Abstract
We have evaluated two synthetic epothilone analogues lacking the 12,13-epoxide functionality, 12,13-desoxyepothilone B (dEpoB), and 12,13-desoxyepothilone F (dEpoF). The concentrations required for 50% growth inhibition (IC50) for a variety of anticancer agents were measured in CCRF-CEM/VBL1000 cells (2,048-fold resistance to vinblastine). By using dEpoB, dEpoF, aza-EpoB, and paclitaxel, the IC50 values were 0.029, 0.092, 2.99, and 5.17 μM, respectively. These values represent 4-, 33.5-, 1,423- and 3,133-fold resistance, respectively, when compared with the corresponding IC50 in the parent [nonmultiple drug-resistant (MDR)] CCRF-CEM cells. We then produced MDR human lung carcinoma A549 cells by continuous exposure of the tumor cells to sublethal concentrations of dEpoB (1.8 yr), vinblastine (1.2 yr), and paclitaxel (1.8 yr). This continued exposure led to the development of 2.1-, 4,848-, and 2,553-fold resistance to each drug, respectively. The therapeutic effect of dEpoB and paclitaxel was also compared in vivo in a mouse model by using various tumor xenografts. dEpoB is much more effective in reducing tumor sizes in all MDR tumors tested. Analysis of dEpoF, an analog possessing greater aqueous solubility than dEpoB, showed curative effects similar to dEpoB against K562, CCRF-CEM, and MX-1 xenografts. These results indicate that dEpoB and dEpoF are efficacious antitumor agents with both a broad chemotherapeutic spectrum and wide safety margins.
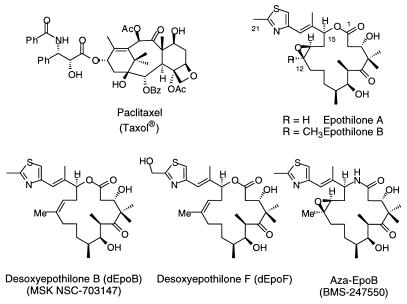
Among naturally occurring molecules that stabilize microtubule assemblies, paclitaxel (Taxol) is by far the best known (Fig. 1). It has been extensively studied and widely used as a front-line cancer chemotherapeutic agent, especially for treating solid tumors (1–3). The clinical successes of paclitaxel have spurred searches for other agents that inhibit the growth of tumors through similar tubulin-based mechanisms of action. More recently discovered compounds with a taxol-like mechanism of action include the discodermolides (4, 5), eleutherobins (6), laulimalides (7), and epothilones (8–14). Despite their supposed common binding site on tubulin assemblies (15–18), their chemical structures and/or pharmacological profiles are drastically different.
Figure 1.
Structures of paclitaxel and epothilones.
Epothilones A and B (Fig. 1), along with other minor related constituents, are macrolides isolated from the cellulose degrading myxobacterium Sorangium cellulosum (8). These cytotoxic compounds have evoked much attention among scientists in various disciplines because of their remarkable antitumor activities. Epothilone B (EpoB) exhibits significantly higher cytotoxicity than does taxol against various cancer cell lines. In particular, the epothilones are apparently not susceptible to deactivation through multiple drug resistance (MDR) and maintain activity against resistant tumor cell lines.
Because of the promising in vitro data associated with epothilones, we have been engaged in their chemical synthesis and biological evaluation. Pharmacological evaluations in xenograft mouse models revealed that EpoB, although the most potent drug in the epothilone series, possessed poor therapeutic efficacy at its maximal-tolerated dose (19). In contrast, we discovered that 12,13-desoxyepothilone B (dEpoB) displays a much more promising therapeutic index despite its slightly decreased in vitro cytotoxicity relative to EpoB (19, 20).
In this paper, we present detailed investigations of the in vitro and in vivo pharmacologic properties of dEpoB (14) and desoxyepothilone F (dEpoF) (21). A comparison of dEpoB and dEpoF relative to 15-desoxy-15-aza-epothilone (aza-EpoB, BMS-247550) (22), a compound that has been advanced to phase I clinical trials by Bristol–Myers Squibb (BMS), is also discussed. The BMS group obtained the lactam version of EpoB through an elegant partial synthesis starting from EpoB. In our lab, we gained access to aza-EpoB and dEpoF through the medium of total synthesis. The synthetic details for our production of dEpoF and aza-EpoB have been published elsewhere (21, 22), hence this paper will focus on the pharmacological properties of these compounds in comparison to dEpoB. It will be shown that dEpoB and dEpoF each manifest a broad spectrum of antitumor properties and exhibit a curative effect in nude mice bearing various human tumor xenografts such as leukemia, mammary, and colon carcinoma. The preliminary toxicological profile of dEpoB has also been assessed and demonstrates that dEpoB is well tolerated at therapeutically effective doses. These findings mark dEpoB and dEpoF as highly promising candidates for advancement to human clinical trials.
Materials and Methods
Chemicals.
Paclitaxel (Taxol), etoposide (VP-16), teniposide (VM-26), camptothecin (CPT), actinomycin D (AD), and vinblastine sulfate (VBL) were purchased from Sigma. All stock solutions of the above compounds were prepared by using DMSO as solvent (except VBL in saline) and were further diluted to desired concentrations for experimental use. The final concentration of DMSO in the tissue cultures was 0.25% (vol/vol) or less to avoid solvent cytotoxicity. For in vivo studies, dEpoB, dEpoF, and aza-EpoB were dissolved in Cremophor/ethanol (1:1) vehicle and then diluted with saline for i.v. infusion over 6 hours. Paclitaxel in Cremophor/ethanol formulation was commercially obtained as the clinical preparation from Bristol–Myers Squibb. VBL (Velban; Eli Lilly), etoposide (VP-16, Vepisid, Bristol–Myers Squibb), Camptosar (Irinotecan or CPT-11; Amersham Pharmacia and Upjohn), and adriamycin (DX or Adr, Doxorubicin-HCl; Amersham Pharmacia and Upjohn) were used at the manufacturer's formulation and diluted with saline.
Tumor and Cell Lines.
The CCRF-CEM human lymphoblastic leukemic cells, its teniposide-resistant subline (CCRF-CEM/VM1), and vinblastine-resistant subline (CCRF-CEM/VBL100) were obtained from W. T. Beck (University of Illinois, Chicago, Il). These sublines were exposed to increasing sublethal concentrations (IC50–IC90) of: vinblastine for 16 months, paclitaxel for 12 months, and teniposide for 12 months, respectively (designated CCRF-CEM/VBL1000, CCRF-CEM/taxol, and CCRF-CEM/VM2, respectively). These nascent resistant cell lines exhibited a 2,048-fold resistance to vinblastine (IC50: 0.973 μM), a 206-fold resistance to paclitaxel (IC50: 0.339 μM), and a 12.5-fold resistance to etoposide (IC50: 19.8 μM), respectively, when compared with naive CCRF-CEM cells. A similar procedure was used for the time course of the development of drug resistance in A549 human lung carcinoma cells. Ovarian adenocarcinoma UL3-C and UL3-B/taxol and hamster lung fibroblasts and its sublines (DC-3F, DC-3F/ADII and DC-3F/ADX) were obtained from the cell bank of this institution.
The following human cancer cells were obtained from American Type Culture Collection (ATCC, Rockville, MD): mammary carcinoma (MX-1), mammary adenocarcinoma (MCF-7), ovarian adenocarcinoma (SK-OV-3), lung carcinoma (A549), colon adenocarcinoma (HT-29), colon carcinoma (HCT-116), prostate adenocarcinoma (PC-3), chronic myeloblastic leukemia (K562), and promyelocytic leukemia (HL-60).
Animals.
Athymic nude mice bearing the nu/nu gene were used for all human tumor xenografts. Outbred Swiss-background mice were obtained from Charles River Breeding Laboratories. Male mice 8 weeks old or older weighing 22 g or more were used for most experiments. Drug was administered via the tail vein for 6 h by i.v. infusion. Tumor volumes were assessed by measuring length × width × height (or width) by using a caliper. A programmable Harvard PHD2000 syringe pump with multitrack was used for i.v. infusion. The typical 6-h infusion volume for each drug in Cremophor/ethanol (1:1) was 100 μl in 2.0 ml of saline. All animal studies were conducted in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Animals and the protocol approved by the Memorial Sloan–Kettering Cancer Center's Institutional Animal Care and Use Committee. In keeping with the policy of this committee for the humane treatment of tumor-bearing animals, mice were euthanized when tumors reached ≥10% of their total body weight.
Cytotoxicity Assays.
In preparation for in vitro cytotoxicity assays, cells were cultured at an initial density of 2–5 × 104 cells per milliliter. They were maintained in a 5% CO2-humidified atmosphere at 37°C in RPMI medium 1640 (GIBCO/BRL) containing penicillin (100 units/mL), streptomycin (100 μg/mL, GIBCO/BRL), and 5% heat-inactivated FBS. For solid tumor cells growing in a monolayer (such as MCF-7/Adr), cytotoxicity of the drug was determined in 96-well microtiter plates by using the sulforhodamine B method as described by Skehan et al. (23). For cells grown in suspension (such as CCRF-CEM and its sublines), cytotoxicity was measured, in duplicate, by using the 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-5-carboxanilide)-2H-terazodium hydroxide (XTT) microculture method (24) in 96-well microtiter plates. For both methods, the absorbance of each well was measured with a microplate reader (EL-340, Bio-Tek, Burlington, VT). Dose–effect relationship data were analyzed with the median-effect plot (25) by using a previously described computer program (26).
HPLC Analysis.
To prepare a plasma sample for HPLC analysis, 30 μl of methanol was added to either human blood plasma or nude mice plasma (300 μl) containing dEpoB (0.05–20 μg/mL) and mixed for 2 min, followed by the addition of an additional 300 μl of methanol. The centrifuged supernatant was analyzed by using a Nova-pak C18 column (3.9 × 300 cm, Waters) with 65% acetonitrile/water containing 0.08% triethylamine and 0.02% phosphoric acid as the mobile phase at 0.8 mL/min. UV absorbance was measured at 250 nm.
Results
In Vitro Cytotoxicity Against Leukemic and Solid Tumor Cells and Their Sublines.
The in vitro cytotoxicities of dEpoB, dEpoF, aza-EpoB, and paclitaxel against various tumor cells were first examined (see Table 1, which is published as supplemental data on the PNAS web site, www.pnas.org). The growth of all parent cell lines tested was strongly inhibited by the above four compounds with similar IC50 values. dEpoB and dEpoF exhibited little drug resistance, whereas both aza-EpoB and paclitaxel showed substantially diminished activities against drug resistance subcell lines. It is noteworthy that the desoxyepothilones consistently outperformed other conventional anticancer agents in inhibiting the growth of the resistant tumor cells.
Therapeutic Efficacy of Various Anticancer Agents Against Human Colon Carcinoma HCT-116 Xenografts.
The therapeutic efficacy of antitumor agents fluctuates with a variety of factors, such as the tumor models used, the dosage of drug, treatment schedules, and routes of administration. To establish a protocol that allows for comparison between various anticancer agents, xenografts of a sensitive tumor (human colon carcinoma HCT-116) were first selected and treated with chemotherapeutic agents. When the same treatment schedule described in Materials and Methods was applied, dEpoB, dEpoF, paclitaxel, and CPT-11 all exhibited curative effects (see Fig. 9, which is published as supplemental data on the PNAS web site). These results indicate that the dose levels of each drug to be used in the present studies are adequate, and the routes of administration are viable. Comparison of the therapeutic effects of differing anticancer agents was performed at near their maximal tolerated doses by measuring the extent of body-weight decreases and/or lethality. Thus, the protocols established in these experiments were extended to subsequent in vivo tests.
Therapeutic Efficacy of Various Anticancer Agents Against Human Leukemia K562 Xenografts.
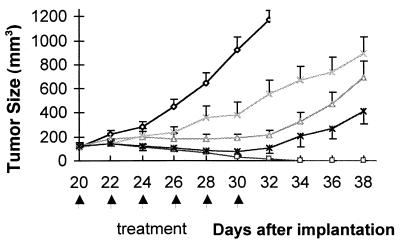
The therapeutic efficacy of dEpoB was compared with paclitaxel, doxorubicin, and vinblastine by using sensitive (non-MDR) human chronic myelocytic leukemia K562 xenografts in nude mice (Fig. 2). In these experiments, dEpoB was shown to be particularly effective, as manifested by complete remission of the tumor mass at the end of the treatment. By contrast, adriamycin, paclitaxel, and vinblastine exhibited only partial suppressive effects. These results provide a differential perspective on our initial findings (19, 27, 28), in which dEpoB showed comparable efficacy to paclitaxel in several sensitive tumors but proved superior over paclitaxel in a broad range of drug-resistant tumors (e.g., tumors resistant to VBL, Adr, or paclitaxel). The striking difference presented by these experiments suggests that even sensitive tumors can be more responsive to dEpoB than conventional anticancer agents.
Figure 2.
Therapeutic effect of dEpoB, paclitaxel (Taxol), doxorubicin (Adriamycin), and vinblastine in nude mice bearing human chronic myelocytic leukemia tumor K562 xenograft. K562 tumor cells (1 × 107, in 0.2 ml/mouse) were implanted s.c. on day 0. Every other day, treatments were given starting on day 20 with 30 mg/kg of dEpoB, 6-h i.v. infusion (□, n = 5); 20 mg/kg of Taxol 6-h i.v. infusion (Δ, n = 3); 3 mg/kg of Adriamycin, i.v. injection, (X, n = 4), and 2.5 mg/kg of vinblastine, i.v. injection (*, n = 4). The control mice (O, n = 4) received vehicle, Cremophor–ethanol (1:1), only. The vertical bars indicate mean tumor size in mm3 ± SE.
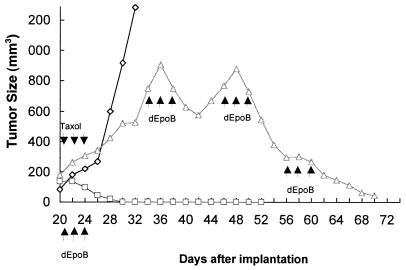
By using the same K562 xenograft, a direct comparison between dEpoB and paclitaxel was made (Fig. 3). Remarkably, a single course of treatment with dEpoB led to the disappearance of the tumor in 4 weeks. On the other hand, the identical schedule of treatment with paclitaxel showed little curative effects. When the paclitaxel-treated mice were subsequently subjected to dEpoB administration, the rapid response of the tumor size was observed. After three cycle treatments, the established tumor mass as large as 880 mm3 dramatically began to shrink to less than 20 mm3 in size.
Figure 3.
Therapeutic effect of single drug treatment with dEpoB or paclitaxel (Taxol) and sequential treatment with Taxol followed by dEpoB in nude mice bearing human chronic myelocytic leukemia tumor K562 xenograft. The tumor tissue (50 mg/mouse) was implanted s.c. on day 0. Every other day, treatments were given starting on day 20 with 30 mg/kg of dEpoB, 6-h i.v. infusion (Q2D × 3, □) and with of 20 mg/kg Taxol, 6-h i.v. infusion (Q2D × 3, Δ). The latter group of mice was then treated with 30 mg/kg of dEpoB for three cycles (Q2D × 3, Δ) as shown by arrows. The control mice (◊) received vehicle (Cremophor–ethanol = 1:1) only. Average tumor size from two mice is shown.
The same sequential experiment was performed by using aza-EpoB and dEpoF (Fig. 4). Treatment of K562 xenografts with the maximal tolerated dose of aza-EpoB (6 mg/kg) strongly suppressed tumor growth but did not result in shrinkage. The same group of mice, which had developed a tumor of ≈500 mm3 in size, were subsequently treated with dEpoF (30 mg/kg). Similar to the observation made in the previous experiment (Fig. 3), the administration of dEpoF led to rapid reduction in the size of the tumor. Furthermore, the tumors continued to shrink after cessation of treatment and advanced to the stage of complete disappearance 3 weeks later.
Figure 4.
Therapeutic effect of sequential treatment of mice bearing K562 xenografts with aza-dEpoB. K562 tumor tissue (1 × 107 in 50 ml/mouse) was inoculated s.c. into nude mice on day 0. Mice were treated with 15 mg/kg of aza-dEpoB (Δ, n = 3), 6-h i.v. infusion on days 20, 22, 24, 26, 28, and 30. The same groups of mice were then treated with 30 mg/kg of dEpoF, 6-h i.v. infusion on days 42, 44, 46, 48, 52, and 54. On day 76, all three mice were tumor free. The control mice (O, n = 3) received vehicle (Cremophore–ethanol = 1:1) only.
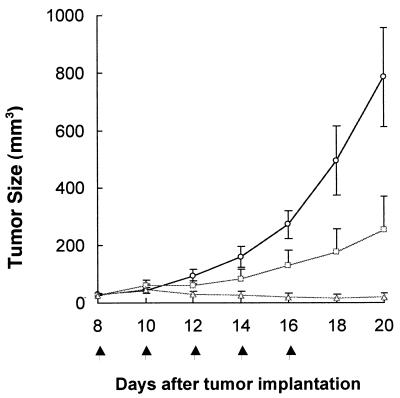
Therapeutic Efficacy of dEpoF Against Human Mammary Carcinoma MX-1 Xenografts.
We had shown earlier that both dEpoB and paclitaxel displayed curative effects against MX-1 xenografts (19, 27, 28). A similar experiment was conducted with dEpoF as shown in Fig. 5. In this experiment, subjects were treated with the drug at two different dose levels. Although partial suppression of tumor growth was observed at a dose of 15 mg/kg, increasing the dose of dEpoF to 30 mg/kg readily reduced the size of the tumor. At the end of the treatment, complete disappearance of the tumor occurred in three of five mice.
Figure 5.
Therapeutic effect of dEpoF in nude mice bearing MX-1 xenografts. MX-1 tumor tissue (50 mg/mouse) was implanted s.c. into nude mice on day 0. The doses of 15 mg/kg (□, n = 4) and 30 mg/kg (Δ, n = 5) of dEpoF were given via 6-h i.v. infusion on days 8, 10, 12, 14, and 16. For the 30 mg/kg group, three of five mice were tumor free on days 16, 18, and 20, respectively. The vertical bars indicate mean tumor size in mm3 ± SE.
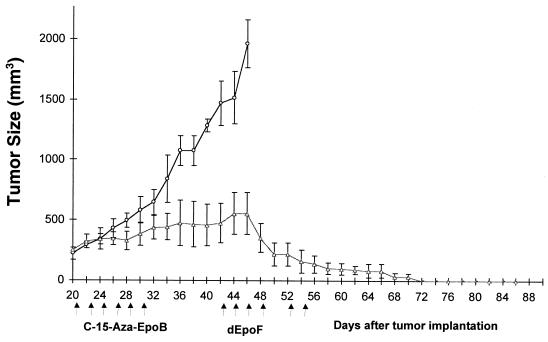
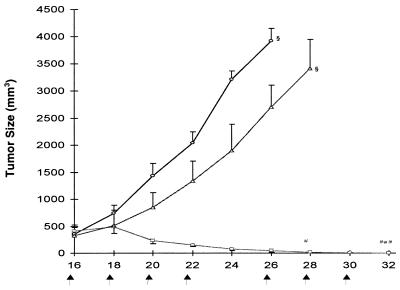
Comparison of the Therapeutic Efficacy of dEpoF and Aza-EpoB Against CCRF-CEM Xenografts.
Nude mice bearing well established CCRF-CEM tumor xenografts were treated with either dEpoF or aza-EpoB. As shown in Fig. 6, only moderate suppression of tumor growth was observed by treatment with aza-EpoB. In sharp contrast, dEpoF markedly reduced tumor size, rendering one of three mice completely tumor free on day 28 (sixth dose). The remaining two mice in the study became tumor free on day 32. The control group showed a slight gain in body weight (0.7 g) on day 26, as determined by the subtraction of the tumor (4 g) from the total body weight. The mice treated with dEpoF showed reduction in their body weight by ≈18% on day 32 yet without lethality. In the case of mice treated with aza-EpoB, a 13% reduction in body weight and 3.4 g of average tumor weight were observed on day 28, after which the mice were killed because of excess tumor burden.
Figure 6.
Therapeutic effect of dEpoF and aza-EpoB against CCRF-CEM tumor xenografts. Mice were treated with 30 mg/kg of dEpoF (□) or 6 mg/kg of aza-EpoB (Δ) via 6-h i.v. infusion on days 16, 18, 20, 22, 26, and 28 (n = 3). On day 28, one of three mice was tumor free, and tumors disappeared in all mice on day 32. The control mice (O, n = 3) received vehicle only. On day 16, when treatment started, the tumors were well established, with an average size of about 400 mm3 for each group. Control mice were scarified on day 26, and the mice treated with aza-EpoB were scarified on day 28 because of excessive tumor burden.
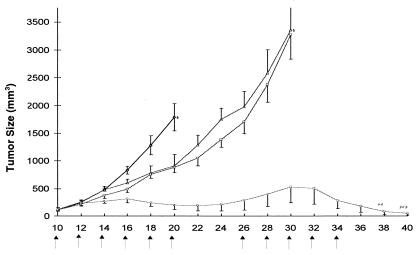
Comparison of the Therapeutic Efficacy of dEpoB vs. Aza-EpoB Using MX-1 Xenografts.
The tumor-suppressive activity of dEpoB and aza-EpoB was assessed in a side-by-side comparison by using MX-1 xenografts of ≈100 mm2 in size. Treatment of the xenograft mice with aza-EpoB effected inhibition of the tumor growth but without tumor shrinkage. Further treatment with aza-EpoB had little effect as tumor progression continued. Eventually, the animals were scarified because of tumor burden (Fig. 7). There was little change in the body weight of mice treated with aza-EpoB at dose levels of 4 mg/kg. However, dose levels of 6 mg/kg resulted in body-weight decreases of about 3 g on day 18, and one of five mice died of toxicity on day 22.
Figure 7.
Therapeutic effect of dEpoB and aza-EpoB against MX-1 xenografts in nude mice. MX-1 tumor tissue (50 mg/mouse) was implanted s.c. onto nude mice on day 0. The control mice (◊, n = 5) received vehicle only. Aza-EpoB 4 mg/kg (□, n = 5), 6 mg/kg (Δ, n = 5), and dEpoB 30 mg/kg (O, n = 5) were given via 6-h i.v. infusion Q2D × 6 on days 10, 12, 14, 16, 18, and 20. On day 10, the average tumor size was 120 mm3. The second cycle of treatments (Q2D × 3) with aza-EpoB (4 mg/kg) and aza-EpoB (6 mg/kg) started on days 26, 28, and 30. On day 30, the mice in these two groups were killed because of excessive tumor burden. The second cycle of treatments for dEpoB (30 mg/kg, Q2D × 5) started on day 26 and ended on day 34. On day 22, one of five mice in the group treated with aza-EpoB (6 mg/kg) died of toxicity. The three of five mice treated with dEpoB exhibited tumor disappearance on days 32, 38, and 40. In the aza-EpoB (6 mg/kg)-treated group, one of five mice died of toxicity on day 22.
In a similar set of experiments, nude mice bearing MX-1 tumor of ≈120 mm3 in size were treated with dEpoB. Initial administration of dEpoB on days 10–20 slowed the rate of tumor growth with subsequent tumor shrinkage (Fig. 7). On continued treatment with dEpoB on days 26–34, complete remission of tumor masses was achieved in three of five mice on days 32, 38, and 40. It was noted that the tumor shrinkage continued even six days after the last dose of dEpoB. Administration of dEpoB caused decrease in body-weight of 3.5 g as measured on day 22. However, the subjects quickly recovered their body weight to the near control level on day 26. After the second cycle of treatment with dEpoB, a similar body-weight loss of ≈4.1 g was noted on day 36. Gratifyingly, as much as 2 g of body-weight was recovered in days 36–40, during which tumor disappearance occurred.
Comparison of the Therapeutic Efficacy of dEpoB vs. Paclitaxel by Using Various Human Tumor Xenografts.
In addition to a comparison with aza-EpoB, the therapeutic effect of dEpoB was evaluated relative to that of paclitaxel in a variety of human tumor xenografts (Table 2, which is published as supplemental data on the PNAS web site). Eight solid tumors (lung carcinoma A549, mammary carcinoma MX-1, colon adenocarcinoma HT-29, colon carcinoma HCT-116, prostate adenocarcinoma PC-3, ovary adenocarcinomas SK-OV-3, and UL3-C) and five leukemic tumors (T-cell acute lymphoblastic leukemia CCRF-CEM and its 73-fold paclitaxel-resistant CCRF-CEM/taxol subline and 699-fold vinblastine-resistant CCRF-CEM/VBL100 subline, chronic myeloblastic leukemia K562, and promyelocytic leukemia HL-60) were used for s.c. implantation. In the cases of all three drug-resistant tumors (MCF-7/Adr, CCRF-CEM/taxol, and CCRF-CEM/VBL100) and leukemia (K562), dEpoB showed a much greater (>>) or very much greater (>>>) therapeutic effect than paclitaxel. dEpoB produced an equal or slightly greater effect (≥) than paclitaxel in the tumor models such as lung A549, mammary MX-1, and leukemia CCRF-CEM. Some tumor models, such as colon HT-29, colon HCT-116, and leukemia HL-60, were more responsive to the treatment with paclitaxel than dEpoB. In the cases of prostate PC-3 and ovarian SK-OV-3 xenografts, paclitaxel demonstrated a greater effect (>>) than dEpoB. Another ovarian tumor UL3-C, however, showed more responsiveness to dEpoB than to paclitaxel.
In the cases of MCF-7/Adr, HT-29, PC-3, and UL3-C, neither dEpoB nor paclitaxel treatment achieved remission, despite the rapid initial reduction in tumor size. In contrast, tumor disappearance has been realized in all or most MX-1 and CCRF-CEM xenografts by both dEpoB and paclitaxel. As was consistently observed in the cases of aza-EpoB treatment, suppression of the growth of CCRF-CEM/taxol and CCRF-CEM/VBL100 and K562 tumors by paclitaxel treatment did not induce tumor shrinkage, whereas treatment with dEpoB resulted in total tumor disappearance in all animals treated.
In Vivo Comparison of Therapeutic Efficacy Between dEpoB, dEpoF, EpoB, and aza-EpoB Against Several Established Anticancer Agents.
To determine the relative therapeutic efficacy of the epothilones, a comparison was made against anticancer agents such as paclitaxel (Taxol), adriamycin (ADR), vinblastine (VBL), camptothecin, Camptosar (CPT-11), and etoposide (VP-16). Different routes of administration (i.p. or i.v. injection and i.v. infusion) and different schedules of treatment were used in these sets of experiments, which used one murine tumor, eight types of human solid tumors, and three types of human leukemias (see Table 3, which is published as supplemental data on the PNAS web site). The experiments were carried out at or near the maximal tolerated doses with 10–20% decreases in body weight without lethality.
Overall, dEpoB exhibited the broadest and most efficacious effects. dEpoF, an analog of dEpoB possessing an additional hydroxyl group at C21, exhibited a therapeutic profile similar to dEpoB. The in vivo efficacy of dEpoF had not yet been directly compared with other established chemotherapeutic agents. Despite potent in vitro cytotoxicities, EpoB and aza-EpoB, both of which contain the 12,13-epoxide, appeared to possess narrow therapeutic windows, as indicated by the occurrence of deaths even with only moderate decreases in body weight (ref. 19 and Figs. 6 and 7).
Development of Drug-Resistant Cell Models.
To assess the proclivity of cell lines to gain resistance to anticancer agents, human lung carcinoma A549 cells were repeatedly exposed to sublethal concentrations of antitumor agents. Exposure of the cells to vinblastine (VBL) for 14.3 months led to a 4,848-fold resistance. Similarly, after exposure for 21.3 months, the tumor cells gained 2,858- and 16.3-fold resistance to paclitaxel and adriamycin, respectively. On the other hand, only 2.1-fold resistance was rendered by exposure to dEpoB for 21.4 months. These experiments indicate that dEpoB is not only more efficacious against drug-resistant tumor cells but also robust with respect to development of drug resistance.
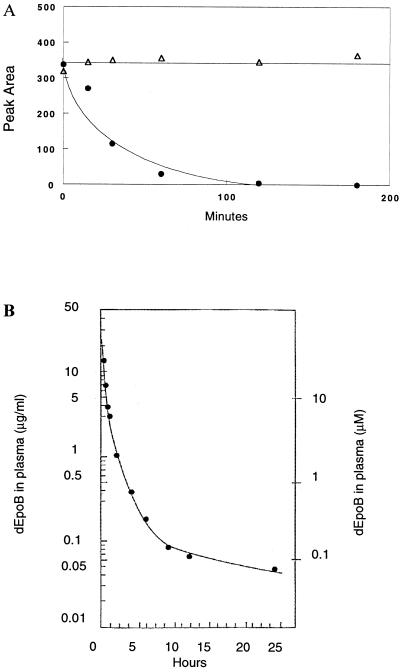
Stability of dEpoB in Plasma.
To exert a desirable pharmacological effect, antitumor agents should retain a reasonable stability in the biological medium. Preliminary studies by using murine plasma suggested that dEpoB, although exhibiting an excellent antitumor profile, had worrisome bioinstability. The approximate in vitro half-life (t1/2) of dEpoB, in murine plasma, was only 20 min (Fig. 8A). However, further studies by using human plasma contradicted these initial findings. In the same experiment with human plasma, dEpoB was found to be stable with a t1/2 of over 3 h, as determined by HPLC analysis. The lower half-life of dEpoB in murine plasma may be a result of elevated esterase levels.
Figure 8.
(A) Stability of dEpoB in mouse and human plasmas in vitro. dEpoB was incubated with nude mice plasma (●) and with human plasma (Δ), and the relative concentrations at various time points were determined by HPLC. (B) The concentration of dEpoB in a beagle dog plasma. dEpoB (6 mg/kg) in Cremophor–ethanol (1:1) was infused i.v. into the femoral vein of a beagle dog over 10 min. Serial samples of blood were collected at different time points up to 24 h. All dEpoB concentrations in plasma in μg/ml on the left scale, and in μM on the right scale were determined by HPLC, as described in Materials and Methods.
Toxicity and Pharmacokinetics of dEpoB in Beagle Dogs.
With the promising in vitro stability and in vivo efficacy of dEpoB, we turned our attention to pharmacokinetic studies. dEpoB was administered to male beagle dogs (11.2–14 kg) via i.v. infusion at doses of 2, 6, 12, and 20 mg/kg. The drug, which was formulated in Cremophor 3%, ethanol 3%, propyleneglycol 40%, and glucose 54%, was administered at a rate of 1 mL/kg for 10 min by using a syringe pump. It was necessary to pretreat the animals at −30 min with Benadryl (5 mg/kg, i.v.), cimetidine (5 mg/kg, i.v.), and dexamethasone (1 mg/kg, i.m.) to minimize allergic reactions because of Cremophor administration.
Dose levels of dEpoB at 2 or 6 mg/kg (40 or 120 mg/m2) produced no observable toxicity. At a dose of 12 mg/kg (240 mg/m2), there was moderate diarrhea (without blood) and a drop in body weight (1.3 kg) on day 3. The body weight slowly recovered in 2–3 weeks. White blood cell counts slightly decreased (13%) after administration of the drug but recovered in 9 days. No significant changes were observed in platelet counts and in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels. Nor were significant histopathologic lesions found in any of the 28 organs or tissues examined, including: heart, lung, kidney, spleen, small and large intestine, liver, muscle, bone marrow, and lymph nodes. When the dose of dEpoB was further increased to 20 mg/kg (400 mg/m2), there was severe bloody diarrhea and dehydration on day 2 with a body-weight loss of 0.9 kg. On day 3, the body-weight loss was 3.6 kg, and the white blood count dropped to 0.6 k/μl accompanied by a 3- to 6-fold increase in ALT and AST by day 4. The subject displayed general weakness, loss of appetite, and slothfulness, and eventually expired on day 4. There was a gross abnormality (reddish discoloration) of the intestinal mucosa from duodenum to the ileum. The small intestines contained blood-tinged mucinous materials. Histopathological examination of bone marrow showed reduction in cellularity. Necrosis of intestinal mucosal epithelium with crypt cells was most severely affected at this lethal dose.
Pharmacokinetic studies were also carried out for dEpoB in beagle dogs at a dose of 6 mg/kg (120 mg/m2 body surface), with 10-min i.v. infusion. Serial blood samples were collected in 5-ml heparinized tubes intermittently from −15 min to 24 h. Plasma concentrations of dEpoB were determined by HPLC, as described in Materials and Methods. dEpoB was shown to have a half-life greater than 5 h (Fig. 8B). At the end of 24-hr collection, dEpoB plasma concentration of 0.045 μg/ml (or 0.092 μM) was considerably higher than the IC50 value of dEpoB (0.0095 μM), which had been required for inhibiting CCRF-CEM cell growth in tissue culture.
Discussion
Despite a similar mechanism of action for tumor inhibition, the epothilones and taxanes show remarkably different MDR profiles. Both the in vitro and in vivo studies described herein clearly indicate that the epothilones are much less susceptible to the onset of MDR. In our comparative experiments, dEpoB has more favorable pharmacological features than paclitaxel, especially in terms of efficacy against drug-resistant cells or tumors (19, 29). Although the naturally occurring congener, EpoB, is the most cytotoxic member of this family, in our assessments in rodents, it possesses a rather narrow therapeutic index (19). In sharp contrast, the less cytotoxic dEpoB exhibited a much enhanced therapeutic range relative to EpoB because of low toxicity toward the host.
Preliminary reports on the in vivo therapeutic effects of dEpoB,** EpoB (30, 31), and aza-EpoB (32) have recently appeared independently, revealing some promising aspects of each compound. In the present article, we have compared dEpoB, dEpoF, aza-EpoB, and paclitaxel in the same experimental settings. It has been demonstrated through these comparative studies that the therapeutic effects of dEpoB and dEpoF are superior to Taxol and aza-EpoB, as well as some currently used cancer therapeutic agents such as etoposide, vinblastine, adriamycin, and Camptostar. The results also indicate that dEpoF and dEpoB have similar chemotherapeutic effects and are curative against human K562 tumor xenografts in nude mice. The high efficacy of dEpoB against a broad spectrum of human tumor xenografts is apparent despite the fact that dEpoB has a short half-life (t1/2 = 15–20 min) in nude mice plasma in vitro. It is likely that the slow infusion protocol may have compensated for the short half-life in mice. Ultimately, the necessity for i.v. infusion may be eliminated by the long half-life of dEpoB in human plasma and in beagle dogs.
Essential features of useful cancer therapeutic agents involve not only high efficiency against various forms of cancer but also low toxicity toward the host [i.e., wide therapeutic window or high therapeutic index (LD50/ED50)]. An important toxicological finding is that dEpoF and dEpoB may render a 23–29% drop in body weight in nude mice during treatment without causing lethality, whereas only a 14–20% drop in body weight caused by administration of EpoB or aza-EpoB led to lethality.** Furthermore, complete tumor disappearances were achieved for dEpoB and dEpoF in the absence of lethality, whereas EpoB (19) or aza-EpoB caused lethality when only marginal therapeutic effects were achieved. Although the origin of the toxicity encountered in the epoxide containing epothilones remains intriguing and warrants further investigation, the current results, as reflected by direct comparisons in murine in vivo models, clearly favor the 12,13-desoxyepothilones as the more promising type of agents. The need for caution in extrapolating results in xenograft mice to human tumors in a clinical setting cannot be overstated.
Supplementary Material
Acknowledgments
We thank Dr. George Sukenick of the NMR core facility for Mass Spectral and NMR analyses, and Quen-Hui Tang and Luan-Ing Chen for technical assistance. This research was supported by National Institutes of Health (NIH) Grants CA-28824 (S.J.D.) and Core Grant CA-08748 (T.C.-C). Postdoctoral fellowship support is gratefully acknowledged [S.J.S. (NIH F32GM1997201) and C.L. (U.S. Army Grant DAMD 17–97-1–7146 and 17–98-1–8155)]. S.J.D. is a member of the Scientific Advisory Board of Kosan Biosciences, Inc., which has a financial interest in potential epothilone drugs.
Abbreviations
- EpoB
Epothilone B
- MDR
multiple drug resistance
- dEpoB
12,13-desoxyepothilone B
- dEpoF
12,13 desoxyepothilone F
- aza-EpoB
15-desoxy-15-aza-epothilone
Footnotes
Chou, T.-C., Guam, Y., Zhang, X.-G., Lee, C., Stachel, S. J., Bertino, J. R. & Danishefsky, S. J. (2001), Proc. Am. Assoc. Cancer Res. 42, 371 (abstr.).
References
- 1.Landino L M, MacDonald T L. In: The Chemistry and Pharmacology of Taxol and Its Derivatives. Favin V, editor. New York: Elsevier; 1995. [Google Scholar]
- 2.Rose W C. Anti-Cancer Drugs. 1992;3:311–321. [PubMed] [Google Scholar]
- 3.Rowinsky E K, Eisenhauer E A, Chaudhry V, Arbuck S G, Donehower R C. Semin Oncol. 1993;20:1–15. [PubMed] [Google Scholar]
- 4.Gunasekera S P, Gunasekera M, Longley R E. J Org Chem. 1990;55:4912–4915. [Google Scholar]
- 5.Haar E T, Kowalski R J, Hamel E, Lin C M, Longley R E, Gunasekera S P, Rosenkranz H S, Day B W. Biochemistry. 1996;35:243–250. doi: 10.1021/bi9515127. [DOI] [PubMed] [Google Scholar]
- 6.Lindel T, Jensen P R, Fenical W, Long B H, Casazza A M, Carboni J, Fairchild C R. J Am Chem Soc. 1997;119:8744–8745. [Google Scholar]
- 7.Mooberry S L, Hernandez T G, Plubrukarn A, Davidson B S. Cancer Res. 1999;59:653–680. [PubMed] [Google Scholar]
- 8.Höfle G, Bedorf N, Steinmetz H, Schumburg D, Gerth K, Reichenbach H. Angew Chem Int Ed Engl. 1996;35:1567–1569. [Google Scholar]
- 9.Balog A, Meng D, Kamenecka T, Bertinato P, Su D-S, Sorensen E J, Danishefsky S J. Angew Chem Int Ed Engl. 1996;35:2801–2803. [Google Scholar]
- 10.Su D-S, Meng D, Bertinato P, Balog A, Soresen E J, Danishefsky S J, Zheng Y-H, Chou T-C, He L, Horwitz S B. Angew Chem Int Ed Engl. 1997;36:757–759. [Google Scholar]
- 11.Nicolaou K C, Winssinger N, Pastor J A, Ninovic S, Sarabia F, He Y, Vourloumis D, Yang Z, Li T, Giannakakou P, Hamel E. Nature (London) 1997;387:268–272. doi: 10.1038/387268a0. [DOI] [PubMed] [Google Scholar]
- 12.Meng D, Su D-S, Balog A, Bertinato P, Sorsensen E J, Danishefsky S J, Zheng Y-H, Chou T-C, He L, Horwitz S B. J Am Chem Soc. 1997;119:2733–2734. [Google Scholar]
- 13.Meng D, Bertinato P, Balog A, Su D-S, Kamenecka T, Sorensen E J, Danishefsky S J. J Am Chem Soc. 1997;119:10073–10092. [Google Scholar]
- 14.Balog A, Harris C R, Savin K, Zhang X-G, Chou T-C, Danishefsky S J. Angew Chem Int Ed Engl. 1998;37:2675–2678. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2675::AID-ANIE2675>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 15.Bollag D M, McQuency P A, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods C M. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 16.Su D-S, Balog A, Meng D, Bertinato P, Danishefsky S J, Zheng Y-H, Chou T-C, He L, Horwitz S B. Angew Chem Int Ed Engl. 1997;36:2093–2096. [Google Scholar]
- 17.Kowalski R J, Giannakakou P, Hamel E. J Biol Chem. 1997;272:2534–2541. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 18.Nicolaou K C, Vourloumis D, Li T, Pastor J, Winssinger N, He Y, Ninkovic S, Sarabia F, Vallberg H, Roschangar F, et al. ChemBioChem. 2001;2:69–78. [Google Scholar]
- 19.Chou T-C, Zhang X-G, Balog A, Su D S, Meng D, Savin K, Bertino J R, Danishefsky S J. Proc Natl Acad Sci USA. 1998;95:9642–9647. doi: 10.1073/pnas.95.16.9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balog A, Harris C R, Savin K, Zhang X-G, Chou T-C, Danishefsky S J. Angew Chem Int Ed Engl. 1998;37:2675–2678. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2675::AID-ANIE2675>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 21.Lee C B, Chou T-C, Zhang X-G, Wang Z-G, Kuduk S D, Chappell M D, Stachel S J, Danishefsky S J. J Org Chem. 2000;65:6525–6533. doi: 10.1021/jo000617z. [DOI] [PubMed] [Google Scholar]
- 22.Stachel S J, Chappell M D, Lee C B, Danishefsky S J, Chou T-C, Horwitz S B. Org Lett. 2000;2:1637–1639. doi: 10.1021/ol005932m. [DOI] [PubMed] [Google Scholar]
- 23.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren J T, Bokesch H, Kenny S, Boyd M R. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 24.Scudiero D A, Shoemaker R H, Paull K D, Monks A, Tierney S, Nofziger T H, Currens M J, Scniff D, Boyd M R. Cancer Res. 1988;46:4827–4833. [PubMed] [Google Scholar]
- 25.Chou T-C, Talalay P T. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 26.Chou T-C, Hayball M. CalcuSyn for Windows. Cambridge, U.K.: Biosoft; 1997. [Google Scholar]
- 27.Chou T-C, Zhang X-G, Danishefsky S J. Proc Am Assoc Cancer Res. 1998;39:163–164. [Google Scholar]
- 28.Chou T-C, Zhang X-G, Harris C R, Kuduk S D, Balog A, Savin K, Danishefsky S J. Proc Natl Acad Sci USA. 1998;95:15798–15802. doi: 10.1073/pnas.95.26.15798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chappell M D, Stachel S J, Lee C B, Danishefsky S J. Org Lett. 2000;2:1633–1636. doi: 10.1021/ol0059302. [DOI] [PubMed] [Google Scholar]
- 30.Altmann K-H, Wartmann K, O'Reilly T. Biochim Biophys Acta. 2000;1479:M79–M91. doi: 10.1016/s0304-419x(00)00009-3. [DOI] [PubMed] [Google Scholar]
- 31.Wartmann M, Muller M, Woods-Cook K, Mett H, Altman K-H, Fabbro D, O'Reilly F. Proc Am Assoc Cancer Res. 1998;39:163. [Google Scholar]
- 32.Borzilleri R M, Zheng X, Schmidt R J, Johnson J A, Kim S-H, DiMarco J D, Fairchild C R, Gougoutas J Z, Lee F Y F, Long B H, Vite G D. J Am Chem Soc. 2000;122:8890–8897. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.