Abstract
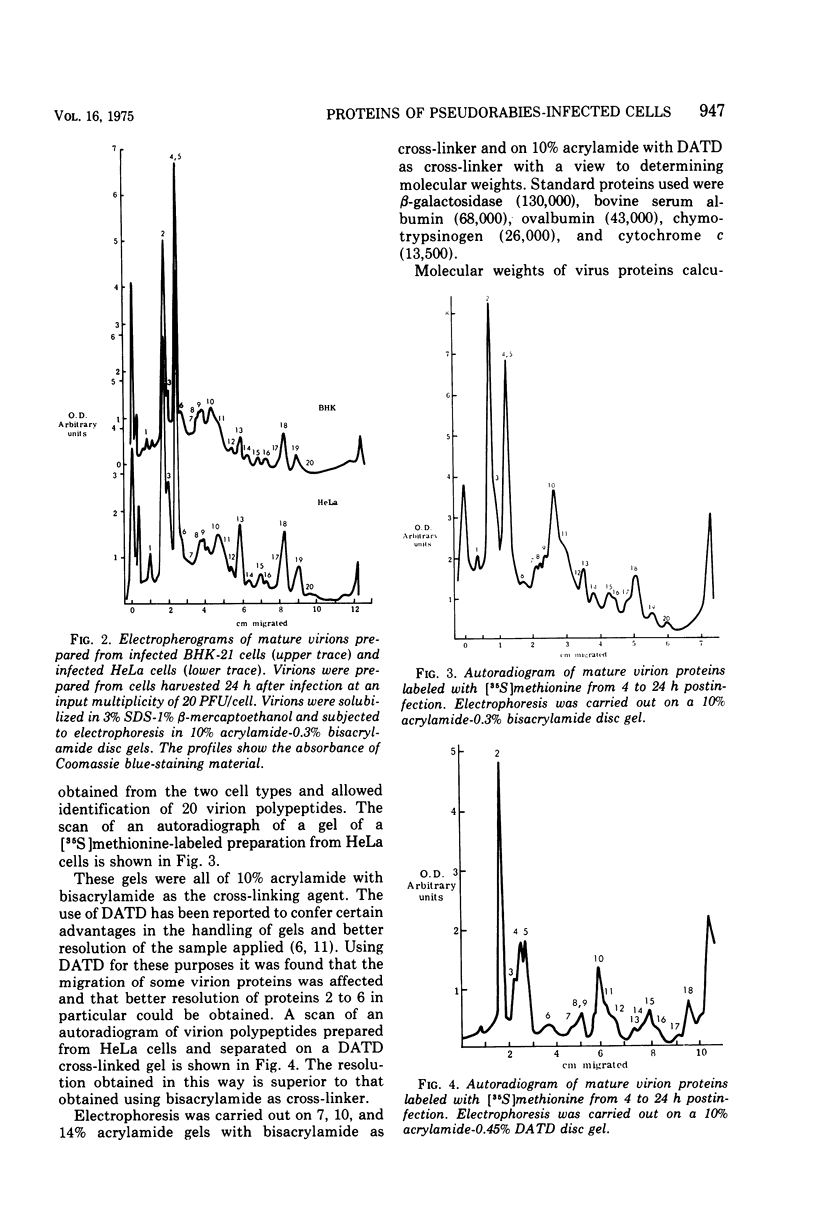
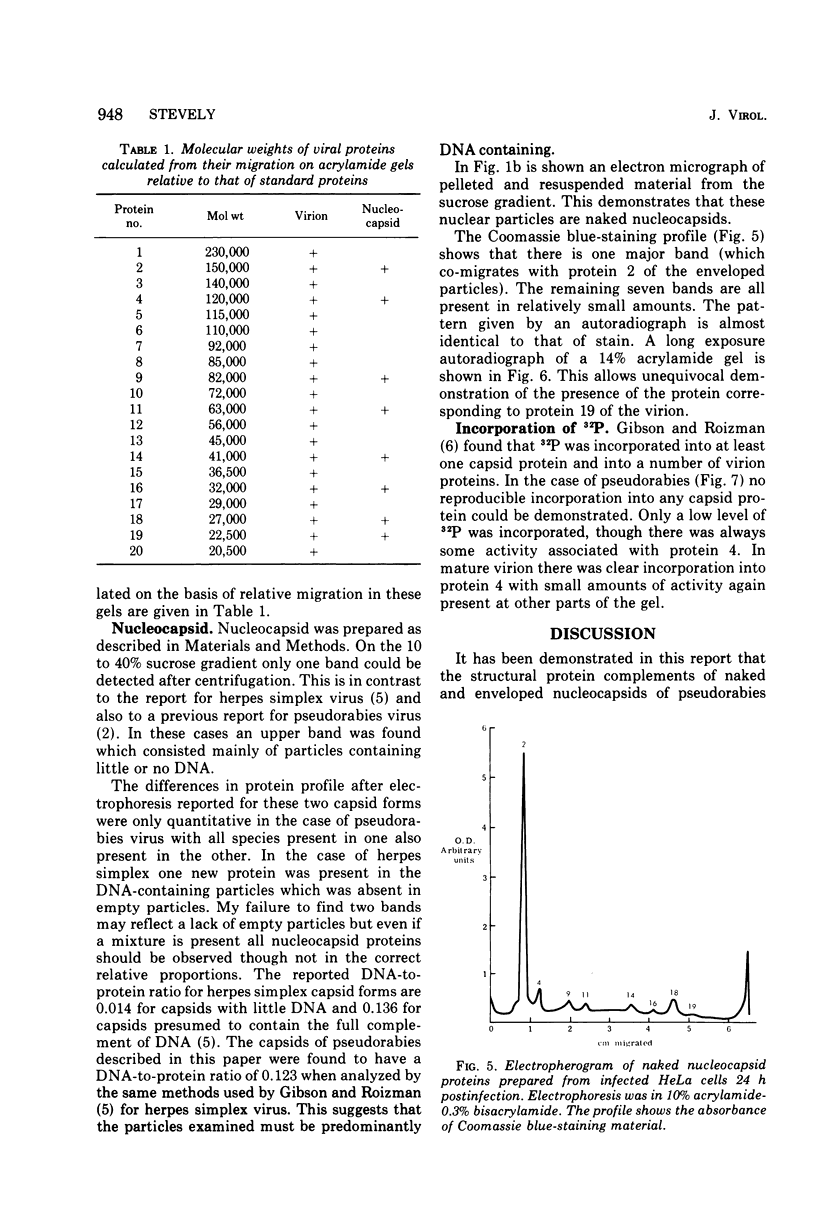
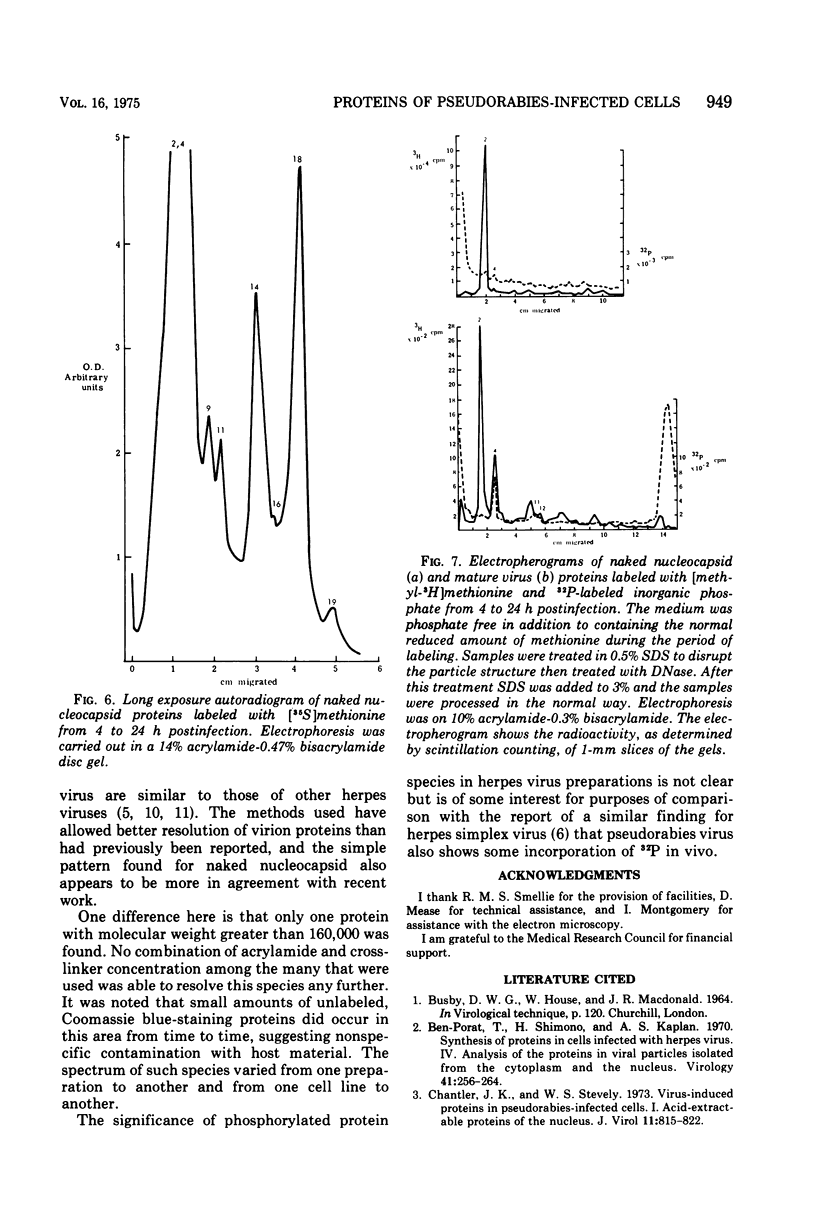
Analysis of purified naked and enveloped nucleocapsids of pseudorabies virus with high-resolution techniques has allowed a reassessment of their protein composition. Enveloped particles are shown to contain at least 20 proteins whose molecular weights are in the range 20,000 to 230,000. Naked nucleocapsids contain one major and seven minor proteins in the molecular weight range 20,000 to 155,000. Phosphorylation of at least one virion protein is shown to take place in vivo. These results demonstrate that pseudorabies virus is similar in its protein complement to other herpesviruses which have recently been examined.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Shimono H., Kaplan A. S. Synthesis of proteins in cells infected with herpesvirus. IV. Analysis of the proteins in viral particles isolated from the cytoplasm and the nucleus. Virology. 1970 Jun;41(2):256–264. doi: 10.1016/0042-6822(70)90077-2. [DOI] [PubMed] [Google Scholar]
- Chantler J. K., Stevely W. S. Virus-induced proteins in pseudorabies-infected cells. I. Acid-extractable proteins of the nucleus. J Virol. 1973 Jun;11(6):815–822. doi: 10.1128/jvi.11.6.815-822.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. Staining and radiolabeling properties of B capsid and virion proteins in polyacrylamide gels. J Virol. 1974 Jan;13(1):155–165. doi: 10.1128/jvi.13.1.155-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN A. S., VATTER A. E. A comparison of herpes simplex and pseudorabies viruses. Virology. 1959 Apr;7(4):394–407. doi: 10.1016/0042-6822(59)90068-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Perdue M. L., Kemp M. C., Randall C. C., O'Callaghan D. J. Studies of the molecular anatomy of the L-M cell strain of equine herpes virus type 1: proteins of the nucleocapsid and intact virion. Virology. 1974 May;59(1):201–216. doi: 10.1016/0042-6822(74)90216-5. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]