Abstract
Postural tachycardia syndrome (POTS) is defined by a heart rate increment of 30 beats/min or more within 10 minutes of standing or head-up tilt in the absence of orthostatic hypotension; the standing heart rate is often 120 beats/min or higher. POTS manifests with symptoms of cerebral hypoperfusion and excessive sympathoexcitation. The pathophysiology of POTS is heterogeneous and includes impaired sympathetically mediated vasoconstriction, excessive sympathetic drive, volume dysregulation, and deconditioning. POTS is frequently included in the differential diagnosis of chronic unexplained symptoms, such as inappropriate sinus tachycardia, chronic fatigue, chronic dizziness, or unexplained spells in otherwise healthy young individuals. Many patients with POTS also report symptoms not attributable to orthostatic intolerance, including those of functional gastrointestinal or bladder disorders, chronic headache, fibromyalgia, and sleep disturbances. In many of these cases, cognitive and behavioral factors, somatic hypervigilance associated with anxiety, depression, and behavioral amplification contribute to symptom chronicity. The aims of evaluation in patients with POTS are to exclude cardiac causes of inappropriate tachycardia; elucidate, if possible, the most likely pathophysiologic basis of postural intolerance; assess for the presence of treatable autonomic neuropathies; exclude endocrine causes of a hyperadrenergic state; evaluate for cardiovascular deconditioning; and determine the contribution of emotional and behavioral factors to the patient's symptoms. Management of POTS includes avoidance of precipitating factors, volume expansion, physical countermaneuvers, exercise training, pharmacotherapy (fludrocortisone, midodrine, β-blockers, and/or pyridostigmine), and behavioral-cognitive therapy. A literature search of PubMed for articles published from January 1, 1990, to June 15, 2012, was performed using the following terms (or combination of terms): POTS; postural tachycardia syndrome, orthostatic; orthostatic; syncope; sympathetic; baroreceptors; vestibulosympathetic; hypovolemia; visceral pain; chronic fatigue; deconditioning; headache; Chiari malformation; Ehlers-Danlos; emotion; amygdala; insula; anterior cingulate; periaqueductal gray; fludrocortisone; midodrine; propranolol; β-adrenergic; and pyridostigmine. Studies were limited to those published in English. Other articles were identified from bibliographies of the retrieved articles.
Abbreviations and Acronyms: EDS, Ehlers-Danlos syndrome; HUT, head-up tilt; NE, norepinephrine; NET, norepinephrine transporter; POTS, postural tachycardia syndrome
Postural tachycardia syndrome (POTS) is one of the most common manifestations of orthostatic intolerance.1,2 According to current criteria for adults,3 POTS is defined by a heart rate increment of 30 beats/min or more within 10 minutes of standing or head-up tilt (HUT) in the absence of orthostatic hypotension; the standing heart rate is often 120 beats/min or higher. These criteria may not be applicable for individuals with low resting heart rate. For individuals aged 12 to 19 years, the required increment is at least 40 beats/min.3 The orthostatic tachycardia may be accompanied by symptoms of cerebral hypoperfusion and sympathetic hyperactivity that are relieved by recumbency. Symptoms of cerebral hypoperfusion include light-headedness, blurred vision, cognitive difficulties, and generalized weakness; symptoms of excessive sympathoexcitation include palpitations, chest pain, and tremulousness. The term orthostatic intolerance is used to describe a condition in which patients develop symptoms on standing or HUT but do not fulfill the heart rate criteria for the diagnosis of POTS.4 The diagnostic criteria for orthostatic intolerance and POTS in adults are unsuitable for children and adolescents, and new criteria for these disorders in this age group have been recently proposed.5 POTS is more frequent in women (female:male ratio, 4.5:1), and most cases occur between the ages of 15 and 25 years. Up to 50% of cases have antecedent viral illness, and 25% have a family history of similar complaints.1,6,7 The experience from extensive series indicates that POTS is pathophysiologically heterogeneous. Many patients with POTS present with multiple chronic symptoms that are not directly related to orthostatic stress, and only a small subgroup of patients with POTS have a defined autonomic disorder. Physical deconditioning and psychological factors have an important role in these patients. Therefore, the evaluation and management of POTS should be multidisciplinary. These patients pose a particular challenge in management, which reflects the pathophysiologic heterogeneity of orthostatic intolerance, the presence of multiple comorbidities not directly related to orthostatic stress, and the lack of recognition that patients with POTS frequently require exercise training and behavioral-cognitive approaches to obtain long-term control of their symptoms. There have been many recent reviews on POTS.2,8,9 This review will focus on (1) the pathophysiologic heterogeneity of POTS, (2) comorbid conditions commonly associated with POTS, (3) an expanded view of POTS pathophysiology, and (4) the evaluation and rationale for a multimodal approach to these patients.
A literature search of PubMed for articles published from January 1, 1990, to June 15, 2012, was performed using the following terms (or combination of terms): POTS; postural tachycardia syndrome, orthostatic; orthostatic; syncope; sympathetic; baroreceptors; vestibulosympathetic; hypovolemia; visceral pain; chronic fatigue; deconditioning; headache; Chiari malformation; Ehlers-Danlos; emotion; amygdala; insula; anterior cingulate; periaqueductal gray; fludrocortisone; midodrine; propranolol; β-adrenergic; and pyridostigmine. Studies were limited to those published in English. Other articles were identified from bibliographies of the retrieved articles.
POTS as a Syndrome of Orthostatic Intolerance
Symptoms Reflecting Orthostatic Intolerance
POTS is one of the most common syndromes of orthostatic intolerance; others include reflex (neurally mediated, vasovagal) syncope and orthostatic hypotension in its several forms.3,10 The manifestations of POTS that reflect orthostatic intolerance (POTS in the strict sense) include those of cerebral hypoperfusion and reflex sympathetic activation. Up to one-third of patients may develop secondary orthostatically triggered vasovagal (reflex, neurally mediated) syncope.11 As in all types of orthostatic intolerance, typical exacerbating factors include heat exposure, physical exertion, heavy meals, prolonged recumbency, menses, and drugs such as diuretics and vasodilators.12
Classic Pathophysiology and POTS Subtypes
Exaggerated postural tachycardia may reflect several pathophysiologically distinct mechanisms2,4,6,8,9,13-16 (Table 1). Based on autonomic testing and plasma norepinephrine (NE) levels, POTS has been classified into several subtypes, including neuropathic and hyperadrenergic POTS2,13 and, based on measurement of leg venous pressure and calf blood flow, as low-flow, high-flow, and normal-flow POTS.8,16 β2-Adrenoreceptor polymorphisms may contribute to the hemodynamic diversity of patients with POTS.14,15
TABLE 1.
Pathophysiologic Mechanisms of Orthostatic Intolerance in POTS
| Mechanism (POTS subtype) | Markers | Examples |
|---|---|---|
| Impaired sympathetically mediated vasoconstriction in the lower limbs (neuropathic POTS) | Impaired distal sweating Blunted late phase II in the VMLow supine blood pressureReduced NE spillover in leg veinsReduced cardiac MIBG uptakeHigh leg blood flow | Restricted postviral or autoimmune neuropathies |
| Excessive cardiac sympathoexcitatory responses (hyperadrenergic POTS) | Standing plasma NE ≥600 pg/mL Fluctuating blood pressure or hypertension during HUT | AnxietyPheochromocytomaMast cell activation disordersVGKC autoimmunity? |
| Volume dysregulation | Elevated plasma angiotensin IIImpairment of RAA systemImpaired renal control of fluid secretion | Conditions associated with hypovolemia |
| Physical deconditioning | V̇o2max <85% on exercise testingReduced left ventricular mass | Prolonged bed restChronic fatigue, pain |
HUT = head-up tilt; MIBG = meta-iodobenzylguanidine; NE = norepinephrine; POTS = postural tachycardia syndrome; RAA = renin-angiotensin-aldosterone; VGKC = voltage-gated potassium channel; VM = Valsalva maneuver; V̇o2max = maximum oxygen consumption.
Neuropathic POTS
The term neuropathic POTS1,17 describes a subgroup of patients with indirect evidence of peripheral sympathetic denervation in the lower limbs. This condition is characterized by loss of sweating in the feet on thermoregulatory sweat tests and quantitative sudomotor axon reflex testing17 and impaired increase of NE release in the lower limb in response to orthostatic stress.17 In a large series,7 54% of patients had evidence of peripheral sudomotor denervation. The primary pathophysiologic mechanism of postural intolerance in this subgroup of patients is presumed to be impaired peripheral vasoconstriction, leading to venous pooling in the lower limbs. Consistent with this possibility, a subgroup of patients with POTS have impaired indirect measures of sympathetic vasoconstriction assessed by the blood pressure profile during the Valsalva maneuver or HUT7 and venous pooling in the absence of venous compliance defects in the lower limbs.18 This neuropathic subgroup corresponds to the so-called high-flow POTS subgroup, characterized by reduced total peripheral resistance while supine and venous pooling in the legs on standing.8,16 Reduced myocardial meta-iodobenzylguanidine uptake has been reported in patients with POTS.19 However, the relationship between this finding and peripheral adrenergic denervation of the lower limb is still undetermined. The frequent onset after a viral illness and the presence of a ganglionic acetylcholine receptor antibody in 14% of patients suggest an autoimmune cause of neuropathic POTS in some cases.7
Hyperadrenergic POTS
Between 30% and 60% of patients with POTS have evidence of increased central sympathetic drive, as reflected by standing plasma NE levels of 600 pg/mL or more (to convert to pmol/L, multiply by 5.911); fluctuating blood pressure or hypertensive response during HUT; and episodes of tachycardia, hypertension, and hyperhidrosis.2,7,20 In these patients, the episodes can be triggered not only by orthostatic stress but also by emotional stimuli and physical activity. This subgroup of patients may correspond to the so-called low-volume POTS characterized by supine vasoconstriction, supine tachycardia, pale and cold skin, and increased supine muscle sympathetic nerve activity.16 These patients have been categorized into a primary or central hyperadrenergic subgroup with plasma NE levels often between 1000 and 2000 pg/mL that compromises approximately 5% to 10% of cases and a heterogeneous group of secondary hyperadrenergic POTS.21 Loss-of-function sequence variation of the norepinephrine transporter (NET) and reduced clearance of synaptic NE were found in a case of hyperadrenergic POTS.22 However, increased NE levels more commonly reflect pharmacological NET blockade by drugs such as tricyclic antidepressants, selective NET inhibitors, and amphetaminelike drugs such as methylphenidate. Secondary hyperadrenergic POTS has also been associated with mast cell activation disorders.23 In patients with hyperadrenergic POTS, the possibility of hyperthyroidism or a catecholamine-secreting tumor, such as pheochromocytoma, should be considered. Laboratory studies should include determination of plasma and urinary metanephrine levels, and if they are elevated, imaging studies to detect pheochromocytoma may be necessary.24
Hyperadrenergic states may also be secondary to immune disorders associated with antibodies against components of the voltage-gated potassium channel complex in the setting of limbic encephalitis or Morvan syndrome.25 However, hyperadrenergic POTS as the only manifestation of anti–voltage-gated potassium channel complex autoimmunity has not yet been convincingly demonstrated.
POTS and Volume Dysregulation
Many patients with POTS have low plasma, red cell, and total blood volumes as assessed using various techniques.26-28 In one study, 28.9% of patients excreted less than 100 mEq/L of sodium in 24 hours, which was interpreted as consistent with a hypovolemic status.7 In many cases, these patients have low levels of standing plasma renin activity and aldosterone compared with controls. However, some patients have a low-flow phenotype associated with inappropriately high plasma angiotensin II levels, reflecting reduced estimated angiotensin-converting enzyme 2 activity.28 Functional gastrointestinal disorders associated with poor water intake due to nausea or excess fluid loss due to diarrhea may result in hypovolemia with secondary orthostatic symptoms and tachycardia. In this case, POTS should be considered a consequence of the gastrointestinal disorder.
POTS and Physical Deconditioning
Most patients with POTS exhibit greater and more persistent tachycardia, reduced stroke volume, reduced left ventricular mass, and reduced peak oxygen uptake when upright and during and after exercise compared with control subjects.29,30 These findings indicate the presence of physical deconditioning, which may be an important factor in the development of orthostatic symptoms, regardless of the underlying pathophysiology.30,31 Bed rest or deconditioning also decreases the gain of the vasoconstrictor baroreceptor reflex32,33 and the vestibulosympathetic reflex,34 which could also predispose patients to orthostatic intolerance. However, there is evidence that bed rest or deconditioning does not primarily affect the reflex control of muscle sympathetic nerve activity.35,36
Comorbidities in POTS
Many patients with POTS experience chronic symptoms that cannot be mechanistically explained by postural intolerance or excessive tachycardia.2,37 Many of these symptoms are also prevalent in patients without orthostatic intolerance; in these cases, excessive postural tachycardia is secondary to hypovolemia, prolonged bed rest, physical deconditioning, and anxiety, in various combinations.
Visceral Pain and Dysmotility
A percentage of patients with POTS experience visceral symptoms referred to the upper or lower gastrointestinal tract, bladder, and other organs. In a large series of adult patients with POTS,7 nausea was present in 39%, bloating in 24%, diarrhea in 18%, constipation in 15%, abdominal pain in 15%, and bladder symptoms in 9% of cases. These symptoms are similar to those typically reported by patients with functional motility disorders such as functional dyspepsia, gastric emptying disorders, irritable bowel syndrome, and interstitial cystitis, among others.38,39 The underlying pathophysiology of these disorders includes mucosal inflammation, visceral hypersensitivity, secondary visceromotor dysfunction, and, in most cases, behavioral amplification.39-42 Although these symptoms may be more frequently reported in patients with neuropathic POTS,2 they should not necessarily be considered a manifestation of a primary underlying autonomic disorder (dysautonomia) because these are disorders of visceral sensitivity mediated by visceral afferents and not due to primary involvement of the enteric nervous system or efferent parasympathetic or sympathetic output via the vagus, pelvic, or splanchnic nerves.
Chronic Fatigue, Insomnia, and Fibromyalgia
Chronic fatigue,43-45 fibromyalgia,46 and sleep disturbances44 have been frequently associated with POTS. In a large series of adult patients with POTS, many reported chronic fatigue (48%), sleep disturbance (32%), and myofascial pain (16%).7 There is an overlap between chronic fatigue syndrome and POTS. Patients with POTS who have chronic fatigue syndrome have higher laboratory markers of sympathetic activation than patients without chronic fatigue.43,45
Ehlers-Danlos Syndrome
Ehlers-Danlos syndrome (EDS) is a heterogeneous disorder that includes several forms, all linked to sequence variations in genes encoding for fibrillar proteins and/or collagen processing enzymes leading to reduced structural integrity of connective tissue. Joint hypermobility, which is characteristic of EDS type III associated with sequence variations in tenascin X, has been frequently associated with POTS.47-49 However, the mechanistic relationship between these 2 entities is incompletely defined. Whereas tenascin X sequence variations affect cardiovascular tissue leading to valvular disease,50 the hypothesis that impaired integrity of vascular connective tissue leads to impaired venous return and secondary orthostatic tachycardia has not yet been convincingly tested. EDS III is also characterized by early onset of chronic pain, particularly in the shoulders, hands, and knees,51 which may be disabling due to associated anxiety, depression, and a somatosensory amplification state52; this may lead to secondary hypersympathetic responses triggered by fear of pain on standing.
Headache and Other Neurologic Conditions
Chronic headache, including migraine, is a common comorbidity in patients with POTS.7,37,53 Orthostatic headaches also occur in patients with POTS,53,54 even in the absence of intracranial hypovolemia or cerebrospinal fluid leak.55 The relationship between POTS and orthostatic headache is uncertain because treatment of orthostatic tachycardia with volume expansion is only partially effective in these patients.55 Orthostatic tachycardia has also been reported in patients with type I Chiari malformation,56,57 in some cases associated with syringomyelia.58 However, a direct relationship between incidental type I Chiari malformation and orthostatic intolerance has not been convincingly demonstrated.59 POTS has also been shown in patients with a remote history of traumatic head injury,60 but this does not necessarily implicate direct damage of central autonomic areas as the primary cause of orthostatic intolerance.
Expanded Pathophysiology of POTS
Potential Mechanisms of Persistence of Symptoms
The persistence of orthostatic symptoms despite adequate control of the heart rate and the coexistence of many nonorthostatic symptoms commonly reported by patients with POTS suggest that impaired processing of viscerosensory (including cardiovascular) information, conditioning, and behavioral amplification also play a contributory role in this disorder. For example, many triggers, such as viral illness (particularly if associated with gastrointestinal fluid loss), prolonged bed rest, or both, may result in relatively rapid development of symptoms of orthostatic intolerance and sympathoexcitation. This pattern of responses may become conditioned, leading to failure of the viscerosensory and sympathetic systems to readapt to the routine demands of standing and exercise after the acute illness. This situation would be akin to the case of chronic subjective dizziness, in which there is failure of the postural and ocular motor control systems to readapt to the routine demands of locomotion after an acute episode of vertigo and postural instability.61,62 Because standing up not only produces activation of the baroreflex but also triggers a vestibulosympathetic reflex,63 exaggerated conditioned responses to vestibular inputs and lack of readaptation of the vestibulosympathetic responses may also contribute to persistence of posture-induced symptoms and tachycardia in these patients. Abnormal processing of sensory information, including somatic hypervigilance64 and behavioral amplification, may also contribute to persistence of symptoms, including those not triggered by orthostatic stress, such as visceral pain,41 fibromyalgia,65,66 and chronic dizziness. Furthermore, deconditioning, poor sleep, and psychological factors such as anxiety or depression may all lead to relative predominance of sympathetic over vagal control the heart rate.67-69
Pathophysiologic Basis of POTS
The central circuits involved in visceral sensation, aversive conditioning, behavioral arousal, stress responses, and pain processing and modulation may all be involved in the maintenance of the symptoms in patients with POTS (Figure). Key components of this network include the brainstem sensory relay nuclei, amygdala, insula, anterior cingulate cortex, hypothalamus (particularly the paraventricular nucleus and lateral hypothalamic area), periaqueductal gray region, medullary raphe, and ventrolateral medulla.70-74 Information from visceral mechanoreceptors (including cardiovascular, gastrointestinal, and genitourinary receptors) as well as visceral and somatic nociceptors is conveyed to this network via the dorsal horn of the spinal cord, nucleus of the solitary tract, and parabrachial nucleus of the pons70; vestibular receptors activate this network via relay in the vestibular nuclei.75 This information is referred to as interoception because it reflects the physiological state of the body.71 These inputs trigger homeostatic reflexes integrated at the level of the medulla, including the baroreflex76 and vestibulosympathetic reflexes63 that are initiated by orthostatic stress and mediated by sympathoexcitatory neurons of the rostral ventrolateral medulla.77 These interoceptive inputs also relay, either directly or via the thalamus, to forebrain areas such as the hypothalamus, amygdala, insular cortex, and anterior cingulate cortex.70-72 All these areas are reciprocally interconnected and control sympathetic cardiovascular output via projections to the rostral ventrolateral medulla.77 The amygdala automatically attributes emotional valence to the stimulus and is involved in associative learning and conditioned fear responses.78 The anterior insular cortex is required for conscious awareness of bodily sensations.71 For example, functional magnetic resonance imaging studies showed increased activity of the anterior insula during tasks in which subjects attend to the timing of their heartbeats.79 The dorsal anterior cingulate (also called the midcingulate) cortex is activated during tasks that involve awareness, attention, and behavioral engagement that are associated with an increase in sympathetic drive.72 These areas project to the hypothalamus and periaqueductal gray region, which orchestrate coordinated autonomic and pain modulatory responses in the setting of emotion and stress. Functional neuroimaging studies also indicate that the insular and anterior cingulate cortex may also be involved in modulation of baroreflex-mediated sympathetic and cardiovascular responses.80
FIGURE.
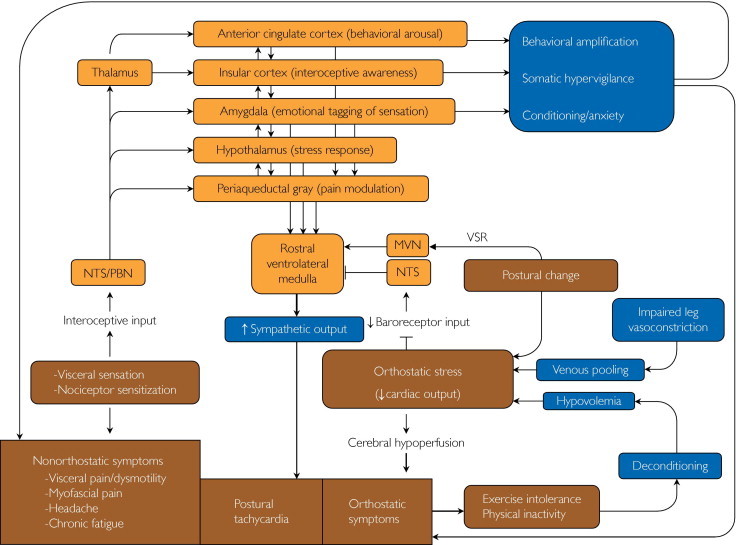
Complex pathophysiology of postural tachycardia syndrome (POTS). The mechanisms of orthostatic intolerance in POTS include impaired sympathetic vasoconstriction leading to venous pooling, hypovolemia, deconditioning, and hyperadrenergic state. Excessive reflex sympathoexcitation may be triggered by orthostatic stress via reduced baroreceptor input to the nucleus of the solitary tract (NTS) and activation of vestibulosympathetic reflexes (VSR) relayed via the medial vestibular nucleus (MVN), resulting in increased activity of sympathoexcitatory neurons of the rostral ventrolateral medulla. Many comorbidities of POTS, including visceral pain and dysmotility, other chronic pain conditions, and dizziness may reflect abnormal processing of interoceptive information, relayed via the NTS and parabrachial nucleus (PBN) via the ventromedial portion of the thalamus to a central network that includes the anterior cingulate cortex, insula, amygdala, hypothalamus, and periaqueductal gray region.
There is a reciprocal interaction between these areas and areas of the prefrontal cortex involved in focused attention and emotional modulation.81 The insular, midcingulate, and dorsolateral prefrontal cortex are also components of the cortical network that is activated in response to pain and visceral sensations, referred to as the pain matrix.82,83 This network exhibits considerable use-dependent synaptic plasticity, which may be maladaptive, for example in the setting of chronic stress or pain.82,83 Functional neuroimaging studies also show that the connectivity among the amygdala, cingulate, and prefrontal cortex is affected in disorders such as anxiety and depression,81 which are common comorbid conditions in patients with symptoms associated with POTS, including those of visceral pain and dysmotility, chronic fatigue, fibromyalgia, and insomnia.
Evaluation and Management of Patients With POTS
Patients with POTS require a multidisciplinary evaluation and multimodality treatment.2,12
Evaluation
Elicitation of the clinical history of patients with POTS should address the trigger, timing of onset, and progression of orthostatic symptoms; precipitating or aggravating factors; presence of associated nonorthostatic symptoms; fluid and caffeine intake; level of physical activity; sleep pattern; response to previous attempted treatments; and current drug therapy. The patient should undergo a comprehensive cardiac and neurologic examination in addition to measurements of supine and standing heart rate and blood pressure. Examination could also reveal indirect evidence of venous pooling (such as lower extremity edema) or excessive sympathetic activity (such as cold, clammy hands). The main aspects of laboratory evaluation in patients with POTS are summarized in Table 2. The aims are to exclude primary cardiac causes of inappropriate tachycardia; determine, if possible, the most likely primary pathophysiologic basis of postural intolerance; identify treatable causes of autonomic neuropathy in patients with neuropathic POTS; exclude endocrine or other systemic causes of a hyperadrenergic state in patients with hyperadrenergic POTS; assess the degree of cardiovascular conditioning; investigate the mechanisms of gastrointestinal and other associated symptoms; and address possible psychiatric comorbidities. The basic evaluation should include a cardiac evaluation, autonomic reflex testing, and measurement of supine and standing plasma catecholamine levels. Screening HUT table testing has been shown to be helpful in the evaluation of patients with syncope of unknown cause, including POTS.84 Noninvasive plethysmographic blood pressure and heart rate monitoring allows a careful examination of the beat-to-beat systolic and diastolic blood pressure and heart rate responses during HUT. Data regarding stroke index and total peripheral resistance, as well as heart rate variability, can also be obtained from these recordings. This information provides a more thorough picture of the hemodynamic and reflex responses associated with orthostatic stress and allows a more precise categorization of patients with POTS into the different pathophysiologic subtypes (neuropathic, hyperadrenergic, or hypovolemic).
TABLE 2.
Laboratory Evaluation in Patients With POTS
| Investigation | Rationale |
|---|---|
| Cardiac evaluation (ECG, echocardiogram, Holter monitoring) | Exclude a primary cardiac cause of inappropriate sinus tachycardia |
| Head-up tilt (at a 60° angle for 10 min) | Distinguish POTS from other forms of OI |
| Cardiovagal and sudomotor function tests | Detect underlying autonomic neuropathy |
| Plasma catecholamine, both supine and during standing or head-up tilt | Assessment of baroreflex sympathoexcitation and possible hyperadrenergic POTS |
| 24-Hour blood pressure and heart rate monitoring | Correlate the timing of the patient's symptoms with the presence of tachycardia |
| Exercise testing with V̇o2max | Detect physical deconditioning |
| Additional tests in individual cases am and pm cortisol Thyroid cascade Plasma and urinary metanephrines Serum tryptase, urinary methylhistamine Autoantibodies (VGKC complex, ganglionic AChR) MRI of the head with gadolinium | Evaluate for chronic fatigue Evaluate for hyperadrenergic state Detect pheochromocytoma Detect mast cell activation disorders Detect autoimmune causes of POTS Patients with orthostatic headache |
| Special additional evaluations | |
| Gastrointestinal or urologic evaluations | POTS patients with suspected functional visceral dysmotility syndromes |
| Behavioral medicine evaluation | POTS patients with multiple associated nonorthostatic symptoms |
AChR = acetylcholine receptor; ECG = electrocardiogram; MRI = magnetic resonance imaging; OI = orthostatic intolerance; POTS = postural tachycardia syndrome; VGKC = voltage-gated potassium channel.
Management
The management of POTS involves nonpharmacological (Table 3) and pharmacological (Table 4) approaches.12
TABLE 3.
Nonpharmacological Management of POTS
| Management | Rationale |
|---|---|
| Avoidance of precipitating factors | |
|
Reduce orthostatic stress Avoid deconditioningReduce peripheral vasodilatationAvoid blood pooling in mesenteric vesselsPrevent vasodilatationPrevent excessive vasodilatationPrevent excessive synaptic NE levels |
Maintenance of intravascular volume
|
Avoid hypovolemia as a trigger of orthostatic stress |
| Physical countermaneuvers | Reduce venous pooling |
| Support garments | Reduce venous pooling |
Exercise program
|
Improve physical conditioningReduced venous pooling |
| Behavioral medicine program | Address and manage other chronic nonorthostatic symptoms |
NE = norepinephrine; POTS = postural tachycardia syndrome.
TABLE 4.
Pharmacological Management of POTS
| Drug | Rationale | Special considerations in patients with POTS |
|---|---|---|
| Fludrocortisone | Volume expansion | May worsen headache |
| Midodrine | Vasoconstriction (reduces venous pooling) | May worsen GI symptoms or urinary retention |
| β-Adrenergic blockers (propranolol, cardioselective drugs) | Reduce sympathetic influence on the sinus node | Propranolol may be potentially useful to treat comorbidities (anxiety, migraine)All β-blockers should be started at low doses because patients may have β-adrenoceptor supersensitivityβ-Blockers may exacerbate fatigue or produce hypotension if used without concomitant nonpharmacological approaches |
| Pyridostigmine | Potentiates ganglionic sympathoexcitation during orthostatic stressMay contribute to reducing heart rate | May help visceral hypomotility but may exacerbate symptoms of visceral hypermotility |
GI = gastrointestinal; POTS = postural tachycardia syndrome.
Patient Education
Patient education is a fundamental aspect of POTS management. Patients should be informed about the differences between the symptoms that are due to orthostatic intolerance and those that are not and about potential aggravating or precipitating factors that may exacerbate their postural and exercise intolerance.
Sodium and Water Intake
Regardless of the basic pathophysiologic mechanism, most patients require volume expansion with adequate daily water (1.5-2 L) and sodium intake. This is particularly important on awakening or before a predictable orthostatic stress. Excessive caffeine intake should be avoided because it may increase diuresis and promote hypovolemia.12
Physical Countermaneuvers
Physical countermaneuvers, such as leg crossing, bending forward and placing a foot on a chair, making a fist, or active contraction of abdominal or buttock muscles may produce small increases in mean arterial pressure to maintain an adequate cerebral blood flow and prevent fainting in patients with all forms of orthostatic intolerance, including neurogenic orthostatic hypotension,85 vasovagal syncope,86 and POTS.87
Support Garments
Because blood pooling on standing occurs both in the abdomen and lower limbs, some patients with POTS may benefit from wearing support garments, such as thigh- or waist-high tight support stockings or an abdominal binder.88
Exercise Training
As in all causes of physical deconditioning, reduced stroke volume is a key component in the pathogenesis of POTS.29 As described by Shibata et al,89 short-term exercise training improves physical fitness and cardiovascular responses during exercise in patients with POTS. Given that deconditioned patients with POTS may rapidly become symptomatic during exercise, they should begin with a very small amount of exercise, using either a recumbent cycle or treadmill and gradually increasing the regimen over the next 6 to 8 weeks. Patients also benefit from light strengthening exercises for the major muscle groups using weight machines.
Pharmacological Therapy
Drug therapy of POTS includes the mineralocorticoid fludrocortisone90 to promote intravascular volume expansion, the α1-adrenergic agonist midodrine87,91 to elicit peripheral vasoconstriction and reduce venous pooling, β-blockers including propranolol87,92 and cardioselective agents such as bisprostol90,93 to control the excessive sinus tachycardia, and the cholinesterase inhibitor pyridostigmine94-96 to prolong the phasic effects of acetylcholine on the autonomic ganglia, leading to peripheral sympathetic vasoconstrictor output (and potentiating vagal effects) on standing (Table 3). However, adverse effects of these drugs can limit their use in patients with POTS because of the presence of comorbid symptoms. Fludrocortisone may exacerbate headache and vertigo, particularly in patients with migraine. Midodrine can increase the risk of urinary retention in patients with functional bladder disorders by increasing contraction of the smooth muscle of the bladder neck. Propranolol and other β-blockers may exacerbate fatigue and exercise intolerance and may produce hypotension in patients with low intravascular volume. Pyridostigmine may potentiate muscarinic receptor activation in the gastrointestinal tract, exacerbating nausea, vomiting, abdominal cramps, and diarrhea, and in the bladder, potentiating the symptoms of detrusor hyperactivity.
Why Are Some Patients With POTS So Difficult to Treat?
Several factors contribute to the difficulties in the management of patients with POTS. First is the lack of patient education (or, worse, patient misinformation) about the disorder. This leads to unrealistic expectations about the beneficial effects of treatment aimed to correct the postural tachycardia and secondary frustration and symptom amplification. A second factor is the failure to recognize the heterogeneous pathophysiologic basis of orthostatic tachycardia in POTS. Drug treatment may need to be individualized, depending on whether impaired peripheral vasoconstriction and venous pooling, hyperadrenergic state, or hypovolemia is thought to be the most prominent trigger. A complicating factor is that the doses of fludrocortisone, midodrine, β-blockers, or pyridostigmine used for treatment of other conditions such as neurogenic orthostatic hypotension or inappropriate sinus tachycardia may not be tolerated by many patients with POTS, particularly those with physical deconditioning and comorbid conditions. Drugs used to treat POTS comorbidities may also exacerbate orthostatic tachycardia; these include amphetaminelike psychostimulants used to treat chronic fatigue and antidepressants such as amitriptyline used to treat insomnia, migraine, fibromyalgia, or visceral pain. Another reason for symptom persistence is the failure to address physical deconditioning with a gradual exercise program. Although many patients are motivated to exercise, they may initially exercise too vigorously, leading to worsening of symptoms. Finally and perhaps most importantly is the fundamental role of somatic hypervigilance, behavioral arousal, and emotional conditioning in the maintenance of the patient's orthostatic, as well as nonorthostatic, symptoms. Both the patient and the physician should realize that, as in many other chronic conditions, realistic expectations and behavioral-cognitive approaches are more likely than pharmacotherapy to improve the patient's quality of life.
Conclusions and Perspective
POTS is a prototypical chronic, potentially disabling condition with no clear pathologic substrate and multiple interacting pathophysiologic mechanisms. Thus, it resembles functional visceral pain/dysmotility disorders, fibromyalgia, chronic headache, and chronic fatigue syndrome. In POTS, as in all these comorbid disorders, symptoms frequently develop after a triggering factor such as a viral illness or surgical procedure and persist despite resolution of the underlying condition. This suggests that whereas each of these conditions is defined by the most prominent symptom, their chronicity depends on common interacting mechanisms that likely reflect plastic changes in central nervous system areas involved in processing of interoceptive (visceral and somatic nociceptive) information, interoceptive awareness, behavioral arousal, and stress responses.
Article Highlights.
-
■
Postural tachycardia syndrome (POTS) is not a unique nosological entity, and its treatment should be individualized.
-
■
The pathophysiologic mechanisms of orthostatic intolerance in POTS are multifactorial and include hypovolemia, venous pooling, hyperadrenergic states, and restricted adrenergic neuropathies in the lower limb.
-
■
Many symptoms reported by patients with POTS, including visceral symptoms and chronic pain disorders, are not due to orthostatic intolerance and therefore are not, in the strict sense, part of the syndrome.
-
■
Most patients with POTS and evidence of a sympathetic neuropathy restricted to the lower limbs have no identifiable cause of neuropathy; pheochromocytoma should be excluded in patients with excessive sympathetic responses.
-
■
Except for effects of chronic pain and physical deconditioning, there is no clear mechanistic relationship between Ehlers-Danlos syndrome or Chiari malformation and POTS.
-
■
The management of POTS includes patient education, volume restitution, physical countermaneuvers, graded exercise training, and pharmacotherapy (fludrocortisone, midodrine, β-blockers, and/or pyridostigmine).
-
■
Cognitive-emotional factors, including behavioral conditioning and amplification, have a key role in POTS.
References
- 1.Low P.A., Opfer-Gehrking T.L., Textor S.C. Postural tachycardia syndrome (POTS) Neurology. 1995;45(4, suppl 5):S19–S25. [PubMed] [Google Scholar]
- 2.Low P.A., Sandroni P., Joyner M., Shen W.K. Postural tachycardia syndrome (POTS) J Cardiovasc Electrophysiol. 2009;20(3):352–358. doi: 10.1111/j.1540-8167.2008.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman R., Wieling W., Axelrod F.B. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 4.Parsaik A.T., Allison T.G., Singer W. Deconditioning in patients with orthostatic intolerance [published online ahead of print September 19, 2012] Neurology. 2012 doi: 10.1212/WNL.0b013e31826d5f95. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer W., Sletten D.M., Opfer-Gehrking T.L., Brands C.K., Fischer P.R., Low P.A. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr. 2012;160(2):222–226. doi: 10.1016/j.jpeds.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandroni P., Opfer-Gehrking T.L., McPhee B.R., Low P.A. Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin Proc. 1999;74(11):1106–1110. doi: 10.4065/74.11.1106. [DOI] [PubMed] [Google Scholar]
- 7.Thieben M.J., Sandroni P., Sletten D.M. Postural orthostatic tachycardia syndrome: the Mayo Clinic experience. Mayo Clin Proc. 2007;82(3):308–313. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- 8.Medow M.S., Stewart J.M. The postural tachycardia syndrome. Cardiol Rev. 2007;15(2):67–75. doi: 10.1097/01.crd.0000233768.68421.40. [DOI] [PubMed] [Google Scholar]
- 9.Carew S., Connor M.O., Cooke J. A review of postural orthostatic tachycardia syndrome. Europace. 2009;11(1):18–25. doi: 10.1093/europace/eun324. [DOI] [PubMed] [Google Scholar]
- 10.Wieling W., Thijs R.D., van Dijk N., Wilde A.A., Benditt D.G., van Dijk J.G. Symptoms and signs of syncope: a review of the link between physiology and clinical clues. Brain. 2009;132(pt 10):2630–2642. doi: 10.1093/brain/awp179. [DOI] [PubMed] [Google Scholar]
- 11.Alshekhlee A., Guerch M., Ridha F., Mcneeley K., Chelimsky T.C. Postural tachycardia syndrome with asystole on head-up tilt. Clin Auton Res. 2008;18(1):36–39. doi: 10.1007/s10286-007-0445-9. [DOI] [PubMed] [Google Scholar]
- 12.Mathias C.J., Low D.A., Iodice V., Owens A.P., Kirbis M., Grahame R. Postural tachycardia syndrome—current experience and concepts. Nat Rev Neurol. 2011;8(1):22–34. doi: 10.1038/nrneurol.2011.187. [DOI] [PubMed] [Google Scholar]
- 13.Garland E.M., Raj S.R., Black B.K., Harris P.A., Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007;69(8):790–798. doi: 10.1212/01.wnl.0000267663.05398.40. [DOI] [PubMed] [Google Scholar]
- 14.Jacob G., Garland E.M., Costa F. Beta2-adrenoceptor genotype and function affect hemodynamic profile heterogeneity in postural tachycardia syndrome. Hypertension. 2006;47(3):421–427. doi: 10.1161/01.HYP.0000205120.46149.34. [DOI] [PubMed] [Google Scholar]
- 15.Nickander K.K., Carlson P.J., Urrutia R.A., Camilleri M., Low P.A. A screen of candidate genes and influence of beta2-adrenergic receptor genotypes in postural tachycardia syndrome. Auton Neurosci. 2005;120(1-2):97–103. doi: 10.1016/j.autneu.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Stewart J.M., Montgomery L.D. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2004;287(3):H1319–H1327. doi: 10.1152/ajpheart.00086.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacob G., Costa F., Shannon J.R. The neuropathic postural tachycardia syndrome. N Engl J Med. 2000;343(14):1008–1014. doi: 10.1056/NEJM200010053431404. [DOI] [PubMed] [Google Scholar]
- 18.Stewart J.M. Pooling in chronic orthostatic intolerance: arterial vasoconstrictive but not venous compliance defects. Circulation. 2002;105(19):2274–2281. doi: 10.1161/01.cir.0000016348.55378.c4. [DOI] [PubMed] [Google Scholar]
- 19.Haensch C.A., Lerch H., Schlemmer H., Jigalin A., Isenmann S. Cardiac neurotransmission imaging with 123I-meta-iodobenzylguanidine in postural tachycardia syndrome. J Neurol Neurosurg Psychiatry. 2010;81(3):339–343. doi: 10.1136/jnnp.2008.168484. [DOI] [PubMed] [Google Scholar]
- 20.Streeten D.H. Pathogenesis of hyperadrenergic orthostatic hypotension: evidence of disordered venous innervation exclusively in the lower limbs. J Clin Invest. 1990;86(5):1582–1588. doi: 10.1172/JCI114878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raj S.R. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6(2):84–99. [PMC free article] [PubMed] [Google Scholar]
- 22.Shannon J.R., Flattem N.L., Jordan J. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. 2000;342(8):541–549. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- 23.Shibao C., Arzubiaga C., Roberts L.J., II Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension. 2005;45(3):385–390. doi: 10.1161/01.HYP.0000158259.68614.40. [DOI] [PubMed] [Google Scholar]
- 24.Manger W.M. The protean manifestations of pheochromocytoma. Horm Metab Res. 2009;41(9):658–663. doi: 10.1055/s-0028-1128139. [DOI] [PubMed] [Google Scholar]
- 25.Misawa T., Mizusawa H. Anti-VGKC antibody-associated limbic encephalitis/Morvan syndrome [in Japanese] Brain Nerve. 2010;62(4):339–345. [PubMed] [Google Scholar]
- 26.Streeten D.H., Thomas D., Bell D.S. The roles of orthostatic hypotension, orthostatic tachycardia, and subnormal erythrocyte volume in the pathogenesis of the chronic fatigue syndrome. Am J Med Sci. 2000;320(1):1–8. doi: 10.1097/00000441-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Raj S.R., Robertson D. Blood volume perturbations in the postural tachycardia syndrome. Am J Med Sci. 2007;334(1):57–60. doi: 10.1097/MAJ.0b013e318063c6c0. [DOI] [PubMed] [Google Scholar]
- 28.Stewart J.M., Ocon A.J., Clarke D., Taneja I., Medow M.S. Defects in cutaneous angiotensin-converting enzyme 2 and angiotensin-(1-7) production in postural tachycardia syndrome. Hypertension. 2009;53(5):767–774. doi: 10.1161/HYPERTENSIONAHA.108.127357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu Q., Vangundy T.B., Galbreath M.M. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2010;55(25):2858–2868. doi: 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masuki S., Eisenach J.H., Schrage W.G. Reduced stroke volume during exercise in postural tachycardia syndrome. J Appl Physiol. 2007;103(4):1128–1135. doi: 10.1152/japplphysiol.00175.2007. [DOI] [PubMed] [Google Scholar]
- 31.Joyner M.J., Masuki S. POTS versus deconditioning: the same or different? Clin Auton Res. 2008;18(6):300–307. doi: 10.1007/s10286-008-0487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasser E.M., Moffitt J.A. Regulation of sympathetic nervous system function after cardiovascular deconditioning. Ann N Y Acad Sci. 2001;940:454–468. doi: 10.1111/j.1749-6632.2001.tb03698.x. [DOI] [PubMed] [Google Scholar]
- 33.Xiao X., Mukkamala R., Sheynberg N., Williams G.H., Cohen R.J. Effects of prolonged bed rest on the total peripheral resistance baroreflex. Comput Cardiol. 2002;29:53–56. [PubMed] [Google Scholar]
- 34.Dyckman D.J., Sauder C.L., Ray C.A. Effects of short-term and prolonged bed rest on the vestibulosympathetic reflex. Am J Physiol Heart Circ Physiol. 2012;302(1):H368–H374. doi: 10.1152/ajpheart.00193.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawelczyk J.A., Zuckerman J.H., Blomqvist C.G., Levine B.D. Regulation of muscle sympathetic nerve activity after bed rest deconditioning. Am J Physiol Heart Circ Physiol. 2001;280(5):H2230–H2239. doi: 10.1152/ajpheart.2001.280.5.H2230. [DOI] [PubMed] [Google Scholar]
- 36.Iwasaki K., Zhang R., Perhonen M.A., Zuckerman J.H., Levine B.D. Reduced baroreflex control of heart period after bed rest is normalized by acute plasma volume restoration. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1256–R1262. doi: 10.1152/ajpregu.00613.2002. [DOI] [PubMed] [Google Scholar]
- 37.Ojha A., Chelimsky T.C., Chelimsky G. Comorbidities in pediatric patients with postural orthostatic tachycardia syndrome. J Pediatr. 2011;158(1):20–23. doi: 10.1016/j.jpeds.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Adibi P., Behzad E., Shafieeyan M., Toghiani A. Upper functional gastrointestinal disorders in young adults. Med Arh. 2012;66(2):89–91. doi: 10.5455/medarh.2012.66.89-91. [DOI] [PubMed] [Google Scholar]
- 39.Fukudo S., Kuwano H., Miwa H. Management and pathophysiology of functional gastrointestinal disorders. Digestion. 2012;85(2):85–89. doi: 10.1159/000334652. [DOI] [PubMed] [Google Scholar]
- 40.Grover M., Drossman D.A. Functional abdominal pain. Curr Gastroenterol Rep. 2010;12(5):391–398. doi: 10.1007/s11894-010-0125-0. [DOI] [PubMed] [Google Scholar]
- 41.Koloski N.A., Jones M., Kalantar J., Weltman M., Zaguirre J., Talley N.J. The brain-gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012;61(9):1284–1290. doi: 10.1136/gutjnl-2011-300474. [DOI] [PubMed] [Google Scholar]
- 42.Wu J.C. Psychological co-morbidity in functional gastrointestinal disorders: epidemiology, mechanisms and management. J Neurogastroenterol Motil. 2012;18(1):13–18. doi: 10.5056/jnm.2012.18.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ocon A.J., Messer Z.R., Medow M.S., Stewart J.M. Increasing orthostatic stress impairs neurocognitive functioning in chronic fatigue syndrome with postural tachycardia syndrome. Clin Sci (Lond) 2012;122(5):227–238. doi: 10.1042/CS20110241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagai K., Song Y., Ling J.F. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med. 2011;7(2):204–210. [PMC free article] [PubMed] [Google Scholar]
- 45.Okamoto L.E., Raj S.R., Peltier A. Neurohumoral and haemodynamic profile in postural tachycardia and chronic fatigue syndromes. Clin Sci (Lond) 2012;122(4):183–192. doi: 10.1042/CS20110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staud R. Autonomic dysfunction in fibromyalgia syndrome: postural orthostatic tachycardia. Curr Rheumatol Rep. 2008;10(6):463–466. doi: 10.1007/s11926-008-0076-8. [DOI] [PubMed] [Google Scholar]
- 47.Gazit Y., Nahir A.M., Grahame R., Jacob G. Dysautonomia in the joint hypermobility syndrome. Am J Med. 2003;115(1):33–40. doi: 10.1016/s0002-9343(03)00235-3. [DOI] [PubMed] [Google Scholar]
- 48.Kanjwal K., Saeed B., Karabin B., Kanjwal Y., Grubb B.P. Comparative clinical profile of postural orthostatic tachycardia patients with and without joint hypermobility syndrome. Indian Pacing Electrophysiol J. 2010;10(4):173–178. [PMC free article] [PubMed] [Google Scholar]
- 49.Keller N.R., Robertson D. Familial orthostatic tachycardia. Curr Opin Cardiol. 2006;21(3):173–179. doi: 10.1097/01.hco.0000221577.41125.c8. [DOI] [PubMed] [Google Scholar]
- 50.O'Connell M., Burrows N.P., van Vlijmen-Willems M.J., Clark S.M., Schalkwijk J. Tenascin-X deficiency and Ehlers-Danlos syndrome: a case report and review of the literature. Br J Dermatol. 2010;163(6):1340–1345. doi: 10.1111/j.1365-2133.2010.09949.x. [DOI] [PubMed] [Google Scholar]
- 51.Sacheti A., Szemere J., Bernstein B., Tafas T., Schechter N., Tsipouras P. Chronic pain is a manifestation of the Ehlers-Danlos syndrome. J Pain Symptom Manage. 1997;14(2):88–93. doi: 10.1016/s0885-3924(97)00007-9. [DOI] [PubMed] [Google Scholar]
- 52.Baeza-Velasco C., Gely-Nargeot M.C., Vilarrasa A.B., Fenetrier C., Bravo J.F. Association between psychopathological factors and joint hypermobility syndrome in a group of undergraduates from a French university. Int J Psychiatry Med. 2011;41(2):187–201. doi: 10.2190/PM.41.2.g. [DOI] [PubMed] [Google Scholar]
- 53.Mack K.J., Johnson J.N., Rowe P.C. Orthostatic intolerance and the headache patient. Semin Pediatr Neurol. 2010;17(2):109–116. doi: 10.1016/j.spen.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Mathys J., Beiser I., Maurer G., Humm A.M. Orthostatic headache with tachycardia [in German] Praxis (Bern 1994) 2011;100(10):613–616. doi: 10.1024/1661-8157/a00534. [DOI] [PubMed] [Google Scholar]
- 55.Mokri B., Low P.A. Orthostatic headaches without CSF leak in postural tachycardia syndrome. Neurology. 2003;61(7):980–982. doi: 10.1212/01.wnl.0000085868.37963.7d. [DOI] [PubMed] [Google Scholar]
- 56.Prilipko O., Dehdashti A.R., Zaim S., Seeck M. Orthostatic intolerance and syncope associated with Chiari type I malformation. J Neurol Neurosurg Psychiatry. 2005;76(7):1034–1036. doi: 10.1136/jnnp.2004.048330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pasupuleti D.V., Vedre A. Postural orthostatic tachycardia warrants investigation of Chiari I malformation as a possible cause. Cardiology. 2005;103(1):55–56. doi: 10.1159/000081854. [DOI] [PubMed] [Google Scholar]
- 58.Nogués M., Delorme R., Saadia D., Heidel K., Benarroch E. Postural tachycardia syndrome in syringomyelia: response to fludrocortisone and beta-blockers. Clin Auton Res. 2001;11(4):265–267. doi: 10.1007/BF02298959. [DOI] [PubMed] [Google Scholar]
- 59.Garland E.M., Robertson D. Chiari I malformation as a cause of orthostatic intolerance symptoms: a media myth? Am J Med. 2001;111(7):546–552. doi: 10.1016/s0002-9343(01)00922-6. [DOI] [PubMed] [Google Scholar]
- 60.Kanjwal K., Karabin B., Kanjwal Y., Grubb B.P. Autonomic dysfunction presenting as postural tachycardia syndrome following traumatic brain injury. Cardiol J. 2010;17(5):482–487. [PubMed] [Google Scholar]
- 61.Ruckenstein M.J., Staab J.P. Chronic subjective dizziness. Otolaryngol Clin North Am. 2009;42(1):71–77. doi: 10.1016/j.otc.2008.09.011. ix. [DOI] [PubMed] [Google Scholar]
- 62.Dieterich M., Krafczyk S., Querner V., Brandt T. Somatoform phobic postural vertigo and psychogenic disorders of stance and gait. Adv Neurol. 2001;87:225–233. [PubMed] [Google Scholar]
- 63.Carter J.R., Ray C.A. Sympathetic responses to vestibular activation in humans. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R681–R688. doi: 10.1152/ajpregu.00896.2007. [DOI] [PubMed] [Google Scholar]
- 64.Benrud-Larson L.M., Dewar M.S., Sandroni P., Rummans T.A., Haythornthwaite J.A., Low P.A. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc. 2002;77(6):531–537. doi: 10.4065/77.6.531. [DOI] [PubMed] [Google Scholar]
- 65.Staud R. Brain imaging in fibromyalgia syndrome. Clin Exp Rheumatol. 2011;29(6, suppl 69):S109–S117. [PubMed] [Google Scholar]
- 66.Clauw D.J., Arnold L.M., McCarberg B.H., FibroCollaborative The science of fibromyalgia. Mayo Clin Proc. 2011;86(9):907–911. doi: 10.4065/mcp.2011.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jurysta F., Lanquart J.P., Sputaels V. The impact of chronic primary insomnia on the heart rate—EEG variability link. Clin Neurophysiol. 2009;120(6):1054–1060. doi: 10.1016/j.clinph.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 68.Barnett K.J., Cooper N.J. The effects of a poor night sleep on mood, cognitive, autonomic and electrophysiological measures. J Integr Neurosci. 2008;7(3):405–420. doi: 10.1142/s0219635208001903. [DOI] [PubMed] [Google Scholar]
- 69.Shoemaker J.K., Usselman C.W., Rothwell A., Wong S.W. Altered cortical activation patterns associated with baroreflex unloading following 24 hours of physical deconditioning [published online ahead of print May 21, 2012] Exp Physiol. 2012 doi: 10.1113/expphysiol.2012.065557. [DOI] [PubMed] [Google Scholar]
- 70.Saper C.B. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 71.Craig A.D. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 72.Critchley H.D. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493(1):154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 73.Critchley H.D., Nagai Y., Gray M.A., Mathias C.J. Dissecting axes of autonomic control in humans: insights from neuroimaging. Auton Neurosci. 2011;161(1-2):34–42. doi: 10.1016/j.autneu.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 74.Holstege G., Bandler R., Saper C.B. The emotional motor system. Prog Brain Res. 1996;107:3–6. doi: 10.1016/s0079-6123(08)61855-5. [DOI] [PubMed] [Google Scholar]
- 75.Fuller P.M., Jones T.A., Jones S.M., Fuller C.A. Evidence for macular gravity receptor modulation of hypothalamic, limbic and autonomic nuclei. Neuroscience. 2004;129(2):461–471. doi: 10.1016/j.neuroscience.2004.05.059. [DOI] [PubMed] [Google Scholar]
- 76.Benarroch E.E. The arterial baroreflex: functional organization and involvement in neurologic disease. Neurology. 2008;71(21):1733–1738. doi: 10.1212/01.wnl.0000335246.93495.92. [DOI] [PubMed] [Google Scholar]
- 77.Guyenet P.G. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7(5):335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 78.LeDoux J. Rethinking the emotional brain [published correction appears in Neuron. 2012;73(5):1052] Neuron. 2012;73(4):653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Critchley H.D. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. Int J Psychophysiol. 2009;73(2):88–94. doi: 10.1016/j.ijpsycho.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kimmerly D.S., O'Leary D.D., Menon R.S., Gati J.S., Shoemaker J.K. Cortical regions associated with autonomic cardiovascular regulation during lower body negative pressure in humans. J Physiol. 2005;569(pt 1):331–345. doi: 10.1113/jphysiol.2005.091637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buckholtz J.W., Meyer-Lindenberg A. Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74(6):990–1004. doi: 10.1016/j.neuron.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 82.Legrain V., Iannetti G.D., Plaghki L., Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol. 2011;93(1):111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 83.Henry D.E., Chiodo A.E., Yang W. Central nervous system reorganization in a variety of chronic pain states: a review. PM R. 2011;3(12):1116–1125. doi: 10.1016/j.pmrj.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 84.Abi-Samra F., Maloney J.D., Fouad-Tarazi F.M., Castle L.W. The usefulness of head-up tilt testing and hemodynamic investigations in the workup of syncope of unknown origin. Pacing Clin Electrophysiol. 1988;11(8):1202–1214. doi: 10.1111/j.1540-8159.1988.tb03973.x. [DOI] [PubMed] [Google Scholar]
- 85.Wieling W., van Lieshout J.J., van Leeuwen A.M. Physical manoeuvres that reduce postural hypotension in autonomic failure. Clin Auton Res. 1993;3(1):57–65. doi: 10.1007/BF01819146. [DOI] [PubMed] [Google Scholar]
- 86.Krediet C.T., de Bruin I.G., Ganzeboom K.S., Linzer M., van Lieshout J.J., Wieling W. Leg crossing, muscle tensing, squatting, and the crash position are effective against vasovagal reactions solely through increases in cardiac output. J Appl Physiol. 2005;99(5):1697–1703. doi: 10.1152/japplphysiol.01250.2004. [DOI] [PubMed] [Google Scholar]
- 87.Gordon V.M., Opfer-Gehrking T.L., Novak V., Low P.A. Hemodynamic and symptomatic effects of acute interventions on tilt in patients with postural tachycardia syndrome. Clin Auton Res. 2000;10(1):29–33. doi: 10.1007/BF02291387. [DOI] [PubMed] [Google Scholar]
- 88.Smit A.A., Wieling W., Fujimura J. Use of lower abdominal compression to combat orthostatic hypotension in patients with autonomic dysfunction. Clin Auton Res. 2004;14(3):167–175. doi: 10.1007/s10286-004-0187-x. [DOI] [PubMed] [Google Scholar]
- 89.Shibata S., Fu Q., Bivens T.B., Hastings J.L., Wang W., Levine B.B. Short-term exercise training improves the cardiovascular response to exercise in the postural orthostatic tachycardia syndrome. J Physiol. 2012;590(pt 15):3495–3505. doi: 10.1113/jphysiol.2012.233858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Freitas J., Santos R., Azevedo E., Costa O., Carvalho M., de Freitas A.F. Clinical improvement in patients with orthostatic intolerance after treatment with bisoprolol and fludrocortisone. Clin Auton Res. 2000;10(5):293–299. doi: 10.1007/BF02281112. [DOI] [PubMed] [Google Scholar]
- 91.Chen L., Wang L., Sun J. Midodrine hydrochloride is effective in the treatment of children with postural orthostatic tachycardia syndrome. Circ J. 2011;75(4):927–931. doi: 10.1253/circj.cj-10-0514. [DOI] [PubMed] [Google Scholar]
- 92.Raj S.R., Black B.K., Biaggioni I. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation. 2009;120(9):725–734. doi: 10.1161/CIRCULATIONAHA.108.846501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yusuf S., Camm A.J. The sinus tachycardias. Nat Clin Pract Cardiovasc Med. 2005;2(1):44–52. doi: 10.1038/ncpcardio0068. [DOI] [PubMed] [Google Scholar]
- 94.Singer W., Opfer-Gehrking T.L., Nickander K.K., Hines S.M., Low P.A. Acetylcholinesterase inhibition in patients with orthostatic intolerance. J Clin Neurophysiol. 2006;23(5):476–481. doi: 10.1097/01.wnp.0000229946.01494.4c. [DOI] [PubMed] [Google Scholar]
- 95.Raj S.R., Black B.K., Biaggioni I., Harris P.A., Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation. 2005;111(21):2734–2740. doi: 10.1161/CIRCULATIONAHA.104.497594. [DOI] [PubMed] [Google Scholar]
- 96.Kanjwal K., Karabin B., Sheikh M. Pyridostigmine in the treatment of postural orthostatic tachycardia: a single-center experience. Pacing Clin Electrophysiol. 2011;34(6):750–755. doi: 10.1111/j.1540-8159.2011.03047.x. [DOI] [PubMed] [Google Scholar]


