Abstract
OBJECTIVES
To investigate the effects of birth trauma and estrogen on urethral elastic fibers and elastin expression
METHODS
Pregnant rats were subjected to sham operation (Delivery-only), DVDO (delivery, vaginl distension and ovariectomy), or DVDO+E2 (estrogen). At 2, 4, 8, or 12 weeks, their urethras were harvested for elastic fiber staining and RT-PCR analysis. Urethral cells were treated with TGF-β1 and/or estrogen and analyzed for elastin mRNA expression. Urethral cells were also examined for the activities of Smad1 and Smad3/4 responsive elements in response to TGF-β1 and estrogen.
RESULTS
At 8 weeks post-treatment, the urethras of DVDO rats had fewer and shorter elastic fibers when compared to Delivery-only rats, and those of DVDO+E2 rats had fewer and shorter elastic fibers when compared to DVDO rats. Elastin mRNA was expressed at low levels in Delivery-only rats and at increasingly higher levels in DVDO rats at 2, 4, and 8 weeks but at sharply lower levels in DVDO+E2 rats when compared to DVDO rats at 8 weeks. Urethral cells expressed increasingly higher levels of elastin mRNA in response to increasing concentrations of TGF-β1 up to 1 ng/ml. At this TGF-β1 concentration, urethral cells expressed significantly lower levels of elastin mRNA when treated with estrogen prior to or after TGF-β1 treatment. Both Smad1 and Smad3/4 responsive elements were activated by TGF-β1 and such activation was suppressed by estrogen.
CONCLUSIONS
Birth trauma appears to activate urethral elastin expression via TGF-β1 signaling. Estrogen interferes with this signaling, resulting in improper assembly of elastic fibers.
Keywords: Urethra, Urinary incontinence, Birth trauma, Elastic fibers, Estrogen, TGF-β
INTRODUCTION
Urinary incontinence (UI) afflicts more than 200 million people worldwide 1 and costs nearly 20 billion dollars in the United States alone in 2000 2. UI is 2 to 3 times more prevalent in women than in men up to age 80, after which it affects both sexes equally 3. In a recent National Health and Nutrition Examination Survey of 4,229 women older than 20 years, 49.6% of these women reported UI symptoms. Of those reporting UI symptoms, 49.8% reported stress urinary incontinence (SUI), 15.9% urge urinary incontinence (UUI), and 34.3% mixed urinary incontinence 4.
Pregnancy, parity, and menopause are known risk factors for female SUI 5,6. Indeed, by combining these 3 risk factors in a DVDO rat model (delivery, vaginal distension and ovariectomy) we have established a highly reproducible animal model for female SUI. By using this model, we have shown that estrogen and selective estrogen receptor modulators (SERMs) had an overall negative impact on the urinary continence mechanism and urethral tissue integrity 7-9. These findings thus provide plausible mechanistic and molecular explanations for the epidemiological data linking estrogen and SERMs with exacerbating urinary incontinence 10-14.
Tissue function depends not only on cellular but also extracellular integrity. Elastic fibers, a major constituent of the extracellular compartment, endow tissues with flexibility and reversible extensibility. The formation of elastic fibers is a complex process that occurs primarily during early development and cannot be recapitulated in adult tissues 15. Thus, damaged elastic fibers in adult tissues are often repaired improperly with the consequence of loss of tissue elasticity. As birth trauma to the urethra is a causative factor for SUI, it has been reported that the periurethral tissue of parous women with SUI had abnormal elastic fibers 16. A subsequent study, which used an ex vivo whole-mount rat bladder/urethra preparation, reported changes in compliance and stiffness of the urethra after birth trauma 17. The authors hypothesized that altered compliance may be due to either altered intrinsic basal urethral tone or changes in the extracellular matrix, specifically elastic fibers. In a more recent study, Lee et al 18 reported that the lysyl oxidase like-1 knockout (LOXL1-KO) mice, which are unable to assemble elastic fibers properly, exhibit SUI symptoms 18. Thus, it appears that urinary continence depends on an intact elastic fiber system, and, if so, it is possible that estrogen might impact the continence mechanism by interfering with elastic fiber formation. In the present study we first investigated the effects of DVDO and estrogen on the urethral elastic fibers. As the results were in agreement with our hypothesis, we went on to investigate the molecular mechanism responsible for such effects. The results show that DVDO might upregulate elastin gene expression through TGF-β1/Smad signaling, and estrogen might interfere with this signaling pathway.
MATERIALS AND METHODS
Animals and Treatments
All animal experiments were approved by the Institutional Animal Care and Use Committee or the University of California San Francisco. Female Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). Twelve of them were 4-month-old virgin used for the preparation of urethral cell cultures. Another 66 rats were 4-month-old pregnant rats at gestational day 16. After spontaneous delivery, these rats were randomly divided into 3 equal groups. The Delivery-only group received no further intervention; the DVDO group received intravaginal balloon dilation and ovariectomy; the DVDO+E2 group was similar to the DVDO group except with additional estrogen treatment. The DVDO procedure has been described previously 7. Briefly, after delivery, rats were anesthetized with ketamine (90 mg/kg intraperitoneal) and xylazine (10 mg/kg intraperitoneal). The balloon of a transurethral catheter (18F; Bard Inc., Covington, GA) with the tip cut off was placed intravaginally and was filled with 3 ml of water. A 130 g weight was placed on the catheter providing a constant pull directing pressure to the pelvic floor. The catheter was left in place for 4 h. One week after birth trauma, bilateral ovariectomy was performed under 2% isoflurane anesthesia. For estrogen treatment, 17β-estradiol (0.1 mg/kg/day; Sigma-Aldrich, St. Louis, MO) was dissolved in 20% DMSO in Alzet Osmotic Pump (Model 2004, 28-day duration, Durect Corp. Cupertino, CA). The pump was implanted subcutaneously in the dorsum of the neck and replaced with a fresh one after 28 days. The rats were sacrificed at 2 weeks, 4 weeks, 8 weeks, or 12 weeks and their urethras harvested for elastin stain and RT-PCR analysis.
Elastic Fiber Staining
Urethral tissue specimens were fixed in cold 2% formaldehyde and 0.002% saturated picric acid in 0.1 μmol/L phosphate buffer (pH 8.0) for 4 hours, followed by overnight immersion in a buffer containing 30% sucrose for cryoprotection. The specimens were embedded in OCT Compound (Sakura Finetic USA, Torrance, Calif.) cut at 10 μm thickness, mounted to SuperFrost Plus charged slides (Fisher Scientific, Pittsburgh, Pa.), and air-dried for 5 minutes. The dried tissue sections were immersed in 0.25% potassium permanganate solution for 5 min, cleared in 5% oxalic acid, and soaked in resorcin-fuchsin solution overnight. After being washed in water, sections were counterstained with Van Gieson solution for 1 min, and then dehydrated in ethanol, cleared in Histo-Clear and mounted with Histomount. To prevent variations in staining, all samples were stained simultaneously using this procedure. For image analysis, five randomly selected fields per tissue per animal for each treatment group were photographed and recorded using a Retiga Q Image digital still camera and ACT-1 software (Nikon Instruments Inc., Melville, NY).
Urethral Cell Culture
Rat urethral cells were cultured as previously described 9. Briefly, the urethra was deepithelialized, washed 3 times in sterile phosphate-buffered saline (PBS), and cut into 2-3 mm3 segments. The segments were placed evenly onto a 100-mm cell culture dish (Falcon-Becton Dickinson Labware, Franklin Lakes, NJ). Ten minutes later, 10 ml of Dulbecco’s Modified Eagle Medium (DMEM) containing penicillin (100 units/ml), streptomycin (100 μg/ml), and 10% FBS was added to the dish, which was then kept undisturbed in a humidified 37°C incubator with 5% CO2. Five days later, tissue segments that had detached from the dish were removed, and the culture medium was replaced. This process was repeated after another five days of culture. When small islands of cells were noticeable, the cells were treated with trypsin and transferred to a fresh dish. Expansion of each cell strain was continued with change of medium every 3 days and passages approximately every 10 days. All cells used in the following experiments were from passages 3 or 4, and examined for calponin expression (smooth muscle specificity) by immunofluorescence staining. All experiments were done in triplicates with cells isolated from each animal. All data are presented as the average of three independent experiments.
Treatment with TGF-β1 and Estrogen
Urethral cells were seeded in 100-mm cell culture dish (Falcon-Becton Dickinson Labware, Franklin Lakes, NJ) and cultured with 10 ml of DMEM containing penicillin (100 units/ml), streptomycin (100 mg/ml), and 10% FBS. The cells were then kept in a humidified 37°C incubator with 5% CO2 till 80% confluent. The cells were then starved in serum-free DMEM overnight and treated with TGF-β1 (R&D Systems, Inc. Minneapolis, MN) and/or estrogen (17β-estradiol, Sigma-Aldrich, St. Louis, MO) in DMEM with 0.1% BSA for indicated durations. For TGF-β1 dose response, concentrations of 0, 0.01, 0.1, 1, and 10 ng/ml were used in a 16-h period. For combinatory treatment, cells were treated either with 10 nM estrogen for 1 h and then with 1 ng/ml TGF-β1 for 16 h or with 1 ng/ ml TGF-β1 for 1 h and then with 10 nM estrogen for 16 h.
RT-PCR Analysis
The RNAs from urethral tissue cells were isolated by the Tri-Reagent RNA extraction method (Molecular Research Center, Cincinnati, OH). To remove the contaminating DNA, each RNA sample (20–50 μg) were treated with 10 units of RNase-free DNase I (Roche Molecular Biochemicals, Pleasanton, CA) at 37°C for 30 min. The RNAs were then purified by phenol/chloroform extraction and ethanol precipitation. Quantity and purity of RNAs were measured by spectrophotometry with UV adsorption at wavelengths 260 and 280. Integrity was visualized by the sharpness of the 28s and 18s ribosomal RNA bands in agarose gels. The RNA (2.5 μg) was reverse transcribed in a reaction volume of 20 μl and the product was diluted 5 fold with TE buffer (10mM Tris–HCl, pH 8, 1mM EDTA). One μl of each dilution was used in a 10 μl PCR to identify the optimal input within the linear amplification range. PCR was performed in DNA Engine thermocycler (MJ Research, Watertown, MA) under calculated temperature control. Primers pairs for elastin gene were Forward: AAAGTTCCTGGTGTCGGTCTTCCA and Reverse: AGCAGCTCCATACTTAGCAGCCTT. Primer pairs for β-actin gene were Forward: TCTACAATGAGCTGCGTGTG and Reverse: AATGTCACGCACGATTTCCC. The cycling program were set for 35 cycles of 94°C, 10 s; 55°C, 10 s; 72°C, 10 s, followed by one cycle of 72°C,5 min. The PCR products were electrophoresed in 1.5% agarose gels in the presence of ethidium bromide, and visualized by UV fluorescence.
Densitometric quantification of the PCR products was determined by the ChemiImager 4000 System (Alpha Innotech, San Leandro, CA). For SYBR Green real-time PCR, all reagents were purchased from Applied Biosystems (Foster City, CA). Primers for elastin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes were obtained from Integrated DNA Technologies (Coralville, IA). Primers pairs for elastin were Forward: ATCGGTGGCTTAGGAGTCTCAACA and Reverse: TGGAAGACCGACACCAGGAACTTT. Primer pairs for GAPDH gene were Forward: GCCAGCCTCGTCTCATAGACA and Reverse: TGGTAACCAGGCGTCCGATA. The reactions were conducted in the Prism 7300HT sequence detection system (Applied Biosystems). Cycling conditions included an initial phase at 95°C for 3 min, 40 cycles at 95°C for 15 s, and 55°C for 60 s, followed by melting curve analysis at 95°C for 15 s, 55°C for 15 s, and 95°C for 15 s. Real-time PCR results were analyzed by SDS 7000 software (Applied Biosystems) to determine the expression level of relative to that of GAPDH. All experiments were performed in triplicates.
Luciferase Assay
Urethral cells were seeded at approximately 1 × 104 cells/well in a 6-well plate. The next day, 0.5 μg of a construct containing the TGF-β1-responsive element (Smad1 or Smad/3/4 19,20), and 10 ng of pRL-SV40 (Promega Inc., Madison, WI) were transfected into the cells with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA). When indicated, cells were treated with 1 ng/ml TGF-β1 and/or 10 nM 17β-estradiol 24 h after transfection. The cells were further incubated for 24 h before being washed and lysed in 100 μl of lysis buffer (Promega). The lysates (20 μl) were assayed for firefly luciferase activity (Smad activity) and Renilla luciferase activity (SV40 promoter activity) with the Dual-Luciferase reporter assay system (Promega Inc. Madison, WI) and the TD-20/20 luminometer (Turner Designs Inc., Sunnyvale, CA). Each experiment was done in triplicates.
Statistical Analysis
Data was analyzed with Prism 4 (GraphPad Software, Inc., San Diego, CA, USA) and expressed as mean ± standard error of the mean for continuous variables. The continuous data was compared using one-way analysis of variance. The Tukey-Kramer test was used for post-hoc comparisons. Statistical significance was set at p < 0.05.
RESULTS
Effects of DVDO and Estrogen on Urethral Elastic Fibers
The results (Fig. 1) show that in the urethra of normal (Delivery-only) rats, the elastic fibers were most abundant in the smooth muscle compartment and generally longest in the striated muscle compartment. In DVDO rats, there were fewer elastic fibers in all three compartments and those in the striated muscle compartment were shorter when compared to normal rats. In DVDO+E2 rats, there were significantly fewer elastic fibers in all three compartments and those in the striated muscle compartment were shorter when compared to DVDO rats.
Figure 1.
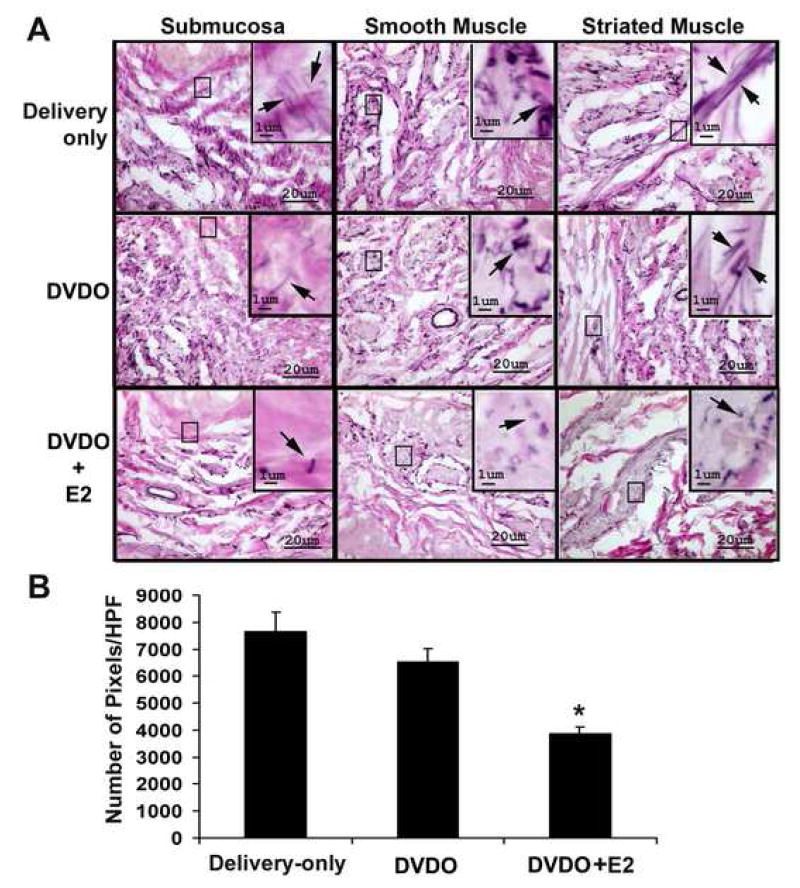
Effects of DVDO and estrogen on urethral elastic fibers. Female rats were sham-operated (Delivery-only), DVDO-treated, or DVDO+E2-treated; 8 weeks later, their urethras were stained for elastic fibers. A. In the submucosa of Delivery-only rats, elastic fibers were scanty and short; in the smooth muscle, they were abundant; in the striated muscle, they were not as abundant but were longer. In DVDO-treated rats, elastic fibers were fewer and shorter in all three compartments; in DVDO+E2-treated rats, elastic fibers were fewest and shortest in all three compartments. Images in the large panels were originally photographed at 400x; those in the inserts at 1000x. Arrows point to representative elastic fibers or fragments. B. The elastic fibers in all three compartments were quantified by counting the number of pixels of positively stained elastic fibers in 5 high-power fields (HPF) per tissue sample. Statistical analysis of the average of the number of pixels/HPF of each treatment group (n=6 per group) indicated significant differences (* p<0.05), between the DVDO+E2 and DVDO groups.
Effects of DVDO and Estrogen on Urethral Elastin mRNA Expression
The results (Fig. 2) show that in the urethra elastin mRNA was expressed at low levels in Delivery-only rats and at increasingly higher levels in DVDO-treated rats at 2, 4, and 8 weeks. At 12 weeks, the expression level was similar to that at 8 weeks (data not shown). More importantly, the expression level was sharply lower in rats treated with estrogen when compared to those without estrogen treatment at 8 weeks (Fig. 2).
Figure 2.
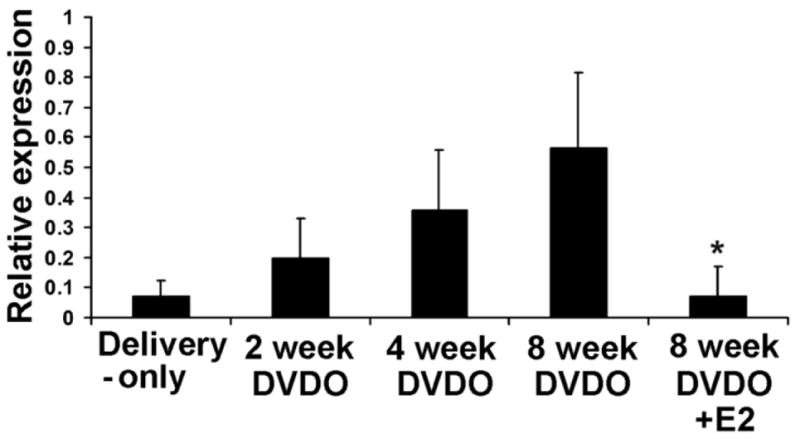
Effects of DVDO and estrogen on urethral elastin mRNA expression. Female rats were sham-operated (Delivery-only; n=6 at each time point), DVDO-treated (n=6 at each time point), or DVDO+E2-treated (n=6 at the 8-week time point); 2, 4, 8, and 12 weeks later, their urethras were examined by RT-PCR for elastin mRNA expression. The PCR products were visualized by gel electrophoresis followed by densitometric and statistical analyses. “Relative expression” is the ratio of elastin versus β-actin expression. * indicates significant difference (p<0.05) compared to 8-week DVDO. For ease of presentation, the 12-week data, which was similar to the 8-week data, is not shown.
Effects of TGF-β1 and Estrogen on Elastin mRNA Expression
Treatment of urethral cells with TGF-β1 for 16 h at different dosages resulted in the highest elastin mRNA expression at 1 ng/ml of TGF-β1, as revealed by both RT-PCR (data not shown) and real-time PCR (Fig. 3A). Thus, this concentration of TGF-β1 was used in subsequent tests for the effects of estrogen. Treatment of urethral cells with estrogen alone had a slight negative effect on elastin mRNA expression (Fig. 3B). Treatment of urethral cells with estrogen 1 h prior to the addition of TGF-β1 effectively blocked the induction of elastin mRNA expression. When estrogen was added 1 h after the addition of TGF-β1, the blocking was still effective although at a lesser extent (Fig. 3B).
Figure 3.
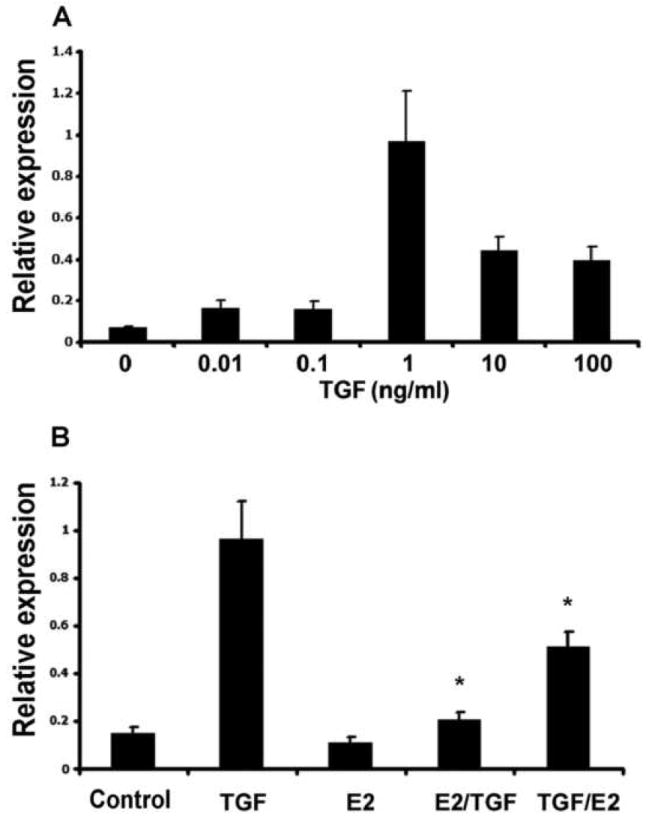
Effects of TGF-β1 and estrogen on urethral elastin mRNA expression. Urethral cells were isolated from female rats, treated with TGF-β1 and/or estrogen, and analyzed by real-time PCR. A. Dosage response of elastin mRNA expression to TGF-β1. “Relative expression” is the ratio of elastin versus GAPDH expression. B. Elastin mRNA expression in urethral cells treated with 1 ng/ml TGF-β1 (TGF), 10 nM estrogen (E2), 10 nM estrogen for 1 h and then 1 ng/ml TGF-β1 for 16 h (E2/TGF), or 1 ng/ ml TGF-β1 for 1 h and then 10 nM estrogen for 16 h (TGF/E2). “Relative expression” is the ratio of elastin versus GAPDH expression. * indicates significant difference (p<0.05) compared to TGF-β1 alone. The data are the average of 3 independent experiments.
TGF-β1 and Estrogen Effect Through Smad Responsive Elements
Treatment of urethral cells with TGF-β1 resulted in upregulated activities of both Smad1 and Smad3/4 responsive elements (Fig. 4). Treatment with estrogen alone had no effects, but the addition of estrogen to TGF-β1-treated cells blocked the effects of TGF-β1 on both Smad1 and Smad3/4 responsive elements (Fig. 4).
Figure 4.
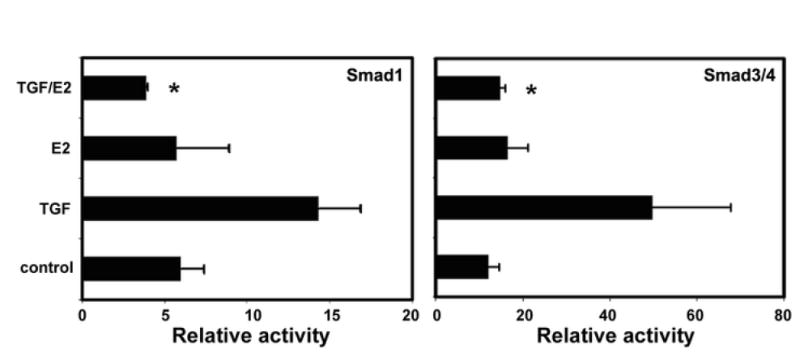
Effects of TGF-β1 and estrogen on Smad responsive elements. Urethral cells were isolated from female rats, transfected with Smad1 or Smad3/4 responsive elements, treated with TGF-β1 and/or estrogen, and analyzed by luciferase assay. “TGF” denotes treatment with 1 ng/ml TGF-β1; “E2” denotes treatment with 10 nM estrogen; “TGF/E2” denotes treatment with 1 ng/ ml TGF-β1 for 1 h and then 10 nM estrogen for 16 h. “Relative activity” is the ratio of firefly luciferase activity (derived from Smad responsive element) versus Renilla luciferase activity (internal control). * indicates significant difference (p<0.05) compared to TGF-β1 alone. The data are the average of 3 independent experiments.
COMMENT
It is well established that childbirth and menopause are two major risk factors for SUI. Although earlier studies considered estrogen deficiency as responsible for the negative effect of menopause on urinary continence, recent epidemiological studies have provided strong evidence that supplemental therapy with estrogen actually worsens UI. However, it remains unclear what mechanisms are responsible for the development of SUI and what additional mechanisms are responsible for the exacerbating effects of estrogen. Therefore, in an effort to gain a better understanding of these pathological processes, we developed a DVDO animal model that displays urinary dysfunctions resembling human SUI. Past studies with this animal model showed that estrogen indeed increased the incidence of abnormal voiding, and which was accompanied with decreased expression of alpha-1A-adrenergic receptor in the urethral smooth muscle 7,9. More recent studies further identified the involvement of the extracellular compartment, including the elastic fibers 21,22. These latter findings thus support earlier studies in which the elastic fiber system was identified as an integral part of the continence mechanism 16,18. In the present study we aimed at identifying the molecular mechanism responsible for estrogen’s effects on the elastic fiber system.
We started the project by examining urethral elastic fibers in Delivery-only, DVDO, and DVDO+E2 rats at 8 weeks post-operation because we have previously determined that DVDO rats exhibited most pronounced UI symptoms at this time point 23. The results show that the elastic fibers were fewer and shorter in the DVDO rats when compared to the Delivery-only rats, and were further reduced in abundance and length in the DVDO+E2 rats. To explore what molecular mechanisms might be responsible for such changes, we examined elastin expression in the urethras of Delivery-only, DVDO, and DVDO+E2 rats at 2, 4, 8, and 12 weeks post-operation. The results show that elastin mRNA was on average expressed at a low level in the Delivery-only rats and at increasingly higher levels in the DVDO rats up to the 8-week time point. The 12-week data, which was not shown for ease of presentation, was similar to the 8-week data, thus suggesting a plateau had reached at around the 8-week time point. More importantly, estrogen treatment essentially completely blocked the upregulated expression in the DVDO rats at 8 weeks. Therefore, it can be concluded that elastin gene expression, which is inactive in the normal urethra, is gradually upregulated following birth trauma (vagina distension) and ovariectomy, and such upregulated expression is suppressed by estrogen.
To further validate the above-mentioned conclusion, we treated urethral cells, which were mostly (~95%) smooth muscle cells, with TGF-β1 and/or estrogen. The choice of TGF-β1 was due to its well-known involvement in injury/repair and thus a possible mediator of birth-induced injury to the urethra. Indeed, the results show that TGF-β1 upregulated elastin mRNA expression in urethral cells, and more importantly, estrogen blocked this TGF-β1 effect. Since it has been shown that TGF-β1 activates elastin gene through transcription factors Smad1 and Smad3/4 24 and interactions between TGF-β1 and estrogen receptor signaling pathways are mediated by Smad3 25, we went another step further to test whether the effects of TGF-β1 and estrogen on elastin mRNA expression in urethral cells were mediated by Smad1 and Smad3/4 responsive elements. Indeed, the results show that both Smad1 and Smad3/4 responsive elements were activated by TGF-β1 and both were blocked by estrogen. As such, from tissue to cells and from elastic fibers to elastin gene, a consistent picture emerges that birth trauma activates TGF-β1 signaling, and which in turn activates elastin gene in urethral smooth muscle cells (and possibly fibroblasts as well). However, estrogen interferes with the TGF-β1 signaling pathway, resulting in the suppression of elastin expression, and which leads to improper assembly of elastic fibers in the urethra. The defective elastic fiber system weakens the urethra’s ability to withstand the abdominal/bladder pressure that is brought upon during laughing, coughing or sneezing, thus resulting in leakage.
The above-described mechanism is obviously simplistic. It is well known that the pathogenesis of SUI is multifactorial and previously studies by us and other groups have implicated the involvement of various mechanisms including adrenergic signaling in the urethral smooth muscle cells. As such, the importance of the present study is the uncovering of yet another pathway, and unlike the better known pathways, this newly discovered pathway concerns an extracellular entity, that is, the elastic fiber and its main component, elastin. Undoubtedly, additional pathways will be discovered in future studies, and together, they may solve the puzzle of the pathogenesis of SUI.
CONCLUSIONS
Elastin fibers were fewer and shorter in the urethra of rats with birth trauma and ovariectomy. Estrogen exacerbated these conditions. Elastin expression in urethral cells was upregulated by TGF-β1 signaling. Estrogen interferes with this signaling.
Acknowledgments
This work was supported by grants from Mr. Arthur Rock, the Rock Foundation, and the National Institutes of Health (DK64538 and DK069655).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Norton P, Brubaker L. Urinary incontinence in women. Lancet. 2006;367:57–67. doi: 10.1016/S0140-6736(06)67925-7. [DOI] [PubMed] [Google Scholar]
- 2.Hu TW, Wagner TH, Bentkover JD, et al. Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology. 2004;63:461–465. doi: 10.1016/j.urology.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 3.Gibbs CF, Johnson TM, 2nd, Ouslander JG. Office management of geriatric urinary incontinence. Am J Med. 2007;120:211–220. doi: 10.1016/j.amjmed.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 4.Dooley Y, Kenton K, Cao G, et al. Urinary Incontinence Prevalence: Results From the National Health and Nutrition Examination Survey. J Urol. 2007 doi: 10.1016/j.juro.2007.09.081. [DOI] [PubMed] [Google Scholar]
- 5.Cheater FM, Castleden CM. Epidemiology and classification of urinary incontinence. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:183–205. doi: 10.1053/beog.1999.0071. [DOI] [PubMed] [Google Scholar]
- 6.Grodstein F, Fretts R, Lifford K, et al. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189:428–434. doi: 10.1067/s0002-9378(03)00361-2. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi N, Bella AJ, Wang G, et al. Effect of extended-term estrogen on voiding in a postpartum ovariectomized rat model. Can Urol Assoc J. 2007;1:256–263. doi: 10.5489/cuaj.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tantiwongse K, Fandel TM, Wang G, et al. The potential of hormones and selective oestrogen receptor modulators in preventing voiding dysfunction in rats. BJU Int. 2008;102:242–246. doi: 10.1111/j.1464-410X.2008.07582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banie L, Lin G, Ning H, et al. Effects of estrogen, raloxifene and levormeloxifene on alpha1A-adrenergic receptor expression. J Urol. 2008;180:2241–2246. doi: 10.1016/j.juro.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Grady D, Brown JS, Vittinghoff E, et al. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol. 2001;97:116–120. doi: 10.1016/s0029-7844(00)01115-7. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187:521–527. doi: 10.1067/mob.2002.123938. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein SR, Johnson S, Watts NB, et al. Incidence of urinary incontinence in postmenopausal women treated with raloxifene or estrogen. Menopause. 2005;12:160–164. doi: 10.1097/00042192-200512020-00010. [DOI] [PubMed] [Google Scholar]
- 13.Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen with and without progestin on urinary incontinence. Jama. 2005;293:935–948. doi: 10.1001/jama.293.8.935. [DOI] [PubMed] [Google Scholar]
- 14.Steinauer JE, Waetjen LE, Vittinghoff E, et al. Postmenopausal hormone therapy: does it cause incontinence? Obstet Gynecol. 2005;106:940–945. doi: 10.1097/01.AOG.0000180394.08406.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today. 2007;81:229–240. doi: 10.1002/bdrc.20111. [DOI] [PubMed] [Google Scholar]
- 16.Goepel C, Thomssen C. Changes in the extracellular matrix in periurethral tissue of women with stress urinary incontinence. Acta Histochem. 2006;108:441–445. doi: 10.1016/j.acthis.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Prantil RL, Jankowski RJ, Kaiho Y, et al. Ex vivo biomechanical properties of the female urethra in a rat model of birth trauma. Am J Physiol Renal Physiol. 2007;292:F1229–1237. doi: 10.1152/ajprenal.00292.2006. [DOI] [PubMed] [Google Scholar]
- 18.Lee UJ, Gustilo-Ashby AM, Daneshgari F, et al. Lower urogenital tract anatomical and functional phenotype in lysyl oxidase like-1 knockout mice resembles female pelvic floor dysfunction in humans. Am J Physiol Renal Physiol. 2008;295:F545–555. doi: 10.1152/ajprenal.00063.2008. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Xin H, Xu X, et al. CHIP mediates degradation of Smad proteins and potentially regulates Smad-induced transcription. Mol Cell Biol. 2004;24:856–864. doi: 10.1128/MCB.24.2.856-864.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xin H, Xu X, Li L, et al. CHIP controls the sensitivity of transforming growth factor-beta signaling by modulating the basal level of Smad3 through ubiquitin-mediated degradation. J Biol Chem. 2005;280:20842–20850. doi: 10.1074/jbc.M412275200. [DOI] [PubMed] [Google Scholar]
- 21.Breyer BN, Wang G, Lin G, et al. The effect of long-term hormonal treatment on voiding patterns during cystometry and on urethral histology in a postpartum, ovariectomized female rat. BJU Int. doi: 10.1111/j.1464-410X.2010.09268.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin G, Wang G, Banie L, et al. Treatment of Stress Urinary Incontinence with Adipose Tissue-Derived Stem Cells. Cytotherapy. 2010;12:88–95. doi: 10.3109/14653240903350265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sievert KD, Emre Bakircioglu M, Tsai T, et al. The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. Part I: functional and structural change. J Urol. 2001;166:311–317. [PubMed] [Google Scholar]
- 24.Kuang PP, Zhang XH, Rich CB, et al. Activation of elastin transcription by transforming growth factor-beta in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2007;292:L944–952. doi: 10.1152/ajplung.00184.2006. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda T, Yamamoto T, Muraguchi A, et al. Cross-talk between transforming growth factor-beta and estrogen receptor signaling through Smad3. J Biol Chem. 2001;276:42908–42914. doi: 10.1074/jbc.M105316200. [DOI] [PubMed] [Google Scholar]


