Abstract
Male sex is an independent risk factor for long-term neurologic deficits in human preterm infants. Using a chronic, sublethal hypoxia (CSH) mouse model of preterm brain injury, we recently demonstrated acute brain volume loss with an increased male susceptibility to hippocampal volume loss and hypomyelination. We now characterize the long-term, sex-specific effects of CSH on cognition and brain growth. Neonatal mice were treated with CSH for 8 days, raised in normoxia thereafter and underwent behavioral testing at 6 weeks of age. Behavioral assays sensitive to hippocampal function were chosen. CSH-treated males had impairments in associative learning, spatial memory and long-term social memory compared to control males. In contrast, CSH-treated females were less impaired. Persistent reductions in hippocampal and cerebellar volumes were found in adult CSH-treated males while regional brain volumes in CSH-treated females were indistinguishable from controls. Similar to human preterm infants, males exposed to hypoxia are especially vulnerable to short-term and long-term deficits in cognition and brain growth.
INTRODUCTION
Long-term cognitive deficits are common adverse sequelae of preterm birth (1, 2). Neuroanatomical differences in regional brain volume and myelination in preterm survivors are increasingly recognized (3, 4). Male sex is a strong independent risk factor for adverse cognitive and neuroanatomical outcomes in preterm infants, but the mechanism for their vulnerability is unknown (1). While rodent models have been developed to study mechanisms of preterm brain injury (5), few studies have looked at males and females separately, limiting our understanding of sex-specific damage.
To elucidate the mechanisms that protect the female developing brain or render males more susceptible to damage, we are exploring sex-specific differences in a rodent model that mimics neuroanatomical findings of preterm brain injury. Although many factors contribute to preterm brain injury, cerebral hypoxia is important due to its frequency in the preterm population from respiratory distress syndrome, chronic lung disease and hypotension (6). A rodent model of chronic, sublethal hypoxia (CSH) has been shown to cause neuroanatomical findings of diffuse brain volume loss, impaired myelination and ventriculomegaly, findings that are strikingly similar to those seen in human preterm infants (7). Using this model, we recently demonstrated sex-specific susceptibility to CSH, with males having greater hippocampal volume loss and less myelination than females (8).
We hypothesized that CSH would also lead to sex-specific effects on long-term cognitive development and brain growth, with greater impairment in males. To test this, we selected a panel of behavioral assays to test learning, memory and social awareness to compare cognitive development in control and CSH-treated mice. Long-term neuroanatomical differences were analyzed at the conclusion of behavioral testing.
MATERIALS AND METHODS
Hypoxia
All experiments were approved by the Administrative Panel on Laboratory Animal Care at Stanford University (Protocol #10829). Hypoxia treatment was carried out as described in (8). C57BL/6 litters of 4 male pups, 4 female pups, a C57BL/6 dam and a CD-1 foster dam were randomized to hypoxia or normoxia from P3-P11. The hypoxic litters with dams were placed in a chamber where a continuous oxygen sensor and controller unit (Biospherix, New York) adjusted nitrogen gas infusion rates to maintain a fraction of inspired oxygen (FiO2) of 0.1. The normoxic litters remained in ambient air with a FiO2 of 0.21. On P11, the litters in the hypoxia chamber were returned to ambient air. While there is a significant mortality rate for pups in hypoxia, there is no sex difference in mortality. Litters were weaned at P21.
IntelliCage testing
Automated behavioral testing for the evaluation of learning and memory was conducted in the IntelliCage (NewBehavior AG, Switzerland). Radiofrequency identification devices (RFID, Datamars SA, Switzerland) were implanted subcutaneously for individual mouse identification. The IntelliCage holds up to 16 mice and provides a group housing environment with unrestricted food access but restricted water access limited to four programmable testing corners. The mice enter testing corners via an opening large enough for only one mouse. Heat sensors in the corners detect mouse entry and the RFID identifies the specific mouse. Each testing corner has two programmable doors that can be open, closed, or openable with a nosepoke to provide water access which is considered reward. A programmable air puff is considered punishment. Above each door are programmable LED lights of varying colors for choice discrimination. Corner visits, nosepokes, licks and visit times were recorded.
Baseline activity level
Testing started at 5 weeks of age. Water access was unrestricted in all testing corners. The average visits to testing corners during light and dark cycles were measured over 3 days.
Place learning and reversal testing
Testing started at 6 weeks of age. Water access was restricted to two 1-hour-long drinking sessions per day. During these sessions, each mouse was assigned one specific corner (place learning corner) where water was accessible in response to a nosepoke. The percentage of nosepokes in each corner was measured for 4 days (8 sessions). After 4 days, corners were reprogrammed for each mouse so water became inaccessible in the place learning corner but became accessible in a different corner (reversal corner). After the switch, the percentage of nosepokes in each corner was measured for another 4 days (8 sessions). Nonparametric permutation test was performed to assess the difference in the probabilities of nosepokes at 3 sessions after reversal between control and hypoxic animals of both sexes.
Cued punishment testing
Testing started at 9 weeks of age. Water access was restricted to two 1-hour-long drinking sessions per day. During the sessions, in all testing corners, one door was transiently labeled with green LEDs and the other with yellow LEDs. Half of the animals were assigned yellow as correct and green as incorrect; the other half were assigned the opposite. A nosepoke at a door with the incorrect LEDs caused an air puff while a nosepoke at a door with the correct LEDs allowed water access. LED light colors above each door were switched every 30 minutes to eliminate place learning as a confounder. The percentage of errors (nosepokes at the incorrect door) was measured for 7 days (14 sessions). Statistical analysis was performed using a generalized mixed effects regression model to evaluate the difference in error rates between control and hypoxic animals of both sexes. Mouse-specific random effect was included to account for within-mice correlations. The regression analysis also adjusted for session variation.
Standard behavioral testing
Exploratory Activity in Novel Environment
Testing started at 17 weeks of age. The Activity Chamber (Med Associates Inc., St. Albans) was used to assess general activity, gross locomotor activity and exploratory behavior. Assessment took place in a 43.2 cm × 43.2 cm arena with infrared detectors within a sound-attenuating chamber under dim light. The animal was placed in the center of the arena and allowed to move freely for 10 minutes while being tracked by an automated tracking system. Ambulatory time, resting time and distance moved were recorded.
Morris water maze (MWM) with delayed matching-to-place (DMP)
Testing started at 17 weeks of age. The DMP water maze task was used to assess learning and memory as described previously (9). A dark-colored tank (170 cm in diameter) was filled with opacified water. Each day, each mouse was placed in the MWM at one of four randomly selected locations and given up to 90 seconds to find a submerged platform. Once the platform was found, the animal was allowed to remain on the platform for 10 seconds. If the mouse failed to find the platform after 90 seconds, the animal was placed on the platform for 10 seconds. 4 trials with an inter-trial interval of 8-12 minutes were conducted daily with the platform in a fixed location. Each subsequent day for 6 days, the submerged platform was moved to a new location and the 4 trials repeated. During each trial, the time to locate the submerged platform (escape latency) was recorded. The mixed effect linear regression analysis was performed in each sex to evaluate the difference in escape latency time savings between trials 1 and 2 on days 2 through 7 between control and hypoxic animals. Mice and day specific random effects were included to account for correlations.
Social testing
Testing started at 10 weeks of age. Due to the sex-specific design of the sociability assays, only male mice were tested. Mice were individually housed 1 week before testing. Prior to social memory testing, randomly-selected, individually-housed, C57BL/6 ovariectomized female mice (OEF) were put into the home cages of test mice for 4 hours per day for 5-7 days to reduce sexual behavior. This test was conducted as described in (10), but modified by adding a sixth trial. The same, never-before-met OEF was placed into the home cage of the test mouse for four 1-minute trials with a 10-minute inter-trial interval. In the fifth trial, a novel OEF was introduced. In the sixth trial, the original OEF from trials 1-4 was reintroduced as control. All trials were videotaped and analyzed for socialization time (anogenital, perioral and body investigation). The mixed effect linear regression model was used to compare socialization time between control and hypoxic animals for the first four sessions. The Wilcoxen test was used for comparisons for the fifth and sixth sessions.
Brain sectioning
Brains were harvested following intracardiac perfusion with 4% paraformaldehyde. Brains were fixed overnight and dehydrated in 30% sucrose with 0.01% sodium azide. Sagittal sections of 50 μm thickness were obtained using a sliding microtome. Every 5th section was mounted on a slide.
Volume analysis
Brain sections obtained at 20 weeks of age were stained with cresyl violet. Sections were imaged using a Leica DFC280 camera on a Leica stereoscope. Cortex, hippocampus and cerebellum areas were measured for each section using ImageJ (NIH, Bethesda), multiplied by section thickness and added to determine regional volumes. A linear regression model was used to compare regional brain volumes between control and hypoxic animals. Comparisons were made on absolute volumes within each sex and for clarity graphed relative to male normoxic volumes.
Cell proliferation
On P11, 5 mg/kg 5-bromodeoxyuridine (BrdU) was injected intraperitoneally. Brains sections were obtained at 6 weeks of age. Immunohistochemistry was done on free-floating sections using 1:1000 anti-BrdU (Abcam, Cambridge) and anti-NeuN antibodies (Abcam, Cambridge). Immunostaining was visualized on a Nikon E800 microscope. All BrdU+ cells within the dentate gyrus were counted for each section and divided by the dentate gyrus area to calculate BrdU+ cell density. The t-test was used to compare the BrdU+ cell density between control and hypoxic animals for each sex.
RESULTS
98 mice (57 males, 41 females) were tested in three cohorts (IntelliCage only, IntelliCage+Activity Chamber+MWM/DMP, social testing). In all cohorts, as noted previously (7, 8), hypoxic mice weighed significantly less than controls immediately after hypoxia (data not shown). However, as in prior studies, gradual somatic catch-up growth was seen.
Baseline activity
In the IntelliCage, visits to testing corners and circadian patterns of exploratory activity were no different between control and hypoxic animals (Figure 1A). In the Activity Chamber, no differences were seen in baseline activity as measured by ambulatory duration, resting duration and distance moved (Figure 1B-D). This is consistent with published data reporting greater locomotor activity in young CSH-treated animals that disappeared by 6 weeks of age (11).
Figure 1. Activity levels.
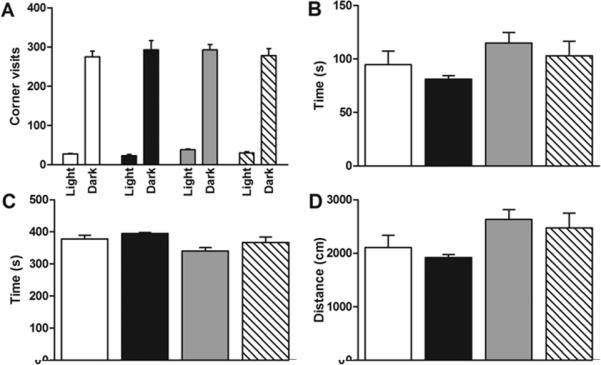
(A) Hypoxia does not affect the number of IntelliCage corner visits in light and dark cycle. n=28 (MC=7,MH=7,FC=8,FH=6). (B,C,D) Hypoxia does not affect ambulatory duration, resting duration or distance moved in the Activity Chamber. n=28 (MC=4,MH=10,FC=5,FH=9). White=MC, black=MH, gray=FC, hatched=FH.
Place learning and reversal of place learning
Location memory was tested with place learning and reversal tasks in the IntelliCage (Figure 2). Place learning was assessed by measuring the percentage of nosepokes in the place learning corner over serial drinking sessions. Over the first eight sessions, no difference in place learning was seen between control and hypoxic mice in either sex. All groups showed an increase percentage of nosepokes in the water-accessible place learning corner over time and a corresponding decrease in the water-inaccessible reversal corner. After water access was changed to the reversal corner following session 8, all groups showed a decline in the percentage of nosepokes in the water-inaccessible place learning corner and a corresponding increase in the water-accessible reversal corner. However, reversal of place learning was significantly slower in hypoxic males than controls (Figure 2A, sessions 10-11). No delay in reversal of place learning was seen in hypoxic females compared to controls (Figure 2B). Eventually, both hypoxic and control males achieved the same percentage of nosepokes at the reversal corner, implying a deficit in the speed of reversal of place learning but not in the eventual ability to learn the reversed location. Impairment in reversal of place learning is considered to be a sensitive test for hippocampal dysfunction (12, 13). Deficits with place learning can be seen with major neurotoxic (14), traumatic (15) or ischemic (16) hippocampal lesions but were not detected in this model.
Figure 2. IntelliCage place learning and reversal.
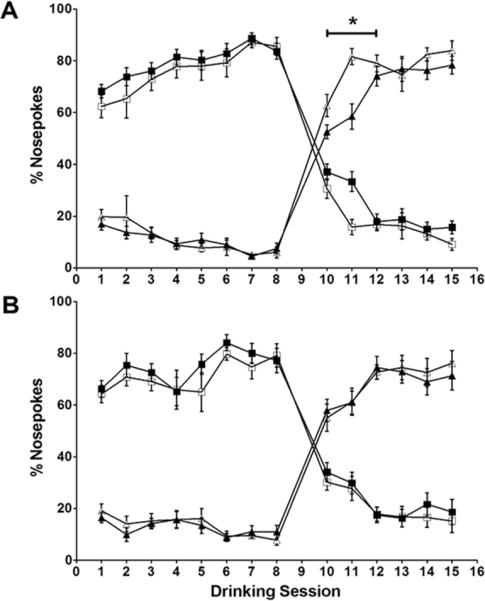
(A) In males, hypoxia does not affect place learning but delays reversal of place learning. (B) In females, hypoxia does not affect place learning or reversal of place learning. n=57 (MC=12,MH=17,FC=13,FH=15). □: normoxia/place learning corner; ■:hypoxia/place learning corner, △: normoxia/reversal corner; ▲:hypoxia/reversal corner. * p<0.05.
Cued punishment
Associative learning was tested with the cued punishment task in the IntelliCage (Figure 3). Associative learning was considered impaired in animals with greater percentages of punished, errant nosepokes. Error rates in both hypoxic and control mice decreased to less than chance by the first session. However, hypoxic males consistently made a higher percentage of errant nosepokes (Figure 3A), with an odds ratio of 1.89 (p<0.05). Hypoxic females also seemed to make more errant nosepokes compared to controls (Figure 3B) but were less significantly affected than males, with an odds ratio of 1.5 (p=0.14).
Figure 3. Cued punishment.
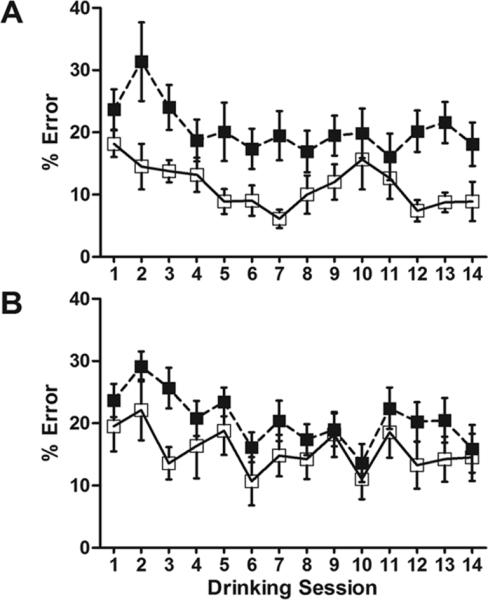
(A) Males exposed to hypoxia make a significantly higher percentange of errant nosepokes than controls (OR 1.89, 95% CI: [1.05-3.39], p<0.05). (B) Females exposed to hypoxia are less affected than males. (OR 1.50, 95% CI: [0.89-2.55], p=0.14). n=56 (MC=11,MH=17,FC=13,FH=15). □: normoxia; ■: hypoxia.
Morris water maze
Spatial learning and working spatial memory were tested with the MWM (Figure 4). The MWM is a well-validated method to assess spatial memory (12). In the DMP variation of the MWM, the submerged platform is relocated every day, specifically testing working spatial memory by measuring time savings from trial 1 to trial 2 over multiple days and platform locations. In the first trial, there was no difference in escape latency between control and hypoxic animals as expected. However, hypoxic males had longer escape latencies in subsequent trials, suggesting spatial learning deficits (Figure 4A, left). Time savings between trials 1 and 2 (Figure 4A, right) were greater in controls compared to hypoxic males, approaching statistical significance (34.8 s vs. 18.9 s, p=0.09). Hypoxic females appeared to have slightly longer escape latencies in subsequent trials compared to controls, although the effect was less pronounced (Figure 4B, left). Time savings between trials 1 and 2 (Figure 4B, right) were not significantly greater in controls compared to hypoxic females (18.1 s vs. 11.7 s, p=0.50).
Figure 4. Morris water maze.
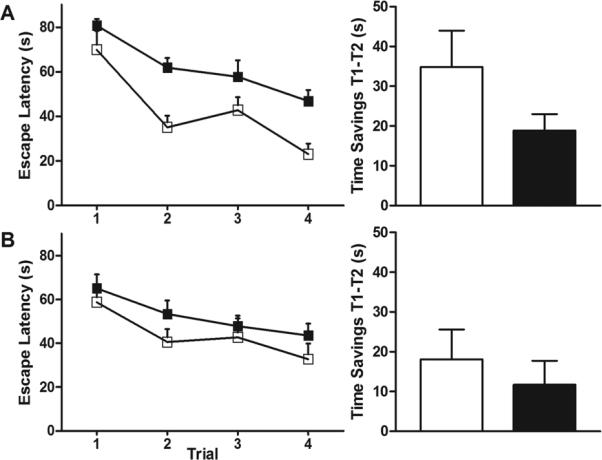
(A) Males exposed to hypoxia have longer escape latencies in subsequent trials and less time savings between trial 1 and trial 2 than controls (18.9 s vs. 34.8 s, p=0.09). (B) Females exposed to hypoxia are less affected than males (11.7 s vs. 18.1 s, p=0.50). n=28 (MC=4,MH=10,FC=5,FH=9). □: normoxia; ■: hypoxia.
Social testing
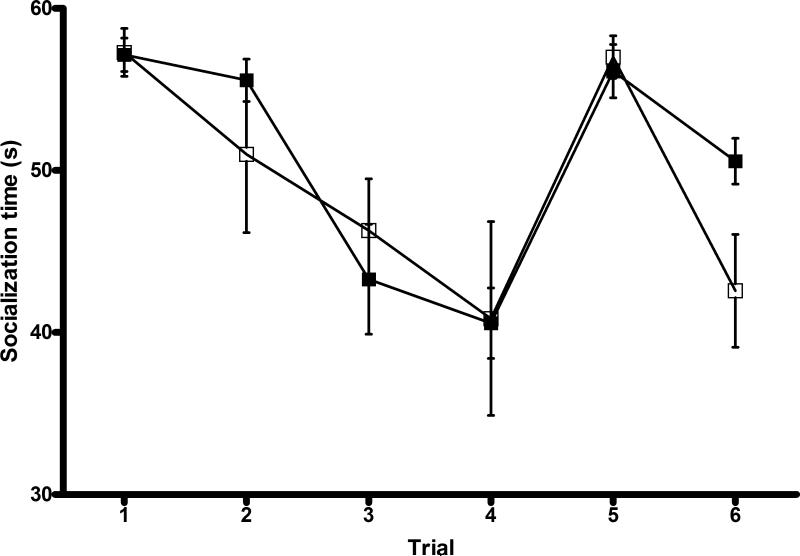
Sociability, social recognition, preference for social novelty and social memory were assessed using a modified 5-trial social memory test (Figure 5). A high socialization time was seen in both groups in trial 1, implying that sociability was unaffected. When the same OEF was reintroduced in trials 2-4, socialization time declined in both groups, implying intact social recognition and memory. When a novel OEF was introduced in trial 5, socialization time increased in both groups, implying intact preference for social novelty. With reintroduction of the original OEF, a decrease in socialization time was seen in both groups. However, hypoxic males had a lesser decline in socialization time (5.5 s vs. 14.4 s, p=0.08), suggesting long-term social memory impairment.
Figure 5. Social testing.
The standard pattern of high socialization time (trial 1) followed by decreased socialization time (trials 2-4) and increased socialization time with a novel OEF (trial 5) demonstrates intact short-term social memory and preference for social novelty in both groups. Reintroduction of the original OEF in trial 6 reveals that hypoxic males have less decline in socialization time than controls, suggesting impairment of long-term social memory (5.5 s vs. 14.4 s, p=0.08). n=14 (MC=7,MH=7). □: normoxia; ■: hypoxia.
Brain volume analysis
Regional brain volumes (cerebrum, cerebellum and hippocampus) were measured in a subset of mice after behavioral testing. In our previous work (8), mice exposed to hypoxia experienced a 31% reduction in cortical volume and a 40% reduction in cerebellar and hippocampal volume at P11. In particular, sex-specific differential volume loss in the hippocampus was seen (43% reduction in males, 36% reduction in females). By 20 weeks of age, cortical volumes in hypoxic males and females recovered completely compared to sex-matched controls (Figure 6A). However, hippocampal volumes remained 25% smaller (p<0.01) and cerebellar volumes remained 23% (p<0.01) smaller in hypoxic males compared to controls (Figure 6B-C). In contrast, hypoxic females recovered hippocampal and cerebellar volumes to control levels.
Figure 6. Brain section analysis.
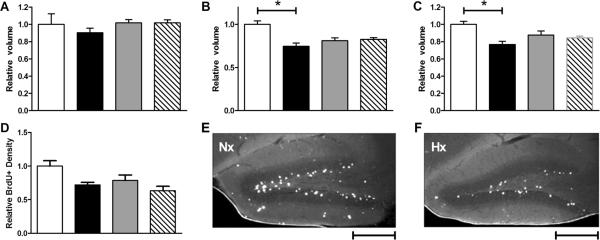
(A) Cortical volumes in hypoxic animals have recovered to control levels. (B) Hippocampal volumes in adult hypoxic males remain significantly smaller than controls (MH 0.75 vs. MC 1, p<0.01) while hippocampal volumes in hypoxic females recover. (C) Cerebellar volumes in adult hypoxic males remain significantly smaller than controls (MH 0.77 vs. MC 1, p<0.01) while cerebellar volumes in hypoxic females have recovered. n=21 (MC=4,MH=7,FC=4,FH=6). (D) BrdU+ cell density in the dentate gyrus of hypoxic males is reduced compared to controls (p=0.08). BrdU+ cell density in the dentate gyrus of hypoxic females trends toward reduction compared to controls but is not significant (p=0.45). (E-F) BrdU immunostaining in the dentate gyrus of normoxic (Nx) and hypoxic (Hx) male hippocampus at 4x. Scale bar = 500 μm. n=16 (MC=3,MH=5,FC=3,FH=5). Volumes and cell densities normalized to male controls. White=MC, black=MH, gray=FC, hatched=FH.
Cell proliferation
In a separate group of mice, BrdU was injected intraperitoneally on P11 to assess the effect of hypoxia on cell proliferation in the hippocampus. Proliferation in the hippocampus was decreased in the dentate gyrus following CSH (Figure 6D). BrdU+ cell density is reduced in hypoxic males compared to controls, approaching statistical significance (p=0.08). BrdU+ cell density also appears slightly decreased in hypoxic females compared to controls, although this was not statistically significant (p=0.45).
DISCUSSION
The CSH rodent model closely mimics preterm brain injury. In preterm infants, chronic, low-grade cerebral hypoxia has been correlated with adverse neurodevelopmental outcomes (17). This cerebral hypoxia is simulated by exposing mice pups to CSH from P3-P11, a period of mouse brain development matching the third trimester in humans. Brain volume loss, reduced subcortical white matter, ventriculomegaly (7) and disorganization of white matter tracts (11) are seen in both CSH-treated rodents and in preterm humans. Male mice exposed to CSH were particularly susceptible to hippocampal volume loss and hypomyelination when measured immediately after hypoxia (8). Here, we present the first study on long-term, sex-specific effects of CSH on neurobehavioral outcomes and brain volume recovery.
The behavioral testing performed was prompted by our observation of greater hippocampal volume loss in males following CSH (8). The role of the hippocampus in associative learning is increasingly appreciated (18) and its role in spatial memory and spatial information processing is well-established (12, 19). Our behavioral assays were selected specifically to test these functions. We used the IntelliCage, a recently developed behavioral screening tool that has been used to detect cognitive impairments related to hippocampal damage (20). With the IntelliCage, programmable delivery of cues, reward and punishment combined with automated tracking of individual mice allows for multiple behavioral assays to be done on a large cohort of mice simultaneously. Environmental conditions affect standard behavioral testing (21) but the IntelliCage requires minimal handling and training. Mice live in a natural, enriched, social environment, reducing anomalous behaviors related to individual housing needed for certain tests (22). Automated data collection increases throughput, reducing the time and cost of behavioral screening. Using the IntelliCage, we detected greater long-term impairments in reversal of place learning and associative learning in males, suggesting that males subjected to CSH are not only more susceptible to short-term hippocampal injury, but also to long-term hippocampal dysfunction.
Although the IntelliCage has been used in several studies (20, 23), its use in this model is novel. To validate the IntelliCage results, we performed MWM testing, a standard behavioral assay for hippocampus-dependent spatial learning and memory. Consistent with IntelliCage results, greater deficits were again seen in males exposed to CSH on the MWM.
Social testing was included since mounting evidence suggests that preterm infants, particularly males, are at risk for socialization abnormalities (2, 24). Sociability, social recognition and short-term social memory were unaffected. The lack of a difference in most measures of social testing may reflect an inability of our assay to detect subtle social deficits, particularly social deficits that are often subjective in human studies (25). Most rodent studies demonstrating significant social deficits have involved knockout mice (10, 26), extreme models whose phenotypes may not be replicated by our model. Long-term social memory appeared impaired in hypoxic males consistent with hippocampal dysfunction.
In this study, long-term hippocampal and cerebellar volume loss persisted only in CSH-treated male. Cell proliferation in the hippocampus was attenuated in CSH-treated males, which may explain in part their inability to recover hippocampal volume. Our data suggests a link between decreased hippocampal size and impaired long-term hippocampal-dependent cognitive function. However, IntelliCage and MWM testing can be affected by changes in brain regions beyond the hippocampus. Volumetric analysis suggests that cerebellar damage may also be involved in the persistent male deficits. Cortical damage is less likely to contribute since cortical volumes recover by adulthood, although recovery of volumes may not equate to recovery of function. This cortical volume recovery is consistent with data describing catch-up cortical growth (7) and enhanced cortical neurogenesis (27) after CSH. Prior work showed a brisk proliferative response to CSH in the cortical subventricular zone (27), in contrast to our observation of decreased proliferation in the post-CSH hippocampus. Regional differences in cell proliferation in CSH-treated males may explain why cortical volumes recover while hippocampal volumes remain reduced in adulthood. The inability of males to completely recover from cerebellar volume loss was unexpected; no sex differences were seen in cerebellar volume loss in our prior study (8). From observation, hypoxic males and females have erratic, jittery movements shortly after hypoxia. These movements become less noticeable over time and are not observed by adulthood, suggesting recovery of gross cerebellar function, if not cerebellar volume.
It is interesting that in very low birth weight human infants imaged by MRI at 15 years of age, males had less hippocampal and cerebellar white matter than females (28), similar to our mice. Strikingly, in that same study, hippocampal and cerebellar white matter volumes were correlated with cognitive and perceptual functions. While we did not observe long-term differences in myelination using histological methods (data not shown), other work has reported DTI abnormalities in adult male mice exposed to CSH (11).
Behavioral studies are often performed on male mice only because effects of potential estrus on behavior are unknown. However, testing for sex differences necessitates study of both sexes. By co-housing females which suppresses estrus cycles (29) and comparing hypoxic to control females, we reduced the potential estrus cycle confounding effects. The neglect of sex-specific analysis in neuroscience research has recently been criticized (30). Our results demonstrate the need to study the effects of brain injury in a sex-specific manner in order to identify male risk factors or female protective factors that could lead to targeted therapeutic modalities. Our study demonstrates the relative ease and importance of sex comparisons.
Although sex differences are noted in many diseases and model systems, the mechanism underlying the differences remains unclear. Sex differences may be related to different sex steroid exposure or receptor expression between males and females or to differential temporal development the male versus female brain, thus shifting the timing of susceptibility (31). Lastly, sex differences may be due to yet-unidentified genetic differences from sex chromosomes (32).
We have established the viability of the CSH mouse model for sex-specific neurodevelopmental outcome studies. Furthermore, we have demonstrated the utility of novel (IntelliCage) and standard (MWM) behavioral assays as methods to test the effects of preterm brain injury in animal models. This model can be utilized to develop and test potential neuroprotective strategies tailored to the sex-specific requirements of vulnerable preterm infants.
Acknowledgements
We thank Ghezal Omar and Jonathan Tran for their histology assistance.
Statement of financial support: Supported by NIH Director's New Innovator Award DP2OD005675, K08 NS044989, John Merck Fund for Developmental Disabilities and Packard Children's Health Initiative (A.A.P.); Lucile Packard Foundation for Children's Health Pediatric Research Fund, Ruth L. Kirschstein National Research Service Award T32-HD007249 (W.J.L.); NIMH T32MH020016, NINDS R01NS053898-03S1 (S.R.M.)
ABBREVIATIONS
- BrdU
5-bromodeoxyuridine
- CSH
chronic, sublethal hypoxia
- DMP
delayed matching-to-place
- FC
female control
- FH
female hypoxia
- MC
male control
- MH
male hypoxia
- MWM
Morris water maze
- OEF
ovariectomized female
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hintz SR, Kendrick DE, Vohr BR, Kenneth Poole W, Higgins RD. For The Nichd Neonatal Research N 2006 Gender differences in neurodevelopmental outcomes among extremely preterm, extremely-low-birthweight infants. Acta Paediatr. 95:1239–1248. doi: 10.1080/08035250600599727. [DOI] [PubMed] [Google Scholar]
- 2.Charkaluk ML, Truffert P, Fily A, Ancel PY, Pierrat V. Neurodevelopment of children born very preterm and free of severe disabilities: the Nord-Pas de Calais Epipage cohort study. Acta Paediatr. 2010;99:684–689. doi: 10.1111/j.0803-5253.2010.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 4.Thompson DK, Warfield SK, Carlin JB, Pavlovic M, Wang HX, Bear M, Kean MJ, Doyle LW, Egan GF, Inder TE. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130:667–677. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- 5.Yager JY, Ashwal S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr Neurol. 2009;40:156–167. doi: 10.1016/j.pediatrneurol.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Back SA, Rivkees SA. Emerging concepts in periventricular white matter injury. Semin Perinatol. 2004;28:405–414. doi: 10.1053/j.semperi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Ment LR, Schwartz M, Makuch RW, Stewart WB. Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Brain Res Dev Brain Res. 1998;111:197–203. doi: 10.1016/s0165-3806(98)00139-4. [DOI] [PubMed] [Google Scholar]
- 8.Mayoral SR, Omar G, Penn AA. Sex differences in a hypoxia model of preterm brain damage. Pediatr Res. 2009;66:248–253. doi: 10.1203/PDR.0b013e3181b1bc34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steele RJ, Morris RG. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 11.Chahboune H, Ment LR, Stewart WB, Rothman DL, Vaccarino FM, Hyder F, Schwartz ML. Hypoxic injury during neonatal development in murine brain: correlation between in vivo DTI findings and behavioral assessment. Cereb Cortex. 2009;19:2891–2901. doi: 10.1093/cercor/bhp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bannerman DM, Deacon RM, Seeburg PH, Rawlins JN. GluR-A-Deficient mice display normal acquisition of a hippocampus-dependent spatial reference memory task but are impaired during spatial reversal. Behav Neurosci. 2003;117:866–870. doi: 10.1037/0735-7044.117.4.866. [DOI] [PubMed] [Google Scholar]
- 14.McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Brain Res Rev. 1993;18:33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- 15.Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- 16.Auer RN, Jensen ML, Whishaw IQ. Neurobehavioral deficit due to ischemic brain damage limited to half of the CA1 sector of the hippocampus. J Neurosci. 1989;9:1641–1647. doi: 10.1523/JNEUROSCI.09-05-01641.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pryds O. Low neonatal cerebral oxygen delivery is associated with brain injury in preterm infants. Acta Paediatr. 1994;83:1233–1236. doi: 10.1111/j.1651-2227.1994.tb13002.x. [DOI] [PubMed] [Google Scholar]
- 18.Brasted PJ, Bussey TJ, Murray EA, Wise SP. Role of the hippocampal system in associative learning beyond the spatial domain. Brain. 2003;126:1202–1223. doi: 10.1093/brain/awg103. [DOI] [PubMed] [Google Scholar]
- 19.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 20.Voikar V, Colacicco G, Gruber O, Vannoni E, Lipp HP, Wolfer DP. Conditioned response suppression in the IntelliCage: assessment of mouse strain differences and effects of hippocampal and striatal lesions on acquisition and retention of memory. Behav Brain Res. 2010;213:304–312. doi: 10.1016/j.bbr.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 22.Wolfer DP, Litvin O, Morf S, Nitsch RM, Lipp HP, Wurbel H. Laboratory animal welfare: cage enrichment and mouse behaviour. Nature. 2004;432:821–822. doi: 10.1038/432821a. [DOI] [PubMed] [Google Scholar]
- 23.Mechan AO, Wyss A, Rieger H, Mohajeri MH. A comparison of learning and memory characteristics of young and middle-aged wild-type mice in the IntelliCage. J Neurosci Methods. 2009;180:43–51. doi: 10.1016/j.jneumeth.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Autism spectrum disorders in extremely preterm children. J Pediatr. 2010;156:525–531. doi: 10.1016/j.jpeds.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt LA, Miskovic V, Boyle MH, Saigal S. Shyness and timidity in young adults who were born at extremely low birth weight. Pediatrics. 2008;122:e181–e187. doi: 10.1542/peds.2007-3747. [DOI] [PubMed] [Google Scholar]
- 26.Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 27.Fagel DM, Ganat Y, Silbereis J, Ebbitt T, Stewart W, Zhang H, Ment LR, Vaccarino FM. Cortical neurogenesis enhanced by chronic perinatal hypoxia. Exp Neurol. 2006;199:77–91. doi: 10.1016/j.expneurol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Martinussen M, Flanders DW, Fischl B, Busa E, Lohaugen GC, Skranes J, Vangberg TR, Brubakk AM, Haraldseth O, Dale AM. Segmental brain volumes and cognitive and perceptual correlates in 15-year-old adolescents with low birth weight. J Pediatr. 2009;155:848–853. doi: 10.1016/j.jpeds.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKinney TD. Estrous cycle in house mice: effects of grouping, preputial gland odors, and handling. J Mammal. 1972;53:391–393. [PubMed] [Google Scholar]
- 30.2010 Putting gender on the agenda. Nature. 465:665. doi: 10.1038/465665a. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy MM. The two faces of estradiol. Neuroscientist. 2009;15:599–610. doi: 10.1177/1073858409340924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mage DT, Donner M. Female resistance to hypoxia: does it explain the sex difference in mortality rates? J Womens Health (Larchmt) 2006;15:786–794. doi: 10.1089/jwh.2006.15.786. [DOI] [PubMed] [Google Scholar]