Abstract
AIM: To investigate the effect of novel probiotics on the clinical characteristics of high-fructose induced metabolic syndrome.
METHODS: Male Wistar rats aged 4 wk were fed a 70% w/w high-fructose diet (n = 27) or chow diet (n = 9) for 3 wk to induce metabolic syndrome, the rats were then randomized into groups and administered probiotic [Lactobacillus curvatus (L. curvatus) HY7601 and Lactobacillus plantarum (L. plantarum) KY1032] at 109 cfu/d or 1010 cfu/d or placebo by oral gavage for 3 wk. Food intake and body weight were measured once a week. After 6 wk, the rats were fasted for 12 h, then anesthetized with diethyl ether and sacrificed. Blood samples were taken from the inferior vena cava for plasma analysis of glucose, insulin, C-peptide, total-cholesterol, triglycerides and thiobarbituric acid-reacting substances. Real-time polymerase chain reaction was performed using mouse-specific Taqman probe sets to assess genes related to fatty acid β-oxidation, lipogenesis and cholesterol metabolism in the liver. Target gene expression was normalized to the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase.
RESULTS: Rodents fed a high-fructose diet developed clinical characteristics of the metabolic syndrome including increased plasma glucose, insulin, triglycerides, total cholesterol and oxidative stress levels, as well as increased liver mass and liver lipids compared to chow fed controls. Probiotic treatment (L. curvatus HY7601 and L. plantarum KY1032) at high (1010 cfu/d) or low dosage (109 cfu/d) lowered plasma glucose, insulin, triglycerides and oxidative stress levels. Only high-dose probiotic treatment reduced liver mass and liver cholesterol. Probiotic treatment reduced lipogenesis via down-regulation of SREBP1, FAS and SCD1 mRNA levels and increased β-oxidation via up-regulation of PPARα and CPT2 mRNA levels.
CONCLUSION: Probiotic L. curvatus HY7601 and L. plantarum KY1032 combined suppressed the clinical characteristics of high-fructose-induced metabolic syndrome, therefore, may provide a natural alternative for the treatment of diet-induced metabolic syndrome.
Keywords: Dyslipidemia, Fasting glucose, Gut microbiota, High-fructose diet, Inflammation, Insulin resistance, Lactobacillus, Metabolic syndrome, Oxidative stress, Probiotic
INTRODUCTION
Metabolic syndrome is a rapidly growing worldwide pandemic, which is associated with a greater risk of multiple chronic pathologies including cardiovascular disease and Type 2 diabetes. Metabolic syndrome is characterised by a cluster of metabolic abnormalities including insulin resistance, elevated fasting glucose, elevated plasma triglycerides, elevated blood pressure, low-grade inflammation, abdominal obesity and reduced high-density lipoprotein (HDL)-cholesterol[1,2]. There is no universal cause of metabolic syndrome, although major underlying risk factors include abdominal obesity, physical inactivity or diabetogenic diets[1].
Diabetogenic diets with high fructose content have been strongly implicated in the development of metabolic syndrome, cardiovascular disease and Type 2 diabetes[3]. Food manufacturers are increasingly using fructose corn syrup to increase sweetness, as well as the palatability of food and beverages. However, there is growing experimental evidence that excessive fructose intake can lead to metabolic abnormalities, including insulin resistance, dyslipidemia as well as abdominal adiposity in both animals and humans[4]. Animals fed a high-fructose diet develop clinical characteristics of metabolic syndrome, therefore, high-fructose fed animals are particularly useful for assessing potential therapeutic interventions against metabolic syndrome[5]. Excess dietary fructose can be converted to triglycerides through de novo lipogenesis, resulting in increased lipid accumulation in the liver and elevated blood lipid levels. Over a period of time prolonged high intracellular and systemic lipid levels can cause increased oxidative stress and inflammation, both of which can trigger insulin resistance, leading to increased blood glucose levels. Due to the lack of effective drugs to treat metabolic syndrome, there is growing interest in natural therapeutics to prevent or manage metabolic syndrome.
Over the past five years, probiotics have rapidly emerged as natural therapeutics with potential to target key risk factors associated with metabolic syndrome[6,7]. Probiotics consist of single or multiple live bacterial species, which may directly or indirectly modulate gut microbial activity and improve host health. The human gut harbours between 1014 bacterial species collectively forming the gut microbiota[8]. Gut microbial communities are proposed to provide the host with the ability to harvest otherwise inaccessible energy from the diet[9-11] and modulate host genes associated with energy storage in adipose tissue[2,12]. Probiotics have been widely assessed in-vivo in diet-induced obesity models[13-17], however, different probiotic species even from the same family can exert variable effects on lipid accumulation and obesity[18], therefore, it remains essential to assess the effectiveness of probiotic strains in different animal disease models in-vivo. Probiotic yogurt containing multiple Lactobacillus strains has been reported to alleviate fasting blood glucose, plasma insulin and triglyceride in high-fructose fed rats[19]. However, few other studies have considered the impact of probiotics on high-fructose diet-induced metabolic syndrome. To our knowledge, the dose-dependent and metabolic effects of probiotic treatment in high-fructose-induced metabolic syndrome remain to be established.
The aim of this study was to assess the dose-dependent effects of a probiotic consisting of Lactobacillus curvatus (L. curvatus) HY7601 and Lactobacillus plantarum (L. plantarum) KY1032 on hyperlipidemia, hyperglycemia, insulin resistance, oxidative stress and hepatic metabolism related gene expression in high-fructose fed rats with metabolic syndrome. We hypothesized that probiotic treatment may protect against dysregulated metabolism induced by a high-fructose diet in a dose-dependent manner.
MATERIALS AND METHODS
Animals, diets and experimental design
Male Wistar rats (n = 36) aged 4 wk were purchased from Jackson Laboratories (Bar Harbor, United States). All rats were individually housed under a constant temperature and humidity (22 ± 1 °C, 55% ± 10%) with a 12 h light/12 h dark cycle. The experimental design consisted of a pretreatment phase (0-3 wk) and a treatment phase (3-6 wk). During the pretreatment phase, male Wistar rats were fed a 70% w/w high-fructose diet (n = 27) to induce metabolic abnormalities or a chow diet (n = 9) for 3 wk. The composition of the high-fructose diet was formulated according to Table 1. During the treatment phase, the placebo (HF) group (n = 9), low dose probiotic (LP) group (n = 9) and high dose probiotic (HP) group (n = 9) were fed the same high-fructose diet with placebo, 109 cfu probiotics or 1010 cfu probiotics, respectively, administered orally each day for a further 3 wk. The chow control group was fed the same chow diet with placebo administered orally each day for a further 3 wk. Freeze-dried Lactobacillus strains were produced by Culture Systems Inc. (United States), and packed with lactose according to Table 2. Each pack was resuspended in 500 μL distilled water prior to administration. Food intake and body weight were measured once a week. Before sacrifice, rats were fasted for 12 h and anesthetized with diethyl ether. Blood samples were taken from the inferior vena cava for plasma analysis. Epididymal adipose tissue and liver tissue were removed, rinsed with phosphate buffered saline, weighed and immediately frozen at -70 °C. The experimental design was approved by the Ethics Committee of Korea Yakult Company Limited R and D Center.
Table 1.
Composition of high fructose diet
| Ingredient | High fructose diet (g) |
| Casein | 200.0 |
| L-cystine | 3.0 |
| Fructose | 700.0 |
| Cellulose powder | 50.0 |
| Corn oil | 25.0 |
| Lard | 20.0 |
| Mineral Mix S10026 | 10.0 |
| DiCalcium Phosphate | 13.0 |
| Calcium Carbonate | 5.5 |
| Potassium Citrate | 16.5 |
| Vitamin Mix V10001 | 10.0 |
| Choline Bitartrate | 2.0 |
| Total | 1000.0 |
Table 2.
The composition of each supplement pack
| Placebo (mg) | 109 probiotics (mg) | 1010 probiotics (mg) | |
| Lactobacillus curvatus HY7601 | - | 5.0 (5 × 108) | 50.0 (5 × 109) |
| Lactobacillus plantarum KY1032 | - | 2.5 (5 × 108) | 25.0 (5 × 109) |
| Lactose | 100.0 | 92.5 | 25. |
| Total | 100.0 | 100.0 | 100.0 |
Freeze-dried Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 concentration was 1 × 108 and 2 × 108 cfu/mg, respectively.
Blood analysis
Plasma glucose, insulin and C-peptide concentrations were determined using the glucose assay kit (Cayman, United States), insulin enzyme-linked immunosorbent assay (ELISA) kit (Millipore, United States) and rat C-peptide ELISA kit (EIAab, China), respectively, according to the manufacturer’s instructions. Insulin resistance was assessed based on homeostasis model assessment of insulin resistance (HOMA-IR), calculated as the product of fasting plasma glucose (FPG) and insulin (FPI), divided by a constant[20]. The equation was [FPG (mg/dL) × FPI (μU/mL)]/2430. Plasma total-cholesterol and triglyceride concentrations were determined using commercial kits (AsanPharm, South Korea). Plasma thiobarbituric acid-reacting substances (TBARS) were measured to assess oxidative stress as described previously[21].
Hepatic lipid profile analysis
Hepatic lipids were extracted as previously reported[22]. The dried lipid residues were dissolved in 1 mL of isopropanol for the triglyceride and cholesterol assays. Hepatic triglyceride and cholesterol concentrations were measured using the same commercial kits (AsanPharm, South Korea) used for the plasma analysis.
RT-qPCR
Total RNA was extracted from liver (15 mg) tissue using an RNAqueous kit (Ambion, United States). DNA was removed with a Turbo DNA-free kit (Ambion, United States). RNA integrity was verified and RNA quantified using a GeneQuant Pro spectrophotometer (GE Healthcare, United States). Total RNA (2 μg) was reverse-transcribed into cDNA with a high-capacity RNA-to-cDNA kit (Applied Biosystems Inc., United States). Then cDNA was amplified on a 7500 Real Time PCR System (Applied Biosystems Inc., United States) using mouse-specific Taqman probe sets (Table 3) under the following conditions: 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s, and 60 °C for 1 min. Target gene expression was normalized to the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (catalog number Rn01775763_g1). Data was analyzed using the ABI 7500 System Sequence Detection software (Applied Biosystems Inc., United States) and presented as mean ± SE.
Table 3.
Catalog numbers of Taqman probes
| Taqman probe | Catalog number |
| Peroxisome proliferative activated receptor a | Rn00566193_m1 |
| Carnitine palmitoyl transferase 1 | Rn00580702_m1 |
| Carnitine palmitoyl transferase 2 | Rn00563995_m1 |
| Acyl-CoA oxidase 1 | Rn01460628_m1 |
| Sterol regulatory element binding protein-1 | Rn01495769_m1 |
| Fatty acid synthase | Rn00569117_m1 |
| Stearoyl-CoA desaturase 1 | Rn00594894_g1 |
| Cholesterol 7 alpha-hydroxylase | Rn00564065_m1 |
| Low density lipoprotein receptor | Rn00598442_m1 |
Statistical analysis
All data were presented as mean ± SE. Statistical analysis was performed using SPSS software (SPSS Inc., United States). Data were analyzed by one way analysis of variance, and the differences between experimental groups were evaluated using Duncan’s multiple range test at the P < 0.05 level. Significant differences between chow control and HF control groups were determined using unpaired Student’s t-test. Significant differences between week 3 and week 6 in each parameter were determined using the paired Student’s t-test, and the values were considered statistically significant when P < 0.05.
RESULTS
Effects of probiotic treatment on food intake, body weight and tissue mass
Metabolic syndrome was induced in rodents by feeding them a high-fructose diet over 3 wk, while a control group was fed a chow diet. Rodents with metabolic syndrome were then randomized into three treatment groups and fed a high-fructose diet for a further 3 wk, with daily treatment of either probiotic (HP) at a dose of 1 × 1010 cfu/d, probiotic (LP) at a dose of 1 × 109 cfu/d or placebo administered by oral gavage. The chow control group were fed the same diet for the same period and administered placebo by oral gavage. Average food intake was suppressed in the high-fructose diet fed rodents compared to the chow fed controls (Figure 1A). However, low or high dose probiotic treatment had no significant effect on food intake (Figure 1A). Body weight gain and epididymal fat mass were not significantly affected by high-fructose feeding or probiotic treatment regardless of dosage (Figure 1B and C). Importantly, average liver mass, which was significantly increased by high-fructose diet, was significantly lower following HP treatment by 10% (P < 0.01) compared to high-fructose fed controls (Figure 1D).
Figure 1.
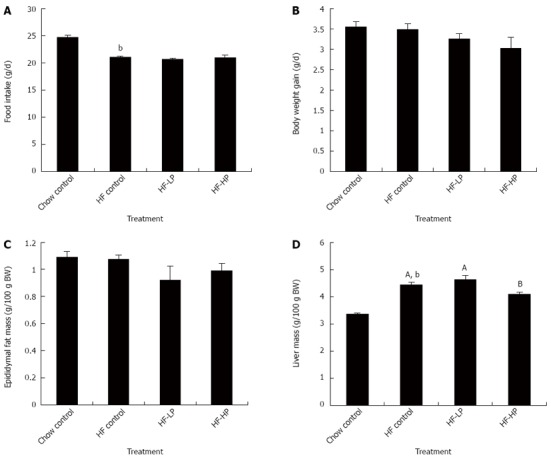
Effects of probiotic treatment on food intake (A), body weight gain (B), epididymal fat mass (C) and liver mass (D) in high-fructose diet-fed rats. Results are expressed as mean ± SE. bP < 0.01 vs chow control by unpaired Student’s t-test; ABBars with different capital letters are significantly different at P < 0.05 by Duncan’s multiple range tests. HP: High dose probiotic; HF: High fructose diet; LP: Low dose probiotic; BW: Body weight.
Effects of probiotic treatment on dyslipidemia
Hypertriglyceridemia was effectively induced by a high-fructose diet within 3 wk (163.91 ± 6.50 mg/dL vs 49.26 ± 3.99 mg/dL, HF and chow group, respectively). However, subsequently 3-wk of probiotic treatment significantly reduced average plasma triglyceride levels by 46% compared to placebo treatment in the high-fructose fed rodents (Figure 2A). The probiotic treatment had no influence on either total cholesterol or HDL cholesterol levels, which were increased by high-fructose diet intake (Figure 2B and C).
Figure 2.
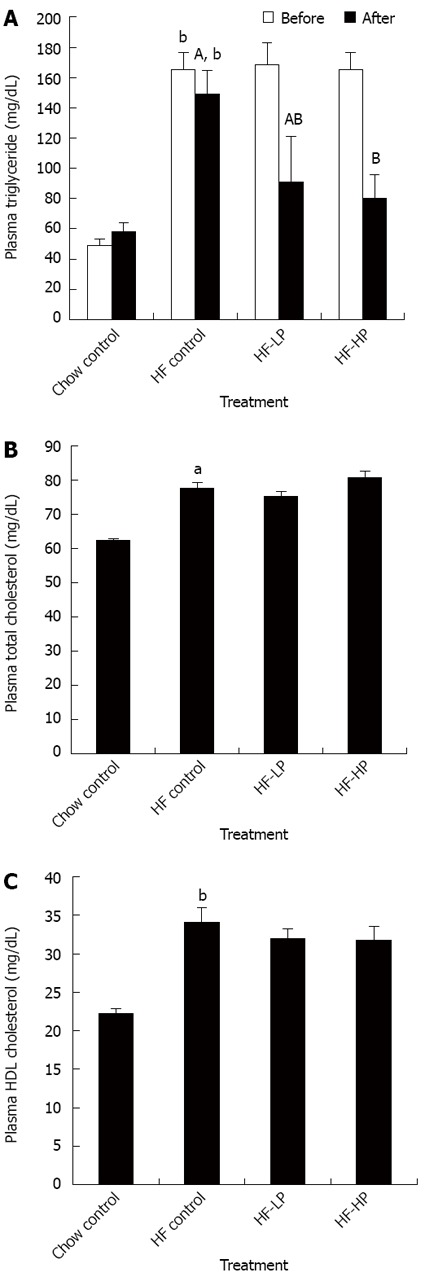
Effects of probiotic treatment on plasma triglyceride (A), plasma total cholesterol (B) and plasma high-density lipoprotein cholesterol (C) in high-fructose diet-fed rats. Results are expressed as mean ± SE. aP < 0.05 and bP < 0.01 vs chow control by unpaired Student’s t-test; ABBars with different capital letters are significantly different at P < 0.05 by Duncan’s multiple range tests. HF: High fructose diet; HP: High dose probiotic; LP: Low dose probiotic; HDL: High-density lipoprotein.
Effects of probiotic treatment on hyperglycemia, insulin resistance and oxidative stress
The average plasma glucose levels of the high-fructose fed rats were significantly higher than that of the chow fed controls at week 6, but were effectively reduced following LP and HP treatment by 24% (P < 0.05) and 14% (P < 0.05), respectively, (Figure 3A). The average plasma insulin levels of the high-fructose fed rats were significantly higher at both weeks 3 and 6 compared to the chow fed controls. However, after HP treatment plasma, insulin levels were substantially lower (31%, P < 0.05) compared to the high-fructose fed rats. Moreover, the plasma insulin levels following LP and HP treatment were reduced by 33% (P < 0.01) and 29% (P < 0.05), respectively, compared to before treatment (Figure 3B). The HOMA-IR, a representative index of insulin resistance, was significantly higher in the high-fructose fed rats compared to the chow fed controls at week 6, but was significantly reduced by LP (35%) and HP (34%) treatment (Figure 3C). Furthermore, HOMA-IR following LP treatment was 25% (P < 0.05) lower compared to before treatment (Figure 3C). Plasma C-peptide is another biomarker associated with insulin resistance. Plasma C-peptide level was 24% (P < 0.05) lower following HP treatment compared to placebo treatment in high-fructose fed rats (Figure 3D). Moreover, plasma C-peptide levels after HP treatment were 28% (P < 0.05) lower compared to before treatment (Figure 3D). In addition, probiotic treatment of either LP or HP decreased plasma TBARS levels by 37% (P < 0.01) and 50% (P < 0.001), respectively (Figure 3E).
Figure 3.
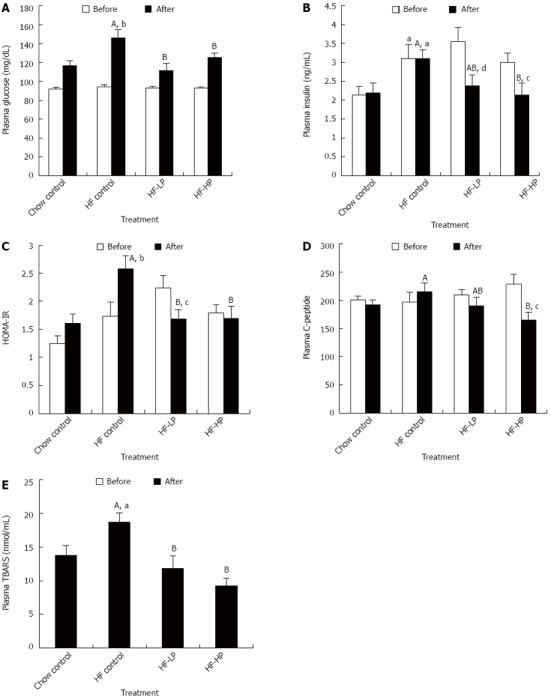
Effects of probiotic treatment on plasma glucose, plasma insulin, insulin resistance, plasma C-peptide and plasma thiobarbituric acid-reacting substances in high-fructose diet-fed rats. Results are expressed as mean ± SE. aP < 0.05 and bP < 0.01 HF control vs chow control by unpaired Student’s t-test; cP < 0.05 and dP < 0.01 week 6 vs week 3 by paired Student’s t-test; ABBars with different capital letters are significantly different at P < 0.05 by Duncan’s multiple range tests. HF: High fructose diet; LP: Low dose probiotic; HP: High dose probiotic; TBARS: Thiobarbituric acid-reacting substances.
Effects of probiotic treatment on hepatic lipid content and gene expression
Both hepatic triglyceride and cholesterol levels in the high-fructose fed rats were significantly higher than those in the chow fed controls. Probiotic treatment with either LP or HP tended to reduce hepatic triglyceride levels (Figure 4A), and HP significantly reduced (27%, P < 0.05) hepatic cholesterol levels compared to the high-fructose fed controls (Figure 4B).
Figure 4.
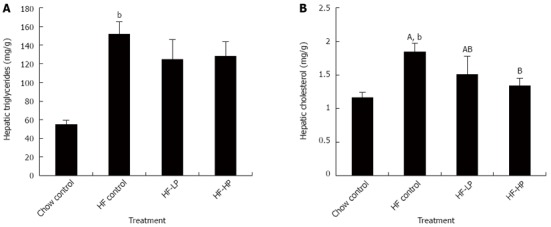
Effects of probiotic treatment on hepatic triglyceride and hepatic total cholesterol in high-fructose diet-fed rats. Results are expressed as mean ± SE. bP < 0.01 HF control vs chow control by unpaired Student’s t-test; ABBars with different capital letters are significantly different at P < 0.05 by Duncan’s multiple range tests. HF: High fructose diet; LP: Low dose probiotic; HP: High dose probiotic.
In order to determine the mechanisms underlying the probiotic effect on hepatic lipid homeostasis, we examined the mRNA levels of hepatic genes associated with lipid metabolism (Figure 5A-C). High-fructose diet intake altered gene expression compared to chow diet intake. The changes in gene expression in the high-fructose group indicated decreases in fatty acid β-oxidation (PPARα, CPT1, CPT2 and ACOX1), cholesterol uptake (LDLR) and bile acid synthesis (CYP7A1), and increases in fatty acid synthesis (SREBP1, FAS, SCD1). Importantly, HP treatment significantly reversed high-fructose-induced gene expression changes including up-regulation of PPARα (+76%), CPT2 (+66%) and CYP7A1 (+71%), and down-regulation of SREBP1 (-30%), FAS (-54%) and SCD1 (-23%), although LP treatment did not cause any significant changes in hepatic lipid metabolism gene expression.
Figure 5.
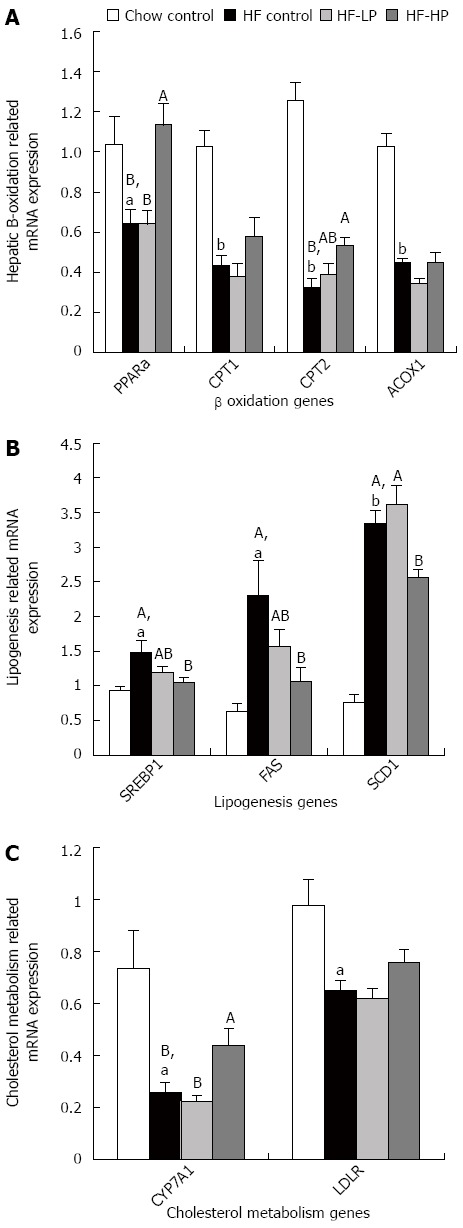
Effects of probiotic treatment on hepatic B-oxidation, lipogenesis and cholesterol metabolism related gene expression in high-fructose diet-fed rats. Results are expressed as mean ± SE. aP < 0.05 and bP < 0.01 HF control vs chow control by unpaired Student’s t-test; ABBars with different capital letters are significantly different at P < 0.05 by Duncan’s multiple range tests. HF: High fructose diet; LP: Low dose probiotic; HP: High dose probiotic; FAS: Fatty acid synthase; SREBP1: Sterol regulatory element-binding protein-1; PPARα: Peroxisome proliferator-activated receptor α; CPT: Carnitine palmitoyltransferase; ACOX: Acyl-coenzyme A oxidase; SCD: Steaoryl-CoA desaturase; CYP7A1: Cholesterol 7α-hydroxylase gene; LDLR: Low-density lipoprotein receptor.
DISCUSSION
Recent studies indicate that the gut microbiota plays an important role in host lipid and glucose metabolism. Therefore, therapeutic probiotics which can manipulate the gut microbiota may also prevent some of the risk factors underlying the development of metabolic syndrome including dyslipidemia, elevated fasting glucose levels and insulin resistance[23]. In the present study, we used a novel probiotic consisting of L. curvatus HY7601 and L. plantarum KY1302 isolated from Korean fermented cabbage. We showed that probiotic administered to high-fructose fed mice reversed the risk factors underlying the metabolic syndrome. Previous evidence from high-fructose diet-fed rat studies indicated that a probiotic-cultured yogurt called Dahi can also improve metabolic abnormalities[19,24]. Importantly, in the present study, we established the dose-dependent effects of a novel combination of probiotic strains on metabolic syndrome.
High-fructose intake is reported to promote lipogenesis and suppress glucose intake[25-27]. Consistent with other studies of high-fructose fed animals, hepatic gene expression analysis indicated that lipogenesis was increased via upregulation of SREBP1, FAS and SCD1, conversely β-oxidation was decreased via downregulation of PPARα and PPARα-regulated CPT1, CPT2 and ACOX1 expression[25-27]. Increased lipogenesis and decreased β-oxidation lead to excess accumulation of cellular lipids, as shown by the liver enlargement and hypertriglyceridemia in the high-fructose fed rats in the present study. Hypertriglyceridemia is known to be an important predictor of cardiovascular disease mortality in subjects with diabetes or impaired glucose tolerance[28], therefore, reducing plasma triglyceride levels may improve long-term health.
Previous studies have shown that probiotics can alter the gut microbiota[29], and direct gut microbiota manipulations in germ-free mice significantly affect host lipid metabolism[2,12]. Here we showed that 1010 cfu/d probiotic treatment led to upregulated PPARα and CPT2 expression reflecting activation of β-oxidation, and downregulated SREBP1, FAS and SCD1 expression reflecting suppression of lipogenesis. Moreover, these probiotics induced transcriptional changes which resulted in a significant reduction in liver mass and plasma triglyceride levels. These findings are consistent with a previous report which showed that L. plantarum KY1032 inhibits lipid droplet accumulation during adipocyte differentiation. In contrast to plasma triglyceride levels, hepatic triglyceride levels were only slightly reduced by probiotic treatment. We hypothesized that the probiotic-induced increase in hepatic β-oxidation related gene expression was partly to clear excess hepatic triglycerides generated through high-fructose-induced de novo lipogenesis.
Contrary to hepatic triglyceride levels, cholesterol levels were significantly reduced by probiotic treatment, whereas plasma cholesterol levels were unchanged. We assessed whether the reduction in hepatic cholesterol following probiotic treatment was due to altered hepatic cholesterol metabolism related gene expression. High-fructose diet suppressed CYP7A1 expression, which encodes cholesterol 7 α-hydroxylase, the rate-limiting enzyme involved in the formation of bile from cholesterol. However, 1010 cfu/d probiotic treatment upregulated CYP7A1 expression indicating increased bile synthesis activity. Some probiotics are also reported to increase bile salt hydrolase activity[30]. Hepatic LDLR expression remained unchanged, suggesting that probiotic treatment did not enhance uptake of plasma cholesterol into the liver, which is consistent with the absence of changes in plasma cholesterol levels. However, the effect of probiotics on liver cholesterol metabolism may be partly dependent on the diet regime used, because in high-fat or high-cholesterol diet fed animals, probiotics are reported to decrease plasma and hepatic cholesterol levels together.
The probiotic effect on host lipid levels may also contribute to the observed improvement in glucose homeostasis. Excess intracellular lipids can inhibit insulin signalling, hence reduce insulin-stimulated glucose uptake leading to hyperglycemia and hyperinsulinemia as shown in this study. We observed that probiotic treatment at 109 or 1010 cfu/d significantly lowered plasma glucose, insulin and C-peptide concentrations, and reduced insulin resistance indicated by the reduced HOMA-IR index[20,31]. Excess fructose intake is also reported to promote lipid peroxidation and oxidative stress is implicated in the pathogenesis of insulin resistance[32,33]. In the present study, probiotic treatment at 109 or 1010 cfu/d significantly reversed the oxidative stress present in the high-fructose fed rats with metabolic syndrome, indicated by lower plasma TBARS levels. Some Lactobacillus strains are also reported to possess anti-oxidative activity. For example, probiotics have been reported to reduce exercise-induced oxidative stress, via increases in anti-oxidative activity, which helps neutralize reactive oxygen species[34]. Furthermore, studies in human Type 2 diabetes show probiotic supplementation can increase superoxide dismutase and glutathione peroxidase activities[35], which are anti-oxidants that help protect against oxidative stress.
While evidence is growing on the effects of probiotic treatment on many chronic pathologies, some issues remain to be addressed. Probiotics are widely assumed to modulate the gut microbiota, and exert health benefits via direct modification of gut microbial communities[8]. Studies on diet-induced mice indicate that probiotics alter the gut microbiota[29], similar to our recent experience with the same probiotic used in this study (unpublished observations). However, in another study using germ-free mice transplanted with a small artificial gut microbial community, a multi-species probiotic failed to change the gut microbiota[36]. Whether multi-species probiotics are more effective than single-species probiotics is not clear, because few studies make this comparison. We used a two species probiotic in the present study, and based on our preliminary data the results suggest that combined species are more effective than either species alone. Finally, whether probiotics exert dose-dependent effects against metabolic syndrome has not been evaluated, nor has the minimum amount of live bacteria that is necessary for functional health benefits. Evidence from the present study indicates that probiotics containing 1010 cfu/d showed the greatest effectiveness against high-fructose-induced metabolic syndrome over a relatively short period, without any adverse effects. In contrast, probiotic treatment at 109 cfu/d exerted minimal effects on hypertriglyceridemia, but effectively improved hyperglycaemia and insulin resistance. In the case of hepatic gene expression, 109 cfu/d probiotic treatment had no modulatory effect on any of the genes tested, but probiotic treatment at 1010 cfu/d modulated lipogenesis and β-oxidation related genes.
In conclusion, probiotic L. curvatus HY7601 and L. plantarum KY1032 combined can suppress the clinical characteristics of high-fructose-induced metabolic syndrome, therefore, may provide a natural alternative for the treatment of diet-induced metabolic syndrome.
ACKNOWLEDGMENTS
Choi MS and McGregor RA declare no conflicts of interest. Park DY, Ahn YT and Huh CS are current or past employees of Korea Yakult Co., Ltd.
COMMENTS
Background
Metabolic syndrome is a growing health problem characterised by elevated blood sugar and blood lipid levels, insulin resistance, high blood pressure and abdominal obesity. Poor diet consisting of high sugar or high fat content is a major risk factor for metabolic syndrome. There is no universal treatment for metabolic syndrome. Probiotics found in fermented foods have emerged as a natural way of protecting against metabolic syndrome, but many probiotic strains have varying metabolic effects. Therefore, a major challenge for scientists is discovering probiotic strains, which may help protect against metabolic syndrome.
Research frontiers
Probiotic strains identified in kimchi, a traditional Korean fermented cabbage consumed regularly in South Asian countries, are reported to have various beneficial properties. Current research aims to determine whether these probiotic strains are effective in different animal models of disease including metabolic syndrome, Type 2 diabetes and obesity.
Innovations and breakthroughs
The authors showed that two probiotic strains, Lactobacillus curvatus (L. curvatus) HY7601 and Lactobacillus plantarum (L. plantarum) KY1032, can suppress metabolic abnormalities such as hypertriglyceridemia, hyperglycemia and insulin resistance in high-fructose-induced metabolic syndrome. These probiotic health benefits were associated with decreased lipogenesis and increased β-oxidation-related gene expression in the liver.
Applications
Probiotic with L. curvatus HY7601 and L. plantarum KY1032 may provide a natural supplement for the management of the underlying risk factors of metabolic syndrome.
Terminology
Probiotics consist of live micro-organisms which confer beneficial effects on host health. Hypertriglyceridemia is prolonged elevated triglyceride levels in blood. Hyperglycemia is prolonged elevated glucose levels in the blood. Insulin resistance is the inability of insulin to stimulate glucose uptake pathways in fat, skeletal muscle and the liver.
Peer review
This paper investigated the effect of novel probiotics on the clinical characteristics of high-fructose induced metabolic syndrome. The authors were suggesting a mechanism. One novel thing of this paper is that no one has looked at these specific microbes in this combination. It is well written.
Footnotes
Supported by The Basic Science Research Program, Center for Food and Nutritional Genomics, the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science and Technology, No. 2011-0000912 and Korea Yakult Co., Ltd.
P- Reviewer Gibson DL S- Editor Xiong L L- Editor Webster JR E- Editor Zhang DN
References
- 1.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw Cardiol. 2005;4:198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik VS, Popkin BM, Bray GA, Després JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121:1356–1364. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Moura RF, Ribeiro C, de Oliveira JA, Stevanato E, de Mello MA. Metabolic syndrome signs in Wistar rats submitted to different high-fructose ingestion protocols. Br J Nutr. 2009;101:1178–1184. doi: 10.1017/S0007114508066774. [DOI] [PubMed] [Google Scholar]
- 6.Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, Possemiers S, Van Holle A, François P, de Vos WM, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 8.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 9.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 11.Bird AR, Brown IL, Topping DL. Starches, resistant starches, the gut microflora and human health. Curr Issues Intest Microbiol. 2000;1:25–37. [PubMed] [Google Scholar]
- 12.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K, Paek K, Lee HY, Park JH, Lee Y. Antiobesity effect of trans-10,cis-12-conjugated linoleic acid-producing Lactobacillus plantarum PL62 on diet-induced obese mice. J Appl Microbiol. 2007;103:1140–1146. doi: 10.1111/j.1365-2672.2007.03336.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee HY, Park JH, Seok SH, Baek MW, Kim DJ, Lee KE, Paek KS, Lee Y, Park JH. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim Biophys Acta. 2006;1761:736–744. doi: 10.1016/j.bbalip.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Kondo S, Xiao JZ, Satoh T, Odamaki T, Takahashi S, Sugahara H, Yaeshima T, Iwatsuki K, Kamei A, Abe K. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci Biotechnol Biochem. 2010;74:1656–1661. doi: 10.1271/bbb.100267. [DOI] [PubMed] [Google Scholar]
- 16.Kang JH, Yun SI, Park HO. Effects of Lactobacillus gasseri BNR17 on body weight and adipose tissue mass in diet-induced overweight rats. J Microbiol. 2010;48:712–714. doi: 10.1007/s12275-010-0363-8. [DOI] [PubMed] [Google Scholar]
- 17.Chen JJ, Wang R, Li XF, Wang RL. Bifidobacterium longum supplementation improved high-fat-fed-induced metabolic syndrome and promoted intestinal Reg I gene expression. Exp Biol Med (Maywood) 2011;236:823–831. doi: 10.1258/ebm.2011.010399. [DOI] [PubMed] [Google Scholar]
- 18.Yin YN, Yu QF, Fu N, Liu XW, Lu FG. Effects of four Bifidobacteria on obesity in high-fat diet induced rats. World J Gastroenterol. 2010;16:3394–3401. doi: 10.3748/wjg.v16.i27.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yadav H, Jain S, Sinha PR. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition. 2007;23:62–68. doi: 10.1016/j.nut.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab. 2008;295:E1269–E1276. doi: 10.1152/ajpendo.90207.2008. [DOI] [PubMed] [Google Scholar]
- 21.Do GM, Oh HY, Kwon EY, Cho YY, Shin SK, Park HJ, Jeon SM, Kim E, Hur CG, Park TS, et al. Long-term adaptation of global transcription and metabolism in the liver of high-fat diet-fed C57BL/6J mice. Mol Nutr Food Res. 2011;55 Suppl 2:S173–S185. doi: 10.1002/mnfr.201100064. [DOI] [PubMed] [Google Scholar]
- 22.Park DY, Ahn YT, Huh CS, Jeon SM, Choi MS. The inhibitory effect of Lactobacillus plantarum KY1032 cell extract on the adipogenesis of 3T3-L1 Cells. J Med Food. 2011;14:670–675. doi: 10.1089/jmf.2010.1355. [DOI] [PubMed] [Google Scholar]
- 23.Kootte RS, Vrieze A, Holleman F, Dallinga-Thie GM, Zoetendal EG, de Vos WM, Groen AK, Hoekstra JB, Stroes ES, Nieuwdorp M. The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:112–120. doi: 10.1111/j.1463-1326.2011.01483.x. [DOI] [PubMed] [Google Scholar]
- 24.Yadav H, Jain S, Sinha PR. Effect of skim milk and dahi (yogurt) on blood glucose, insulin, and lipid profile in rats fed with high fructose diet. J Med Food. 2006;9:328–335. doi: 10.1089/jmf.2006.9.328. [DOI] [PubMed] [Google Scholar]
- 25.Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond) 2005;2:5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim HJ, Ntambi JM. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem. 2004;279:25164–25171. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- 27.Nagai Y, Nishio Y, Nakamura T, Maegawa H, Kikkawa R, Kashiwagi A. Amelioration of high fructose-induced metabolic derangements by activation of PPARalpha. Am J Physiol Endocrinol Metab. 2002;282:E1180–E1190. doi: 10.1152/ajpendo.00471.2001. [DOI] [PubMed] [Google Scholar]
- 28.Fontbonne A, Eschwège E, Cambien F, Richard JL, Ducimetière P, Thibult N, Warnet JM, Claude JR, Rosselin GE. Hypertriglyceridaemia as a risk factor of coronary heart disease mortality in subjects with impaired glucose tolerance or diabetes. Results from the 11-year follow-up of the Paris Prospective Study. Diabetologia. 1989;32:300–304. doi: 10.1007/BF00265546. [DOI] [PubMed] [Google Scholar]
- 29.Murphy EF, Cotter PD, Hogan A, O'Sullivan O, Joyce A, Fouhy F, Clarke SF, Marques TM, O'Toole PW, Stanton C, et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut. 2012:Epub ahead of print. doi: 10.1136/gutjnl-2011-300705. [DOI] [PubMed] [Google Scholar]
- 30.Begley M, Hill C, Gahan CG. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol. 2006;72:1729–1738. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu T, Giovannucci E, Pischon T, Hankinson SE, Ma J, Rifai N, Rimm EB. Fructose, glycemic load, and quantity and quality of carbohydrate in relation to plasma C-peptide concentrations in US women. Am J Clin Nutr. 2004;80:1043–1049. doi: 10.1093/ajcn/80.4.1043. [DOI] [PubMed] [Google Scholar]
- 32.Kelley GL, Allan G, Azhar S. High dietary fructose induces a hepatic stress response resulting in cholesterol and lipid dysregulation. Endocrinology. 2004;145:548–555. doi: 10.1210/en.2003-1167. [DOI] [PubMed] [Google Scholar]
- 33.Busserolles J, Rock E, Gueux E, Mazur A, Grolier P, Rayssiguier Y. Short-term consumption of a high-sucrose diet has a pro-oxidant effect in rats. Br J Nutr. 2002;87:337–342. doi: 10.1079/BJNBJN2002524. [DOI] [PubMed] [Google Scholar]
- 34.Martarelli D, Verdenelli MC, Scuri S, Cocchioni M, Silvi S, Cecchini C, Pompei P. Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr Microbiol. 2011;62:1689–1696. doi: 10.1007/s00284-011-9915-3. [DOI] [PubMed] [Google Scholar]
- 35.Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28:539–543. doi: 10.1016/j.nut.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 36.McNulty NP, Yatsunenko T, Hsiao A, Faith JJ, Muegge BD, Goodman AL, Henrissat B, Oozeer R, Cools-Portier S, Gobert G, et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med. 2011;3:106ra106. doi: 10.1126/scitranslmed.3002701. [DOI] [PMC free article] [PubMed] [Google Scholar]


