Abstract
Nitroglycerin, which helps impaired cardiac function as it is converted to nitric oxide, is used worldwide to treat patients with various ischemic and congestive cardiac diseases, including angina pectoris. Nevertheless, after continuous treatment, the benefits of nitroglycerin are limited by the development of tolerance to the drug. Nitroglycerin tolerance is a result of inactivation of aldehyde dehydrogenase 2 (ALDH2), an enzyme essential for cardioprotection in animals subjected to myocardial infarction (MI). Here we tested the hypothesis that the tolerance that develops as a result of sustained nitroglycerin treatment increases cardiac injury by subsequent MI. In a rat model of MI, 16 hours of prior, sustained nitroglycerin treatment (7.2 mg/kg/day) resulted in infarcts that were twice as large as those in untreated control animals and in diminished cardiac function at 3 days and 2 weeks after the MI. We also sought to identify a potential treatment to protect against this increased cardiac damage. Nitroglycerin inhibited ALDH2 activity in vitro, an effect that was blocked by Alda-1, an activator of ALDH2. Co-administration of Alda-1 (16 mg/kg/day) with the nitroglycerin prevented the nitroglycerin-induced increase in cardiac dysfunction after MI in rats, at least in part by enhancing metabolism of reactive aldehyde adducts that impair normal protein functions. If our animal studies showing that nitroglycerin tolerance increases cardiac injury upon ischemic insult are corroborated in humans, activators of ALDH2 such as Alda-1 may help to protect MI patients from this nitroglycerin-induced increase in cardiac injury, while maintaining the cardiac benefits of the increased nitric oxide concentrations produced by nitroglycerin.
Introduction
Nitroglycerin and other organic nitrates remain the most common treatments for patients with stable and unstable angina pectoris, myocardial infarction (MI) and heart failure (1). The beneficial effect of nitroglycerin stems from its ability to be converted to NO, thereby increasing blood flow to the heart by dilating coronary arteries, and decreasing cardiac preload by venodilation (2). However, nitroglycerin’s benefits are limited by tolerance that develops after continuous treatment (3). Mechanisms contributing to nitroglycerin tolerance include increased oxidative stress, endothelial dysfunction and increased sensitivity to vasoconstrictors (3). Molecular insight into the mechanism of nitroglycerin tolerance came from the work of Chen et al. (4), who demonstrated that the mitochondrial enzyme aldehyde dehydrogenase 2 (ALDH2) is required for bioconversion of nitroglycerin to release nitric oxide (NO) and achieve vasodilation. Prolonged exposure to nitroglycerin, however, inactivates ALDH2 and leads to diminished bioactivation of nitroglycerin, resulting in a loss of nitroglycerin’s vasodilating effect.
ALDH2 is best known for its role in metabolizing the ethanol intermediate, acetaldehyde. However, ALDH2 is also critical for the metabolism of lipid peroxidation products, such as 4-hydroxynonenal, 4-HNE (5). These highly toxic, reactive aldehydes can create aldehydic adducts with proteins, causing protein dysfunction and tissue injury (6), and have been linked to various diseases, such as cancer and MI, in humans (7-9). Recently we showed that activation of ALDH2 by Alda-1, an allosteric activator of ALDH2, reduced cardiac damage caused by ischemic insult (10), suggesting a cardioprotective role for ALDH2. Moreover, inactivation of ALDH2 associated with nitroglycerin tolerance resulted in an increase in infarct size, ex vivo (10). These data indicated a potential risk to patients who experience an MI while on continuous nitroglycerin treatment. Because continuous nitroglycerin infusion is commonly administered to patients with an acute coronary syndrome, for instance, prior to angioplasty in the emergency department, these patients could be at risk for increased cardiac injury if they develop an MI during this treatment. Here, using a rat model, we tested the risk of sustained nitroglycerin treatment for increasing MI severity in vivo.
Results
Alda-1 prevents inactivation of ALDH2 by nitroglycerin
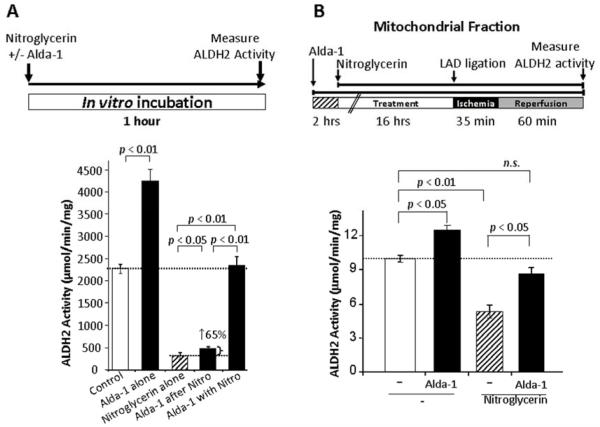
Using purified recombinant ALDH2, we first carried out in vitro studies to determine the effect of nitroglycerin and Alda-1 on the enzymatic activity of recombinant ALDH2 by measuring the conversion of NAD to NADH (seen as increased absorbance at A340 nm). A 1-hour incubation with nitroglycerin (1 μM) inhibited ALDH2 dehydrogenase activity by 85±3% (Fig. 1A). Subsequent treatment with Alda-1 slightly increased ALDH2 activity by 65% (p<0.05 vs. nitroglycerin alone). However, the increase was modest in scale (to 25% of basal ALDH2 activity after Alda-1 treatment). In contrast, co-treatment with nitroglycerin and Alda-1 completely abrogated nitroglycerin-induced ALDH2 inactivation (Fig. 1A).
Fig. 1. Sustained treatment with nitroglycerin inhibited ALDH2 activity.
(A) Effect of nitroglycerin and Alda-1 on ALDH2 activity in vitro. One-hour treatment with nitroglycerin (1 μM) inhibited the dehydrogenase activity of recombinant ALDH2 by 85±3% (p<0.01), as determined by an ALDH2 enzymatic activity assay in vitro. Subsequent addition of Alda-1 (20 μM) resulted in a moderate increase (65±27%) in ALDH2 activity. Co-incubation with nitroglycerin and Alda-1 completely prevented nitroglycerin-induced ALDH2 inactivation. (Top inset) Treatment protocol. Data are mean±SEM (n=3-6). Nitro, nitroglycerin. (B) Effect of nitroglycerin and Alda-1 on mitochondrial ALDH2 activity in vivo. Sustained nitroglycerin treatment (7.2 mg/kg/day) delivered through a transdermal patch resulted in reduced ALDH2 activity (mitochondrial fraction) in control animals after 35 min of LAD occlusion followed by 60 min of reperfusion in Wistar rats in vivo (p<0.05 vs. control). Alda-1 (16 mg/kg/day) delivered continuously through an Alzet pump in the presence of nitroglycerin partially restored ALDH2 activity (p<0.05 nitroglycerin vs. nitroglycerin and Alda-1). (Top inset) Treatment protocol. Data are mean±SEM (n=4). LAD, ligation of the left anterior descending coronary artery
Sustained nitroglycerin treatment inactivates ALDH2 in vivo
We have previously shown that a 16-hour sustained nitroglycerin treatment (7.2 mg/kg/day) in vivo, followed by MI ex vivo (ischemia was induced in isolated rat hearts using a Langendorff apparatus), caused a significant decrease in myocardial ALDH activity (10). This treatment mimicked a clinically relevant dose and duration of nitroglycerin, such as intravenous treatment of acute angina pectoris in an emergency room. We used an in vivo model of MI, in which male Wistar rats were subjected to ischemia-reperfusion by ligating the left anterior descending (LAD) coronary artery in vivo, and showed that nitroglycerin pretreatment reduced ALDH2 activity by 47% (Fig. 1B). As nitroglycerin treatment was continued throughout the course of the MI (ischemia and the subsequent reperfusion), the data suggest that the myocardium had diminished ALDH2 activity at the onset of and during MI. In contrast, sustained, simultaneous co-treatment with Alda-1 (16 mg/kg/day) inhibited the nitroglycerin-induced decrease in ALDH2 activity in the myocardium, suggesting that Alda-1 could protect ALDH2 from nitroglycerin-induced inactivation in vivo (Fig. 1B).
Continuous nitroglycerin treatment increases infarct size
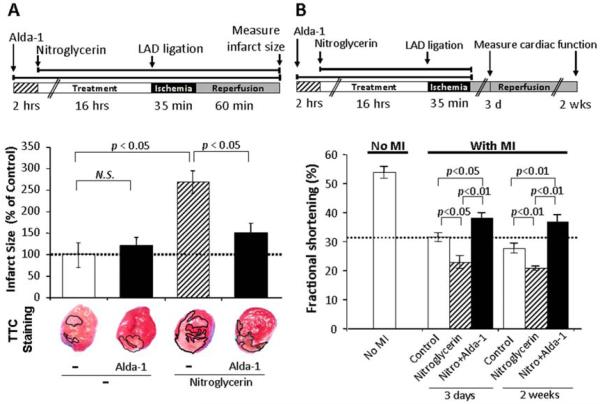
What are the physiological consequences of nitroglycerin-induced inactivation of ALDH2? Using an in vivo MI model in which rats were subjected to 35 minutes of ischemia followed by 60 minutes of reperfusion, we found that 16-hour sustained treatment with nitroglycerin (7.2 mg/kg/day) resulted in an infarct size twice as large as that produced in rats without nitroglycerin treatment (Fig. 2A). Although we have previously shown that Alda-1 alone protects from ischemic injury in vivo, whether Alda-1 also protects from nitroglycerin-induced ischemic injury remained to be determined. Co-administration of Alda-1 decreased the nitroglycerin-induced increase in infarct size by nearly 50%, suggesting that Alda-1 protection of ALDH2 from nitroglycerin-induced inactivation is sufficient to largely prevent the increased MI damage associated with sustained nitroglycerin treatment (Fig. 2A). Nitroglycerin-induced cardiac damage could be mediated by mechanisms that are independent of ALDH2. To rule out this possibility, we cultured primary cardiomyocytes with nitroglycerin for 16 hours (100 μM; Fig.S1A). This treatment increased cell death after hypoxia-reoxygenation, compared to hypoxia-reoxygenation only. In contrast, if cyanamide was included to inhibit ALDH2 (5mM, for 1 hour prior to hypoxia-reoxygenation; causing 60% increased cell death), nitroglycerin no longer increased cell death by hypoxia-reoxygenation (Fig. S1B), as compared to treatment with cyanamide alone. These findings suggest that nitroglycerin-mediated cytotoxicity occurs mainly as a result of ALDH2 inactivation. Furthermore, similar results were obtained with another, structurally unrelated ALDH2 inhibitor, daidzin (1μM; Fig. S1C). However, because cyanamide and daidzin caused a much higher increase in myocyte death than did nitroglycerin, additive effects of nitroglycerin and cyanamide or daidzin treatment should not necessarily be expected. The data suggest that ALDH2 activity is required for Alda-1 induced protection from nitroglycerin.
Fig. 2. Alda-1 prevented nitroglycerin-induced increase in infarct size and restored nitroglycerin-induced decrease in cardiac function after MI in vivo.
(A) Sustained nitroglycerin treatment (7.2 mg/kg/day, 16 hours) delivered through a transdermal patch caused more than a two-fold increase in infarct size after 35 min of LAD occlusion followed by 60 min of reperfusion in Wistar rats in vivo (p<0.05 vs. control). Alda-1 (16 mg/kg/day) delivered continuously through an Alzet pump in the presence of nitroglycerin almost completely abolished the nitroglycerin-induced increase in infarct size (p<0.05 vs. the nitroglycerin-treated group). (Top inset) Treatment protocol. (Lower row) Cross-sections of hearts after MI stained by triphenyltetrazolium chloride (TTC) White area (outlined) corresponds to the infarcted region. Data are mean±SEM (n=6-7). (B) Rats were subjected to the same MI treatment as in (A), followed by 3 days or 2 weeks of reperfusion to determine cardiac function by echocardiography. The group receiving nitroglycerin demonstrated a significantly lower fractional shortening, as determined by echocardiography, at 3 days and 2 weeks after MI (p<0.05 vs. control at 3 days; p<0.01 vs. control at 2 weeks). Co-treatment with Alda-1 prevented the deterioration of cardiac function caused by sustained nitroglycerin treatment (p<0.01 vs. control at 3 days and at 2 weeks). (Top inset) Treatment protocol. Data are mean±SEM (n=5-8).
Nitroglycerin induces vasodilatation and reduction in blood pressure; this may affect injury by myocardial ischemia. As expected, a 2-hour treatment with nitroglycerin (7.2 mg/kg/day) significantly reduced systolic and diastolic blood pressures by approximately 20% and 30%, respectively, demonstrating nitroglycerin’s vasodilating effect (Table S1). However, blood pressure reduction was not observed after the 16-hour sustained nitroglycerin treatment, indicating tolerance to nitroglycerin (Table S2). Therefore, the increase in MI-induced injury after sustained nitroglycerin treatment was not due to hypotension.
Alda-1 treatment prevents nitroglycerin-induced decrease in cardiac function after MI
To confirm the benefit of Alda-1 on nitroglycerin-induced increase in cardiac injury after MI, we next examined cardiac function. Rats receiving sustained nitroglycerin (7.2 mg/kg/day) treatment alone for 16 hours preceding MI demonstrated reduced fractional shortening at 3 days (23±5% vs. 31±4%, for nitroglycerin and control, respectively) and 2 weeks (21±2% vs. 28±5%, respectively) after MI, relative to the control MI group (Fig. 2B). In contrast, co-treatment with Alda-1 (16 mg/kg/day) prior to MI prevented nitroglycerin-induced deterioration of fractional shortening at both time points (Fig. 2B; p<0.01 for both 3 days and 2 weeks). These data corroborate the benefit of Alda-1 in preventing nitroglycerin-induced increases in cardiac injury after MI in vivo.
Alda-1 inhibits nitroglycerin-induced increase in protein carbonylation in ischemic myocardium
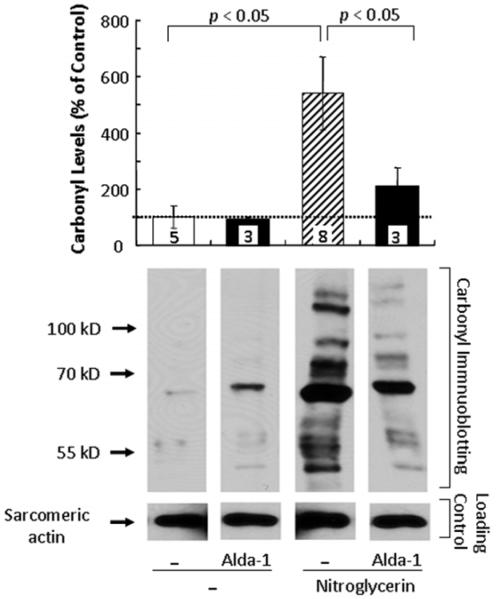
Increased protein carbonylation contributes to ischemic cardiac injury (6). We have suggested that the cardioprotective effect of ALDH2 activation is a result of preservation of protein function by reduction of the aldehydic load in the cell (10). Active ALDH2 metabolizes reactive aldehydes that otherwise accumulate under oxidative stress; this reduces the production of aldehydic adducts (carbonylation) that inactivate cellular enzymes and proteins (5). We measured total protein carbonylation in the infarcted myocardium one hour after MI (see Fig. 2B for protocol). Rats receiving 16-hour sustained nitroglycerin treatment prior to MI had more than five times higher levels of protein carbonylation than did the control MI group (541±130% vs. 100±39%, for nitroglycerin and control, respectively; Fig. 3). Co-treatment with nitroglycerin and Alda-1 drastically reduced the overall protein carbonylation levels (208±69% vs. 541±130%, for combined treatment and nitroglycerin alone, respectively; Fig. 3). Therefore, sustained nitroglycerin treatment exacerbates ischemia-induced accumulation of toxic aldehydic adducts in the myocardium, which may lead to increased cardiac injury. Alda-1 treatment mitigates this nitroglycerin-induced cardiac injury by protecting ALDH2 from inactivation (10) and thus inhibiting the increased aldehydic adduct accumulation associated with sustained nitroglycerin treatment.
Fig. 3. Activation of ALDH2 by Alda-1 inhibited nitroglycerin-induced increase in protein carbonylation in the myocardium after MI in vivo.
Sustained treatment with nitroglycerin (protocol as in Fig. 2A) increased carbonyl levels in rat myocardium by 5 times after 35 min of ischemia and 60 min of reperfusion (p<0.05 vs. control). Co-treatment with Alda-1 prevented the nitroglycerin-induced increase in total carbonyl levels after MI (p<0.05 vs. the nitroglycerin-treated group). Sarcomeric actin was used as the internal loading control. Top histogram shows quantitation of α-carbonyl immunoblots with ImageJ. Data are mean±SEM (n=3-8). Bottom shows a representative Western blot.
An alternative nitrate spares myocardial ALDH2 and cardiac function after MI
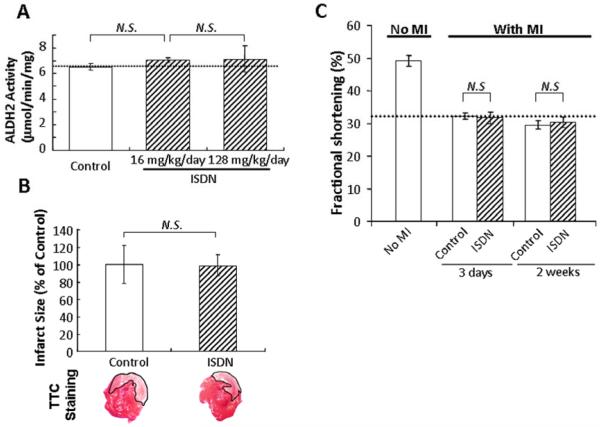
Isosorbide dinitrate (ISDN) is an alternative NO donor often used in a sustained fashion to treat angina (1). We sought to determine the effect of ISDN on ALDH2 activity and MI-induced cardiac injury in vivo. Sustained treatment with ISDN for 16 hours at two doses (16 and 128 mg/kg/day, comparable to and in high excess, respectively, of human doses, taking body surface into account) did not alter ALDH2 activity (Fig. 4A). These data are expected, because NO production from ISDN is independent of ALDH2 activity. Furthermore, a 16-hour sustained ISDN treatment did not affect infarct size induced by transient MI (Fig. 4B) and did not decrease cardiac function at 3 days and 2 weeks after MI (Fig. 4C). The data demonstrate that nitroglycerin, but potentially not other organic nitrates, induces increased cardiac injury after MI.
Fig. 4. Sustained treatment with ISDN did not cause inactivation of ALDH2 and did not induce an increase in cardiac injury after MI in vivo.
(A) Sustained treatment with ISDN (protocol as in Fig. 1B) for 16 hours at two doses (16 or 128 mg/kg/day) did not inhibit ALDH2 activity, as compared to the control group. Data are mean±SEM (n=3). (B) Sustained ISDN treatment (16 mg/kg/day; protocol as in Fig. 2A) did not affect infarct size induced by transient MI, as compared to the control group. (Bottom) Cross-sections of hearts after MI stained by TTC. White area (outlined) corresponds to the infarcted region. Data on infarct size are mean±SEM (n=5). (C) Sustained ISDN treatment (16 hours) prior to MI did not exacerbate cardiac function at 3 days and 2 weeks after MI (protocol as in Fig. 2B). N.S.; non significant difference compared with control rats subjected to MI. Data are mean±SEM (n=4-6).
Discussion
Nearly 10 million Americans suffer from acute coronary syndrome, angina, acute MI, or related illnesses. Because many of these patients are prescribed nitroglycerin for the management of these diseases, any risk associated with the use of nitroglycerin can potentially affect millions of people. Although clinical strategies such as intermittent use of nitroglycerin are effective in minimizing tolerance to the vasodilative effect of the drug, other consequences of nitroglycerin tolerance, such as a risk of increased injury by MI as a result of inactivation of ALDH2, are unclear (1). There have been no double-blinded, placebo-controlled trials examining the long-term safety and efficacy of nitrates. In a retrospective study, Nakamura et al. analyzed records of nearly 3000 patients in North America, Israel and Japan who had recovered from an acute coronary event. Long-term nitrate use (with average follow-up duration of 26 months) was associated with increased mortality rate and a higher risk of cardiac death. However, as noted by the authors, this study was limited by the lack of proper control subjects and a possible tendency towards heavier nitrate use in patients with more severe disease (11).
Here, we have demonstrated that sustained treatment with nitroglycerin resulted in an increase in infarct size and a decrease in fractional shortening after MI in rats. If confirmed in humans, these data suggest that patients receiving continuous nitroglycerin treatment (for example, intravenous administration to manage acute coronary syndrome in an emergency room context) may be at risk for increased cardiac injury if they develop an MI during the nitroglycerin treatment. The benefit of sustained nitroglycerin treatment in various clinical settings should therefore be re-examined. We also demonstrated that inactivation of ALDH2, an enzyme that clears toxic aldehydic adducts in the cell, contributes to nitroglycerin-induced cardiac toxicity. We also demonstrate a targeted means to protect against the deleterious effect of nitroglycerin on the ischemic myocardium. Specifically, the ALDH2 activator Alda-1 inhibited the nitroglycerin-induced increase in infarct size and the associated decrease in cardiac functions after MI. If corroborated in humans, co-administration of an ALDH2 activator such as Alda-1 to patients on chronic nitroglycerin treatment may reduce the potential injury associated with nitroglycerin tolerance.
We chose the length of the sustained treatment (16 hours) to mimic that of human patients hospitalized to receive continuous nitroglycerin infusion after an acute coronary syndrome or MI. As demonstrated in table S2, this sustained treatment induced tolerance to the nitroglycerin-mediated reduction in blood pressure. However, more rapid onset of nitroglycerin tolerance may occur in some patients and the consequence of tolerance in these patients should be examined in the future. In contrast, although acute ISDN treatment (2 hours) caused a reduction in blood pressure similar to that induced by nitroglycerin (table S1B and S2B), sustained ISDN treatment (16 hours) continued to reduce both systolic and diastolic blood pressures by over 10%, indicating that ISDN induced less tolerance to vasodilation than did nitroglycerin after 16 hours of continuous treatment. These data suggest that increased ischemic injury may be associated with greater tolerance to nitrates. Furthermore, unlike nitroglycerin, ISDN did not inhibit ALDH2 activity and did not increase cardiac injury by MI in vivo. Therefore, measurement of ALDH2 activity after sustained treatment with nitroglycerin or other nitrates, in for example peripheral blood cells (12), may predict the cardiac outcome after an ischemic event. If applicable in humans, these differential effects of clinically used organic nitrates on the ischemic myocardium would favor the use of ISDN for chronic treatment.
How does this study fit with other clinical findings? Leesar and colleagues showed that terminating sustained nitroglycerin treatment 24 hours before a coronary angioplasty–induced ischemic event in humans reduced ischemic damage (as measured by ST-segment elevation) by 65% (13). Gori et al. reported opposing effects of short and long treatment with nitroglycerin, respectively, on forearm blood flow after transient limb ischemia in human subjects (14). Similar to the angioplasty study by Leesar et al., Gori et al. demonstrated that a 2-hour treatment with nitroglycerin protected against ischemia-induced impairment in forearm blood flow, if ischemia was induced 24 hours after the nitroglycerin treatment. However, if nitroglycerin was given continuously for 7 days until just before the ischemia was induced, forearm blood flow was impaired relative to the control subjects undergoing limb ischemia without nitroglycerin treatment (15). Together, these and our data demonstrate that sustained treatment with nitroglycerin over many hours up until the ischemic event increases damage induced by oxidative stress and leads to increased tissue injury. In contrast, short-duration nitroglycerin treatment or treatment terminated a period of time before the ischemic event likely activates cytoprotective pathways (13). The implication of these results for patients is that continuous exposure to nitroglycerin may be undesirable, not only because its benefit wears out (tolerance), but more importantly because it reduces the activity of a critical cardioprotective enzyme, ALDH2, and thus exacerbates injury associated with ischemia.
We also examined how sustained nitroglycerin treatment induces ALDH2 inactivation. Since ALDH2 can be inactivated by prolonged treatment with nitroglycerin and other structurally unrelated NO donors, such as ISDN and S-nitrosoglutathione (GSNO) in vitro, ALDH2 inactivation is likely mediated by NO. Furthermore, because ISDN did not inhibit ALDH2 as nitroglycerin did in vivo, we speculate that this NO-mediated ALDH2 inactivation is dependent on access to the ALDH2 catalytic tunnel by NO. Because nitroglycerin is a substrate for and is metabolized by ALDH2, NO generated in the catalytic tunnel may inactivate ALDH2 by interacting with critical amino acids at that site. In contrast, ISDN-derived NO reaches ALDH2 by diffusion, with lower concentrations likely at the ALDH2 catalytic tunnel, compared to those produced by nitroglycerin. This would diminish the probability of NO-mediated ALDH2 inactivation by ISDN and may explain why sustained treatment with ISDN neither inactivated ALDH2 nor increased cardiac injury after MI in vivo.
How does NO inactivate ALDH2? Since ALDH2 inactivation induced by nitroglycerin and ISDN was completely prevented by the reducing agent, dithiolthreitol (DTT) in vitro, we suggest that ALDH2 inhibition was achieved by NO-mediated oxidation, such as SNO formation on cysteines (16) that are critical for the catalytic activity of ALDH2 (e.g., cysteine 302) (17). Previous studies have demonstrated critical roles for cysteines in NO-induced enzyme inactivation (18). Loss of ALDH2 activity as a result of cysteine oxidation is consistent with the established findings that oxidative stress contributes to the development of nitroglycerin tolerance (3, 4). Crystallographic studies of ALDH2–Alda-1 complexes show that Alda-1 reduces access to these cysteines in the catalytic tunnel, suggesting a mechanism by which Alda-1 may protect against nitroglycerin-induced ALDH2 inactivation (17). However, it is formally possible that nitroglycerin affects another site in ALDH2. The exact mechanism needs to be further elucidated.
Alda-1 may slightly inhibit nitroglycerin bioconversion to NO in vitro (19). This is not desirable because of the medical benefit of NO in patients with angina pectoris. However, we found that Alda-1 given concomitantly with nitroglycerin in vivo did not inhibit vasodilatation in rats, indicating that Alda-1 does not inhibit nitroglycerin bioconversion to NO in vivo. Furthermore, we demonstrated that Alda-1 prevented ALDH2 inactivation caused by prolonged treatment with nitroglycerin and two other NO donors, including those that are not bioactivated by ALDH2. Therefore, Alda-1 is unlikely to protect ALDH2 activity and produce cardiac benefit by preventing ALDH2-mediated bioactivation of nitroglycerin. Rather, our data favor the possibility that Alda-1 protects from NO-induced ALDH2 inactivation by preventing S-nitrosylation of the critical cysteines (SNO formation)in the catalytic tunnel of ALDH2.
Finally, we found that sustained nitroglycerin treatment elevated protein carbonylation (toxic aldehydic protein adducts) by more than 5 times in the myocardium after MI, compared to rats with MI alone. As demonstrated by Stamler and colleagues (4), nitroglycerin inactivates ALDH2, a key enzyme that clears these toxic aldehydes. These aldehydic adducts often cause irreversible dysfunction of critical enzymes, such as 3-phosphate dehydrogenase (20) and 20S proteasome complex (21). Nitroglycerin tolerance is associated with various forms of oxidative stress (3), and here we have identified a link between nitroglycerin tolerance and harmful aldehydic protein adducts in the ischemic myocardium. Alternatively, nitroglycerin may build up in mitochondria after ALDH2 inhibition, causing protein oxidation and impairing respiration, directly. The precise mechanism linking nitroglycerin tolerance to increased aldehydic load and cardiac damage remains to be elucidated. We further showed that co-treatment with Alda-1 and nitroglycerin prevented nitroglycerin-induced increases in protein carbonylation, suggesting that activation of ALDH2 protects the myocardium from oxidative stress. Transgenic mice carrying mutant ALDH2*2 (Asian mutation) have higher glutathione levels and are more resistant to oxidative stress than the wild-type mice, likely as a result of compensatory metabolic remodeling (22). This result and our findings suggest that ALDH2 may regulate oxidative stress in the cell through multiple pathways; the mechanisms remain to be further elucidated.
Our studies show that nitroglycerin tolerance in animals exacerbates MI-induced damage to the myocardium. That this finding applies to humans as well is suggested by the fact that an inactivating ALDH2 mutation is found in 40% of the East Asian population (23) and carriers of this mutation have a higher risk for cardiovascular diseases (8, 24). In our study we have shown that Alda-1 rescues the diminished activity of mutant ALDH2 almost to that of wild-type ALDH2 (10), and prevents ALDH2 inactivation by nitroglycerin. In addition to testing the benefit of ALDH2 activators such as Alda-1 in patients with MI, clinical studies reassessing the use of sustained nitroglycerin treatment in patients with cardiovascular diseases generally, and in East Asian patients in particular, are needed.
Methods
In vivo sustained treatment regimen
Animal care and husbandry procedures were in accordance with established institutional and National Institutes of Health guidelines. Nitroglycerin (7.2 mg/kg/day) was delivered in a sustained fashion using a Nitrek transdermal patch (0.2 mg/hr, Bertek Pharmaceuticals) for 16 hours to induce nitroglycerin tolerance in male Wistar rats (250–300g) (10). Continuous infusion of Alda-1 (16 mg/kg/day) was achieved using an Alzet osmotic pump (2001D, Durect, CA) and began 2 hours prior to nitroglycerin treatment and ended after 18 hrs. ISDN (16 or 126 mg/kg/day) was delivered continuously for 16 hours using an Alzet osmotic pump (2001D, Durect, CA). A group implanted with pumps containing the solvent alone (50% polyethylene glycol and 50% dimethyl sulfoxide by volume) served as the control.
In vivo model of left anterior descending coronary artery (LAD) ligation
Male Wistar rats (250-300 g) were anesthetized with 3% isoflurane. Anesthesia was maintained with 1.5% isoflurane. The surgical procedures used for LAD ligation were performed as described (25). Briefly, animals were intubated with a rodent ventilator at 70 breaths/min. Body temperature was maintained at 37°C with an appropriate heating blanket. A ligature was placed around the LAD coronary artery, using a 5-0 polyethylene suture. Coronary occlusion was achieved by tightening a suture against the tube with a clamp, for 35 min. Occlusion was confirmed by immediate blanching of the infarcted area. Reperfusion was achieved by release of the ligature for 60 min. At the end of reperfusion, hearts were excised and infarct size determined by triphenyltetrazolium chloride (TTC) staining (10). In the survival study, a 4-0 vicryl absorbable suture was used to close the soft tissue and nylon suture to stitch the skin. Buprenorphine (0.05 mg/kg) was given subcutaneously every 8 hours for 2 days post-operatively. Fractional shortening was determined at 3 days and 2 weeks post-MI by M-mode echocardiography (GE i13L probe), as described (26).
Enzymatic activity of ALDH2
ALDH2 enzymatic activity was determined by measuring the conversion of NAD+ to NADH at A340 nm, as described (10). The assays were carried out at 25°C in 50 mM sodium pyrophosphate buffer (pH 9.5) in the presence of 10 mM acetaldehyde and 2 μg of recombinant ALDH2 protein. Prior to measurement, the above reaction was incubated with nitroglycerin (1 μM), isosorbide dinitrate (ISDN, 50 μM) or GSNO (40 μM) with or without Alda-1 (20 μM) for 1 hour. To start the reaction, 2.5 mM NAD+ was added and the accumulation of NADH was monitored for 10 min with measurements being taken every 30 sec. During the 10-min measurement, Alda-1 (20 μM) was added to specific groups at 5 min to determine the effect of Alda-1 on ALDH2 after one-hour treatment with nitroglycerin or ISDN. Measurement of mitochondrial ALDH2 activity in the rat myocardium was determined by directly adding 400 μg of the mitochondrial fraction of the myocardium to the reaction mix and reading absorbance at 340 nm for 10 min. Where indicated, dithiothreitol (DTT; 50 mM) was added to the reaction.
Protein Carbonylation
Protein carbonylation levels in the infarcted regions were determined using OxyBlot Protein Oxidation Detection Kit (Millipore), according to the manufacturer’s manual. Bands at different molecular weights were identified that correspond to carbonylated proteins in the myocardium.
Measurement of cell death after hypoxia-reoxygenation, in vitro
In vitro hypoxia-reoxygenation of cultured cardiomyocytes was conducted, as described (27). Briefly, cells were grown to confluence in 24 well plates and the assay was performed in quadruplicate. Cells were washed twice with phosphate-buffered saline, followed by buffer exchange to mimic ischemia and incubated in an anaerobic environment using EZ gas pak pouches (BD Biosciences) for 2.5 hrs at 37°C. The ischemia buffer was then exchanged for normoxic Krebs buffer and cells were incubated for an additional 3 hrs in a humidified, 5% CO2 incubator. Cell death was determined by measuring the release of lactate dehydrogenase (LDH), modified from the procedures previously described (28). LDH activity in the medium and lysate were measured using the CytoTox 96 cytotoxicity assay kit (Promega, WI), according to the manufacturer’s instructions. The plate was read at absorbance λ = 490 nm within 15 min of adding the substrates. In vitro hypoxia-reoxygenation in cultured cardiomyocytes partially mimics in vivo ischemia-reperfusion in rats.
Measurement of blood pressure and heart rate
Systolic and diastolic blood pressures and heart rate were measured under anesthesia (3% isoflurane) by the tail-cuff method (BP-2000, Visitech Systems), as described (29), before and after acute or sustained treatment with nitroglycerin and/or Alda-1, as specified in relevant experiments.
Statistical Analysis
All data are expressed as mean±SEM. Statistical analyses between two groups were performed using the unpaired Student’s t-test. A p-value of <0.05 was considered statistically significant.
Supplementary Material
Funding
This study was supported by National Institute of Health Grant AA11147. J.C.B.F. holds a post-doctoral fellowship from Fundação de Amparo a Pesquisa do Estado de São Paulo - Brasil (FAPESP 2009/03143-1).
Footnotes
Author contributions: L.S. and J.C.B.F. designed and performed all experiments. D.M.R. directed and designed the study. L.S., J.C.B.F. and D.M.R. wrote the manuscript.
Competing interests D.M.R. is the founder of KAI Pharmaceuticals Inc, a company that aims to bring PKC regulators to the clinic. None of the research performed in her laboratory is in collaboration with or supported by the company. The other authors declare that they have no competing interests. A patent has been filed by Stanford University for the therapeutic use of Alda-1 to target ALDH2 and treat myocardial ischemia.
References and Notes
- 1.Parker JD, Parker JO. Nitrate therapy for stable angina pectoris. N Engl J Med. 1998;338:520–531. doi: 10.1056/NEJM199802193380807. [DOI] [PubMed] [Google Scholar]
- 2.Abrams J. Hemodynamic effects of nitroglycerin and long-acting nitrates. Am Heart J. 1985;110:216–224. [PubMed] [Google Scholar]
- 3.Munzel T, Daiber A, Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005;97:618–628. doi: 10.1161/01.RES.0000184694.03262.6d. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Foster MW, Zhang J, Mao L, Rockman HA, Kawamoto T, Kitagawa K, Nakayama KI, Hess DT, Stamler JS. An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation. Proc Natl Acad Sci U S A. 2005;102:12159–12164. doi: 10.1073/pnas.0503723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Lucas DT, Szweda LI. Cardiac reperfusion injury: aging, lipid peroxidation, and mitochondrial dysfunction. Proc Natl Acad Sci U S A. 1998;95:510–514. doi: 10.1073/pnas.95.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantero AV, Portero-Otin M, Ayala V, Auge N, Sanson M, Elbaz M, Thiers JC, Pamplona R, Salvayre R, Negre-Salvayre A. Methylglyoxal induces advanced glycation end product (AGEs) formation and dysfunction of PDGF receptor-beta: implications for diabetic atherosclerosis. Faseb J. 2007;21:3096–3106. doi: 10.1096/fj.06-7536com. [DOI] [PubMed] [Google Scholar]
- 8.Takagi S, Iwai N, Yamauchi R, Kojima S, Yasuno S, Baba T, Terashima M, Tsutsumi Y, Suzuki S, Morii I, Hanai S, Ono K, Baba S, Tomoike H, Kawamura A, Miyazaki S, Nonogi H, Goto Y. Aldehyde dehydrogenase 2 gene is a risk factor for myocardial infarction in Japanese men. Hypertens Res. 2002;25:677–681. doi: 10.1291/hypres.25.677. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama A, Omori T. Genetic polymorphisms of alcohol and aldehyde dehydrogenases and risk for esophageal and head and neck cancers. Jpn J Clin Oncol. 2003;33:111–121. doi: 10.1093/jjco/hyg026. [DOI] [PubMed] [Google Scholar]
- 10.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura Y, Moss AJ, Brown MW, Kinoshita M, Kawai C, Multicenter Myocardial Ischemia Research Group Long-term nitrate use may be deleterious in ischemic heart disease: A study using the databases from two large-scale postinfarction studies. Am Heart J. 1999;138:577–585. doi: 10.1016/s0002-8703(99)70163-8. [DOI] [PubMed] [Google Scholar]
- 12.Wenzel P, Schulz E, Gori T, Ostad MA, Mathner F, Schildknecht S, Gobel S, Oelze M, Stalleicken D, Warnholtz A, Munzel T, Daiber A. Monitoring white blood cell mitochondrial aldehyde dehydrogenase activity: implications for nitrate therapy in humans. J Pharmacol Exp Ther. 2009;330:63–71. doi: 10.1124/jpet.108.149716. [DOI] [PubMed] [Google Scholar]
- 13.Leesar MA, Stoddard MF, Dawn B, Jasti VG, Masden R, Bolli R. Delayed preconditioning-mimetic action of nitroglycerin in patients undergoing coronary angioplasty. Circulation. 2001;103:2935–2941. doi: 10.1161/01.cir.103.24.2935. [DOI] [PubMed] [Google Scholar]
- 14.Gori T, Dragoni S, Di Stolfo G, Sicuro S, Liuni A, Luca MC, Thomas G, Oelze M, Daiber A, Parker JD. Tolerance to nitroglycerin-induced preconditioning of the endothelium: a human in vivo study. Am J Physiol Heart Circ Physiol. 2010;298:H340–345. doi: 10.1152/ajpheart.01324.2008. [DOI] [PubMed] [Google Scholar]
- 15.Gori T, Dragoni S, Di Stolfo G, Sicuro S, Liuni A, Luca MC, Thomas G, Oelze M, Daiber A, Parker JD. Tolerance to nitroglycerin-induced preconditioning of the endothelium: a human in vivo study. Am J Physiol Heart Circ Physiol. 298:H340–345. doi: 10.1152/ajpheart.01324.2008. [DOI] [PubMed] [Google Scholar]
- 16.Moon KH, Kim BJ, Song BJ. Inhibition of mitochondrial aldehyde dehydrogenase by nitric oxide-mediated S-nitrosylation. FEBS Lett. 2005;579:6115–6120. doi: 10.1016/j.febslet.2005.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Miller S, Younus H, Vanam R, Chen CH, Mochly-Rosen D, Hurley TD. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nat Struct Mol Biol. 17:159–164. doi: 10.1038/nsmb.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beretta M, Gorren AC, Wenzl MV, Weis R, Russwurm M, Koesling D, Schmidt K, Mayer B. Characterization of the East Asian variant of aldehyde dehydrogenase-2: bioactivation of nitroglycerin and effects of Alda-1. J Biol Chem. 285:943–952. doi: 10.1074/jbc.M109.014548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida K, Stadtman ER. Covalent attachment of 4-hydroxynonenal to glyceraldehyde-3-phosphate dehydrogenase. A possible involvement of intra- and intermolecular cross-linking reaction. J Biol Chem. 1993;268:6388–6393. [PubMed] [Google Scholar]
- 21.Ferrington DA, Kapphahn RJ. Catalytic site-specific inhibition of the 20S proteasome by 4-hydroxynonenal. FEBS Lett. 2004;578:217–223. doi: 10.1016/j.febslet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Endo J, Sano M, Katayama T, Hishiki T, Shinmura K, Morizane S, Matsuhashi T, Katsumata Y, Zhang Y, Ito H, Nagahata Y, Marchitti S, Nishimaki K, Wolf AM, Nakanishi H, Hattori F, Vasiliou V, Adachi T, Ohsawa I, Taguchi R, Hirabayashi Y, Ohta S, Suematsu M, Ogawa S, Fukuda K. Metabolic remodeling induced by mitochondrial aldehyde stress stimulates tolerance to oxidative stress in the heart. Circ Res. 2009;105:1118–1127. doi: 10.1161/CIRCRESAHA.109.206607. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida A, Huang IY, Ikawa M. Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals. Proc Natl Acad Sci U S A. 1984;81:258–261. doi: 10.1073/pnas.81.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, Tay WT, Chen CH, Zhang Y, Yamamoto K, Katsuya T, Yokota M, Kim YJ, Ong RT, Nabika T, Gu D, Chang LC, Kokubo Y, Huang W, Ohnaka K, Yamori Y, Nakashima E, Jaquish CE, Lee JY, Seielstad M, Isono M, Hixson JE, Chen YT, Miki T, Zhou X, Sugiyama T, Jeon JP, Liu JJ, Takayanagi R, Kim SS, Aung T, Sung YJ, Zhang X, Wong TY, Han BG, Kobayashi S, Ogihara T, Zhu D, Iwai N, Wu JY, Teo YY, Tai ES, Cho YS, He J. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet. 2011;43:531–538. doi: 10.1038/ng.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Churchill EN, Disatnik MH, Mochly-Rosen D. Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of varepsilonPKC and activation of aldehyde dehydrogenase 2. J Mol Cell Cardiol. 2009;46:278–284. doi: 10.1016/j.yjmcc.2008.09.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Tan J, Wang Y, Meldrum KK, Dinarello CA, Meldrum DR. IL-18 binding protein-expressing mesenchymal stem cells improve myocardial protection after ischemia or infarction. Proc Natl Acad Sci U S A. 2009;106:17499–17504. doi: 10.1073/pnas.0908924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 28.Tang YQ, Jaganath IB, Sekaran SD. Phyllanthus spp. induces selective growth inhibition of PC-3 and MeWo human cancer cells through modulation of cell cycle and induction of apoptosis. PLoS One. 5:e12644. doi: 10.1371/journal.pone.0012644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inagaki K, Koyanagi T, Berry NC, Sun L, Mochly-Rosen D. Pharmacological inhibition of epsilon-protein kinase C attenuates cardiac fibrosis and dysfunction in hypertension-induced heart failure. Hypertension. 2008;51:1565–1569. doi: 10.1161/HYPERTENSIONAHA.107.109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.