Abstract
Introduction
Mutations in the gene ALPL in hypophosphatasia (HPP) reduce the function of tissue nonspecific alkaline phosphatase, and the resulting increase in pyrophosphate (PPi) contributes to bone and tooth mineralization defects by inhibiting physiologic calcium-phosphate (Pi) precipitation. Although periodontal phenotypes are well documented, pulp/dentin abnormalities have been suggested in the clinical literature although reports are variable and underlying mechanisms remains unclear. In vitro analyses were used to identify mechanisms involved in HPP-associated pulp/dentin phenotypes.
Methods
Primary pulp cells cultured from HPP subjects were established to assay alkaline phosphatase (ALP) activity, mineralization, and gene expression compared with cells from healthy controls. Exogenous Pi was provided to the correct Pi/PPi ratio in cell culture.
Results
HPP cells exhibited significantly reduced ALP activity (by 50%) and mineral nodule formation (by 60%) compared with the controls. The expression of PPi regulatory genes was altered in HPP pulp cells, including reduction in the progressive ankylosis gene (ANKH) and increased ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1). Odontoblast marker gene expression was disrupted in HPP cells, including reduced osteopontin (OPN), dentin matrix protein 1 (DMP1), dentin sialophosphoprotein (DSPP), and matrix extracellular phosphoprotein (MEPE). The addition of Pi provided a corrective measure for mineralization and partially rescued the expression of some genes although cells retained altered messenger RNA levels for PPi-associated genes.
Conclusions
These studies suggest that under HPP conditions pulp cells have the compromised ability to mineralize and feature a disrupted odontoblast profile, providing a first step toward understanding the molecular mechanisms for dentin phenotypes observed in HPP.
Keywords: Alkaline phosphatase, dental pulp cells, dentin, hypophosphatasia, pyrophosphate
Phosphate (Pi) homeostasis is essential for the normal development and maintenance of skeletal tissues, including dentition (1). Pyrophosphate (PPi) is a potent inhibitor of hydroxyapatite mineral growth (2). Hydrolysis of PPi liberates Pi and relieves the inhibition of mineralization. Thus, a balance in the local regulation of Pi and PPi levels dictates propensity for mineralization. The enzyme tissue nonspecific alkaline phosphatase (TNAP) hydrolyzes PPi to Pi and is expressed by mineralized tissue cells including osteoblasts, odontoblasts, and cementoblasts. In the heritable condition hypophosphatasia (HPP), the deficiency of serum alkaline phosphatase (ALP) activity results from mutations in the TNAP-encoding liver/bone/kidney alkaline phosphatase gene (ALPL, Online Mendelian Inheritance in Man - OMIM 171760) (3, 4). One consequence of HPP is the severely defective formation of acellular cementum, resulting in poor periodontal attachment and premature tooth exfoliation (5), a phenotype recapitulated in the Akp2-null mouse model for HPP (6). Yet, in both humans and mice with reduced ALP, dentin has been reported to be unaffected or less affected than cementum (6–9) although clinical case reports of thin dentin and wide pulp cavities have been described (8–11).
In order to define how cells of the dentin-pulp complex are affected by HPP, primary pulp cells obtained from HPP-diagnosed subjects with observed dentin phenotypes were cultured and assayed for ALP activity, mineralization, and the expression of PPi-associated and odontoblast marker genes. Additionally, we determined whether exogenous Pi, by normalizing the Pi/PPi ratio, would correct functional differences in HPP versus control cells.
Materials and Methods
Human Subjects
A total of seven human subjects (four men and three women) 18 to 22 years old were enrolled in this study, which received Institutional Review Board approval (University of Campinas, School of Dentistry, #065/2005). Inclusion criteria included no history of smoking, diabetes, bone metabolic disorders, or other systemic disease except for HPP in the HPP group. The control subjects (n = 5) were periodontally healthy with erupted teeth scheduled for extraction for orthodontic reasons and with ALP serum levels within the normal adult range (25–100 U/L). HPP-diagnosed subjects included male monozygotic twins (n = 2) with a previous diagnosis of odontohypophosphatasia (12). HPP subjects were monitored and treated as needed, and tooth extractions were performed as a consequence of HPP-related pathology. HPP subjects were seen in the clinic from 1991 to the present day, whereas cells were harvested from HPP and normal subjects from 2004 to 2005.
Cell Isolation and Culture
Extracted teeth were placed in biopsy media, and pulp was harvested by cracking open teeth using a dental chisel and hammer and removing soft tissue with sterile forceps. Pulp cells were obtained by enzymatic digestion with 3 mg/mL collagenase type I and 4 mg/mL dispase (Gibco BRL, Carlsbad, CA) for 1 hour at 37°C. Cells were maintained in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS), 1% L-glutamine, and 1% penicillin/streptomycin (Gibco BRL) and incubated at 37°C in a 5% CO2 atmosphere. Cells from passages 2 to 4 were used for all experiments.
Cell Proliferation
Cells were seeded at 1.5 × 104 cells/cm2 in 96-well plates in DMEM with 2% FBS. From 24 hours to 6 days after plating, cells were counted by a hemacytometer and analyzed by a colorimetric formazan-based cell proliferation assay [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] (Promega, Madison, WI).
ALP Activity
Cells were seeded at 2.0 × 104 cells/cm2 in 60-mm tissue culture dishes in DMEM with 2% FBS and 50 μg/mL ascorbic acid (AA) for up to 21 days. The relative ALP activity was measured using a commercial kit (Labtest Diagnostica, Lagoa Santa, MG, Brazil) as previously reported (13). Briefly, media were removed, cells were rinsed with PBS, and the release of thymolphthalein from thymolphthalein monophosphate substrate was measured by absorbance readings at 590 nm after 30 minutes of incubation at 37°C. The relative ALP activity was normalized to the total protein content per well as determined by the Bradford assay (14) following the manufacturer's recommendation (Bio-Rad Protein Assay; Bio-Rad, Hercules, CA).
Mineralization Assay
Cells were seeded at 2.0 × 104 cells/cm2 in 24-well plates under nonmineralizing (2% FBS and AA) or mineralizing conditions up to 28 days. Mineralizing conditions included 2% FBS and AA plus a phosphate source, 10 mmol/L β-glycerophosphate (βGP), or 1 mmol/L inorganic Pi (a solution of monobasic and dibasic sodium phosphates, pH = 7.4). The dose selected, 1 mmol/L Pi, was based on previous work with cementoblasts (15) as well as preliminary experiments with pulp cells. Mineral nodules were detected by the von Kossa assay and alizarin red S (AR) staining (40 mmol/L, pH = 4.2). AR stain was quantified by measuring the absorbance of bound dye (570 nm) solubilized in 10% cetylpyridinium chloride (MP Biomedicals, Solon, OH) as previously described (16).
Quantitative Polymerase Chain Reaction
Cells were seeded at 2.0 × 104 cells/cm2 in 60-mm plates in non-mineralizing and mineralizing media (with 1 mmol/L Pi), as outlined previously, for up to 20 days. The total RNA was isolated by TRIZOL reagent (Gibco BRL), DNase treated (Turbo DNA-free; Ambion, Austin, TX), and used for complementary DNA synthesis (Transcriptor Reverse Transcription Kit; Roche Diagnostic, Indianapolis, IN). Quantitative real-time polymerase chain reactions were performed using a SYBR green-based hot start polymerase chain reaction kit (FastStart DNA Masterplus, Roche Applied Science; Indianapolis, IN). Relative quantification was performed using amplification efficiency correction with glyceraldehyde-3-phosphate dehydrogenase as the reference gene. The assessed genes and primers are listed in Table 1.
TABLE 1. Polymerase Chain Reaction Primer Sequences.
| Gene | Name | Primer sequence (5′ → 3′) | Genbank number |
|---|---|---|---|
| ALPL | Tissue nonspecific alkaline phosphatase | CGGGCACCATGAAGGAAAG GCCAGACCAAAGATAGAGTT | NM_000478 |
| ANKH | Progressive ankylosis protein | GAGGTGACAGACATCGTGG CCTTTAAATCAAGGCCTCTTTCATTAC | NM_054027.4 |
| ENPP1 | Ectonucleotide phosphodiesterase 1 | AAATATGCAAGCCCTCTTTGT TTTAGAAGGTGGTTAAGACTTCCATGA | NM_006208.2 |
| DMP1 | Dentin matrix protein 1 | AGCCATTCTGAGGAAGACGA TGTTGTGATAGGCATCAACTGTTA | NM_004407 |
| DSPP | Dentin sialophosphoprotein | GCATTCAGGGACAAGTAAGCA CTTGGACAACAGCGACATCCT | NM_014208.3 |
| OPN | Osteopontin | AAAGCCAATGATGAGAGCAA ATTTCAGGTGTTTATCTTCTTCCTTAC | NC_000004 |
| MEPE | Matrix extracellular phosphoglycoprotein | ACCTAGAAGGCAAAGATATTCAAACA TTCGCAGTTTCATCCCTAGT | NM_001184694.1 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | GAAGGTGAAGGTCGGAGTC GAAGATGGTGATGGGATTTC | NM_002046 |
Statistical Analysis
Experiments were performed in triplicate and repeated at least twice. Values are given as means and standard deviations. Intragroup and intergroup comparisons were performed using the Kruskal-Wallis one-way analysis of variance followed by the Student-Newman-Keuls method (α = 0.05) for proliferation and mineralization assays and gene expression. The Student's t test was used for intergroup comparisons for ALP activity. Statistical power was at least 0.08 with α = 0.05 for any statistical test reported.
Results
Diagnosis of HPP with Dentin Phenotype
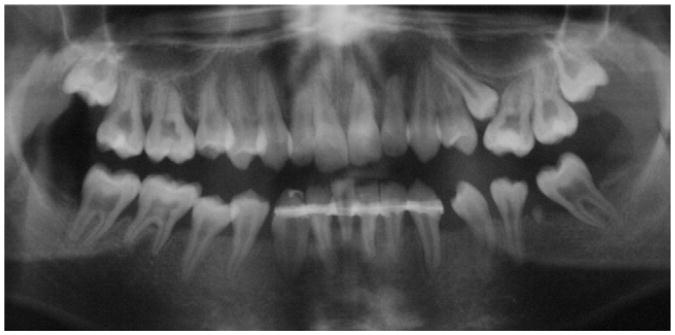
White male identical twins were diagnosed with the HPP subtype odontohypophosphatasia. Genomic sequencing revealed a heterozygous transition 454C > T in exon 5 of the ALPL gene of both siblings, leading to the substitution of cysteine for arginine at position 135 (R135C) as described in Rodrigues et al (12). Their dental history revealed premature exfoliation of primary and permanent teeth in both siblings. Notably, the permanent dentition featured a dentin phenotype including short roots and wide pulp chambers in addition to reduced alveolar bone height (Fig. 1). Cells were harvested for this study when HPP subjects were 22 years old. Serum ALP activity remained low at 8 U/L and 6 U/L; the normal adult range is 25 to 100 U/L.
Figure 1.
HPP with a dentin phenotype: a corresponding panoramic radiograph from a patient with odontohypophosphatasia showing effects of the disease on the permanent dentition, including short roots, enlarged pulp chambers, and thin dentin in addition to reduced alveolar bone height.
HPP Pulp Cells Feature Reduced ALP and Deficient Mineralization in Vitro
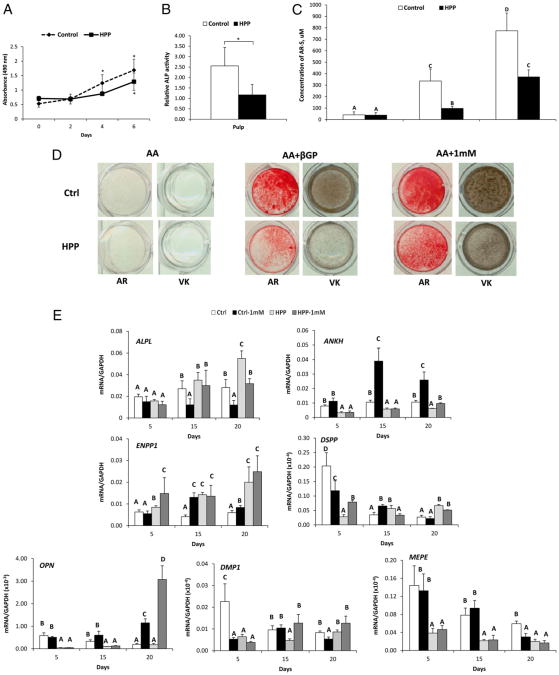
Pulp cells from controls and HPP patients exhibited a typical spindle-shaped fibroblastic morphology and monolayer attachment (data not shown). Cell counting and MTS assay indicated that control and HPP cells presented a similar pattern of proliferation although HPP cells were found to have a lower growth rate at days 4 and 6 (P < .05) (Fig. 2A). In agreement with serum biochemical results, the ALP activity in HPP cells was significantly decreased (P < .05) to approximately 50% of the control levels (Fig. 2B).
Figure 2.
HPP pulp cells exhibit deficient ALP activity and mineralization capacity. (A) Control and HPP cells grown in 2% FBS were counted by the MTS method. Values corresponding to relative cell numbers are shown as mean ± standard deviation (absorbance at 490 nm). *Intragroup significant differences versus day 0 by Kruskal-Wallis one-way analysis of variance followed by the Student-Newman-Keuls method (α = 0.05). (B) The relative ALP was significantly lower in HPP cells versus controls. *Statistically different by the Student t test (α = 0.05). (C and D) AR and von Kossa assays for in vitro mineralization. Compared with controls, HPP cells displayed a severely limited mineralization capacity with βGP as a phosphate source. The HPP mineralization deficiency was partially rescued when 1 mmol/L Pi was used as the phosphate source. Different capital letters indicate intergroup statistical differences (control vs HPP) for α = 0.05 using the Kruskal-Wallis test followed by the Student-Newman-Keuls method. (E) HPP pulp cells feature dysregulation of PPi-associated and odontoblast marker genes. Gene expression for PPi regulating factors (ALPL, ANKH, and ENPP1) and odontoblast markers (DSPP, OPN, DMP1, an MEPE) were dysregulated in HPP cells compared with controls. The addition of Pi showed a limited ability to correct altered gene expression. Different capital letters indicate intergroup statistical differences (control vs HPP, ±Pi treatment) within the same time point (day 5, 15, or 20) for α = 0.05 using the Kruskal-Wallis test followed by the Student-Newman-Keuls method.
Heterogeneous pulp cell populations harbor cells with the potential to acquire an odontoblast-like phenotype, including mineralization potential and gene expression profile, when cultured under appropriate conditions (17). An in vitro mineralization assay was performed to determine the effect of reduced ALP activity on HPP pulp cells' ability to promote mineral nodule formation. Mineralizing conditions were created using either of two phosphate sources: βGP or Pi. Although ionic Pi is available for direct incorporation into hydroxyapatite crystals, βGP provides Pi only with phosphatase activity, such as by ALP. In the absence of Pi or βGP, mineral nodules were not produced by either control or HPP cultures (Fig. 2C and D). The addition of βGP resulted in mineral nodule formation by 28 days. However, HPP cells displayed a severely limited mineralization capacity compared with the controls. The quantification of AR staining revealed three-fold greater mineral formation in control versus HPP pulp cells (P < .05, Fig. 2B and C). However, when 1 mmol/L Pi was included as the phosphate source, the mineralizing deficiency of HPP cells was partially rescued. HPP cells with Pi achieved mineral deposition comparable with the control cells with βGP, suggesting the mineralizing deficit of the HPP cells was the inability to hydrolyze βGP. This is consistent with the low ALP activity resulting from the ALPL mutation. However, AR staining additionally showed that control cells treated with 1 mmol/L Pi presented two-fold greater mineral formation than HPP cells under the same experimental conditions, suggesting a remaining mineralizing deficit or delay in HPP cells.
Altered Gene Expression in HPP Pulp Cells
To provide insight into the mineralization deficiency of HPP cells, gene expression over 20 days was determined for PPi-associated genes as well as odontoblast markers (Fig. 2E). In order to determine the extent that local Pi insufficiency or reduced Pi/PPi ratio may affect cell phenotype, 1 mmol/L exogenous Pi was added to cell cultures. Interestingly, HPP cells featured significantly increased levels of messenger RNA for ALPL on day 20, suggesting a feedback mechanism in an attempt to compensate for ALP deficiency. The expression of the PPi regulatory gene ANKH was reduced in HPP versus control cells over all time points. The addition of exogenous Pi enhanced ANKH expression in controls but had little effect on HPP cells. Conversely, ENPP1 expression was higher in HPP versus control cells and was slightly increased over time in response to Pi in both cell populations.
Over the culture period, HPP cells exhibited a significantly reduced expression of odontoblast markers DMP1 (days 5 and 15), DSPP (day 5), OPN (days 5 and 15), and MEPE (days 5, 15, and 20). The addition of Pi had a limited ability to rescue expression, observed as increased DMP1 on day 15 and OPN on day 20, with little or no effect on DSPP and MEPE expression. As an overall trend, both PPi- and odontoblast-related genes were disrupted in HPP cells, and added Pi was not able to rescue the odontoblast cell phenotype as defined by control pulp cells here.
Discussion
In HPP, mutations in ALPL reduce TNAP function, resulting in skeletal mineralization defects including rickets and osteomalacia (3, 4). Dental case reports indicate defective cementum (5, 7, 18, 19), whereas dentin has been described as normal or variably affected (8–11). Here we began to bridge the gap between case reports on dentin pathology and a molecular understanding of how odontoblasts are affected by HPP. Pulp cells from HPP subjects featured reduced ALP, compromised mineralization potential, and a disturbance in gene expression of PPi regulatory factors and odontoblast markers. Adding back Pi to normalize the Pi/PPi ratio provided some measure of correction for mineralization and odontoblast marker genes; however, PPi-associated genes remained dysregulated.
The connection between HPP and defective cementogenesis has long been established. Compromised periodontal attachment and exfoliation of teeth because of aplasia or severe hypoplasia of acellular cementum is consistently described in case reports and is well documented (5, 7–9, 11, 18–25). Reports on pulp and dentin in cases of HPP are much more variable, with authors frequently citing no observed pathology. Although variable, HPP pulp/dentin phenotypes have been described in the clinical literature, including thin dentin, wide pulp chambers, “shell teeth,” and mineralization defects (8–11, 26, 27). In studies of mice deficient for ALP, acellular cementum was inhibited, whereas dentin was reported to be unaffected (6), and transgenic mice featuring PPi deficiency (Ank or Enpp1 loss of function) also exhibit a dramatic cementum phenotype with no discernible alteration in dentin (28, 29). Studies to identify the mechanism for disparity in mineral metabolism of cementum versus dentin have yielded some clues. Van den Bos et al (7) reported higher ENPP1 gene expression, ectonucleotide pyro-phosphatase phosphodiesterase 1 protein (NPP)-like and ALP activity, and PPi concentrations in periodontal ligament (PDL) versus pulp tissues from human subjects (7), and following on this work, we have confirmed a higher basal gene expression of the PPi regulatory factors ALPL, ANKH, and ENPP1 in PDL versus pulp tissues in a sample population of healthy patients (12). These results suggest that PPi metabolism in the PDL is a more dynamically regulated process, predisposing the periodontia to be more sensitive to changes in PPi regulators than dentin.
However, increasing evidence supports that odontoblasts are also sensitive to and dependent on PPi regulation for proper dentin formation. Liu et al (9) showed reduced ALP and capacity for mineralization in dental pulp cells from deciduous teeth extracted from HPP-diagnosed subjects (9). In addition to human case reports already cited, Akp2-null mice, a model for infantile-type HPP, feature thin and hypomineralized dentin (30), and in ongoing studies with these mice, we have identified odontoblast cell function defects including the disruption of odonto-blast gene markers (manuscript in preparation). In the current study, we explored the effects of HPP on the cell profile by using primary dental pulp cell cultures from extracted permanent teeth. The HPP subjects described here were diagnosed with an odonto-specific subtype of HPP in which the clinical phenotype is primarily limited to abnormal serum biochemistry and dental defects (3, 4, 12). To our knowledge, this is the first report examining the gene expression profile of dental pulp cells isolated from HPP patients.
Control pulp cell populations exhibited an odontoblast-like phenotype after culturing under mineralizing conditions, including mineral nodule formation and characteristic gene expression. ALP activity and mineralization capacity in HPP pulp cells were detected but significantly impaired as previously reported for HPP dental pulp cells from deciduous teeth (9) and osteoblasts from Akp2-null mice (31), and they were in agreement with studies of genotype-phenotype correlation indicating that human HPP patients with milder forms of HPP (eg, odontohypophosphatasia) retain some residual ALP activity (4, 19). When 1 mmol/L exogenous Pi was added to correct the Pi/PPi ratio and provide a TNAP-independent phosphate source, the HPP cell mineralization deficiency was partially corrected to the level of control cells. This result suggests that pulp cells lacking TNAP function are otherwise competent to promote mineralization.
HPP pulp cells were assayed for genes involved in PPi regulation (ALPL, ANKH, and ENPP1) as well as odontoblast markers (DMP1, DSPP, OPN, and MEPE). Compared with controls over 20 days, HPP cells increased ENPP1 and decreased ANKH, both of which were poorly corrected by added Pi. Although decreasing PPi transporter ANKH may be an attempt by cells to correct the Pi/PPi ratio, the intriguing increase in ENPP1 would be expected to increase PPi levels and exacerbate the Pi/PPi imbalance and may explain the remaining inequality in HPP cells' ability to mineralize compared with the controls. PDL cells from HPP patients also exhibited depressed ANKH although ENPP1 was unchanged compared with controls (12), which is an interesting difference in PPi metabolism of pulp versus PDL cells that may be related to differences in dentin versus cementum pathology in HPP patients.
Over the culture period, HPP cells exhibited a significantly reduced expression of odontoblast markers, which was only partly ameliorated with added Pi. The expression of OPN in response to Pi and in HPP cells was of particular interest. In the control cells, added Pi tended to increase OPN from days 15 to 20. This agrees with previous studies identifying Pi as a potent inducer of OPN expression in osteoblasts and cementoblasts (15, 32). HPP cells displayed no OPN increase in response to Pi until the late time point, day 20, when the increase was more exaggerated than in the control cells. Previous work with osteoblasts indicated that OPN expression can be altered under conditions of disrupted PPi homeostasis (33, 34), and as an inhibitor of mineral deposition, OPN protein can also influence skeletal and dental development.
Although these observations on functional mineralization and gene expression in HPP pulp cells may reflect an imperfect or insufficient correction of the Pi/PPi ratio, it might also be hypothesized that TNAP plays a more complex or nuanced role in odontoblast cell function or that excess PPi functions as more than a denominator in the Pi/PPi ratio. Ultimately, as a result of ALPL mutation and the loss of ALP enzymatic activity, odontoblast cell function was disturbed in terms of cell expression profile and mineralization, corresponding to clinical reports of pulp/dentin pathology associated with HPP.
Odontoblast secretion of extracellular matrix proteins such as DMP1, OPN, dentin sialoprotein, and dentin phosphoprotein is thought to be importantin the formation and proper mineralizationof circumpulpal dentin, and, in particular, mutations in the gene DSPP have been linked to the conditions dentinogenesis imperfecta and dentin dysplasia (35). Therefore, the reduced expression of such genes in HPP cells may point to additional cell deficiencies contributing to dentin defects observed in the teeth of HPP patients. Alternatively, the loss of odontoblast markers may be the effect of the loss of TNAP and the failure of proper dentin matrix mineralization. Ongoing mechanistic studies in animal models should provide insight into this question. These studies may additionally provide more information on pulp cell differentiation and the relation of odontoblast cell function to dentin mineralization and, ultimately, may inform efforts at dentin regeneration. In addition to current attempts emphasizing the delivery of growth and differentiation factors to promote odontoblast differentiation, the important influence of PPi metabolism on dentin development suggests that targeted modulation of Pi/PPi may provide a novel approach for achieving dentin regeneration.
Acknowledgments
The authors thank Mrs Ana Paula Giorgetti (State University of Campinas, School of Dentistry, Piracicaba, SP, Brazil) for clinical support and Dr Marisi Aidar (State University of Campinas, School of Dentistry, Piracicaba, SP, Brazil) for genetic analyses. The studies were performed while M.J.S. and B.L.F. were affiliated with the Department of Periodontics, University of Washington School of Dentistry.
Supported by São Paulo State Research Foundation (FAPESP, Brazil, grant #07/08192-5 and 08/00534-7 (FHNJ), National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) DE15109 (M.J.S.), and NIH Fogarty International Research Collaboration Award (FIRCA) grant 5R03TW007590-03 (M.J.S. and F.H.N.).
Footnotes
The authors deny any conflicts of interest related to this study.
References
- 1.Foster BL, Tompkins KA, Rutherford RB, et al. Phosphate: known and potential roles during development and regeneration of teeth and supporting structures. Birth Defects Res C Embryo Today. 2008;84:281–314. doi: 10.1002/bdrc.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleisch H, Bisaz S. Mechanism of calcification: inhibitory role of pyrophosphate. Nature. 1962;195:911. doi: 10.1038/195911a0. [DOI] [PubMed] [Google Scholar]
- 3.Whyte M. Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev. 1994;15:439–61. doi: 10.1210/edrv-15-4-439. [DOI] [PubMed] [Google Scholar]
- 4.Mornet E. Hypophosphatasia. Orphanet J Rare Dis. 2007;2:40. doi: 10.1186/1750-1172-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapple IL. Hypophosphatasia: dental aspects and mode of inheritance. J Clin Periodontol. 1993;20:615–22. doi: 10.1111/j.1600-051x.1993.tb00705.x. [DOI] [PubMed] [Google Scholar]
- 6.Beertsen W, VandenBos T, Everts V. Root development in mice lacking functional tissue non-specific alkaline phosphatase gene: inhibition of acellular cementum formation. J Dent Res. 1999;78:1221–9. doi: 10.1177/00220345990780060501. [DOI] [PubMed] [Google Scholar]
- 7.van den Bos T, Handoko G, Niehof A, et al. Cementum and dentin in hypophosphatasia. J Dent Res. 2005;84:1021–5. doi: 10.1177/154405910508401110. [DOI] [PubMed] [Google Scholar]
- 8.Olsson A, Matsson L, Blomquist HK, Larsson A, Sjodin B. Hypophosphatasia affecting the permanent dentition. J Oral Pathol Med. 1996;25:343–7. doi: 10.1111/j.1600-0714.1996.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Li J, Lei H, Zhu T, Gan Y, Ge L. Genetic etiology and dental pulp cell deficiency of hypophosphatasia. J Dent Res. 2010;89:1373–7. doi: 10.1177/0022034510379017. [DOI] [PubMed] [Google Scholar]
- 10.Beumer J, 3rd, Trowbridge HO, Silverman S, Jr, Eisenberg E. Childhood hypophosphatasia and the premature loss of teeth. A clinical and laboratory study of seven cases. Oral Surg Oral Med Oral Pathol. 1973;35:631–40. doi: 10.1016/0030-4220(73)90028-5. [DOI] [PubMed] [Google Scholar]
- 11.Wei KW, Xuan K, Liu YL, et al. Clinical, pathological and genetic evaluations of Chinese patients with autosomal-dominant hypophosphatasia. Arch Oral Biol. 2010;55:1017–23. doi: 10.1016/j.archoralbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues TL, Foster BL, Silverio KG, et al. Correction of hypophosphatasia (Hpp) associated mineralization deficiencies in vitro by phosphate/pyrophosphate modulation in periodontal ligament cells. J Periodontol. 2011 doi: 10.1902/jop.2011.110310. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues TL, Marchesan JT, Coletta RD, et al. Effects of enamel matrix derivative and transforming growth factor-beta1 on human periodontal ligament fibroblasts. J Clin Periodontol. 2007;34:514–22. doi: 10.1111/j.1600-051X.2007.01090.x. [DOI] [PubMed] [Google Scholar]
- 14.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 15.Foster BL, Nociti FH, Jr, Swanson EC, et al. Regulation of cementoblast gene expression by inorganic phosphate in vitro. Calcif Tissue Int. 2006;78:103–12. doi: 10.1007/s00223-005-0184-7. [DOI] [PubMed] [Google Scholar]
- 16.Hessle L, Johnson KA, Anderson HC, et al. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tziafas D, Kodonas K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J Endod. 2010;36:781–9. doi: 10.1016/j.joen.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Bruckner RJ, Rickles NH, Porter DR. Hypophosphatasia with premature shedding of teeth and aplasia of cementum. Oral Surg Oral Med Oral Pathol. 1962;15:1351–69. doi: 10.1016/0030-4220(62)90356-0. [DOI] [PubMed] [Google Scholar]
- 19.Hu JC, Plaetke R, Mornet E, et al. Characterization of a family with dominant hypophosphatasia. Eur J Oral Sci. 2000;108:189–94. doi: 10.1034/j.1600-0722.2000.108003189.x. [DOI] [PubMed] [Google Scholar]
- 20.el-Labban N, Lee K, Rule D. Permanent teeth in hypophosphatasia: light and electron microscopic study. J Oral Pathol Med. 1991;20:352–60. doi: 10.1111/j.1600-0714.1991.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 21.Chapple I. Hypophosphatasia: dental aspects and mode of inheritance. J Clin Periodontol. 1993;20:615–22. doi: 10.1111/j.1600-051x.1993.tb00705.x. [DOI] [PubMed] [Google Scholar]
- 22.Plagmann H, Kocher T, Kuhrau N, Caliebe A. Periodontal manifestation of hypophosphatasia. A family case report. J Clin Periodontol. 1994;21:710–6. doi: 10.1111/j.1600-051x.1994.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 23.Baab D, Page R, Ebersole J, Williams B, Scott C. Laboratory studies of a family manifesting premature exfoliation of deciduous teeth. J Clin Periodontol. 1986;13:677–83. doi: 10.1111/j.1600-051x.1986.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 24.Lundgren T, Westphal O, Bolme P, Modéer T, Norén J. Retrospective study of children with hypophosphatasia with reference to dental changes. Scand J Dent Res. 1991;99:357–64. doi: 10.1111/j.1600-0722.1991.tb01041.x. [DOI] [PubMed] [Google Scholar]
- 25.Lynch C, Ziada H, Buckley L, O'Sullivan V, Aherne T, Aherne S. Prosthodontic rehabilitation of hypophosphatasia using dental implants: a review of the literature and two case reports. J Oral Rehabil. 2009;36:462–8. doi: 10.1111/j.1365-2842.2009.01948.x. [DOI] [PubMed] [Google Scholar]
- 26.Macfarlane J, Swart J. Dental aspects of hypophosphatasia: a case report, family study, and literature review. Oral Surg Oral Med Oral Pathol. 1989;67:521–6. doi: 10.1016/0030-4220(89)90266-1. [DOI] [PubMed] [Google Scholar]
- 27.Reibel A, Manière M, Clauss F, et al. Orodental phenotype and genotype findings in all subtypes of hypophosphatasia. Orphanet J Rare Dis. 2009;4:6. doi: 10.1186/1750-1172-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nociti FH, Jr, Berry JE, Foster BL, et al. Cementum: a phosphate-sensitive tissue. J Dent Res. 2002;81:817–21. doi: 10.1177/154405910208101204. [DOI] [PubMed] [Google Scholar]
- 29.Foster BL, Nagatomo KJ, Bamashmous SO, et al. The progressive ankylosis protein regulates cementum apposition and extracellular matrix composition. Cells Tissues Organs. 2011;194:382–405. doi: 10.1159/000323457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millán J, Narisawa S, Lemire I, et al. Enzyme replacement therapy for murine hypophosphatasia. J Bone Miner Res. 2008;23:777–87. doi: 10.1359/JBMR.071213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wennberg C, Hessle L, Lundberg P, et al. Functional characterization of osteoblasts and osteoclasts from alkaline phosphatase knockout mice. J Bone Miner Res. 2000;15:1879–88. doi: 10.1359/jbmr.2000.15.10.1879. [DOI] [PubMed] [Google Scholar]
- 32.Beck GR, Zerler B, Moran E. Phosphate is a specific signal for induction of osteo-pontin gene expression. Proc Natl Acad Sci U S A. 2000;97:8352–7. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harmey D, Hessle L, Narisawa S, Johnson K, Terkeltaub R, Millán J. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. 2004;164:1199–209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Addison W, Azari F, Sørensen E, Kaartinen M, McKee M. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem. 2007;282:15872–3. doi: 10.1074/jbc.M701116200. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Simmer J. Hereditary dentin defects. J Dent Res. 2007;86:392–9. doi: 10.1177/154405910708600502. [DOI] [PubMed] [Google Scholar]