Abstract
Upon activation by NGF, TrkA is internalized, trafficked and sorted through different endosomal compartments. Proper TrkA trafficking and sorting are crucial events since alteration of these processes hinders NGF-mediated functions. However, it is not fully known which proteins are involved in the trafficking and sorting of TrkA. Here we report that Nedd4-2 regulates the trafficking of TrkA and NGF functions in sensory neurons. Depletion of Nedd4-2 disrupts the correct sorting of activated TrkA at the early and late endosome stage, resulting in an accumulation of TrkA in these compartments and, as a result of the reduced trafficking to the degradative pathway, TrkA is either reverted to the cell surface through the recycling pathway or retrogradely transported to the cell body. In addition, Nedd4-2 depletion enhances TrkA signaling and the survival of NGF-dependent DRG neurons, but not those of BDNF-dependent neurons. Furthermore, neurons from a knock-in mouse expressing a TrkA mutant that does not bind Nedd4-2 protein exhibits increased NGF-mediated signaling and cell survival. Our data indicate that TrkA trafficking and sorting are regulated by Nedd4-2 protein.
Keywords: Nedd4-2, neurons, NGF, trafficking, TrkA
Introduction
Neurotrophins are growth factors involved in different functions in the nervous system, including survival, proliferation, differentiation, axonal growth and synaptic plasticity. All neurotrophins, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4), exert their functions by binding to two different receptors; namely the neurotrophin receptors Trk, which belong to the receptor tyrosine kinase (RTK) superfamily, and the neurotrophin receptor p75, which belongs to the tumor necrosis factor receptor superfamily. All neurotrophins bind to p75 with similar affinity, whereas there is preferential binding to specific Trk receptors: NGF to TrkA, BDNF and NT-4 to TrkB and NT-3 to TrkC (1, 2).
The trafficking and sorting of activated RTKs is a complex and highly regulated process that is seminal for the regulation of the specificity and duration of RTK-generated responses (3–7). The first stage of RTKs upon internalization in response to ligand binding is the early endosome, where their cargo can follow different fates; receptors that are mainly recycled back to the plasma membrane (i.e. transferring receptor) are sent to recycling endosomes, whereas those destined mainly for degradation (i.e. the EGF receptor) are sorted to late endosomes and then to lysosomes (5). The trafficking of Trks is critical for neurotrophin-mediated functions. Trk proteins represent a class of RTKs that are both recycled and degraded upon neurotrophin-induced internalization (8–10). However, different Trks are differentially regulated; whereas TrkA is mainly recycled, TrkB is predominantly degraded (11, 12). In contrast to other RTKs, Trks also undergo retrograde transport from the tip of the axon to the soma, TrkA being the most widely studied neurotrophin receptor (13–19). Retrogradely transported TrkA modulates the survival of NGF-dependent neurons (13–19). While the trafficking of activated TrkA upon NGF binding needs to be tightly regulated, little is known about the molecular machinery involved in the trafficking and sorting of activated TrkA.
The ubiquitination of RTKs has emerged as a critical event for appropriate receptor trafficking and degradation (20). Ubiquitination is a reversible protein modification that is achieved by two opposite families of enzymes: E3 ubiquitin ligases, which add the ubiquitin protein to other proteins, and deubiquitinases (DUBs), which remove the ubiquitin (21). Three independent groups have reported the ubiquitination of Trk proteins (22–24). We have previously reported that TrkA, but not TrkB, binds through a PPXY motif and is ubiquitinated by Nedd4-2 (22), an E3 ubiquitin ligase that belongs to the family of Nedd4 ubiqutin E3 ligases (25). Overexpression of Nedd4-2 in heterologous cells leads specifically to the down-regulation of TrkA protein without altering the internalization rate of the receptor (22). Additional support for our findings comes from an unbiased study using protein microarrays that identified TrkA as a binding partner of Nedd4-2 (26). Although it is clear that Nedd4-2 regulates the levels of TrkA, the mechanism by which Nedd4-2 exerts its function is unknown.
Here we report a new function for Nedd4-2 protein. In addition to modulating TrkA levels (22), Nedd4-2 regulates the trafficking of TrkA protein in response to NGF in dorsal root ganglion (DRG) neurons. We observed that Nedd4-2 depletion delays the arrival of active TrkA to late endosomes, but not to early endosomes, with a subsequent increase in the recycling and retrograde transport of TrkA. As a result, Nedd4-2 affects TrkA signaling and the survival of NGF-dependent sensory neurons; this is a specific effect because Nedd4-2 depletion does not alter the survival of BDNF-dependent DRG neurons. Furthermore, we have generated a knock-in mouse that expresses a TrkA mutant protein that does not bind Nedd4-2. Sensory neurons obtained from this knock-in show increased NGF-mediated signaling and survival. Our data identify Nedd4-2 as a critical protein in the trafficking of TrkA and, therefore, in NGF-mediated functions.
Results
Nedd4-2 regulates TrkA receptor levels
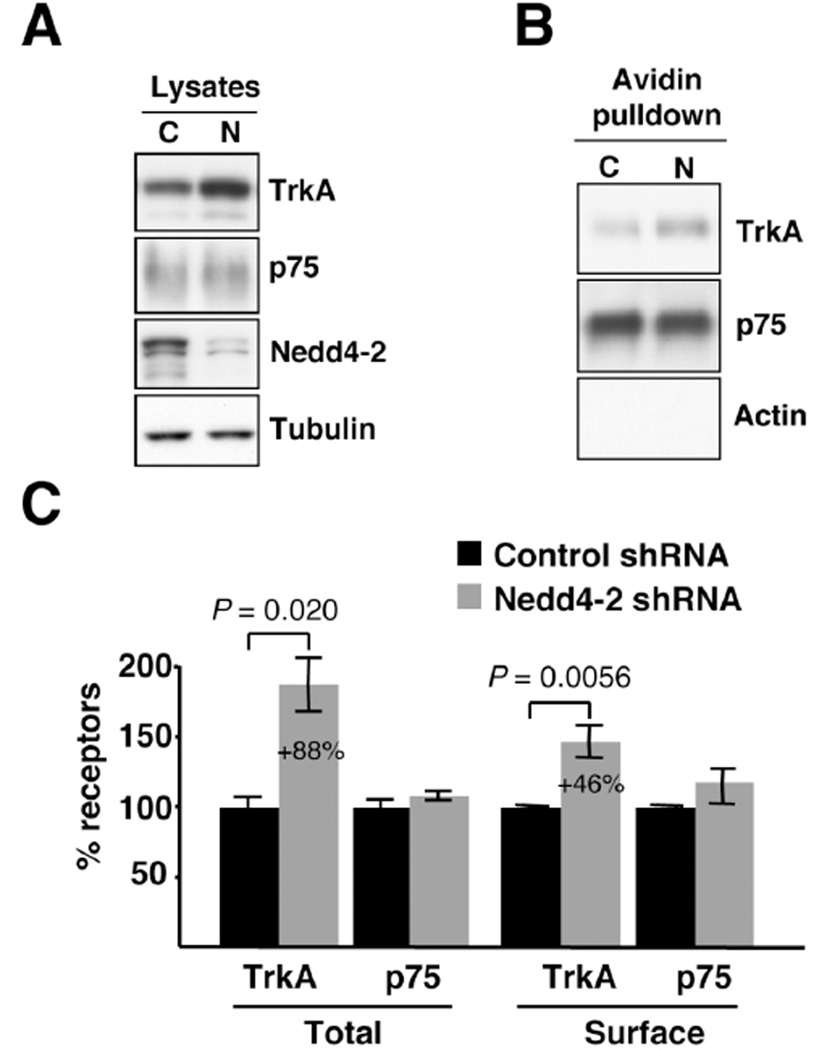
To address whether the endogenous expression of Nedd4-2 modulates the levels of TrkA, we infected DRG neurons with lentivirus expressing specific shRNA to deplete Nedd4-2. Most DRG neurons express TrkA during development and depend on NGF for their survival (27), suggesting that these neurons offer an excellent model to study NGF and its receptor TrkA in vitro. Accordingly, we obtained DRG neurons from E15.5 rat embryos and cultured them in the presence of NGF (50 ng/ml) to select TrkA-expressing neurons. Then, the neurons were infected with control and Nedd4-2 shRNA lentivirus at day in vitro (DIV) 4 to modulate Nedd4-2 protein levels. The rate of infection to perform all the biochemical experiments reported here was at least 90%, as seen from GFP expression (Supplementary Figure S1). Cell lysates from infected neurons were subjected to western blot analysis to assess Nedd4-2 protein levels. Infection with Nedd4-2 shRNA lentivirus rendered a dramatic decrease in the levels of endogenous Nedd4-2 protein (Fig. 1A). Concomitantly to Nedd4-2 depletion, the amount of total TrkA was increased, whereas p75 levels were not affected (Fig. 1A). To assess whether Nedd4-2 depletion might influence the surface expression of NGF receptors, we performed biotinylation experiments. Surface proteins from infected DRG neurons were labeled with biotin and pulled-down with Neutroavidin beads. Surface TrkA levels, but not surface p75 levels, were increased following Nedd4-2 depletion (Fig. 1B). Quantification of the data corresponding to the different experiments indicated an 88% increase in total TrkA levels and a 46% increase in surface TrkA levels in Nedd4-2-depleted neurons as compared to control neurons (Fig. 1C). No significant differences in p75 levels were observed. These results suggest that endogenous Nedd4-2 in sensory neurons regulates the amount of total and surface-bound TrkA, but not p75.
Figure 1. Nedd4-2 depletion increases TrkA levels.
(A) Increased levels of TrkA upon Nedd4-2 depletion. Cultured DRG neurons were infected on DIV4 with control or Nedd4-2 shRNA lentivirus and extracts were obtained on DIV10 to analyze the expression of different proteins using western blot analysis. Tubulin was used as a loading control. Representative western blots are shown. C: Control shRNA lentivirus; N: Nedd4-2 shRNA lentivirus.
(B) Enhanced surface TrkA in Nedd4-2-depleted neurons. Cell surface proteins from NGF-starved DRG neurons infected with control and Nedd4-2 shRNA lentivirus were labeled with biotin and the same amount of proteins was pulled down with Neutroavidin. Western blot analyses were performed with TrkA and p75 antibodies. Representative western blots are shown. C: Control shRNA lentivirus; N: Nedd4-2 shRNA lentivirus.
(C) Quantification of TrkA and p75 proteins in response to Nedd4-2 depletion. Western blots were scanned and quantified using ImageJ software. Data are presented as means ± s.e.m. P values were calculated using a two-tailed Student’s t-test (n = 6–8).
Nedd4-2 modulates the degradation of TrkA in response to NGF
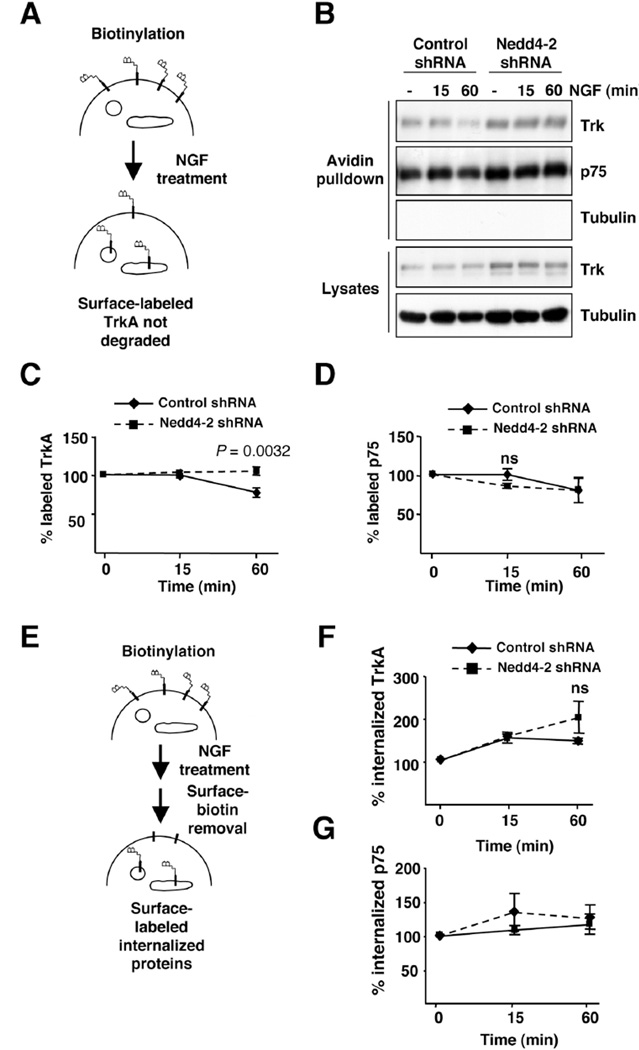
Upon activation, TrkA receptors are ubiquitinated and terminated by degradation (22–24). To address whether Nedd4-2 modulates the degradation rate of TrkA, we performed degradation assays in response to NGF using biotinylation. NGF-dependent DRG neurons infected with control and Nedd4-2 shRNA lentivirus were NGF-starved overnight in the presence of the anti-apoptotic inhibitor Z-VAD-FMK, and then the cell surface proteins were biotinylated, followed by NGF stimulation (50 ng/ml), as described in Material and Methods and outlined in Fig. 2A. After 1 hour of NGF treatment, there was a clear decrease in the amount of biotin-labeled TrkA in the control neurons as a result of TrkA degradation (Fig. 2B). However, the amount of biotinylated TrkA receptors from Nedd4-2-depleted neurons was not altered (Fig 2B). Quantification of multiple experiments indicated no significant degradation of surface TrkA in Nedd4-2-depleted neurons upon NGF stimulation for 1 hour, whereas a 30% reduction in surface TrkA was noted in the control cells (Fig. 2C). The levels of biotinylated TrkA were similar in the control and Nedd4-2 shRNA neurons after 15 minutes of NGF treatment (Fig. 2B,C). No differences were detected in the degradation rate of p75 in response to NGF from the control and Nedd4-2 depleted neurons (Fig. 2B,D). Therefore, Nedd4-2 levels modulate the degradation rate of TrkA in response to NGF.
Figure 2. Nedd4-2 regulates TrkA degradation.
(A) A receptor degradation assay was performed as described in Material and Methods and is depicted in the schematic diagram. Infected DRG neurons were biotinylated to label surface proteins and treated or not with NGF (50 ng/ml) for 15 and 60 minutes. An aliquot of the cell lysates was used as loading control and the biotinylated proteins were precipitated with Neutroavidin-agarose. Surface proteins were subsequently subjected to western blot analysis with Trk, p75 and tubulin antibodies.
(B) Representative western blots are shown. Tubulin was used as a negative control for biotinylated proteins and as a loading control for lysates.
(C) Quantification of surface-labeled TrkA. Data are normalized to the amount of biotinylated TrkA in control and in Nedd4-2-depleted neurons without NGF treatment. Data are presented as means ± s.e.m. P values were calculated using two-tailed Student’s t-test (n = 4).
(D) Quantification of surface-labeled p75. Data are normalized to the amount of biotinylated p75 in control and in Nedd4-2-depleted neurons without NGF treatment. Data are presented as means ± s.e.m. (n = 4); ns, not significant.
(E) A schematic diagram of the internalization assay using biotinylation procedure is shown. DRG neurons were infected with control and Nedd4-2 shRNA lentivirus. Cells were biotinylated as described in Experimental Procedures and then treated with NGF (50 ng/ml) for 15 and 60 minutes. Biotin from non-internalized proteins was removed using reducing conditions as described in Material and Methods. Cell lysates were prepared, surface proteins were subjected to precipitation with Neutroavidin and western blot analysis were performed with the corresponding antibodies. Tubulin was used as a negative control for biotinylated proteins and as a loading control for lysates.
(F) Quantification of internalized TrkA receptor. Data are presented as average ± s.e.m. (n = 3); ns, no significant.
(G) Quantification of internalized p75 receptor. Data are presented as average ± s.e.m. (n = 3).
The degradation of TrkA protein in response to NGF requires the internalization and trafficking of the receptor to the lysosomes. To assess whether Nedd4-2 protein modulates the internalization rate of TrkA in response to NGF, we performed internalization assays in infected DRG neurons using biotinylation. Neurons were NGF-starved as described above and cell surface biotinylation was performed using cleavable biotin, as outlined in Figure 2E. NGF (50 ng/ml) was then applied for 15 or 60 minutes and reducing conditions were applied to strip the biotin from surface proteins that had not been internalized. This treatment allows the detection of internalized biotinylated proteins, which remained protected from cleavage, upon pulling them down using Neutroavidin beads. We did not observe any significant differences in the rate of internalization of TrkA or p75 in the control and Nedd4-2 depleted cells (Fig. 2F,G). Thus, these data suggest that Nedd4-2 protein does not regulate the internalization rate of TrkA in response to NGF. These results are consistent with previous observations regarding the lack of differences in the internalization rate of wild-type TrkA and TrkAP782S, a mutant receptor that does not bind Nedd4-2 (22).
TrkA trafficking at early and late endosomes is regulated by Nedd4-2
To address whether Nedd4-2 regulates TrkA trafficking, we performed immunofluorescence analysis to detect the localization of activated TrkA upon depletion of Nedd4-2 in sensory neurons. First, we identified the localization of Nedd4-2 in neurons using a previously described antibody (22). Staining of NGF-dependent DRG neurons indicated a strong co-localization of Nedd4-2 with the early endosomal markers EEA1 and Rab5 and with the late endosomal marker Rab7 (Fig. 3A). This localization resembles the localization of Itch, another member of the Nedd4 family (28, 29). The specificity of Nedd4-2 antibody was confirmed using Nedd4-2-depleted neurons, which showed almost no signal from the Nedd4-2 antibody as compared with the control neurons (Supplementary Figure S2). These data indicate that Nedd4-2 is located in early and late endosomes.
Figure 3. Upon NGF treatment pTrkA localization is regulated by Nedd4-2.
(A) Co-localization of Nedd4-2 with early endosomes (EEA1 and Rab5) and late endosomes (Rab7). Scale bar, 20 µm.
(B) Co-localization of pTrkA with Rab5 compartments upon NGF treatment in control and in Nedd4-2 shRNA-infected DRG neurons. Infected DRG neurons were identified by GFP expression (not shown) as described in Supplementary Figure S1. Immunofluorescence was performed as described in Material and Methods. Images were taken with a confocal microscope. Scale bar, 20 µm.
(C) Quantification of pTrkA co-localization in Rab5 endosomes. Images were processed using ImageJ and the percentage of co-localization was normalized to the surface levels of TrkA in control and in Nedd4-2-depleted neurons in the absence of NGF. Data are presented as means ± s.e.m. (n = 6–9 neurons/time point).
(D) Co-localization of pTrkA with Rab7 compartments upon NGF treatment in control and in Nedd4-2 shRNA-infected DRG neurons was performed as described in Fig. 3B. Scale bar, 20 µm.
(E) Quantification of pTrkA co-localization in Rab7 endosomes was performed as described in Fig. 3C. Data are presented as means ± s.e.m. P values were calculated using a two-tailed Student’s t-test (n = 5–7 neurons/time point).
(F) Immunoprecipitation of Rab5 and Rab7 endomembranes. Infected DRG neurons were treated with NGF for 10 or 30 min and lysates were immunoprecipitated with Rab5 or Rab7 antibodies, respectively. Representative blots are shown (n = 3). C: Control shRNA lentivirus; N: Nedd4-2 shRNA lentivirus.
(G) Reduced ubiquitination of activated TrkA in endosomal compartments. Infected DRG neurons were homogenized, immunoprecipitated with Rab5 antibodies and, after solubilization, immunoprecipitated with TrkA antibodies. Representative blots are shown (n = 2). C: Control shRNA lentivirus; N: Nedd4-2 shRNA lentivirus.
To address whether Nedd4-2 modulates the location of activated TrkA, we performed stainings with antibodies against phosphorylated TrkA (22, 30) and different endosomal markers. Infected NGF-dependent neurons were starved as described above, stimulated with NGF (50 ng/ml) for different times, and stained. Upon NGF treatment, internalized TrkA co-localized with Rab5 in both the control and Nedd4-2-depleted cells (Fig. 3B). No staining with pTrkA antibody was observed in the absence of NGF (data not shown). To quantify the co-localization, we used a custom program, as described in Material and Methods, and the data corresponding to the surface levels of TrkA at steady-state in control and Nedd4-2 depleted neurons were normalized. No significant differences in the co-localization of pTrkA with Rab5 were observed between the control and Nedd4-2-depleted neurons at 5 or 30 min after normalization (Fig. 3C). Therefore, these results indicate that depletion of Nedd4-2 does not alter the arrival of activated TrkA at early endosomes, in support of previous data indicating that Nedd4-2 does not modify the internalization of TrkA in response to NGF.
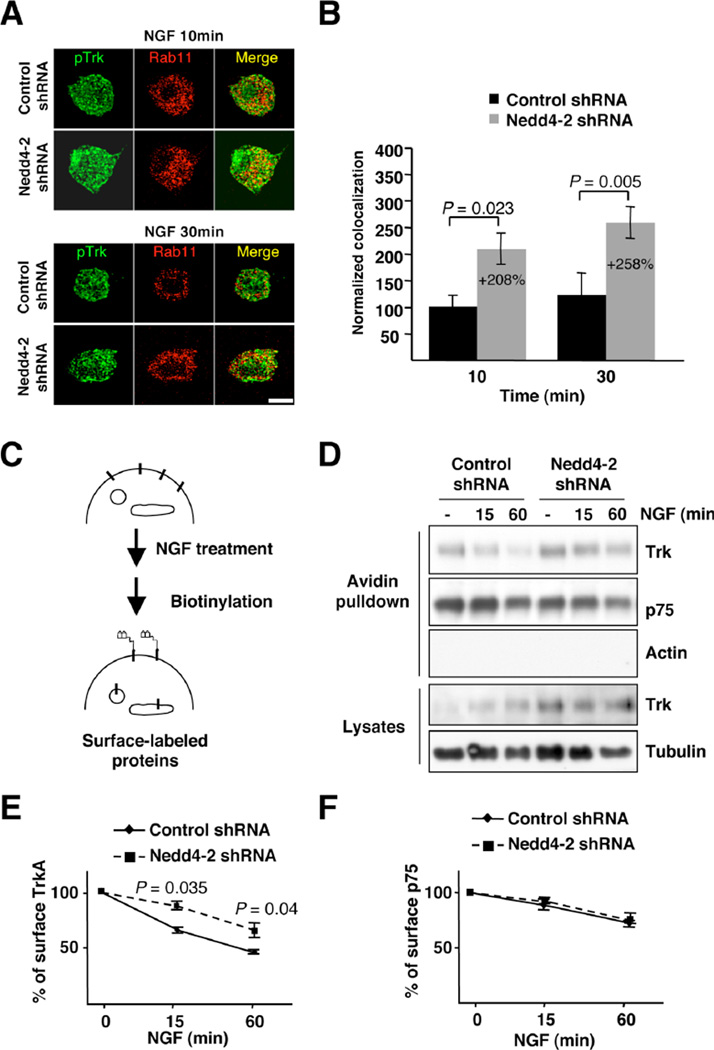
In addition, we performed immunofluorescence studies of activated TrkA with the late endosomal marker Rab7 in response to Nedd4-2 depletion (Fig. 3D). Our data normalized as stated above indicated that after 10 min of NGF treatment there was a 40% decrease in the amount of pTrkA present in the late endosomes of Nedd4-2-depleted cells (Fig. 3E). However, at later time points along NGF treatment (30 min) there was a massive accumulation of pTrkA receptors in late endosomes from Nedd4-2-depleted neurons as compared with the control neurons (300% increase) (Fig. 3E). Accordingly, the sorting of activated TrkA between early and late endosmes and between late endosomes and lysosomes seems to be regulated by Nedd4-2. These data were further confirmed using a biochemical approach. Neurons infected with control and Nedd4-2 shRNA lentivirus were NGF-starved overnight, followed by stimulation with NGF (50 ng/ml) for 10 or 30 min, and the cells were homogenized without detergent to obtain crude membranes. Rab5 and Rab7 antibodies were used to immunoprecipitate the same amount of endomembranes containing these two proteins from control and Nedd4-2-depleted neurons treated with NGF for 10 min (Rab5 immunoprecipitation) or for 30 min (Rab7 immunoprecipitation) and the amount of TrkA protein in the immunoprecipitated membranes was assessed. An increase in TrkA in Rab5-and Rab7-endomembranes from Nedd4-2 depleted neurons as compared to the control neurons was observed after NGF treatment for 10 and 30 min, respectively (Fig. 3F). Taken together, the co-localization and the biochemical experiments suggest that Nedd4-2 regulates the trafficking of activated TrkA at the level of early endosomes and late endosomes.
To address whether Nedd4-2 depletion altered specifically the ubiquitination status of TrkA in early endosomes, we performed immunoprecipitation of activated TrkA from Rab5-immunoprecipitated endomembranes. Western blots using ubiquitin antibodies indicated that TrkA ubiquitination was reduced in Nedd4-2 depleted neurons (Fig. 3G). Therefore, these data indicate that Nedd4-2 ubiquitinates TrkA at the early endosomes supporting further the role of Nedd4-2 protein in these compartments.
Increased TrkA recycling to the plasma membrane by Nedd4-2 depletion
Activated TrkA that reaches early endosomes can also recycle to the plasma membrane via recycling endosomes (31). To address whether Nedd4-2 modulates the recycling of activated receptors to the plasma membrane, we performed immunofluorescence analysis in DRG neurons using the recycling endosomal marker Rab11. Our data suggested that there might be more co-localization of pTrkA with recycling endosomes in Nedd4-2-depleted neurons than in control neurons (Fig. 4A). Indeed, quantification analyses of different cells indicated that in Nedd4-2-depleted neurons there was an increased co-localization of pTrkA with Rab11 as compared to control neurons (208% at 10 min and 258% at 30 min) (Fig. 4B). These data suggest that more TrkA protein recycles to the cell surface when Nedd4-2 is reduced. Further confirmation for this hypothesis came from biotinylation experiments aimed at detecting the amount of TrkA at the cell surface at different time points upon NGF treatment, as outlined in Fig. 4C. Upon NGF treatment (50 ng/ml) we observed a reduction over time in the amount of surface TrkA in control cells (Fig. 4D,E). However, in Nedd4-2-depleted neurons this reduction in TrkA surface levels in response to NGF stimulation was attenuated (Fig. 4D,E), suggesting that TrkA recycling may be enhanced. In contrast, the reduction in p75 from the cell surface in response to NGF was not affected by Nedd4-2 depletion (Fig. 4D,F). Therefore, it may be surmised that TrkA recycles more and is increased in the plasma membrane when Nedd4-2 is depleted, further supporting a potential role for Nedd4-2 in TrkA sorting at early endosomes.
Figure 4. Enhanced pTrkA recycling and surface expression of TrkA in Nedd4-2-depleted DRG neurons.
(A) Co-localization of pTrkA with Rab11 compartments upon NGF treatment in control and in Nedd4-2 shRNA infected DRG neurons was performed as described in Fig. 3B. Scale bar, 20 µm.
(B) Quantification of pTrkA co-localization in Rab11 endosomes was done as described in Fig. 3C. Data are presented as means ± s.e.m. P values were calculated using a two-tailed Student’s t-test (n = 5–7 neurons/time point).
(C) A schematic diagram of the surface expression assay using the biotinylation procedure in response to NGF is shown. DRG neurons were infected with control and Nedd4-2 shRNA lentivirus. Cells were treated with NGF (50 ng/ml) for 15 and 60 minutes and then biotinylated as described in Material and Methods. Cell lysates were prepared; surface proteins were subjected to precipitation with neutroavidin-agarose, and western blot analyses were performed with the corresponding antibodies.
(D) Representative western blots are shown. Tubulin and actin were used as a loading control for lysates and as a negative control for biotinylated proteins, respectively.
(E) Quantification of surface TrkA upon NGF treatment. Data are presented as means ± s.e.m. P values were calculated using a two-tailed Student’s t-test (n = 3).
(F) Quantification of surface p75 upon NGF treatment. Data are presented as means ± s.e.m. (n = 3).
Nedd4-2 depletion enhances the retrograde transport of TrkA in DRG neurons
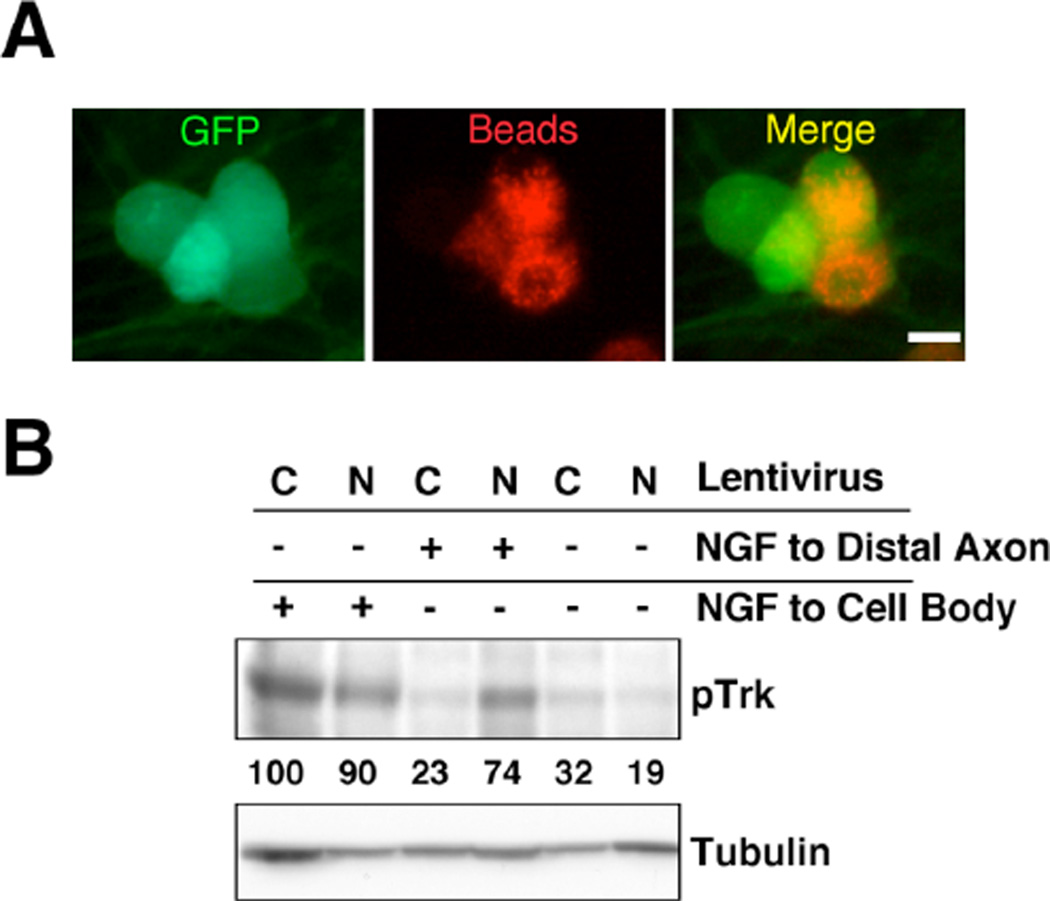
An additional fate of internalized TrkA protein that escapes degradation is retrograde transport to the cell body (19). To address whether the depletion of Nedd4-2 affected the retrograde transport of activated TrkA, we set up compartmentalized NGF-dependent DRG cultures using microfluidic compartments. The rate of retrograde transport in neurons was monitored by the uptake and retrograde transport of red fluorescent beads by healthy neurons projecting axons to the beads-containing distal compartment (Fig. 5A). These beads do not bind to TrkA protein. DRG neurons were infected with lentivirus expressing control or Nedd4-2 shRNA on DIV4, NGF-starved overnight at DIV7, and then stimulated with NGF (50 ng/ml) at the distal axons for 15 hours. Nedd4-2 depletion resulted in an increased amount of activated TrkA in the cell body compartment as compared with control neurons (Fig. 5B). Therefore, the retrograde transport of activated TrkA is also affected by Nedd4-2 protein.
Figure 5. Enhanced NGF-mediated retrograde transport of pTrkA in Nedd4-2-depleted DRG neurons.
(A) DRG neurons growing in microfluidic chambers. Retrograde transport in infected neurons (GFP-positive) was monitored by the presence of red fluorescence in the cell body compartment. Red-fluorescent beads were added to the distal compartment 15 hours before subsequent experiments were performed. Scale bar, 20 µm.
(B) Increased retrograde transport of pTrkA upon Nedd4-2 depletion. Neurons were infected with control or Nedd4-2 shRNA lentivirus and either not stimulated or stimulated with NGF for 10 min in the cell body compartment or for 15 hours in the distal compartment. Lysates were analyzed by western blot with pTrkA antibodies. Tubulin was used as a loading control. Numbers represent pTrk intensity normalized with respect to tubulin loading. C: Control shRNA lentivirus; N: Nedd4-2 shRNA lentivirus.
Nedd4-2 depletion increases TrkA signaling and the survival of NGF-dependent DRG neurons
What are the functional consequences of Nedd4-2 depletion on DRG neurons? To address whether TrkA-mediated signaling was affected by Nedd4-2 expression, we infected NGF-dependent DRG neurons with control and Nedd4-2 shRNA lentivirus and performed western blot analysis in response to NGF. Infected neurons were NGF-starved overnight in the presence of the anti-apoptotic inhibitor Z-VAD-FMK, after which NGF (50 ng/ml) was applied for different times. Cell lysates were collected and analyzed by western blot using antibodies that recognized activated forms of TrkA, PLCγ, Akt and MAPK. Our results indicate that the depletion of Nedd4-2 resulted in an enhancement of the phosphorylation of TrkA and, subsequently, the PLCγ, Akt and MAPK signaling pathways (Fig. 6A). Thus, these data suggested that Nedd4-2 regulates NGF-mediated signaling.
Figure 6. Nedd4-2 modulates NGF-mediated signaling and cell survival.
(A) Nedd4-2 depletion enhances NGF-mediated signaling. DRG neurons were infected with control and Nedd4-2 shRNA lentivirus, and cell extracts were analyzed upon NGF treatment. Active TrkA, PLCγ, MAPK and Akt were assessed using antibodies that recognized specific phosphorylated residues. Representative blots are shown (n = 5). C: Control shRNA lentivirus; N: Nedd4-2 shRNA lentivirus.
(B) Images showing NGF-dependent DRG neurons transfected with control and Nedd4-2 shRNA plasmids. Transfected cells were visualized by GFP expression, and non-apoptotic cells (arrowheads) and apoptotic cells (arrows) were identified by staining with Hoechst3342. Transfected neurons in the Hoechst3342 panel are indicated in red. Scale bar, 20 µm.
(C) Quantification of apoptosis in NGF- and BDNF-dependent neurons transfected with control and Nedd4-2 shRNA plasmids upon withdrawal of the corresponding neurotrophin for 72–120h. Data are presented as means ± s.e.m. P values were calculated using a two-tailed Student’s t-test [n = number of cells from 6 (NGF) and 3 (BDNF) independent experiments].
DRG neurons depend on neurotrophins through Trk-mediated signaling for their survival during development (27). To address whether Nedd4-2 modulates the dependence of sensory neuron survival on neurotrophins, we obtained NGF- and BDNF-dependent neurons and modified their levels of Nedd4-2. The dissected neurons were transfected on DIV4-5 with plasmids that expressed GFP and control shRNA or GFP and Nedd4-2 shRNA. The presence of GFP allows transfected neurons to be identified unambiguously. Apoptotic and non-apoptotic cells were scored at 72–120h after NGF or BDNF withdrawal (Fig. 6B). Non-transfected neurons showed approximately 60% of apoptosis upon neurotrophin deprivation for 72–120h (data not shown). The percentage of apoptosis observed upon NGF withdrawal in the Nedd4-2-depleted neurons was only 34.9%; i.e., significantly reduced in comparison with the 60.8% of control transfected neurons (Fig. 6C). Upon BDNF deprivation, no differences in apoptosis between control (58.7%) and Nedd4-2 shRNA (58.6%) transfected cells were observed in BDNF-dependent neurons (Fig. 6C). Therefore, these data indicate that Nedd4-2 is able to specifically influence the survival of NGF-dependent sensory neurons through the modulation of NGF-mediated signaling.
Enhanced TrkA signaling and survival in DRG neurons from TrkAP782S mutant mice
Since Nedd4-2 can ubiquitinate several different proteins (32), shRNA could exert multiple direct and indirect effects on TrkA protein. To rule out the possibility that our results might be the consequence of an indirect effect, we followed a different experimental approach. Thus, we disrupted the binding of Nedd4-2 to TrkA. Previously, we have reported that the PPVY785 motif present in TrkA, but not in TrkB, is required for the binding of Nedd4-2 (22). Therefore, we generated a knock-in mouse carrying a point mutation in TrkA protein in the codon that encodes for proline 782, which was replaced by serine (Fig. 7A,B,C,D), the amino acid present in TrkB. This mutant TrkA protein (TrkAP782S) lacks the PPVY motif and does not bind Nedd4-2 (22), but is still able to engage PLC-g (Supplementary Figure S3). The targeting vector was constructed by introducing two changes in the proline 782 codon to code for serine in exon 17 (Fig. 7A). The presence of these changes was confirmed by sequencing and by the disappearance of a BstXI restriction site. Targeted ES cells were monitored by southern blot with an external probe (Fig. 7B) and with an internal (neo) probe (data not shown), as described in Material and Methods. Knock-in TrkAP782S mice (KI) were viable and fertile, with a normal life span. Genotyping of the mice was performed by PCR, amplifying 1185 bp and 1385 bp fragments from the WT and KI, respectively (Fig. 7C). The presence of the mutation was assessed by the lack of digestion of the PCR fragment with BstXI in the KI mice (Fig. 7D). To confirm that the mutant amplified DNA could be digested, we used the PstI restriction site (Fig. 7D).
Figure 7. DRG neurons from TrkAP782S mouse display enhanced NGF-mediated signaling and survival.
(A) Schematics of the wild-type TrkA allele, the targeting vector, and the targeted allele before and after Flp recombination. Neo, neomycin resistance gene; DT-A, diphtheria toxin. E, B, and S denote the sites for the restriction enzymes EcoRV, BstXI, and SmaI; black rectangles denote exons; triangles denote loxP sites; and grey rectangles flanking neo cassette denote FRT sites.
(B) Southern blot analysis of genomic DNA from WT and targeted ES cells after digestion with EcoRV. As indicated in the schematics in (A), the 5´ probe detects an 8.8-kb fragment from WT alleles, and a 10.8-kb fragment from the targeted ES cells.
(C) PCR genotyping using mouse tail DNAs is shown. The primer sites used for genotyping are shown (arrows). B: BstXI; P: PstI.
(D) The presence of the mutation that changes the proline to alanine was assessed by BstXI digestion in the PCR product obtained from the KI animals. The upper panel shows the expected DNA fragments from digestion of the amplified DNA from the WT and KI mice. Note the lack of digestion with BstXI, but not with PstI, in the amplified DNA fragment from the KI mouse.
(E) Enhanced NGF-mediated signaling in DRG neurons from TrkAP782S mice. DRG neurons were obtained and cultured for 6–8 days, as described in Material and Methods. NGF was withdrawn and Z-VAD-FMK (20µM) was added to the neurons overnight. Stimulation with NGF (50ng/ml) for different times was performed and cell extracts were assessed using antibodies that recognized specific phosphorylated residues for TrkA, PLCγ, MAPK and Akt. Representative western blots are shown (n = 4). WT: wild type TrkA; KI: TrkAP782S.
(F) Increased survival of DRG neurons from TrkAP782S mice in limiting amounts of NGF. DRG neurons from WT TrkA and TrkAP782S embryos were plated on different amounts of NGF (25, 20, 10, 5, and 1 ng/ml). The number of live neurons present in medium supplemented with 25 ng/ml of NGF was considered 100% and the rest were normalized with respect to this percentage. Data are presented as means ± s.e.m. P values were calculated using two-tailed Student’s t-test (n = 5). WT: wild type TrkA; KI: TrkAP782
To assess whether the NGF-mediated signaling emanating from TrkAP782S protein was altered, we obtained DRG neurons from E13.5 WT and KI embryos. Neurons were cultured and processed as described previously for rat DRG neurons. NGF-dependent neurons from KI mice did present an increase in TrkA, PLCγ, Akt and MAPK activation as compared with WT neurons (Fig. 7E). Therefore, TrkAP782S protein, which does not bind Nedd4-2, is more active upon NGF binding, leading to a higher activation of the downstream signaling cascades.
During development, DRG neurons project to their targets and receive trophic support from the neurotrophins provided by the tissues innervated. Neurons that do not receive enough signaling will die (27). To address the functional consequences of TrkAP782S expression in DRGs, we performed survival assays in NGF-dependent neurons obtained from WT and KI mice. Dissected neurons were plated on different amounts of NGF (25, 20, 5, 1 ng/ml) and survival was monitored on DIV6-9. We observed similar survival percentages in neurons from the WT and KI when 25, 10 and 5 ng/ml of NGF were applied to cultured neurons (Fig. 7F), although at a concentration of 5 ng/ml the trend was for there to be lower survival in WT neurons than in KI neurons (71% vs. 99.8% survival). Using 1 ng/ml of NGF, the survival of WT neurons was compromised; only 20.9% of WT neurons survived at 1 ng/ml in comparison with 100% in 25 ng/ml (Fig. 7F). In contrast, the percentage of KI neurons that survived at 1 ng/ml was very similar to that obtained with KI neurons cultured with 25 ng/ml of NGF (Fig. 7F). Accordingly, mutant TrkAP782S confers a survival advantage to DRG neurons in limiting amounts of NGF, probably due to the increased TrkA signaling.
Discussion
Here we provide evidence that Nedd4-2 protein regulates the degradation of TrkA through modulation of the trafficking of the receptor. Depletion of Nedd4-2 caused impairment in the ubiquitination of TrkA in the early endosomes and in the sorting of activated TrkA at the early endosomes and the late endosomes, the compartments where Nedd4-2 is located, which resulted in enhanced TrkA recycling and retrograde transport. In addition, TrkA signaling and the survival of NGF-dependent DRG neurons were enhanced in response to reduced levels of Nedd4-2. Moreover, neurons from a knock-in mouse expressing a TrkA mutant that does not bind Nedd4-2 behaved in the same way as regards NGF-mediated signaling and the survival effects of Nedd4-2-depleted neurons.
Nedd4-2 regulates TrkA trafficking at the early and late endosome compartments
The trafficking of activated RTKs is a complex and highly regulated process that modulates the magnitude and controls the specificity of the ligand-elicited response. Alterations of RTK trafficking have profound consequences as regards the amount of receptors and therefore in the final outcome of each specific receptor. Our experiments involving decreasing the levels of Nedd4-2 protein in TrkA-dependent neurons indicate an increase in the levels of TrkA through an alteration of receptor trafficking. Our co-localization and biochemical experiments suggest that activated TrkA in Nedd4-2-depleted neurons arrive at the early endosomes as efficiently as in control neurons. However, once there TrkA is not sorted properly to the late endosomes in Nedd4-2-depleted neurons; instead, there is increased cell surface recycling and an intracellular accumulation of active TrkA protein. The accumulation of activated TrkA in endosomal compartments has been reported previously when Rab7 activity was impaired (33).
The decision to recycle RTKs is made at the early endosome compartment (5, 6). Nedd4-2 depletion leads to increased recycling of TrkA to the plasma membrane (Fig. 4A,B) and subsequent accumulation of surface TrkA (Fig. 1C; 4D,E). Since Nedd4-2 is located at the early endosomes (Fig. 3A) and considering the delay of activated TrkA in reaching late endosomes when Nedd4-2 is depleted (Fig. 3E), we believe that Nedd4-2 must play a role in TrkA trafficking at the early endosomes. In addition, Nedd4-2 is also located in the late endosomes (Fig. 3A) and considering the accumulation of activated TrkA at later time points in late endosomes (Fig. 3E), it is very plausible that Nedd4-2 is functioning at the late endosome stage as well. All together, our results indicate that Nedd4-2 is functioning at the early and late endosomal compartments. Nedd4-2 depletion leads to an increased recycling and to a slight accumulation of activated TrkA at the early endosomes in early time points. At later time points upon NGF treatment, Nedd4-2 depletion results in an accumulation of activated TrkA at the late endosomes. To sum up, Nedd4-2 seems to regulate the trafficking and sorting of active TrkA to the degradative pathway acting at the early and late endosome stages. Our data also demonstrated that Nedd4-2 ubiquitinates activated TrkA receptors in early endosomes (Fig. 3G), suggesting that this may be a mechanism by which TrkA trafficking is regulated. In addition, the effect is specific for TrkA in response to NGF, since p75 trafficking kinetics was not affected. The availability of extra TrkA protein at the cell surface and at intracellular compartments in Nedd4-2-depleted cells renders these neurons more responsive to NGF treatment. This may contribute in part to the increased TrkA signaling observed following NGF treatment (Fig. 6A).
Nedd4-2 belongs to the family of Nedd4 E3 ubiquitin ligase proteins (25, 32). All members of the family share structural homology, with a C2 domain in the N-terminus, several WW domains and a HECT catalytic domain at the C-terminus (25, 34, 35). The localization of Nedd4 proteins depends on the C2 domain, which is a phospholipid membrane interaction motif. According to different reports using genetic approaches to knock out their expression, Nedd4 family members exert specific functions (36–40). However, other studies have indicated that there is also functional compensation and redundancy among different Nedd4 proteins (38, 41, 42). Here, we have described a specific function for Nedd4-2 in the modulation of TrkA trafficking. In absence of Nedd4-2 TrkA turnover, albeit at lower kinetics, still proceeds, suggesting that other E3 ubiquitin ligases can compensate for Nedd4-2 depletion. Future work should address the role of other members of the Nedd4 family on TrkA protein, mainly those ones that might be upregulated or compensate in response to Nedd4-2 depletion.
The endosomal sorting of RTKs upon activation is initiated by their ubiquitination by specific E3 ubiqutin ligases, which often begins at the plasma membrane and continues on endosomal membranes. In the endosome membrane, ubiquitinated RTKs are recognized and captured by the endosomal sorting complex for transport (ESCRT) machinery. Alteration of the ubiquitination of TrkA mediated by Nedd4-2 impairs the sorting and trafficking of the receptor, leading to an accumulation of activated TrkA in compartments where it is not normally accumulated. The level of ubiquitination and/or specific ubiquitination mediated by Nedd4-2 on the TrkA receptor may be required for proper endosomal sorting and degradation, as previously described for EGFR (43). Further analysis of the residues that are ubiquitinated in TrkA in response to NGF should shed light on the type and levels of ubiquitination provided by Nedd4-2 that are required for correct TrkA trafficking and sorting.
Implications of alterations of proper TrkA trafficking
In neurons, TrkA signaling may emanate from the cell surface as well as from intracellular compartments (4, 44). There are reports in the literature supporting the notion that activated TrkA exerts its signaling function as from the early endosome (16, 18, 45, 46) as well as from late endosomes (33, 47, 48). The impaired trafficking of activated TrkA protein in response to Nedd4-2 depletion in sensory neurons results in enhanced signaling, as assessed by the activation of PLC-g, Akt and MAPK and, finally, in increased survival of these neurons (Fig. 6). These data are further supported by the use of sensory neurons obtained from a newly generated knock-in mouse that expresses a mutant TrkA that does not bind Nedd4-2 (Fig. 7). These results suggest an important role for Nedd4-2 and its interaction with TrkA in the modulation of NGF-mediated signaling pathways.
The results reported here highlight the notion that correct modulation of the trafficking of the neurotrophin receptor TrkA in response to NGF is a critical event for its functions. It is believed that NGF and TrkA may play important roles in the etiology of neurodegenerative diseases, such as Alzheimer’s. A hypothesis of impaired NGF transport and the subsequent degeneration of cholinergic neurons may underlie one of the earliest events in Alzheimer’s disease (49). Future experiments involving the modulation of TrkA protein levels and NGF-mediated signaling may help to further understand the role of NGF in neurodegenerative diseases. The identification of Nedd4-2 as a molecular determinant of TrkA trafficking represents a potential target for control of the level of TrkA activation.
Material and Methods
Materials
The following antibodies were used: rabbit Nedd4-2 polyclonal and phosphoTrkA (Y785) have been used previously (22); p75 (9992); Trk (C-14) and P4D1 (ubiquitin) (Santa Cruz); Rab7 and bIII-Tubulin (Sigma); phosphoTrk (Y490), phosphoPLCγ and phosphoMAPK (Cell Signaling); EEA1, Rab5 and Rab11 (Beckton Dickinson); phosphoAkt (Ser473) (Upstate); GFP (Molecular Probes and Chemicon).
Plasmids
The sequence 5’ aagtggacaatttaggccgaa 3’ of Nedd4-2 cDNA corresponding to nucleotides 727–747 was used to generate Nedd4-2 shRNA using the lentiviral vector pLVTHM. Control shRNA lentivirus were generated using the sequence 5’ gcgcgctttgtaggattcg 3’ from Euglena gracilis chloroplast DNA between s16 S and 16 S rRNA (50).
Dorsal root ganglia (DRG) neuron culture
DRGs were dissected from E15.5 rat embryos, incubated, and dissociated with 0.25% trypsin in L-15 media for 45 min at 37°C. Cells were centrifuged to eliminate trypsin and resuspended in plating medium (MEM, 10% FBS, 0.4% glucose, 2 mM glutamine, 100 U/ml Pen/Strep). A single-cell suspension was obtained by pipetting up and down 30 times with a 200-µl tip. After counting, cells were plated using plating medium and the corresponding neurotrophin, NGF (50 ng/ml) or BDNF (25 ng/ml) overnight on plastic plates coated with Growth Factor Reduced Matrigel (BD Biosciences) as substrate. On the following day, the medium was changed to NB (neurobasal medium, B-27, 0.4% glucose, 2 mM glutamine), NGF or BDNF and 5-fluorodeoxyuridine (5FU) (2.44 µg/ml) and uridine (2.44 µg/ml). Proliferating cells disappeared after 3–4 days and more than 95% of cells at DIV8 were neurons.
Lentivirus generation and infection
The lentivirus used in this study was generated by co-transfection using calcium phosphate in 293FT cells. 2.5 × 106 293FT cells seeded in a 10 cm plate the day before were transfected with 9 µg of pLVTHM control shRNA or pLVTHM-Nedd4-2 shRNA together with 6 µg of psPAX2 and 5 µg of pMD.2G plasmids. The medium was changed to fresh medium without antibiotics after 16 hours. 48 hours later, the supernatant containing the lentivirus was collected, centrifuged at 3500 rpm for 10 min, filtered through a 0.45 µM filter, and stored in aliquots at −80 °C. To infect 1×105 DRG cells, 100 µl of supernatant containing-lentivirus was added to the NB medium. Infected neurons were monitored by the expression of GFP. According to western blot analysis, Nedd4-2 levels decreased by 80–90 % within 3–4 days after infection.
Western Blot analysis
Cells were lysed in a lysis buffer (10 mM Tris pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 1 mM PMSF, 1 µg/ml Aprotinin, 2 µg/ml Leueptine, 1 mM Vanadate, 10 mM NaF and 20 mM β-glycerophosphate) for 40 min at 4 °C with gentle shaking and centrifuged at 12,000 rpm for 15 min to eliminate the debris. Lysates were added 5 × SDS-buffer and boiled for 7 min to denature the proteins. Proteins were resolved by SDS-PAGE, and western blots were performed with antibodies against the different proteins. To avoid problems with the Ig chains we used ProtA- or ProtG-conjugated HRP when same species antibodies were used for both immunoprecipitation and western blot as previously described (51).
NGF-mediated signaling
Rat NGF- and BDNF-dependent DRGs at DIV3 were infected with lentivirus expressing control or Nedd4-2 shRNA for 5 days. After overnight starvation of neurotrophins in the presence of 20 µM Z-VAD-FMK, DRGs were either not stimulated or stimulated with NGF (50 ng/ml) for 5 min, 15 min, 30 min, 45 min and 60 min at 37 °C and then immediately chilled on ice. Cells were washed with cold PBS and lysed in 2 × SDS loading buffer (100 mM Tris pH 7.5, 25% glycerol, 2% SDS, 0.01% bromophenol blue). Lysates were denatured by heating at 100 °C for 7 minutes and then used for SDS-PAGE. Western blots were performed to analyze pTrk, pAkt Ser473, p-pMAPK, pPLCγ, Nedd4-2 and β-Tubulin III.
Surface Biotinylation Assays
Infected DRGs with control and Nedd4-2 shRNA lentivirus were NGF-starved as indicated above. Cells were stimulated with or without NGF (50 ng/ml) for different times (15 min and 60 min) to induce the internalization of biotinylated surface proteins. Subsequently, cells were washed sequentially using room temperature PBS and cold PBS, chilled on ice, and incubated in 0.5 µg/ml Sulfo-NHS-SS-biotin (Pierce) dissolved in Biotinylation Buffer (PBS, 1 mM CaCl2, 0.5 mM MgCl2) for 20 min at 4 °C to label the membrane proteins. Free biotin was quenched with 0.1M Glycine for 15 min at 4 °C. Cells were then washed twice with cold PBS and lysed as indicated above. Biotinylated proteins were isolated from the total cell lysate by immobilization on NeutroAvidin-beads (Pierce) for at least 3 hours at 4°C. Beads were washed three times with lysis buffer and 20 µl of 2 × SDS sample buffer was added before boiling for 7 min. Proteins were subjected to SDS-PAGE and immunoblotted with the corresponding antibodies.
Internalization Assay
Unlike the Surface Biotinylation Assay, which tests surface protein after NGF (50 ng/ml) induction, the internalization assay tested internalized surface protein in response to NGF. Briefly, infected DRG neurons were grown in mass cultures for 8–10 days and then NGF-starved overnight, as indicated previously. Neurons were subjected to biotinylation on ice with the reversible membrane-impermeable derivative of biotin (sulfo-NHS-S-S-biotin from Pierce; 0.5 mg/ml in PBS). Cells were then washed twice to eliminate free biotin and incubated in pre-warmed medium at 37 °C with or without NGF for different times (15 min and 60 min) to induce the internalization of biotinylated surface protein. To address the rate of internalization, the remaining cell-surface biotin was cleaved by reducing its disulfide linkage with 50 mM DTT for 15 min at 4 °C to assess only the internalized protein. Cells were lysed and biotinylated proteins were precipitated, eluted from the beads, resolved by SDS-PAGE, and immunoblotted with the corresponding antibodies.
Degradation Assays
Infected DRGs with control and Nedd4-2 shRNA lentivirus were NGF-starved as indicated above. The following day, neurons were washed with PBS, chilled on ice and biotinylated using 0.5 µg/ml Sulfo-NHS-SS-biotin for 20 min at 4 °C. Cells were sequentially washed with cold and room-temperature PBS and incubated in pre-warmed medium at 37 °C with or without NGF (50 ng/ml) for different times (15 min and 60 min) to allow the biotinylated receptors to become internalized and degraded. Subsequently, cells were washed and lysed using lysis buffer and the biotinylated proteins were precipitated with NeutroAvidin-beads, washed and subjected to SDS-PAGE, and immunoblotted using different antibodies. With this method it was possible to detect surface-labeled proteins that had not been degraded at different time points, regardless of whether the proteins had been internalized or whether they had returned to the plasma membrane.
Immunofluorescence and quantification
The DRG neurons used to perform immunofluorescence analysis were cultured on coverslips coated with 1 mg/ml PDL and Growth Factor Reduced Matrigel and then infected with 10 µl of lentivirus expressing control or Nedd4-2 shRNA for 5 days. After NGF starvation as described above, 50 ng/ml NGF was added to induce TrkA activation and internalization. Cells were then fixed with 4% paraformaldehyde (PFA) in PBS for 5 min, quenched with 50 mM NH4Cl for 10 min, blocked and permeabilized with PBS containing 5% NGS, 0.1% Tween-20, 0.1% TritonX-100 for 30 min, and incubated with primary antibodies overnight at 4 °C. The following day, cells were washed three times with PBS, incubated with the corresponding fluorescent secondary antibody at room temperature for 40 min, and washed with PBS three times. Images were collected with a Leica confocal microscope. To avoid bleed-through between channels, each channel was acquired separately in the co-localization experiments. For co-localization analyses, images of the cells were processed with the functions of ImageJ (NIH), using similar threshold settings for all pictures analyzed, as described (52). Quantification of the processed images was performed with a custom-written program in Matlab.
Retrograde transport
DRGs neurons were obtained from E.15.5 embryos as described above. Compartmentalized cultures using microfluidic chambers (Standard Neuron Device 450 µM, Xona Microfluidics) were used to assess retrograde transport. The microfluidic chambers were assembled on glass coverslips previously treated with 1 mg/mL PDL the day before dissection of the DRGs, as indicated by the manufacturer. 200 µl of NB media was added to the top wells and 150 µl to the bottom wells and incubated O/N in the incubator. The following day, any chamber with signs of leakage was discarded. The chambers were coated with Matrigel and 2 × 105 cells in 10 µl of plating medium with NGF were plated in each chamber. Cells were allowed to adhere to the coverslip for 10 min and additional plating medium was added to each well of the chamber. To isolate each chamber, the volumes in the wells on one side of the device were higher than the other side. The difference in volume creates hydrostatic pressure, thus isolating each compartment fluidically. The plating medium was replaced on the following day with NB medium with NGF (50 ng/ml) and 5FU. Lentivirus infections were performed as described previously. To assess that there was retrograde transport, we have used red fluorescent beads (40 nm) from Invitrogen.
DRG neuron survival
NGF- and BDNF-dependent rat DRG neurons plated on 24 well-plates were transfected at DIV4-5 with plasmids expressing GFP and control or Nedd4-2 shRNA using lipofectamine 2000 (Invitrogen) overnight. The following day, the medium was replaced, keeping NGF or BDNF for 48 hours, after which both neurotrophins were withdrawn. Transfected cells were recognized by GFP expression and apoptotic neurons, identified by fragmented or condensed nuclei using Hoechst 33342, were scored 72–120 hours after the corresponding withdrawal of neurotrophin. Images were taken at random fields using a Leica DMI3000B microscope equipped with a Leica DFC300FX camera. To avoid variability among the independent experiments, a figure of 100 % was given to the percentage of apoptosis after counting all cells.
DRGs from E13.5 WT and KI mice were dissected as indicated above. 10.000 cells/well in a 24-well plate were plated with 25, 10, 5, 1 or 0 ng/ml of NGF. The following day, the medium was changed to NB medium and 5FU, keeping the same amount of NGF as when they had been plated. Images were taken from 4 random fields after 6–9 days and live cells were counted. The number of live cells growing in 25 ng/ml of NGF was considered 100%.
TrkAP782S Knock-in mice
To generate a targeting construct for TrkA P782S knock-in mice, a genomic clone from 129SV/J mice containing exon 17, which encodes the C-terminus of TrkA, was used. The triplet coding for proline (CCA) was mutated to serine (TCG) using directed mutagenesis, and this was checked by sequencing and the disappearance of a BstXI restriction site. A neomycin-resistance gene flanked by FRT sites containing a loxP site was inserted downstream from exon 17. The truncated diphtheria toxin gene was placed at the end of the short arm for negative selection. The targeting vector was linearized at a unique upstream BstXI site and was electroporated into CJ7 embryonic stem (ES) cells, as previously described (53). Ninetyseven G418-resistant colonies were screened for homologous recombination by Southern blot analysis with an external probe. This probe was a 1.2Kb DNA fragment that was excised from the pmA5 plasmid by SacI digestion. A total of 7 clones showed the correct band pattern with the external probe (Fig. 8B) and a Neo probe (data not shown). Three ES clones were injected into C57BL/6 blastocysts to generate chimeric mice. The chimeras were bred with C57BL/6 mice that expressed the Flp recombinase driven by the actin promoter to remove the Neo cassette and all three gave germline transmission. TrkAP782S genotyping was performed by PCR with tail genomic DNA and the following two primers: TrkA-PvuI-F (5’-ggcgcaggcgatcgagtgtatc-3’), and mTrkA-1-B (5’-cctccgcaatggacaggag-3’). A 1.2 Kb product was amplified from the WT allele, and a 1.4 Kb product was amplified from the mutant allele. Confirmation of the genotyping was accomplished by digestion of the PCR product with the BstXI restriction enzyme, which digests the wt alleles but not the mutant alleles. In addition, digestion with the PstI restriction enzyme was performed to confirm that the lack of digestion of mutant TrkA DNA with BstXI was not due to problems with the amplified DNA.
All animals were housed and bred in the SPF Animal Facility of the University of Salamanca. Proper measures were taken to reduce the pain or discomfort of experimental animals. All animal care and procedures were done in accordance with protocols approved by the Bioethics Committee of the University of Salamanca and following the European Community guidelines.
Supplementary Material
Acknowledgements
We thank D. Trono for the lentiviral plasmids; Dionisio Martín-Zanca and Raquel Rodríguez for critical review of the manuscript and helpful discussions and Pilar Pérez for helpful discussions; Enrique López-Poveda for his help writing the MatLab custom-program to quantify immunofluorescence images. We also thank a reviewer for his/her perseverance helping us to interpret the results. This work was supported by Ministerio de Ciencia e Innovación Grant (BFU2008-00162), by a Marie Curie International Reintegration Grant within the 7th European Community Framework Programme, by Conserjería de Educación (SA074A08) and Conserjeria de Sanidad de Junta Castilla y León to J.C.A. J.C.A. is a “Ramón y Cajal” Investigator from the University of Salamanca and NARSAD 2009 Young Investigator Awardee. This research was supported by the Intramural Research Program of the NIH, Center for Cancer Research, National Cancer Institute (for ES and LT).
References
- 1.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annual review of biochemistry. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 2.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nature reviews. 2003;4(4):299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 3.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science (New York, NY. 1996;274(5295):2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Moheban DB, Conway BR, Bhattacharyya A, Segal RA. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J Neurosci. 2000;20(15):5671–5678. doi: 10.1523/JNEUROSCI.20-15-05671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10(9):609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould GW, Lippincott-Schwartz J. New roles for endosomes: from vesicular carriers to multi-purpose platforms. Nat Rev Mol Cell Biol. 2009;10(4):287–292. doi: 10.1038/nrm2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadowski L, Pilecka I, Miaczynska M. Signaling from endosomes: location makes a difference. Experimental cell research. 2009;315(9):1601–1609. doi: 10.1016/j.yexcr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Knusel B, Gao H, Okazaki T, Yoshida T, Mori N, Hefti F, Kaplan DR. Ligand-induced down-regulation of Trk messenger RNA, protein and tyrosine phosphorylation in rat cortical neurons. Neuroscience. 1997;78(3):851–862. doi: 10.1016/s0306-4522(96)00616-1. [DOI] [PubMed] [Google Scholar]
- 9.Jullien J, Guili V, Reichardt LF, Rudkin BB. Molecular kinetics of nerve growth factor receptor trafficking and activation. The Journal of biological chemistry. 2002;277(41):38700–38708. doi: 10.1074/jbc.M202348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saxena S, Howe CL, Cosgaya JM, Steiner P, Hirling H, Chan JR, Weis J, Kruttgen A. Differential endocytic sorting of p75NTR and TrkA in response to NGF: a role for late endosomes in TrkA trafficking. Mol Cell Neurosci. 2005;28(3):571–587. doi: 10.1016/j.mcn.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Sommerfeld MT, Schweigreiter R, Barde YA, Hoppe E. Down-regulation of the neurotrophin receptor TrkB following ligand binding. Evidence for an involvement of the proteasome and differential regulation of TrkA and TrkB. The Journal of biological chemistry. 2000;275(12):8982–8990. doi: 10.1074/jbc.275.12.8982. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci. 2005;25(26):6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ure DR, Campenot RB. Retrograde transport and steady-state distribution of 125I-nerve growth factor in rat sympathetic neurons in compartmented cultures. J Neurosci. 1997;17(4):1282–1290. doi: 10.1523/JNEUROSCI.17-04-01282.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuruvilla R, Ye H, Ginty DD. Spatially and functionally distinct roles of the PI3-K effector pathway during NGF signaling in sympathetic neurons. Neuron. 2000;27(3):499–512. doi: 10.1016/s0896-6273(00)00061-1. [DOI] [PubMed] [Google Scholar]
- 15.Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118(2):243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Ye H, Kuruvilla R, Zweifel LS, Ginty DD. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron. 2003;39(1):57–68. doi: 10.1016/s0896-6273(03)00266-6. [DOI] [PubMed] [Google Scholar]
- 17.Ginty DD, Segal RA. Retrograde neurotrophin signaling: Trk-ing along the axon. Current opinion in neurobiology. 2002;12(3):268–274. doi: 10.1016/s0959-4388(02)00326-4. [DOI] [PubMed] [Google Scholar]
- 18.Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39(1):69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- 19.Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nature reviews. 2005;6(8):615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
- 20.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annual review of cell and developmental biology. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 21.Komander D. The emerging complexity of protein ubiquitination. Biochemical Society transactions. 2009;37(Pt 5):937–953. doi: 10.1042/BST0370937. [DOI] [PubMed] [Google Scholar]
- 22.Arevalo JC, Waite J, Rajagopal R, Beyna M, Chen ZY, Lee FS, Chao MV. Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron. 2006;50(4):549–559. doi: 10.1016/j.neuron.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 23.Geetha T, Jiang J, Wooten MW. Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling. Molecular cell. 2005;20(2):301–312. doi: 10.1016/j.molcel.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Makkerh JP, Ceni C, Auld DS, Vaillancourt F, Dorval G, Barker PA. p75 neurotrophin receptor reduces ligand-induced Trk receptor ubiquitination and delays Trk receptor internalization and degradation. EMBO Rep. 2005;6(10):936–941. doi: 10.1038/sj.embor.7400503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingham RJ, Gish G, Pawson T. The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene. 2004;23(11):1972–1984. doi: 10.1038/sj.onc.1207436. [DOI] [PubMed] [Google Scholar]
- 26.Persaud A, Alberts P, Amsen EM, Xiong X, Wasmuth J, Saadon Z, Fladd C, Parkinson J, Rotin D. Comparison of substrate specificity of the ubiquitin ligases Nedd4 and Nedd4-2 using proteome arrays. Molecular systems biology. 2009;5:333. doi: 10.1038/msb.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77(5):627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 28.Marchese A, Raiborg C, Santini F, Keen JH, Stenmark H, Benovic JL. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Developmental cell. 2003;5(5):709–722. doi: 10.1016/s1534-5807(03)00321-6. [DOI] [PubMed] [Google Scholar]
- 29.Angers A, Ramjaun AR, McPherson PS. The HECT domain ligase itch ubiquitinates endophilin and localizes to the trans-Golgi network and endosomal system. The Journal of biological chemistry. 2004;279(12):11471–11479. doi: 10.1074/jbc.M309934200. [DOI] [PubMed] [Google Scholar]
- 30.Rajagopal R, Chen ZY, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24(30):6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen ZY, Ieraci A, Tanowitz M, Lee FS. A novel endocytic recycling signal distinguishes biological responses of Trk neurotrophin receptors. Molecular biology of the cell. 2005;16(12):5761–5772. doi: 10.1091/mbc.E05-07-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang B, Kumar S. Nedd4 and Nedd4-2: closely related ubiquitin-protein ligases with distinct physiological functions. Cell death and differentiation. 2010;17(1):68–77. doi: 10.1038/cdd.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saxena S, Bucci C, Weis J, Kruttgen A. The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA. J Neurosci. 2005;25(47):10930–10940. doi: 10.1523/JNEUROSCI.2029-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey KF, Kumar S. Nedd4-like proteins: an emerging family of ubiquitin-protein ligases implicated in diverse cellular functions. Trends in cell biology. 1999;9(5):166–169. doi: 10.1016/s0962-8924(99)01541-x. [DOI] [PubMed] [Google Scholar]
- 35.Rotin D, Staub O, Haguenauer-Tsapis R. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. The Journal of membrane biology. 2000;176(1):1–17. doi: 10.1007/s00232001079. [DOI] [PubMed] [Google Scholar]
- 36.Fang D, Elly C, Gao B, Fang N, Altman Y, Joazeiro C, Hunter T, Copeland N, Jenkins N, Liu YC. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nature immunology. 2002;3(3):281–287. doi: 10.1038/ni763. [DOI] [PubMed] [Google Scholar]
- 37.Yang B, Gay DL, MacLeod MK, Cao X, Hala T, Sweezer EM, Kappler J, Marrack P, Oliver PM. Nedd4 augments the adaptive immune response by promoting ubiquitin-mediated degradation of Cbl-b in activated T cells. Nature immunology. 2008;9(12):1356–1363. doi: 10.1038/ni.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamashita M, Ying SX, Zhang GM, Li C, Cheng SY, Deng CX, Zhang YE. Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell. 2005;121(1):101–113. doi: 10.1016/j.cell.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi PP, Cao XR, Sweezer EM, Kinney TS, Williams NR, Husted RF, Nair R, Weiss RM, Williamson RA, Sigmund CD, Snyder PM, Staub O, Stokes JB, Yang B. Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2. American journal of physiology. 2008;295(2):F462–F470. doi: 10.1152/ajprenal.90300.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P, Sakuma R, Luga V, Roncari L, Attisano L, Wrana JL. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009;137(2):295–307. doi: 10.1016/j.cell.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Myat A, Henry P, McCabe V, Flintoft L, Rotin D, Tear G. Drosophila Nedd4, a ubiquitin ligase, is recruited by Commissureless to control cell surface levels of the roundabout receptor. Neuron. 2002;35(3):447–459. doi: 10.1016/s0896-6273(02)00795-x. [DOI] [PubMed] [Google Scholar]
- 42.Wilkin MB, Carbery AM, Fostier M, Aslam H, Mazaleyrat SL, Higgs J, Myat A, Evans DA, Cornell M, Baron M. Regulation of notch endosomal sorting and signaling by Drosophila Nedd4 family proteins. Curr Biol. 2004;14(24):2237–2244. doi: 10.1016/j.cub.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 43.Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Molecular cell. 2006;21(6):737–748. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 44.MacInnis BL, Campenot RB. Retrograde support of neuronal survival without retrograde transport of nerve growth factor. Science (New York, NY. 2002;295(5559):1536–1539. doi: 10.1126/science.1064913. [DOI] [PubMed] [Google Scholar]
- 45.Howe CL, Valletta JS, Rusnak AS, Mobley WC. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron. 2001;32(5):801–814. doi: 10.1016/s0896-6273(01)00526-8. [DOI] [PubMed] [Google Scholar]
- 46.Wu C, Lai CF, Mobley WC. Nerve growth factor activates persistent Rap1 signaling in endosomes. J Neurosci. 2001;21(15):5406–5416. doi: 10.1523/JNEUROSCI.21-15-05406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52(2):293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Hisata S, Sakisaka T, Baba T, Yamada T, Aoki K, Matsuda M, Takai Y. Rap1-PDZ-GEF1 interacts with a neurotrophin receptor at late endosomes, leading to sustained activation of Rap1 and ERK and neurite outgrowth. The Journal of cell biology. 2007;178(5):843–860. doi: 10.1083/jcb.200610073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calissano P, Matrone C, Amadoro G. Nerve growth factor as a paradigm of neurotrophins related to Alzheimer's disease. Developmental neurobiology. 2010;70(5):372–383. doi: 10.1002/dneu.20759. [DOI] [PubMed] [Google Scholar]
- 50.Kuratomi G, Komuro A, Goto K, Shinozaki M, Miyazawa K, Miyazono K, Imamura T. NEDD4-2 (neural precursor cell expressed, developmentally down-regulated 4-2) negatively regulates TGF-beta (transforming growth factor-beta) signalling by inducing ubiquitin-mediated degradation of Smad2 and TGF-beta type I receptor. The Biochemical journal. 2005;386(Pt 3):461–470. doi: 10.1042/BJ20040738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lal A, Haynes SR, Gorospe M. Clean western blot signals form immunoprecipitated samples. Molecular and cellular probes. 2005;19(6):385–388. doi: 10.1016/j.mcp.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tai CY, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron. 2007;54(5):771–785. doi: 10.1016/j.neuron.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 53.Tessarollo L. Manipulating mouse embryonic stem cells. Methods in molecular biology (Clifton, NJ. 2001;158:47–63. doi: 10.1385/1-59259-220-1:47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.