Abstract
AIM: To evaluate whether metabolic factors are related to distant recurrence of hepatocellular carcinoma (HCC) and survival after curative treatment.
METHODS: This retrospective study included 344 patients whose HCC was treated curatively by radiofrequency ablation (RFA) therapy. The mean age was 67.6 years and the mean observation period was 4.04 years. The etiological background of liver disease was hepatitis B virus infection in 30, hepatitis C virus infection in 278, excessive alcohol drinking in 9, and other in 27 patients. The Child-Pugh classification grade was A (n = 307) or B (n = 37). The number of HCC nodules was one in 260, two in 61, and three in 23 patients. For surveillance of HCC recurrence after curative therapy with RFA, patients were radiologically evaluated every 3 mo. Factors associated with distant recurrence of HCC or survival were studied.
RESULTS: Inadequate maintenance of blood glucose in diabetic patients was associated with higher incidence of distant recurrence. The 1-, 2-, and 3-year recurrence rates were significantly higher in diabetic patients with inadequate maintenance of blood glucose compared with the others: 50.6% vs 26.8%, 83.5% vs 54.4%, and 93.8% vs 73.0%, respectively (P = 0.0001). Inadequate maintenance of blood glucose was an independent predictor of distant recurrence [adjusted relative risk 1.97 (95%CI, 1.33-2.91), (P = 0.0007)] after adjustment for other risk factors, such as number of HCC nodules [2.03 (95%CI, 1.51-2.73), P < 0.0001] and initial level of serum alpha fetoprotein (AFP) [1.43 (95%CI, 1.04-1.97), P = 0.028]. Obesity was not an independent predictor of recurrence. The incidence of distant recurrence did not differ between diabetic patients with adequate maintenance of blood glucose and non-diabetic patients. Among 232 patients who had HCC recurrence, 138 had a second recurrence. The 1-, 2-, and 3-year rates of second recurrence were significantly higher in diabetic patients with inadequate maintenance of blood glucose than in the others: 9.0% vs 5.9%, 53.1% vs 24.3%, and 69.6% vs 42.3%, respectively (P = 0.0021). Inadequate maintenance of blood glucose in diabetic patients [1.99 (95%CI, 1.23-3.22), P = 0.0049] and presence of multiple HCC nodules [1.53 (95%CI, 1.06-2.22), P = 0.024] were again significantly associated with second HCC recurrence. Inadequate maintenance of blood glucose in diabetic patients was also a significant predictor of poor survival [2.77 (95%CI, 1.38-5.57), P = 0.0046] independent of excessive alcohol drinking [6.34 (95%CI, 1.35-29.7), P = 0.019], initial level of serum AFP [3.40 (95%CI, 1.88-6.18), P < 0.0001] and Child-Pugh classification grade B [2.24 (95%CI, 1.12-4.46), P = 0.022]. Comparing diabetic patients with inadequate maintenance of blood glucose vs the others, the 1-, 2-, and 3-year survival rates were significantly lower in diabetic patients with inadequate maintenance of blood glucose: 92% vs 99%, 85% vs 96%, and 70% vs 92%, respectively (P = 0.0003).
CONCLUSION: Inadequate maintenance of blood glucose in diabetic patients is a significant risk factor for recurrence of HCC and for poor survival after curative RFA therapy.
Keywords: Hyperglycemia, Hepatocellular carcinoma, Recurrence, Radio frequency ablation, Survival
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide[1] and its incidence has been increasing in many countries[2]. Surgical resection, liver transplantation, and local ablation therapy, such as radiofrequency ablation (RFA) therapy, have been considered as efficient curative therapies for HCC. RFA therapy is now widely performed in patients with small HCC[3] and a randomized controlled study demonstrated that the survival rates were similar in patients with small HCC receiving RFA or surgical resection[4]. A characteristic of HCC is its high rate of recurrence after curative resection or local ablation therapy, reaching approximately 80% within 5 years[5-7]. Identification of factors related to recurrence of HCC and therapeutic intervention targeting these factors may lead to prevention of frequent recurrence of HCC and improved survival.
Tumor factors, such as the number of HCC nodules and their size, are associated with the recurrence of HCC and survival prognosis[8-10]. Another factor that is associated with the recurrence of HCC and survival is the hepatic reserve function at the time of HCC therapy[8,10,11]. Hepatitis C virus (HCV) and hepatitis B virus (HBV) infection are the major causes responsible for 80% of HCC cases[2] and antiviral therapy targeting HCV[12,13] or HBV[14] has been shown to decrease HCC recurrence, and improve hepatic reserve function and survival. Non-alcoholic steatohepatitis (NASH) has also received attention as a cause of HCC[15]. Metabolic factors, such as obesity and diabetes, are closely linked to the etiology of NASH. These metabolic factors have also been identified as risk factors for several other types of cancer. Obesity is associated with increased mortality rates of several cancers[16,17] and diabetes is also reported as a risk factor for liver, pancreatic, renal, and colon cancers[18,19]. If these metabolic factors are related to the recurrence of HCC, therapeutic intervention targeting these factors may lead to prevention of frequent recurrence of HCC and improved survival. The impact of diabetes on the recurrence of HCC after treatment has been discussed, but with conflicting results[20-23].
In this study, factors contributing to the recurrence and prognosis of HCC after curative treatment were analyzed. We found that inadequate maintenance of blood glucose was related to the high rate of HCC recurrence and poor survival.
MATERIALS AND METHODS
Patients whose HCC was treated by RFA at the Musashino Red Cross Hospital were studied retrospectively for factors associated with recurrence of HCC and survival. The inclusion criteria were as follows: (1) HCC treated curatively with RFA at the Musashino Red Cross Hospital between 1999 and 2007; (2) maximum diameter of HCC nodule ≤ 3 cm; (3) number of HCC nodules ≤ 3; (4) no previous history of treatment for HCC; and (5) follow-up observation for at least 6 mo after RFA therapy. 344 patients met these criteria, including 140 women and 204 men, with a mean age of 67.6 years and mean observation time of 4.04 years. The clinical characteristics of the patients are summarized in Table 1. The etiological background of liver disease was HBV infection in 30, HCV infection in 278, excessive alcohol drinking (intake of ethanol ≥ 60 g/d for ≥ 5 years continuously) in 9, and non-B non-C non-alcoholic etiology in 27 patients. The Child-Pugh classification grade was either A (n = 307) or B (n = 37). The number of HCC nodules was one in 260, two in 61, and three in 23 patients. Thus, 260 patients had a single lesion, and 84 had multiple lesions. The maximum diameter of HCC nodules was 19.9 ± 0.3 mm.
Table 1.
Characteristics of patients undergoing curative radiofrequency ablation for hepatocellular carcinoma n (%)
| Variable | Value |
| Sex (male/female) | 204/140 |
| Age(yr) | 67.6 ± 8.4 |
| Etiology of liver disease: HBV/HCV/NBNC | 30/278/36 |
| AST (IU/L) | 84.0 ± 34.5 |
| ALT (IU/L) | 73.2 ± 36.5 |
| GGT (IU/L) | 82.9 ± 96.8 |
| T-Chol (mg/dL) | 157.8 ± 32.0 |
| TG (mg/dL) | 112.3 ± 55.7 |
| Mean blood sugar (mg/dL) | 139.3 ± 44.0 |
| Diabetes mellitus | 159 (48) |
| BMI > 25 kg/m2 | 86 (25) |
| Maximum diameter of HCC nodule (mm) | 19.9 ± 0.3 |
| Number of HCC nodules: single/2 or 3 | 260/84 |
| AFP (ng/mL) | 214 ± 1025 |
| Alcohol drinking > 60 g/d | 9 (2.6) |
| Child-Pugh grade: A/B | 307/37 |
HBV: Hepatitis B virus; HCV: Hepatitis C virus; NBNV: Neither HBV nor HCV; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; GGT: γ-glutamyltransferase; T-Chol: Total cholesterol; TG: Triglyceride; BMI: Body mass index; AFP: α-fetoprotein; HCC: Hepatocellular carcinoma.
Obesity was defined as a body mass index > 25 kg/m2 according to the definition of the Japan Society for the Study of Obesity[24]. Blood glucose was measured monthly for 6 mo after HCC treatment and the average value was determined. Inadequate maintenance of blood glucose was defined as an average value of blood glucose ≥ 200 mg/dL. The level of hemoglobin A1c (HbA1c) was not used in the present study because the lifespan of erythrocytes is shortened due to hypersplenism in patients with chronic hepatitis or cirrhosis, leading to lower HbA1c levels relative to the blood glucose level[25]. Diagnosis of type 2 diabetes was made according to the American Diabetes Association criteria of a fasting blood glucose level ≥ 126 mg/dL (≥ 7.0 mmol/L) and/or HbA1c level ≥ 6.5[26]. After initial treatment of HCC by RFA, the ablated area was confirmed by contrast-enhanced computed tomography (CT) within one week. If the ablated area was not sufficient, then RFA therapy was repeated until the HCC nodule was completely ablated.
HCC surveillance and diagnosis of recurrence
Diagnosis of HCC was based on abdominal ultrasonography, contrast-enhanced CT, magnetic resonance imaging (MRI), or angiography. Classical HCC was diagnosed for tumors showing vascular enhancement with washout on at least two types of diagnostic imaging. Tumor biopsy was used to diagnose tumors with non-classical imaging findings.
For surveillance of HCC recurrence after curative therapy with RFA, patients were evaluated by abdominal ultrasonography, contrast-enhanced CT, or contrast-enhanced MRI every three months. Recurrence of HCC was diagnosed based on a new lesion detected by ultrasonography showing vascular enhancement with washout on CT or MRI. If the tumor was not hypervascular, a tumor biopsy was performed to confirm the diagnosis.
Statistical analysis
For analysis of survival and recurrence, the time of initial RFA treatment was defined as day zero. Survival rate and recurrence rate were analyzed by the Kaplan-Meier method and log rank test. Multivariate analysis was performed using a Cox proportional hazard model. Data were analyzed using StatView Version 5.0 (SAS Institute Inc, Cary, North Carolina, United States) and IBM-SPSS statistics version 18 (IBM SPSS Inc, Chicago, IL, United States). Statistical significance was set at P < 0.05.
RESULTS
Factors associated with HCC recurrence
Of the 344 patients whose HCC was curatively treated by RFA, 232 had HCC recurrence. The 1-, 2-, and 3-year recurrence rates were 29.3%, 57.5%, and 75.2%, respectively. On univariate analysis, inadequate maintenance of blood glucose, higher initial level of serum AFP and multiple HCC nodules were significantly associated with HCC recurrence. Obesity (P = 0.06) and diabetes (P = 0.65) were not significantly associated with HCC recurrence.
Thirty-seven patients had diabetes with inadequate maintenance of blood glucose, 122 patients had diabetes with adequate maintenance of blood glucose, and 185 patients did not have diabetes. The HCC recurrence rate was significantly higher in diabetic patients with inadequate maintenance of blood glucose than in the others (P = 0.0001) (Figure 1A).
Figure 1.
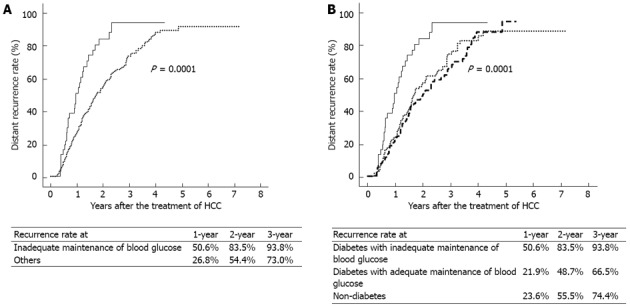
Kaplan-Meier curves showing a higher rate of hepatocellular carcinoma recurrence in diabetic patients with hyperglycemia. A: The cumulative incidence of the recurrence of hepatocellular carcinoma (HCC) was significantly higher in diabetic patients with inadequate maintenance of blood glucose (blood glucose ≥ 200 mg/dL solid line) than in the others (dotted line) (P = 0.0001); B: The HCC recurrence rate was significantly higher in diabetic patients with inadequate maintenance of blood glucose (solid line) than in diabetic patients with adequate maintenance of blood glucose (blood glucose < 200 mg/dL, broken line) or non-diabetic patients (dotted line) (P = 0.0001). There was no significant difference in HCC recurrence rate between diabetic patients with adequate maintenance of blood glucose and non-diabetic patients.
Comparing patients with diabetes (n = 159) and patients who did not have diabetes (n = 185), there was no significant difference in the recurrence rate (P = 0.65). Upon comparison of the three groups, i.e., the diabetes with inadequate maintenance of blood glucose group, the diabetes with adequate maintenance of blood glucose group, and the non-diabetes group, the recurrence rate was significantly higher in the diabetes with inadequate maintenance of blood glucose group than in the other two groups (P = 0.0001) (Figure 1B). On the other hand, there was no significant difference in the HCC recurrence rate between the diabetes patients with adequate maintenance of blood glucose group and the non-diabetes group.
In terms of the number of HCC nodules, namely, single (n = 260) vs multiple (n = 84), the recurrence rate was significantly higher in patients with multiple HCC nodules (P = 0.0001). Within each subgroup of patients with single and multiple HCC nodules, diabetes with inadequate maintenance of blood glucose was significantly associated with recurrence of HCC (single, P = 0.006; multiple, P = 0.025) (Figure 2A, B). In terms of the initial level of serum AFP ≥100 ng/mL (n = 70) vs < 100 ng/mL (n = 274), the recurrence rate was significantly higher in patients with AFP ≥ 100 g/mL (P = 0.018). Within each subgroup of patients with AFP ≥ 100 ng/mL and < 100 ng/mL, diabetes with inadequate maintenance of blood glucose was associated with a higher rate of recurrence (AFP ≥ 100 ng/mL, P = 0.005; AFP < 100 ng/mL, P = 0.017) (Figure 2C, D).
Figure 2.
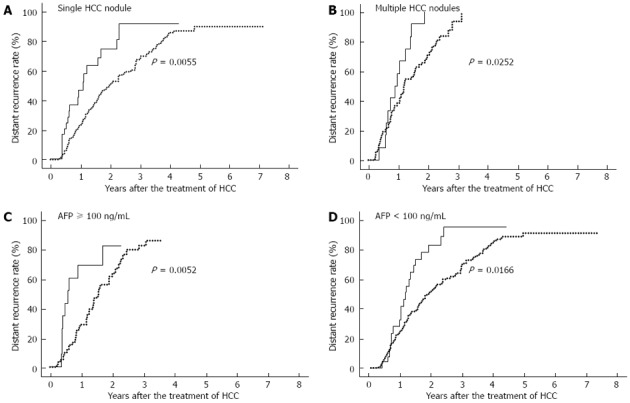
Diabetic patients with inadequate maintenance of blood glucose have higher rate of hepatocellular carcinoma recurrence after stratification by other risk factors. A: P = 0.006 for single hepatocellular carcinoma (HCC) nodule; B: P = 0.025 for multiple HCC nodules; C: P = 0.005 for AFP ≥ 100 ng/mL; D: P = 0.017 for α-fetoprotein (AFP) < 100 ng/mL. The cumulative incidence of the recurrence of HCC was significantly higher in diabetic patients with inadequate maintenance of blood glucose (solid line) than in the others (dotted line). after stratification by number of HCC nodules and by initial level of AFP.
Independent risk factors for distant recurrence of HCC on multivariate analysis were inadequate maintenance of blood glucose in diabetic patients [adjusted relative risk, 1.97 (95%CI, 1.33-2.91), P = 0.0007], multiple HCC nodules [2.03 (1.51-2.73), P < 0.0001], and AFP ≥ 100 ng/mL [1.43 (1.04-1.97), P = 0.028] (Table 2).
Table 2.
Multivariate analysis of factors associated with recurrence of hepatocellular carcinoma
| Factors | Odds ratio (95%CI) | P-value |
| First recurrence | ||
| Inadequate maintenance of blood glucose | 1.97 (1.33-2.91) | 0.0007 |
| Multiple HCC nodules | 2.03 (1.51-2.73) | < 0.0001 |
| AFP ≥ 100 ng/mL | 1.43 (1.04-1.97) | 0.028 |
| Second recurrence | ||
| Inadequate maintenance of blood glucose (mg/dL) | 1.99 (1.23-3.22) | 0.0049 |
| Multiple HCC nodules | 1.53 (1.06-2.22) | 0.024 |
Inadequate maintenance of blood glucose was defined as an average of casual blood glucose of ≥ 200 mg/dL. HCC: Hepatocellular carcinoma; AFP: α-fetoprotein.
Factors associated with second recurrence
Among the 232 patients who had HCC recurrence, 138 had a second recurrence. Regarding second recurrence, inadequate maintenance of blood glucose in diabetic patients and multiple HCC nodules were again significantly associated with HCC recurrence. Obesity (P = 0.18), diabetes (P = 0.31) and initial level of serum AFP (P = 0.08) were not associated with second recurrence. In terms of the number of HCC nodules, namely, single vs multiple, the 1-, 2-, and 3-year recurrence rates were significantly higher in patients with multiple lesions (6.4% vs 6.1%, 39.3% vs 23.1%, and 52.5% vs 42.3%, respectively, P = 0.013). Upon comparing diabetic patients with inadequate maintenance of blood glucose vs the others, the rate of second recurrence was significantly higher in diabetic patients with inadequate maintenance of blood glucose (P = 0.0021) (Figure 3A). Upon comparing patients with diabetes vs patients who did not have diabetes, the rates of second recurrence were not significantly different (P = 0.31). Upon comparison of the three groups, i.e., the diabetes with inadequate maintenance of blood glucose group, the diabetes with adequate maintenance of blood glucose group, and the non-diabetes group, the second recurrence rate was again significantly higher in the diabetes with inadequate maintenance of blood glucose group than in the other two groups (P = 0.0035) (Figure 3B). On the other hand, there was no significant difference in the second recurrence rate between the diabetes with adequate maintenance of blood glucose group and the non-diabetes group.
Figure 3.
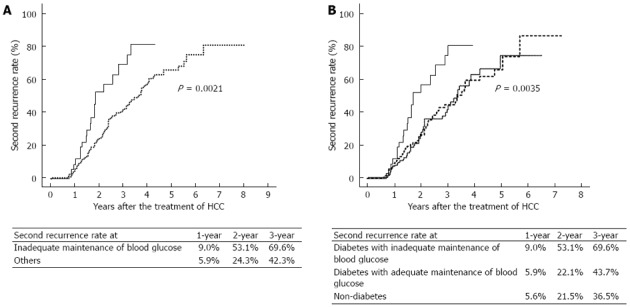
Kaplan-Meier curves showing a higher rate of second recurrence of hepatocellular carcinoma in diabetic patients with inadequate maintenance of blood glucose. A: The cumulative incidence of the second recurrence of hepatocellular carcinoma (HCC) was significantly higher in diabetic patients with inadequate maintenance of blood glucose (blood glucose ≥ 200 mg/dL solid line) than in the others (dotted line) (P = 0.002); B: The rate of second recurrence of HCC was significantly higher in diabetic patients with inadequate maintenance of blood glucose (solid line) than in diabetic patients with adequate maintenance of blood glucose (blood glucose < 200 mg/dL, broken line) or non-diabetic patients (dotted line) (P = 0.004). There was no significant difference in the rate of second recurrence of HCC between diabetic patients with adequate maintenance of blood glucose and non-diabetic patients.
Independent risk factors for second recurrence of HCC on multivariate analysis were inadequate maintenance of blood glucose [1.99 (95%CI, 1.23-3.22), P = 0.0049] and multiple HCC nodules [1.53 (95%CI, 1.06-2.22), P = 0.024] (Table 2).
Factors associated with survival
There were 52 HCC-related or hepatic failure deaths. On univariate analysis, inadequate maintenance of blood glucose, excessive alcohol drinking, higher initial level of serum AFP and Child-Pugh classification grade B were significantly associated with survival. Obesity (P = 0.81) and diabetes (P = 0.11) were not significantly associated with survival.
Upon comparing diabetic patients with inadequate maintenance of blood glucose vs the others, the survival rate was significantly lower in patients with inadequate maintenance of blood glucose (P = 0.0003) (Figure 4A). Upon comparing diabetic patients vs non-diabetic patients, the survival rates were not significantly different (P = 0.11). of the survival rate was compared among the three groups, i.e., the diabetes with inadequate maintenance of blood glucose group, the diabetes with adequate maintenance of blood glucose group, and the non-diabetes group. The survival rate was significantly poorer in the diabetes with inadequate maintenance of blood glucose group than in the other two groups (P = 0.0003) (Figure 4B), while it did not differ between the diabetes with adequate maintenance of blood glucose group and the non-diabetes group.
Figure 4.
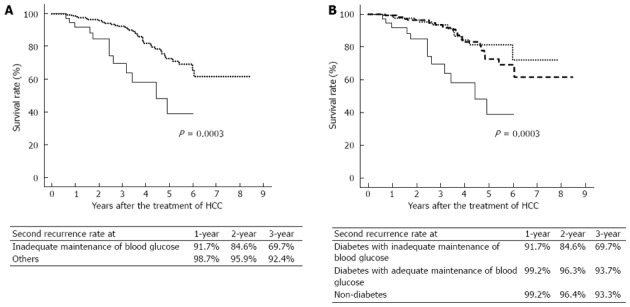
Patients with inadequate maintenance of blood glucose have a lower survival rate. A: The survival rate after curative local ablation therapy for hepatocellular carcinoma (HCC) was significantly lower in diabetic patients with inadequate maintenance of blood glucose (blood glucose ≥ 200 mg/dL solid line) than in the others (dotted line) (P = 0.0003); B: The survival rate was significantly lower in diabetic patients with inadequate maintenance of blood glucose (solid line) than in diabetic patients with adequate maintenance of blood glucose (blood glucose < 200 mg/dL, broken line) or non-diabetic patients (dotted line) (P = 0.0003). There was no significant difference in survival rate between diabetic patients with adequate maintenance of blood glucose and non-diabetic patients.
The number of HCC nodules, which was a significant factor for HCC recurrence, was not related to survival (P = 0.34). Patients with excessive alcohol drinking had poor survival prognosis compared to those with non-excessive or no alcohol drinking (P = 0.046). Survival was better in patients in Child-Pugh A class than in patients in Child-Pugh B class (P = 0.0082). AFP ≥ 100 ng/mL was associated with poor survival compared with AFP < 100 ng/mL (P < 0.0001).
On multivariate analysis, inadequate maintenance of blood glucose was a significant predictor of poor survival [2.77 (95%CI, 1.38-5.57), P = 0.0046] independent of excessive alcohol drinking [6.34 (95%CI, 1.35-29.7), P = 0.019], initial level of serum AFP ≥ 100 ng/mL [3.40 (95%CI, 1.88-6.18), P < 0.0001] and Child-Pugh classification grade B [2.24 (95%CI, 1.12-4.46), P = 0.022] (Table 3).
Table 3.
Multivariable analysis of factors associated with survival
| Factors | Odds ratio (95%CI) | P-value |
| Inadequate maintenance of blood glucose | 2.77 (1.38-5.57) | 0.0046 |
| Alcohol drinking ≥ 60 g/d | 6.34 (1.35-29.7) | 0.019 |
| Child Pugh grade B | 2.24 (1.12-4.46) | 0.022 |
| AFP ≥ 100 ng/mL | 3.40 (1.88-6.18) | < 0.0001 |
Inadequate maintenance of blood glucose was defined as an average of casual blood glucose of ≥ 200 mg/dL. AFP: α-fetoprotein.
DISCUSSION
The impact of metabolic factors, such as hyperglycemia, diabetes and obesity, on distant recurrence and survival after curative RFA therapy for HCC was analyzed retrospectively. We identified that inadequate maintenance of blood glucose in diabetic patients was a significant and independent risk factor for early recurrence of HCC and a risk factor for poor survival, whereas obesity and diabetes were not. Diabetic patients with inadequate maintenance of blood glucose had a higher rate of HCC recurrence and poorer survival compared with diabetic patients with adequate maintenance of blood glucose and non-diabetic patients. In other words, even in patients with diabetes, if the blood glucose was adequately maintained, the HCC recurrence rate and survival did not differ significantly compared with those in non-diabetic patients. These results indicate the possibility that adequate management of hyperglycemia may lead to reduction in the risk of HCC recurrence and improvement of overall survival.
The contribution of diabetes to the development of HCC has been confirmed in several reports[27-30]. The impact of diabetes on the recurrence of HCC after treatment has also been discussed, but with conflicting results[20-22]. A recent study from Taiwan demonstrated that diabetes may not affect the intra-hepatic HCC recurrence and survival after RFA[23]. The results of the present study also indicated that diabetes itself is not a significant risk factor if the level of blood glucose is adequately managed. Rather, hyperglycemia was a significant risk factor for the recurrence of HCC. There may be several mechanisms involved in the relationship between hyperglycemia and HCC recurrence. Hyperglycemia promotes cancer cell proliferation in pancreatic cancer cells and breast cancer cells[31-33] through accelerated cell cycle progression or through the production of reactive oxygen species, leading to activation of protein kinase C and increased DNA synthesis in cancer cells[34]. A previous study in hepatitis C patients indicated that hyperglycemia after challenge with 75-g oral glucose tolerance test was associated with the risk for HCC while hyperglycemia at fasting was not[35]. A possible reason for this result may be that patients with post-challenge hyperglycemia may have higher fluctuations in daily glucose levels that lead to oxidative stress[35], because it was reported that acute fluctuations in blood glucose levels cause greater oxidative stress than sustained chronic hyperglycemia[36]. Taken together, a possible mechanism for the relationship between higher level of casual blood glucose and development of HCC in the present study may be that daily fluctuations in serum glucose levels caused greater oxidative stress. Alternatively, hyper-insulinemia or increased level of insulin-like growth factor, which are caused by hyperglycemia, may be related to carcinogenesis[37-39]. Insulin levels were not measured in our study; therefore, the effects of insulin could not be identified.
Discussions are now taking place on methods of treating diabetes from the standpoint of cancer prevention. Control of hyperglycemia could reduce cancer incidence, which means that hyperglycemia could directly contribute to the development of cancer[39]. The results of our study also showed that adequate management of hyperglycemia may lead to reduction in the risk of HCC recurrence and improvement of overall survival. Improvement in insulin resistance is undoubtedly the most important factor for the treatment of diabetes, but glycemic control is often difficult to achieve with dietary therapy, exercise, or insulin resistance-improving drugs alone. It was reported that metformin may be associated with a lower risk of cancer[38] and there is a theoretical concern that exogenous insulin may be associated with an increased risk of cancer[40]. In fact, a recent study reported that insulin therapy in patients with HCV infection is linked with the development of HCC[41]. On the other hand, with insulin treatment, concomitant use of metformin has been reported to offset the carcinogenic risk of insulin[42]. Whether glycemic control should be a priority, or whether avoiding hyper-insulinemia because of therapy should be a priority, is an issue for future investigation.
In terms of survival of HCC patients, associations with liver function and tumor factors have been reported[10], but conflicting results have been reported for the relationship with diabetes[20,21]. These two studies involved heterogeneous groups of HCC patients treated with various therapies, including surgery, local ablation therapy and transcatheter arterial embolization. This heterogeneity may have led to the conflicting results, because the survival of HCC patients may be strongly affected by the initial treatment. Our study involved a homogeneous patient population, i.e., all patients were initially treated curatively by RFA. The results of our study suggest that glycemic control in diabetic patients, more so than diabetes itself, plays a role in survival. The mechanism by which glycemic control and survival are related is unknown, but frequent recurrence of HCC in hyperglycemic patients and the accumulation of damage in liver function because of repeated treatment intervention for HCC may lead to worsening survival.
In conclusion, inadequate maintenance of blood glucose in diabetic patients was a significant and independent risk factor for early recurrence of HCC and for poor survival. Adequate management of hyperglycemia in diabetic patients may lead to reduction in the risk of HCC recurrence and improvement in overall survival.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide. Radiofrequency ablation (RFA) therapy is an efficient curative therapy for HCC, but long-term survival is limited because of the high rate of distant recurrence of approximately 80% within 5 years. Identification of factors related to recurrence of HCC and therapeutic intervention targeting these factors may lead to prevention of frequent recurrence of HCC and improved survival.
Research frontiers
Metabolic factors, such as obesity and diabetes, have been identified as risk factors for several types of cancer, such as cancer of the liver, pancreas, kidney, and colon. These metabolic factors may be related to recurrence of HCC. The impact of diabetes on the recurrence of HCC after treatment has been discussed, but with conflicting results.
Innovations and breakthroughs
The authors identified that inadequate maintenance of blood glucose in diabetic patients was a significant and independent risk factor for early recurrence of HCC and a risk factor for poor survival, whereas diabetes was not. In other words, even in patients with diabetes, if the blood glucose was adequately maintained, then the HCC recurrence rate and survival did not differ significantly from those in non-diabetic patients.
Applications
The results of the present study indicate the possibility that adequate management of hyperglycemia in diabetic patients may lead to reduction in the risk of HCC recurrence and improvement of overall survival.
Peer review
This is an important study in which the effect of inadequate maintenance of blood glucose in diabetes has been shown as a significant risk factor for distant recurrence of hepatocellular carcinoma and poor survival after curative radiofrequency ablation therapy.
Footnotes
Supported by A Grant-in-Aid from the Ministry of Health, Labor and Welfare, Japan
P- Reviewer Rana SV S- Editor Gou SX L- Editor Stewart GJ E- Editor Zhang DN
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Arii S, Sata M, Sakamoto M, Shimada M, Kumada T, Shiina S, Yamashita T, Kokudo N, Tanaka M, Takayama T, et al. Management of hepatocellular carcinoma: Report of Consensus Meeting in the 45th Annual Meeting of the Japan Society of Hepatology (2009) Hepatol Res. 2010;40:667–685. doi: 10.1111/j.1872-034X.2010.00673.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 6.Izumi N, Asahina Y, Noguchi O, Uchihara M, Kanazawa N, Itakura J, Himeno Y, Miyake S, Sakai T, Enomoto N. Risk factors for distant recurrence of hepatocellular carcinoma in the liver after complete coagulation by microwave or radiofrequency ablation. Cancer. 2001;91:949–956. [PubMed] [Google Scholar]
- 7.Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. doi: 10.1097/00000658-200009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216–222. doi: 10.1097/00000658-199902000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396–1405. doi: 10.1002/hep.20486. [DOI] [PubMed] [Google Scholar]
- 11.Fuke H, Sugimoto K, Shiraki K, Tanaka J, Beppu T, Yoneda K, Yamamoto N, Ito K, Takaki H, Nakatsuka A, et al. Predictive factors for distant recurrence of HCV-related hepatocellular carcinoma after radiofrequency ablation combined with chemoembolization. Aliment Pharmacol Ther. 2008;27:1253–1260. doi: 10.1111/j.1365-2036.2008.03627.x. [DOI] [PubMed] [Google Scholar]
- 12.Hagihara H, Nouso K, Kobayashi Y, Iwasaki Y, Nakamura S, Kuwaki K, Toshimori J, Miyatake H, Ohnishi H, Shiraha H, et al. Effect of pegylated interferon therapy on intrahepatic recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. Int J Clin Oncol. 2011;16:210–220. doi: 10.1007/s10147-010-0150-x. [DOI] [PubMed] [Google Scholar]
- 13.Miyake Y, Takaki A, Iwasaki Y, Yamamoto K. Meta-analysis: interferon-alpha prevents the recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. J Viral Hepat. 2010;17:287–292. doi: 10.1111/j.1365-2893.2009.01181.x. [DOI] [PubMed] [Google Scholar]
- 14.Wong JS, Wong GL, Tsoi KK, Wong VW, Cheung SY, Chong CN, Wong J, Lee KF, Lai PB, Chan HL. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;33:1104–1112. doi: 10.1111/j.1365-2036.2011.04634.x. [DOI] [PubMed] [Google Scholar]
- 15.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 16.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 17.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 18.Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med. 2006;166:1871–1877. doi: 10.1001/archinte.166.17.1871. [DOI] [PubMed] [Google Scholar]
- 19.Hu FB, Manson JE, Liu S, Hunter D, Colditz GA, Michels KB, Speizer FE, Giovannucci E. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J Natl Cancer Inst. 1999;91:542–547. doi: 10.1093/jnci/91.6.542. [DOI] [PubMed] [Google Scholar]
- 20.Toyoda H, Kumada T, Nakano S, Takeda I, Sugiyama K, Kiriyama S, Tanikawa M, Sone Y, Hisanaga Y. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma. Cancer. 2001;91:957–963. [PubMed] [Google Scholar]
- 21.Huo TI, Wu JC, Lui WY, Huang YH, Lee PC, Chiang JH, Chang FY, Lee SD. Differential mechanism and prognostic impact of diabetes mellitus on patients with hepatocellular carcinoma undergoing surgical and nonsurgical treatment. Am J Gastroenterol. 2004;99:1479–1487. doi: 10.1111/j.1572-0241.2004.30024.x. [DOI] [PubMed] [Google Scholar]
- 22.Komura T, Mizukoshi E, Kita Y, Sakurai M, Takata Y, Arai K, Yamashita T, Ohta T, Shimizu K, Nakamoto Y, et al. Impact of diabetes on recurrence of hepatocellular carcinoma after surgical treatment in patients with viral hepatitis. Am J Gastroenterol. 2007;102:1939–1946. doi: 10.1111/j.1572-0241.2007.01354.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen WT, Macatula TC, Lin CC, Lin CJ, Lin SM. Diabetes may not affect outcomes in hepatocellular carcinoma after radio-frequency ablation. Hepatogastroenterology. 2011;58:551–557. [PubMed] [Google Scholar]
- 24.Youkou A, Hasegawa T, Suzuki K, Koya T, Sakagami T, Toyabe S, Arakawa M, Gejyo F, Narita I, Suzuki E. Influence of obesity on control in asthmatic Japanese patients defined by the Japanese definition of obesity. Intern Med. 2011;50:1911–1916. doi: 10.2169/internalmedicine.50.5474. [DOI] [PubMed] [Google Scholar]
- 25.Aizawa N, Enomoto H, Imanishi H, Saito M, Iwata Y, Tanaka H, Ikeda N, Sakai Y, Takashima T, Iwai T, et al. Elevation of the glycated albumin to glycated hemoglobin ratio during the progression of hepatitis C virus related liver fibrosis. World J Hepatol. 2012;4:11–17. doi: 10.4254/wjh.v4.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33 Suppl 1:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai MS, Hsieh MS, Chiu YH, Chen TH. Type 2 diabetes and hepatocellular carcinoma: A cohort study in high prevalence area of hepatitis virus infection. Hepatology. 2006;43:1295–1302. doi: 10.1002/hep.21208. [DOI] [PubMed] [Google Scholar]
- 29.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Ma Q, Li J. High glucose promotes cell proliferation and enhances GDNF and RET expression in pancreatic cancer cells. Mol Cell Biochem. 2011;347:95–101. doi: 10.1007/s11010-010-0617-0. [DOI] [PubMed] [Google Scholar]
- 32.Okumura M, Yamamoto M, Sakuma H, Kojima T, Maruyama T, Jamali M, Cooper DR, Yasuda K. Leptin and high glucose stimulate cell proliferation in MCF-7 human breast cancer cells: reciprocal involvement of PKC-alpha and PPAR expression. Biochim Biophys Acta. 2002;1592:107–116. doi: 10.1016/s0167-4889(02)00276-8. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto M, Patel NA, Taggart J, Sridhar R, Cooper DR. A shift from normal to high glucose levels stimulates cell proliferation in drug sensitive MCF-7 human breast cancer cells but not in multidrug resistant MCF-7/ADR cells which overproduce PKC-betaII. Int J Cancer. 1999;83:98–106. doi: 10.1002/(sici)1097-0215(19990924)83:1<98::aid-ijc18>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi H, Mizuta T, Eguchi Y, Kawaguchi Y, Kuwashiro T, Oeda S, Isoda H, Oza N, Iwane S, Izumi K, et al. Post-challenge hyperglycemia is a significant risk factor for the development of hepatocellular carcinoma in patients with chronic hepatitis C. J Gastroenterol. 2011;46:790–798. doi: 10.1007/s00535-011-0381-2. [DOI] [PubMed] [Google Scholar]
- 36.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 37.Fisher WE, Boros LG, Schirmer WJ. Insulin promotes pancreatic cancer: evidence for endocrine influence on exocrine pancreatic tumors. J Surg Res. 1996;63:310–313. doi: 10.1006/jsre.1996.0266. [DOI] [PubMed] [Google Scholar]
- 38.Renehan A, Smith U, Kirkman MS. Linking diabetes and cancer: a consensus on complexity. Lancet. 2010;375:2201–2202. doi: 10.1016/S0140-6736(10)60706-4. [DOI] [PubMed] [Google Scholar]
- 39.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 40.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawaguchi T, Taniguchi E, Morita Y, Shirachi M, Tateishi I, Nagata E, Sata M. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int. 2010;30:479–486. doi: 10.1111/j.1478-3231.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 42.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]


