrioventricular canal defect
Introduction
Left ventricular noncompaction (LVNC) is a rare genetic cardiomyopathy characterized by a progressive left ventricular systolic dysfunction possibly due to arrest of compaction of myocardial fibers and meshwork during intrauterine life.1 It may occur alone or in association with other congenital cardiac or noncardiac anomalies.2 The average time from onset of symptoms to diagnosis of isolated left ventricular noncompaction according to a study by Ritter et al was 3.5 ± 5.7 years.3 In this study, the most common presentations for this cardiomyopathy were heart failure, ventricular arrhythmias, and thromboembolic events.
Partial atrioventricular canal defect (PAVD) is part of a spectrum of atrioventricular canal defects. These defects are characterized by a common atrioventricular junction and are associated with (1) a trileaflet left AV (atrioventricular) valve, (2) a defect in the atrial component of the AV septum, and (3) an unwedged aorta caused by the common AV orifice displacing anteriorly the aortic root. In contrast to the complete AV defect, PAVD has 2 separate valves. Its presentation is variable and can present later in life.4
LVNC is commonly associated with other congenital anomalies; however, a combination with PAVD to our knowledge has not been previously described.
Case Presentation
A 21 year old young man with a childhood history of repeated sore throats and polyarthritis and with no known family history of cardiomyopathy presented at our center with dyspnea (NYHA functional class IV), bilateral leg edema, and dry nocturnal coughs. Clinical examination found a borderline systolo- diastolic hypertension (blood pressure = 140/90 mmHg), regular heart sounds with a 3–4/6 mesocardiac systolic murmur radiating to areas all of the precordium, signs of decompensated biventricular heart failure, a splenomegaly, and finger clubbing.
Laboratory analysis showed a slight normochromic normocytic anemia of 12.1 g/dL and a SaO2 of 88%.
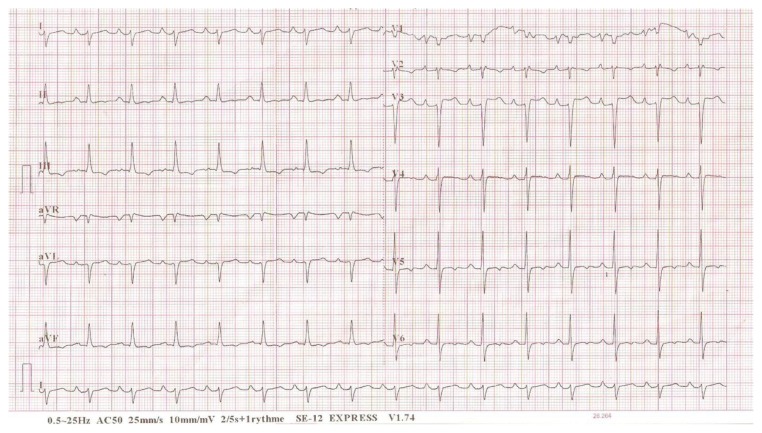
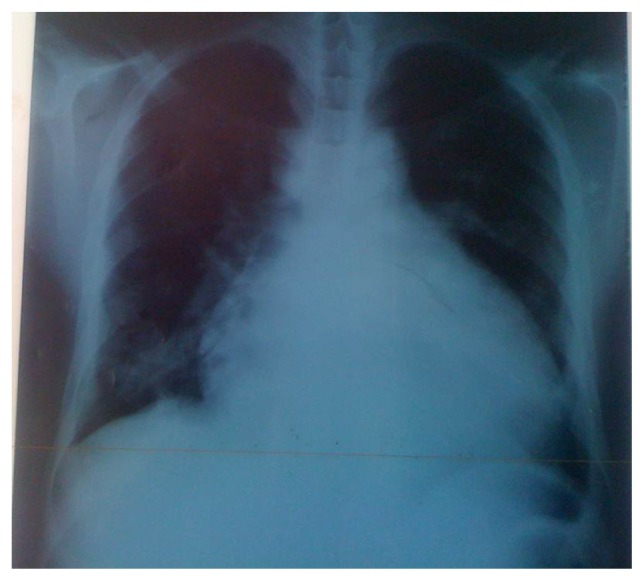
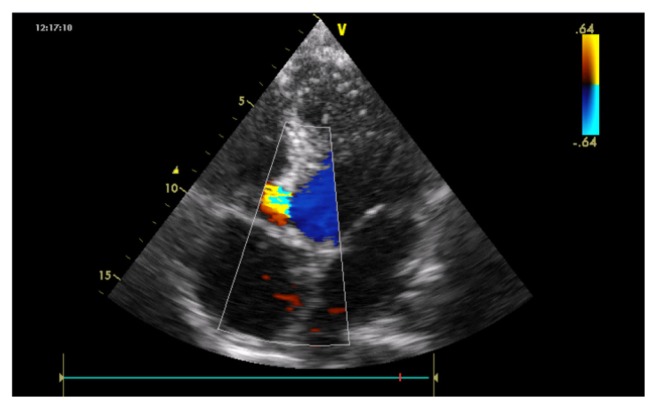
Electrocardiogram (ECG) inscribed a regular sinus rhythm with a heart rate of 94 cycles per minute, right QRS axis deviation at +130°, bi-atrial hypertrophy, a double heart block (first degree AV block and an incomplete right bundle branch block [RBBB]). There was also an inferior and a lateral subepicardial ischemia (Fig. 1). Chest X-ray showed a cardiomegaly with the apex above the diaphragm, a rectitude left middle heart border, bulged right lower heart border, and prominent hilar vessels (Fig. 2). Transthoracic echocardiography showed characteristics consistent with a hypokinetic dilated cardiomyopathy due to a left ventricular noncompaction characterized by the presence of prominent trabeculations predominantly in the left ventricle involving the apical lateral and mid anterolateral segments (Fig. 3) using the American Heart Association standardized segmentation of the left ventricle.5 There was a severe left ventricular systolic dysfunction (LVEF of 28% using biplane Simpson’s method and mitral annular plane systolic excursion (MAPSE) = 8 mm) and moderate dysfunction of the right ventricle (tricuspid annular plane systolic excursion (TAPSE) = 10 mm). A partial atrioventricular canal defect (PAVD) was also noted (Fig. 4). There was also a pulmonary hypertension of 62 mmHg.
Figure 1.
ECG on admission demonstrating bi-atrial hypertrophy, first degree AV block and an incomplete RBBB.
Figure 2.
Chest X-ray of patient at admission.
Figure 3.
Apical two chamber view shows zones of noncompaction and compaction by 2D echocardiography.
Figure 4.
Apical four chamber view shows PAVD by 2D echocardiography.
The patient was admitted for a period of 9 days in a general male cardiology ward with the goals of treating the heart failure, establishing the underlying pathology and mechanism of the heart failure, as well as preventing thromboembolic complications. The patient was sensitized about the disease and the need to adhere to treatment. Two outcomes were measured. One was a test of the patient’s understanding on how to correctly take the drugs and his adherence to the treatment prescribed, and the other was an assessment of the clinical (symptoms and signs) evolution. The patient is at present seen on an outpatient basis to assess his International Normalized Ration (INR), cardiac status (using clinical, electrocardiographic, and echocardiographic parameters), as well as to check on his intake of oral drugs.
Medical treatment involved both drug and nondrug therapies. The former involved salt and fluid restriction during the acute phase of treatment, and the patient was also encouraged to self-administer oral drugs. The drug therapy, on the other hand, involved intravenous furosemide (Lasilix) (which was changed to oral on day 7 of admission), oral spironolactone (Aldactone), and oral captopril. Acenocoumarol (Sintrom) and subcutaneous enoxaparine (lovenox) were given to prevent thromboembolic events. The latter was stopped after a good INR (of 2.14) was obtained with 2 mg of acenocoumarol.
The outcomes measured were favorable with the patient adhering to treatment given and with remarkable clinical improvement noted at the time of discharge and during follow-up care. Transthoracic echocardiographies were performed again on the day of discharge and at two months of follow-up care during which LVEFs of 32% and 48% respectively (using biplane Simpson’s method) were obtained. However, the patient has not yet undergone surgical intervention for the PAVD.
Discussion
LVNC is classified by the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases as an unclassified cardiomyopathy, while the American Heart Association classifies LVNC as a primary genetic cardiomyopathy.1,6 It is characterized by the presence of an extensive noncompacted myocardial layer lining the cavity of the left ventricle.7 Echocardiography is the most widely used imaging modality for the diagnosis of LVNC. However, its use is sometimes challenging. The apical region is poorly visualized by echocardiography and can lead to underestimation of the degree of the left ventricular non-compaction.8 Poor acoustic windows could mislead even experienced echocardiographers the diagnosis of this disease, resulting in an erroneous label such as dilated or hypertrophic cardiomyopathy.9 Cardiovascular magnetic resonance (CMR) has become the method of choice to confirm or rule out LVNC and may outperform echocardiography in defining the morphology and extension of myocardial noncompaction.7,10 This was demonstrated in a study by Yousef et al.11 CMR can also give valuable diagnostic and prognostic information about the disease by depicting fibrosis on delayed contrast-enhanced images.8
Both isolated forms of LVNC or forms associated with another congenital anomaly (or other congenital anomalies), cardiac or noncardiac, have been described. Commonly associated anomalies include ventricular septal defect, coarctation of the aorta, transposition of the great vessels, and atrial septal defect. The precise pathophysiologic mechanism of the association of LVNC and other congenital heart diseases (including PAVD) has not been elucidated, and more studies are needed in this area.
Patients at the time of diagnosis may be asymptomatic or may present with complications that include heart failure, arrhythmias, and thromboembolic events. Rhythm disturbances include atrial fibrillation, Wolff-Parkinson-White syndrome (WPW syndrome), and ventricular tachycardia and, hence, the need for electrocardiograms to diagnose and monitor the occurrence of these potentially lethal conditions7,12 and hence the indispensability of electrocardiogram in both the short- and long-term management. Thromboembolic events that occur are due either to atrial fibrillation or to thrombus formation within the intratrabecular recesses in the noncompacted myocardium.9
In this patient who presented with signs of biventricular heart failure and a relatively low arterial oxygen saturation, there was no arrhythmia; however, there were ECG features of a double block (a first degree AV block and an incomplete RBBB). Also in this case, we saw a very rare combination of LVNC associated with PAVD, of which we did not find a description in the literature.
Our long-term management plan involved monitoring his anticoagulation through periodic INR testing, close watch on the occurrence of arrhythmias by clinical and periodic ECG monitoring, subsequent evaluation for implantation of an implantable cardioverter-defibrillator, and exploration of eventual surgery of the PAVD.
Conclusion
The coexistence of LVNC and PAVD is rare. Echocardiography and, more recently, cardiac magnetic resonance are the main methods of diagnosis. Once diagnosis of LVNC is made, it is important to look for other congenital anomalies that might occur in association with LVNC in order to institute proper management strategies. The importance of other tests such as an ECG should not be overlooked, as this can be useful in identifying potentially life-threatening problems.
Consent
A written informed consent was obtained from the patient after careful consideration for publication of this case report and any accompanying images.
Footnotes
Author Contributions
MB and MJ conducted the literature search, drafted the first manuscript, performed language correction, and participated in article design and coordination. ML and MBN conducted the echocardiography and participated in manuscript draft. AK, SAS, and AM cared for the patient in the ward and during follow-up and contributed to drafting of manuscript. MD, FGB, and AAN critically revised the manuscript for important intellectual content. SMC and AT participated in investigation studies and critically evaluated the article. MS and SAB conceived the case study and participated in its design and coordination. All authors read and approved the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
Funding
Author(s) disclose no funding sources.
References
- 1.Ranjit SH, Rabi M. Left ventricular noncompaction. Iranian Cardiovascular Research Journal. 2009;3(1):1–7. [Google Scholar]
- 2.Maron BJ, Towbin JA, Thiene G, et al. AHA Scientific Statement. Contemporary definition and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807–16. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 3.Ritter M, Oechslin E, Sutsch G, Attenhofer C, Schneider J, Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc. 1997;72(1):26–31. doi: 10.4065/72.1.26. [DOI] [PubMed] [Google Scholar]
- 4.Gatzoulis MA, Hechter S, Webb GD, Williams WG. Surgery for partial atrioventricular septal defect in the adult. Ann Thorac Surg. 1999;67(2):504–10. doi: 10.1016/s0003-4975(98)01137-0. [DOI] [PubMed] [Google Scholar]
- 5.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 6.Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–6. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 7.Kohli SK, Pantazis AA, Shah JS, et al. Diagnosis of left-ventricular noncompaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur Heart J. 2008;29(1):89–95. doi: 10.1093/eurheartj/ehm481. [DOI] [PubMed] [Google Scholar]
- 8.Rosa LV, Salemi VMC, Alexandre LM, Mady Noncompaction cardiomyopathy—a Current View. Arq Bras Cardiol. 2011;97(1):13–9. doi: 10.1590/s0066-782x2011000900021. [DOI] [PubMed] [Google Scholar]
- 9.Dursun M, Agayev A, Nisli K, et al. MR imaging features of ventricular noncompaction: emphasis on distribution and pattern of fibrosis. Eur J Radiol. 2010;74(1):147–51. doi: 10.1016/j.ejrad.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Uribe S, Cadavid L, Hussain T, et al. Cardiovascular magnetic resonance findings in a pediatric population with isolated left ventricular non-compaction. J Cardiovasc Magn Reson. 2012 Jan;14:9. doi: 10.1186/1532-429X-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yousef ZR, Foley PWX, Khadjooi K, et al. Left ventricular non-compaction: clinical features and cardiovascular magnetic resonance imaging. BMC Cardiovasc Disord. 2009 Aug;9:37. doi: 10.1186/1471-2261-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pignatelli RH, McMahon CJ, Dreyer WJ, et al. Clinical characterization of left ventricular noncompaction in children: a relatively common form of cardiomyopathy. Circulation. 2003;108(21):2672–8. doi: 10.1161/01.CIR.0000100664.10777.B8. [DOI] [PubMed] [Google Scholar]