Abstract
Several lines of evidence indicate group III metabotropic glutamate receptors (mGluRs) have systemic anti-hyperalgesic effects. We hypothesized this could occur through modulation of TRPV1 receptors on nociceptors. To address this question we performed anatomical studies to determine if group III mGluRs were expressed on cutaneous axons and if they co-localized with TRPV1. Immunostaining at the electron microscopic level demonstrated that 22% of unmyelinated axons labeled for mGluR8. Immunostaining at the light microscopic level in lumbar dorsal root ganglia (DRG) demonstrated that 80% and 28% of neurons labeled for mGluR8 or TRPV1, respectively. Of those neurons labeled for mGluR8, 25% labeled for TRPV1; of those labeled for TRPV1, 71% labeled for mGluR8. In behavior studies intraplantar injection of the group III mGluR agonist, L-AP-4 (0.1 1.0, 10.0 µM) had no effect on paw withdrawal latency (PWL) to heat in naïve rats but administration of 10 µM L-AP-4 prior to 0.05% capsaicin (CAP), significantly attenuated CAP-induced lifting/licking and reduced flinching behavior. The L-AP-4 effect was specific since administration of a group III antagonist UBP1112 (100 µM) blocked the L-AP-4 effect on CAP, resulting in behaviors similar to CAP alone. Intraplantar injection of UBP1112 alone did not result in nociceptive behaviors, indicating group III mGluRs are not tonically active. Finally, the anti-hyperalgesic effect of group III in this paradigm was local and not systemic since intraplantar administration of L-AP-4 in one hind paw did not attenuate nociceptive behaviors following CAP injection in the contralateral hind paw. Adenyl cyclase/cAMP/PKA may be the second messenger pathway linking these two receptor families because intraplantar injection of forskolin (FSK, 10 µM) reduced PWL to heat and L-AP-4 reversed this FSK effect. Taken together, these results suggest group III mGluRs can negatively modulate TRPV1 through inhibition of AC and downstream intracellular activity, blocking TRPV1-induced activation of nociceptors.
Keywords: L-AP-4, mGluR8, capsaicin, anti-hyperalgesia, primary afferents, sensory neurons
Glutamate, a major excitatory neurotransmitter in the nervous system, is important in peripheral pain transmission and acts on both ionotropic and metabotropic receptors (Carlton, 2001; Goudet et al., 2009). The eight cloned metabotropic glutamate receptors (mGluRs) are G-protein coupled receptors and are divided into three groups depending on homology and pharmacological properties. Group I (mGluRs 1 and 5) are excitatory and coupled to Gq, thus associated with the stimulation of phospholipase C (PLC) and intracellular calcium signals. Group II (mGluRs 2 and 3) and III (mGluRs 4, 6, 7, and 8) are inhibitory and coupled to Gi/o, thus negatively coupled to adenyl cyclase (AC) (Goudet et al., 2009). The inhibitory functions of group II and III make these receptors attractive targets for peripheral pain modulation. Several lines of evidence have implicated group II receptor agonists in the attenuation of peripheral pain (Anjaneyulu et al., 2008; Du et al., 2008). Data indicating that systemic administration of group III mGluR agonists are also effective in treatment of pain are accumulating. For example, administration of the group III agonist, L-(+)-2-Amino-4-phosphonobutyric acid (L-AP-4), in the primate dorsal horn, inhibits capsaicin (CAP)-induced central sensitization (Neugebauer et al., 2000; Goudet et al., 2009). Additionally, application of L-AP-4 to spinal lamina II in nerve injured rats results in greater inhibition of evoked excitatory postsynaptic currents (EPSCs) compared to control rats (Zhang et al., 2009). Finally, systemic administration of (S)-3,4-dicarboxyphenylglycine (DCPG), a selective mGluR8 agonist, attenuates formalin and carrageenan-induced hyperalgesia (Marabese et al., 2007). Currently, studies investigating the effects of group III mGluR activation in the periphery are limited (Walker et al., 2001; Jin et al., 2009). For example, group III mGluR activation has been shown to be anti-hyperalgesic on bee venom-induced nociception in the rat hind paw (Chen et al., 2010). Furthermore, no studies have examined the relationship between peripheral group III mGluR agonists and the transient receptor potential ion channels.
Transient receptor potential vanilloid 1 (TRPV1) is most widely known as the CAP receptor. CAP is an ingredient found in hot chili peppers and activates the channel (Caterina et al., 1997). In addition to CAP, TRPV1 can be activated by a variety of noxious stimuli: heat (above 40°C) (Caterina et al., 1997), acidic pH (Tominaga et al., 1998; Jordt et al., 2000), cannabinoid anandamide (Zygmunt et al., 1999; Smart et al., 2000), HETE (Hwang et al., 2000), and spider toxins (Costa et al., 1997; Siemens et al., 2006). Thus, TRPV1 is a polymodal receptor. Several studies have demonstrated that TRPV1 knockout mice maintain responses to acute noxious stimuli while showing attenuated development of hyperalgesia in the inflammatory state (Caterina et al., 2000; Davis et al., 2000; Bölcskei et al., 2005). These studies highlight the role TRPV1 plays in development of hyperalgesia and the importance of targeting TRPV1 in the treatment of pain.
Pain modulation at its source is an attractive strategy, especially in aiming to reduce side-effects common to most widely available analgesics (Patapoutian et al., 2009; McDougall, 2011). Since the TRP family makes up a large group of temperature sensing ion channels (Patapoutian et al., 2009), it is of great interest to examine the modulation of TRP channels, TRPV1 in particular, in the periphery. In the present study, we examined the effects of group III mGluR activation by L-AP-4 on CAP-induced nociception in the periphery.
Experimental Procedures
All experiments were approved by the University Animal Care and Use committee and followed the guidelines for the ethical care and use of laboratory animals (Zimmermann, 1983). Steps were taken to minimize both the number of animals used and their discomfort.
Immunostaining of the digital nerve at the electron microscopic level
Naïve male Sprague-Dawley rats (n = 3, 250–300 g) were deeply anesthetized with pentobarbital (100 mg/kg) and perfused transcardially with heparinized saline followed by a mixture of 2.5 % glutaraldehyde, 1.0 % paraformaldehyde and 0.1 % picric acid in 0.1 M phosphate buffer (PB) at 4° C. The digital nerves were dissected and cut into 2.0–2.5 mm segments. Prior to immunostaining, all tissue was placed for 1 hr in 1 % sodium borohydride to remove excess glutaraldehydes and rinsed in graded alcohols to increase antibody penetration. The tissue was placed in 10 % normal goat serum (NGS) for 1 hr and incubated in antiserum made in guinea pig directed against mGluR8 (1:2000, Chemicon, Temecula, CA) for 72 hr at 4° C. Following incubation, the tissues were rinsed in phosphate buffered saline (PBS), followed by 3 % NGS for 30 min, incubated in biotinylated goat anti-guinea pig IgG in 1 % NGS for 1 hr, rinsed in 1 % NGS followed by 3 % NGS for 30 min each and incubated in the ABC reagent (Vector Laboratories, Burlingame, CA) for 2 hr. The tissue was incubated in a solution of diaminobenzidine (0.05 %) containing 0.01 % hydrogen peroxide for approximately 4–6 min. After immunostaining, blocks were placed in 1 % phosphate buffered osmium tetroxide for 2 hr, dehydrated and embedded in plastic. For all blocks, ultrathin sections of the digital nerves were cut at right angles to the long axis of the fibers. The sections were mounted on formvar coated slot grids and viewed on a JEOL 100CX electron microscope. A digital nerve cut in cross section from each animal was photographed and montaged, and all labeled and unlabeled axons counted in order to obtain a percentage of mGluR8-labeled axons. For some tissues, the primary antiserum was omitted and the tissue was otherwise treated as described above. Absorption controls were also run with the peptide obtained from Chemicon. Tissue was incubated in a solution containing antibody (1:2000) that was preabsorbed with 100 µg/ml of peptide. Specific immunoreaction product was absent in both of these controls.
Immunostaining the dorsal root ganglia at the light microscopic level
Tissue Collection
Naïve male Sprague-Dawley rats (n = 3, 250–300 g) were deeply anesthetized with pentobarbital (100 mg/kg) and perfused transcardially with 4 % paraformaldehyde and 0.1 % picric acid in 0.1 M PB at 4° C. The L4 dorsal root ganglia (DRG) were dissected out. The ganglia were fast frozen using liquid nitrogen in a cryoembedding compound. The tissue was sectioned (8 µm thick) on a cryostat (Microm International, GmbH), along the short axis and placed on gelatin-dipped 3-well, Teflon-printed slides (Electron Microscopy Sciences, Fort Washington, PA). Serial section sets (3 – 5 sets per ganglia) were collected with a defined section separation so that cell counts could be determined by stereological analysis. Tissue sections were allowed to dry overnight at room temperature (RT°).
Immunohistochemistry
Slides containing tissue were first incubated in PB + 0.2 % TritonX for 10 min. Sections were blocked in 10 % normal donkey serum (NDS) for 1 hr. Sections were incubated in guinea pig polyclonal anti-mGluR8 (1:200,000, Chemicon) for 48 hr at RT° and rinsed with PBS. Next, sections were incubated in biotinylated goat anti-guinea pig IgG (1:200, Vector Laboratories) for 1 hr. After rinsing in PBS, sections were incubated in Vectastain Elite ABC peroxidase reagent (avidin-biotin complex, Vector Laboratories) for 1 hr and rinsed in PBS. Sections were incubated in Cyanine 3-labeled tyramide (1:75, Perkin-Elmer Life Science, Waltham, MA) for 7 min, using the TSA (Tyramide Signal Amplification) protocol, rinsed in PBS, and incubated in 0.03 % H2O2 for 20 min to inactivate residual peroxidase, followed by a rinse in PBS. Next, sections were incubated in goat anti-TRPV1 (1:500 Santa Cruz, Santa Cruz, CA) for 24 hr. Sections were rinsed in PBS and incubated in a Cyanine 2-conjugated mouse anti-goat IgG (1:300 Jackson Laboratories, West Grove, PA) for 1 hr and were rinsed in PBS, then dH2O. Slides were cover slipped using VectaShield mounting media (Vector Laboratories). In separate sets of tissue used for controls, the primary antiserum was omitted and the tissue was otherwise treated as described above. Absorption controls for TRPV1 (Carlton et al., 2009) and mGluR8 (Carlton and Hargett, 2007) have been reported previously from our lab.
Cresyl Violet
To obtain total cell counts, one serial section set representing each DRG was stained with cresyl violet for 2 min.
Analysis
Pairs of serial sections (disector pairs) were photographed and analyzed using the physical disector method (Coggeshall, 1992; Coggeshall and Lekan, 1996; West, 1999). Total cell counts were determined for each DRG by multiplying the number of Tops (cells only present in 1 of the 2 disector sections) by section separation, and dividing by 2 (the number of disector sections). Tissue labeled with mGluR8 and TRPV1 were used to determine percentages of single- and double-labeled cells. Percentages were calculated by dividing the number of single- and double-labeled Tops by the total cell count (obtained from the cresyl violet stained sections) and multiplying by 100. Cell diameters were determined by taking the sum of the length and width of neurons containing a visible nucleus and dividing by 2.
Behavior
Drug Preparations
A 10 % CAP stock solution was diluted with the CAP vehicle (7 % Tween-80 in saline) in order to produce a working solution of 0.05 % CAP. The following drugs were dissolved in 1N NaOH and made up as a 100 mM stock solution: L-AP-4 (Tocris Cookson Ltd., Ellisville, MO, USA), a selective group III mGluR agonist and α-methyl-3-methyl-4-phosphonophenylglycine (UBP1112 [UBP], Tocris), a potent group III mGluR antagonist. Forskolin (FSK) was dissolved in distilled water to a 1 mM stock solution. L-AP-4, UBP and FSK stock solutions were diluted to desired concentrations using PBS (pH 7.4) and the pH was adjusted to 7.4.
Drug Injections
Intraplantar drug injections were administered using a 28-gauge needle attached to a 50 µl Hamilton syringe using PE20 tubing. Since spontaneous nociceptive behaviors are most robust during the first 5–10 min following CAP injection, rats were not anesthetized during these drug injections. In contrast, rats receiving injections for the FSK paradigm were anesthetized with 3% isofluorane (anesthesia could be used since it would not interfere with the evoked heat responses being measured). The needle punctured the plantar skin and was guided through the subcutaneous space to a site just proximal to the pads. All drugs were administered into the same place in the chosen hind paw and all injected volumes were kept constant within an experimental paradigm. Each animal was only used once and the investigator was unaware of the drug combinations injected.
Habituation for behavioral testing
After arrival at the Animal Care Facility, animals were acclimated for at least 3 days before behavioral testing began. Animals were housed in groups of 3 in plastic cages with soft bedding under a reversed light/dark cycle of 12 hr/12 hr. All behavioral testing was performed between 8AM and 5PM, during the animal’s dark cycle. Care was taken to keep the animals in low lighting conditions during transport, habituation and testing. Rats were habituated for thermal testing for 1 hr on each of 5 days by placing them in plexiglass cages (8x8x18 cm) on a glass plate which was ¼ inch thick and maintained at 23° C. During the habituation period, each hind paw was tested twice 10 min apart. A modified Hargreaves et al (1988) method was used, however, the radiant heat source was replaced by a laser. The laser system was custom-made in house and consisted of a 980 nm (near infrared) continuous wave, solid-state laser (4W) with a spot size of 2 mm. Rats were habituated for CAP-induced behavioral testing by placing them on a wire screen platform in plexiglass cages for 1 hr. Each rat was habituated twice before being placed into an experimental group.
Testing for thermal and CAP-induced behaviors
Paw withdrawal latency (PWL) was assessed by examining changes in the length of time the hind paw remained on the glass, when the laser heat stimulus was applied. For the L-AP-4 dose response relationship, PWL was tested at 10 min intervals, for 60 min post-injection and plotted as the percent change from baseline. For the FSK paradigm, PWL for each group was tested at 30 and 60 min post-injection. CAP-induced spontaneous behaviors were assessed by counting the number of flinches and the number of seconds an animal spent lifting and/or licking (L/L) the injected paw at 5 min intervals for 60 min. A flinch was defined as a spontaneous, rapid jerk of the hind paw whether it was on the screen or in the air. The time course of the nociceptive behaviors was plotted as the mean number of flinches and amount of time spent L/L in 5-min intervals for 60 min.
Data Analysis
Data are presented a Mean ± SE (unless noted otherwise). Differences between groups were evaluated with a one way ANOVA. Differences between groups over a time course were evaluated with a two way RM ANOVA (if a normality test was passed). *P < 0.05 was considered significant.
Results
mGluR8-labeled digital axons
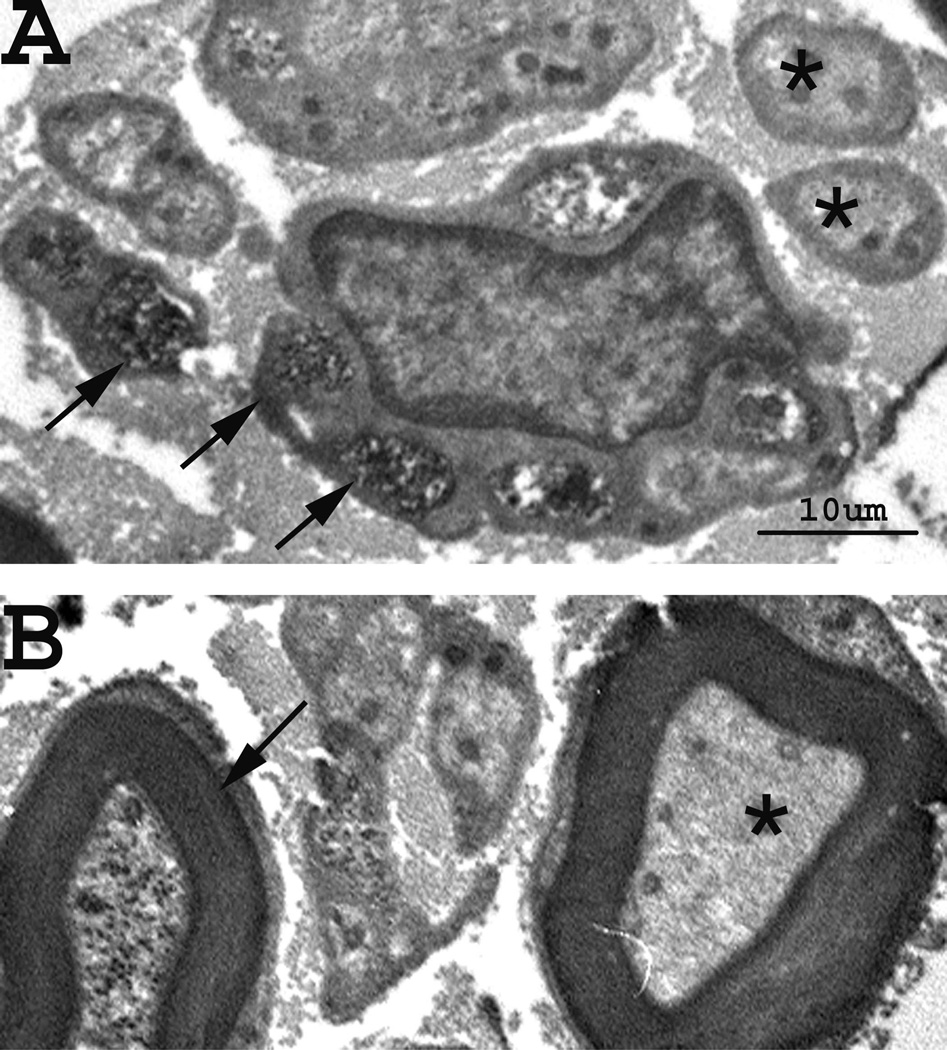
Analysis of montages of hind paw digital nerves demonstrated mGluR8-labeled axons. An axon was considered labeled when dense reaction product was observed associated with the axonal membrane or with microtubules in the axoplasm (Fig. 1). In naïve rats 21.9 ± 6.2 % of unmyelinated axons and 19.4 ± 6.9 % of myelinated axons were labeled for mGluR8 in the digital nerves (Table 1).
Figure 1.
Localization of mGluR8 on axons in the digital nerves. Electron microscopic plate of labeled (arrows) and unlabeled (asterisks) axons. The top panel (A) contains unmyelinated axons while lower panel (B) demonstrates myelinated axons. Scale bar = 10 µm.
Table 1.
Percent of mGluR8-labeled axons in hindpaw digital nerves
| Rat # | Unmyelinated axon Labeled/total |
Percent | Myelinated axons Labeled /total |
Percent |
|---|---|---|---|---|
| # 195 | 53/195 | 27.2 | 20/136 | 14.7 |
| # 196 | 38/251 | 15.1 | 21/129 | 16.2 |
| # 197 | 57/245 | 23.3 | 29/106 | 27.4 |
| Mean ± S.D. | 21.9 ± 6.2 | 19.4 ± 6.9 |
mGluR8 and TRPV1 are co-localized on rat DRG cells
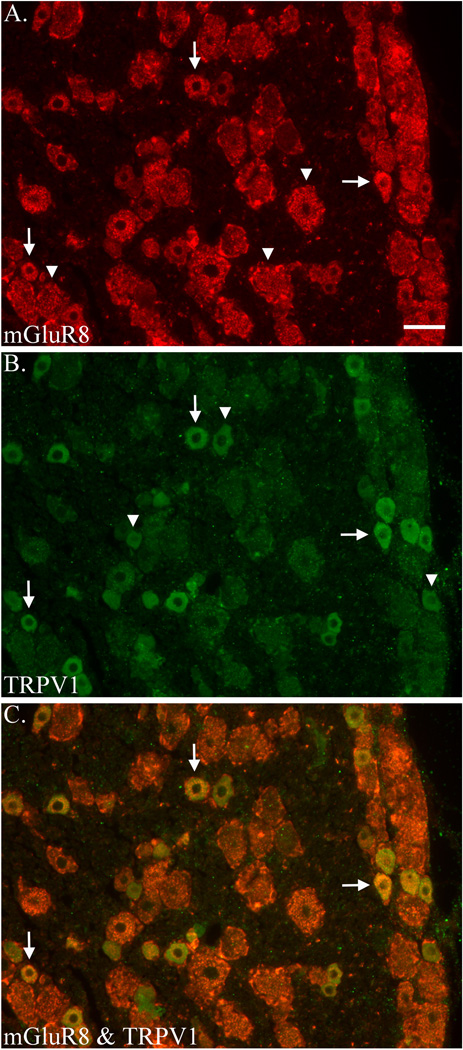
Initial observations indicated a large number of mGluR8-labeled cells and a much smaller number of TRPV1-labeled cells in the DRG. Cells labeled for mGluR8 displayed numerous (red) fluorescent puncta in the cytoplasm but the nuclei were unlabeled (Fig. 2A). Among the DRG cells, the degree of mGluR8 fluorescent label varied from light to heavy. In some cells the puncta were distributed throughout the cytoplasm while in others the puncta were concentrated in one area of the cell. Cells were considered labeled when they contained several fluorescently labeled puncta in the cytoplasm. Control sections contained no puncta. mGluR8 label was also found in satellite glial cells (SGC), the support cells surrounding DRG cells.
Figure 2.
Co-localization of mGluR8 and TRPV1 in rat DRG. Immunostaining for mGluR8 (A) and TRPV1 (B) and merged (C) in the same section. Examples of single- (arrowhead) and double-labeled (arrows) DRG neurons are indicated. Scale bar = 50 µm.
Unlike mGluR8, cells labeled for TRPV1 displayed a homogeneous (non-punctate green) fluorescence that filled the cytoplasm but the nuclei were unlabeled (Fig. 2B). Cells were considered labeled when the fluorescent cytoplasm was distinctly brighter than the background level. Control sections did not contain any level of fluorescence. Cells were considered double-labeled when they contained both the red fluorescent puncta for mGluR8 and the green fluorescent cytoplasm for TRPV1 (merged image resulted in a yellow fluorescence, Fig. 2C). Analysis demonstrated that 80.0 ± 23.3 % and 28.5 ± 8.9 % of cells in the L4 DRG were labeled for mGluR8 and TRPV1, respectively. Results also demonstrated that 25.1 ± 7.1 % of cells labeled for mGluR8 were double-labeled for TRPV1 and 71.0 ± 21.9 % of cells labeled for TRPV1 were double-labeled for mGluR8 (Table 2).
Table 2.
Percent mGluR8- and TRPV1-labeled cells in the DRG
| Rat # | % mGluR8 single-labeled |
% mGluR8 double-labeled with TRPV1 |
% TRPV1 single-labeled |
% TRPV1 double-labeled with mGluR8 |
|---|---|---|---|---|
| # 2 | 60.6 | 25.3 | 28.3 | 54.2 |
| # 3 | 73.6 | 32.1 | 37.5 | 63.0 |
| # 4 | 105.9 | 17.9 | 19.8 | 95.7 |
| Mean ± S.D. | 80.0 ± 23.3 | 25.1 ± 7.1 | 28.5 ± 8.9 | 71.0 ± 21.9 |
When nuclei were present, cell diameters were measured by placing 2 lines perpendicular to each other through the center of the cell. Line measurements were added together and divided by 2. The average diameter of mGluR8-labeled cells was 30.9 µm (range of 12.5 - 58.5 µm) and for TPRV1-labeled cells was 23 µm (range 12.5 - 39.0 µm). Double-labeled cells had an average diameter of 23.3 µm (range 12.5 – 39.0 µm).
Group III mGluR agonist L-AP-4 attenuates CAP-induced nociceptive behaviors
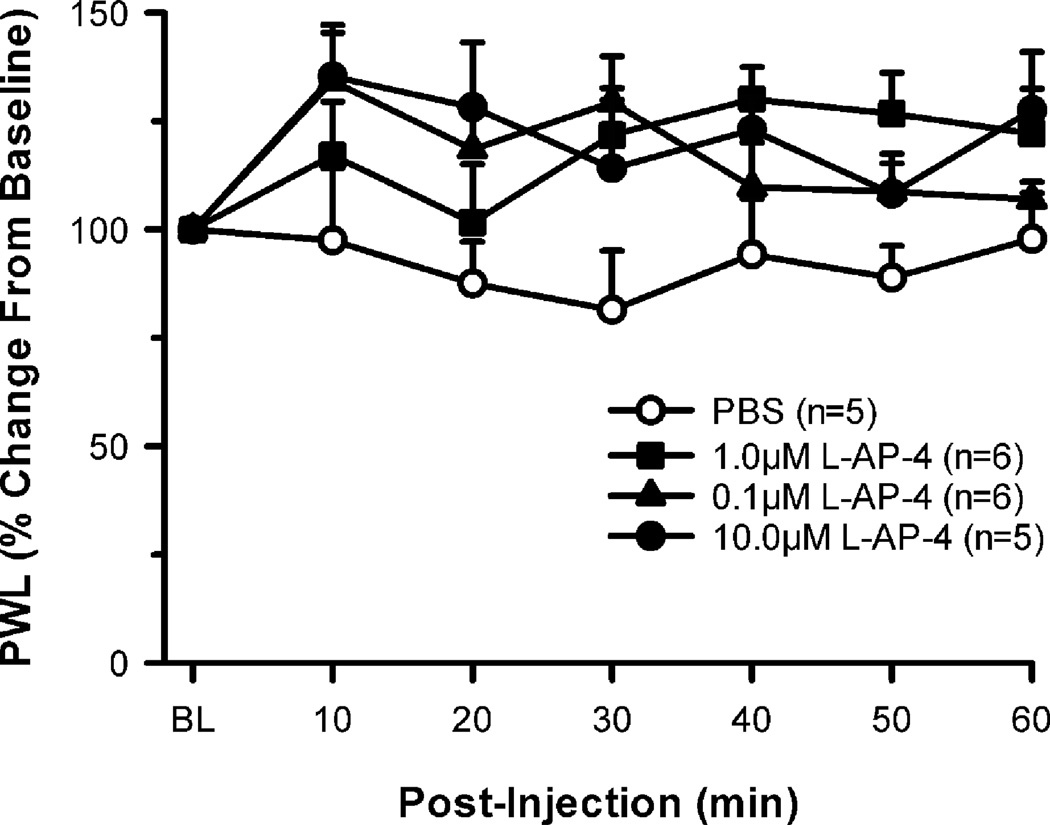
In the behavioral studies, we first examined the effects of L-AP-4 on naïve rats. Separate groups of rats were injected with one of the following: 0.1, 1.0 or 10.0 µM L-AP-4, or PBS and tested for sensitivity to heat every 10 min. Compared to baseline, there was no significant change in thermal PWL following these injections, (although L-AP-4 did tend to increase PWL), demonstrating that administration of L-AP-4 in this dose range had little or no effect in the naïve state (Fig. 3).
Figure 3.
L-AP-4, a group III agonist, has no effect in the naïve state. Intraplantar administration of L-AP-4 (0.1, 1.0, and 10.0 µM) in the naïve rat does not produce any significant change in paw withdrawal latency (PWL) to heat when compared to intraplantar PBS.
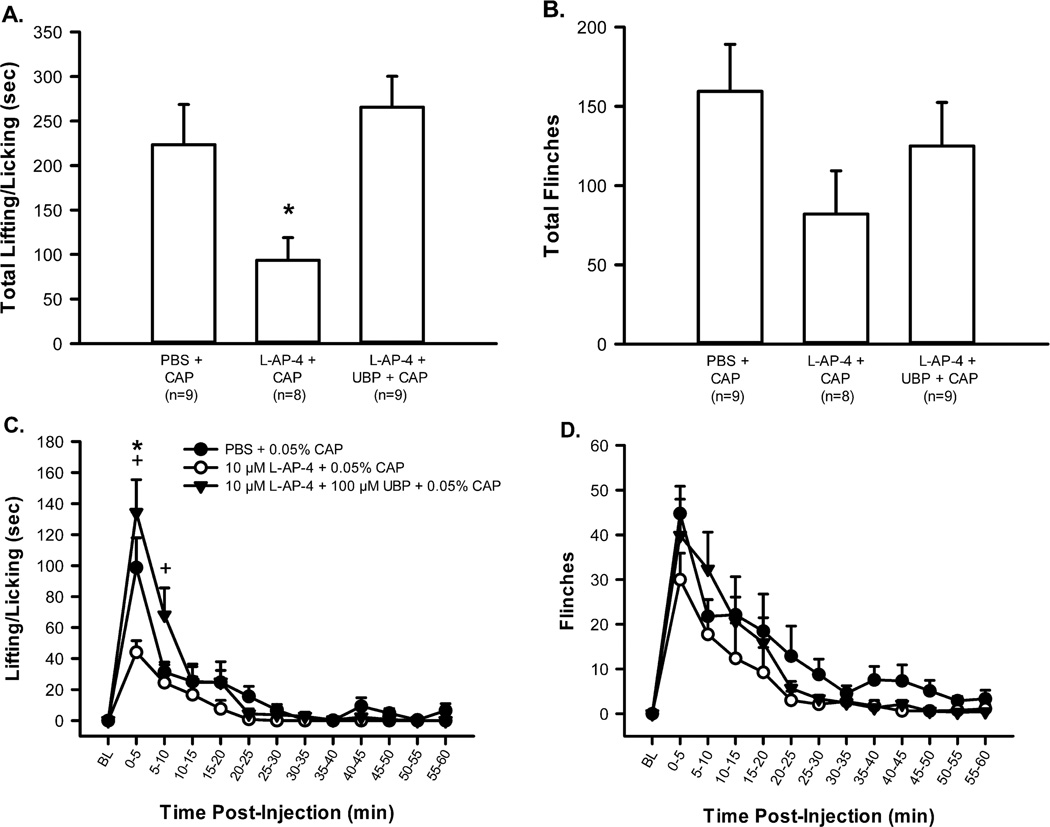
Next, the effects of L-AP-4 on CAP-induced nociception were examined. Separate groups of rats received consecutive intraplantar injections of one of the following drug combinations with a 10 min interval between the 1st and 2nd drug injection: 10 µM L-AP-4 (30 µl, n = 8) + 0.05 % CAP (30 µl); PBS (30 µl, n = 9) + 0.05 % CAP (30 µl). Our lab has previously determined that 2 consecutive injections of PBS do not result in L/L or flinching (data not shown) thus double injections did not contribute to the nociceptive behaviors observed here. The PBS + CAP group responded robustly with L/L and flinching behaviors during the first 5 min following CAP injection (Fig. 4C and D). Responses returned to baseline 40 to 50 min post-injection. Rats that received L-AP-4 prior to CAP showed attenuated nociceptive behaviors: L-AP-4 significantly reduced L/L during the first 5 min interval and significantly reduced the total time engaged in this behavior (Fig. 4A and C); however, while there was a downward trend, the L-AP-4-induced reduction in flinching did not reach significance (Fig. 4B and D).
Figure 4.
L-AP-4 produces an anti-hyperalgesic effect on CAP-induced nociception. Intraplantar administration of CAP (0.05 %) produces lifting/licking (A, total time spent lifting/licking in 1 hr; C, time course of lifting/licking) and flinches (B, total flinches in 1 hr; D, time course of flinching). L-AP-4 (10 µM) significantly reduces the time spent lifting/licking (A, *p < 0.05, significantly different from all other groups, one way ANOVA, Holm-Sidak post hoc test) and there is a tendency for a reduction in the number of flinches (B). L-AP-4 (10 µM) + UBP (100 µM), a group III antagonist, demonstrates selective group III activation by L-AP-4 since UBP inhibits the anti-hyperalgesic effects of L-AP-4 (C, +p<0.05, significantly different from L-AP-4 + CAP, two way RM ANOVA, Holm-Sidak post hoc test).
In order to demonstrate receptor specificity of L-AP-4, this group III agonist was co-injected with a selective group III antagonist, UBP. A separate group of rats received intraplantar injections with the following drug combination with a 10 min interval between the 1st and 2nd drug injections: cocktail of 100 µM UBP + 10 µM L-AP-4 (30 µl, n = 9), 0.05 % CAP (30µl). UBP blocked the inhibitory effect of L-AP-4 such that this group showed behavior that was not significantly different from the group receiving CAP alone (Fig. 4A). UBP significantly reversed the L-AP-4 inhibitory effect on CAP during the 0 - 10 min interval (Fig. 4C).
In order to show that L-AP-4 was acting locally, a separate group of rats received 10 µM L-AP-4 (30 µl, n = 8) in one hind paw + PBS (30 µl) followed 10 min later by 0.05 % CAP (20 µl) in the contralateral hind paw. This group showed behavioral responses that were no different from the PBS + CAP group, indicating L-AP-4 was not having a systemic effect (data not shown).
If group III mGluRs were tonically active, blocking activity with an antagonist would result in nociceptive behaviors (Carlton et al., 2001). To determine if group III mGluRs were tonically active, rats received an intraplantar injection of 100 µM UBP (30 µl, n = 7) alone. This injection did not result in the generation of nociceptive behaviors, demonstrating that group III mGluRs are not tonically active (data not shown).
Group III mGluR activation modulates the cyclic AMP (cAMP)/Protein Kinase A (PKA) pathway
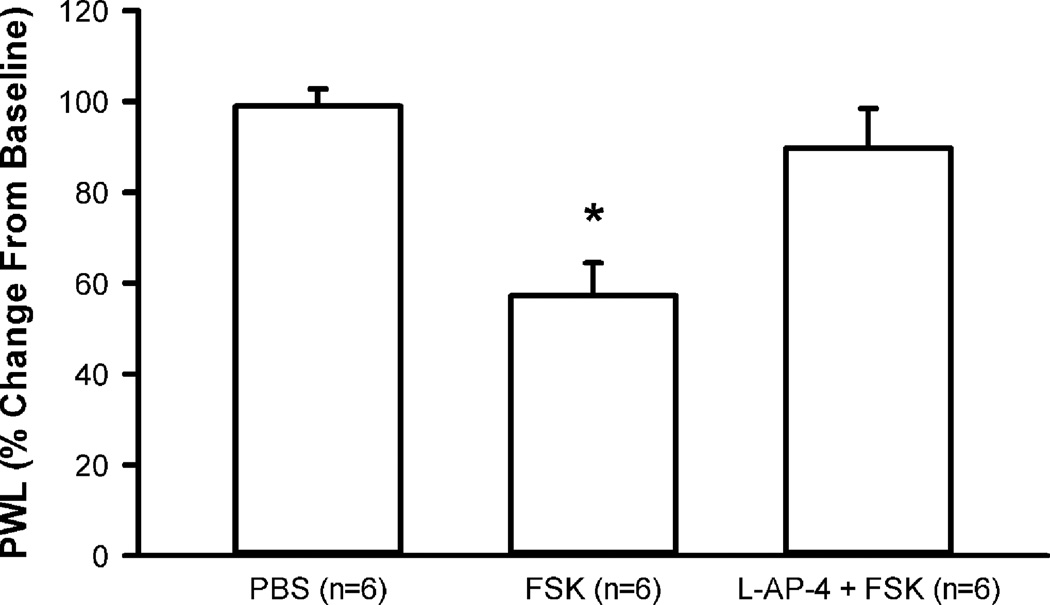
In order to examine whether group III mGluR activation inhibits the cAMP/PKA pathway, the AC activator, FSK was used. Separate groups of rats received one of the following drugs: PBS alone (20 µl, n = 6), 10 µM FSK alone (20 µl, n = 6), or 10 µM L-AP-4 + 10 µM FSK (20 µl, n = 6). Compared to the PBS group, PWL to heat was significantly lowered in the FSK group at both the 30 min (data not shown) and 60 min time points (Fig.5). Administration of L-AP-4 reversed the effects of FSK such that the L-AP-4 + FSK group was not significantly different from the PBS group (Fig. 5).
Figure 5.
L-AP-4 inhibits FSK-induced heat sensitization. Intraplantar administration of FSK (10 µM) significantly lowers PWL, compared to PBS at both the 30 min (data not shown) and 60 min time points (shown above). Administration of L-AP-4 (10 µM) + FSK (10 µM) reverses the effects of FSK, bringing PWL back to baseline. *p < 0.05, significantly different from all other groups, one way ANOVA, Holm-Sidak post hoc test.
Discussion
The present study examined the effects of group III mGluR activation on CAP-induced nociception in the periphery. Our results demonstrate that 1) mGluR8 is localized on unmyelinated axons in digital nerves, 2) mGluR8 and TRPV1 are co-localized in rat DRGs, 3) intraplantar administration of L-AP-4, a selective group III mGluR agonist, has an anti-hyperalgesic effect on CAP-induced nociceptive behaviors, and 4) group III mGluR activation inhibits forskolin-induced heat sensitization.
Our data demonstrate for the first time, the localization of group III mGluR8 in peripheral unmyelinated axons, presumably nociceptors, which would allow mGluR8 agonists and/or antagonists to have a direct effect on nerve terminals. We confirmed TRPV1 localization on small diameter sensory neurons (Carlton and Coggeshall, 2001; Carlton et al., 2009), suggesting that a direct interaction between group III mGluRs and TRPV1 is possible. Analysis indicated approximately 40 % of digital axons labeled for mGluR8 in the periphery, while 80 % of cells labeled for mGluR8 in the DRG. There are several possible explanations for this disparate finding. First, mGluR8-labeled cells in the DRG may have been overestimated due to the fact that background staining was variable in each case. Second, the cells contained within the DRG send axons to various regions of the body, including viscera, joints, and muscles in addition to cutaneous tissue, many of which could express mGluR8. Therefore, those DRG cells involved in cutaneous innervations make up only a subpopulation of the total mGluR8 population. Third, in contrast to paraformaldehyde (LM fixative for DRG), conformational changes caused by glutaraldehyde (EM fixative for digital nerves) can cause tighter cross-linking of the proteins which could reduce antibody-antigen recognition, resulting in fewer axons being labeled. Fourth, the receptor is not continuously expressed along the length of the axon (Carlton and Coggeshall, 2002). Thus, the location of the cut/section may or may not display label even though the axon is in fact labeled. Finally, it is unknown if postganglionic sympathetic neurons express mGluR8 so we cannot exclude the possibility that some of the labeled unmyelinated axons represent sympathetic fibers. Therefore, due to these caveats, the estimates of labeled axons in the digital nerves are conservative compared to estimates in the DRG.
Our results provide the first anatomical evidence for the co-localization of mGluR8 and TRPV1 receptors in the rat DRG. The percentage of DRG cells that single-labeled for each receptor is consistent with previous reports (Guo et al., 1999; Carlton et al., 2004; Carlton and Hargett, 2007; Carlton et al., 2009; however, see Hoffman et al., 2010). These data provide further support for a possible direct interaction between mGluR8 and TRPV1 receptors. Additionally, mGluR8 is only one of four group III mGluRs; thus it is highly possible that there is a higher percentage of co-localization of TRPV1 with at least two other group III mGluRs (mGluR4 and 7). The results confirm that group III is also expressed in SGCs (Carlton and Hargett, 2007), however their function in this glial population remains unknown (however, see reviews Pocock and Kettenmann, 2007; D'Antoni et al., 2008). While mGluR1α and mGluR2/3 have been localized on keratinocytes (Genever et al., 1999), expression of mGluR8 by these cells has not been investigated. Since TRPV1 is expressed by keratinocytes (Ständer et al., 2004), we cannot completely rule out that the effect of L-AP-4 on nociceptors could be indirect.
In the present study, L-AP-4 was chosen because it is a potent nonselective agonist for group III mGluRs. Although nonselective, studies demonstrate greater affinity of this drug at mGluR8, mGluR4 and mGluR6 (localization appears to be limited to retina cells [Nakajima et al., 1993; Nomura et al., 1994]) compared to mGluR7. Receptor potency for L-AP-4 appears to be as follows: mGluR8≥ mGluR4≥mGluR6>>mGluR7 (Conn and Pin, 1997; Saugstad et al., 1997; Wu et al., 1998; Cartmell and Schoepp, 2000; Yang, 2005; Selvam et al., 2007; Niswender et al., 2008).
The lack of an L-AP-4 effect in naïve is consistent with studies that demonstrated sub-cutaneous injections of this drug, in the mM dose range, had no effect on PWL (Jin et al., 2009) or paw withdrawal threshold (PWT) (Walker et al., 2001). The anti-hyperalgesic effects of group III mGluR activation have been demonstrated through central administration of the agonists DCPG and L-AP-4 (Neugebauer et al., 2000; Marabese et al., 2007; Zhang et al., 2009). In the isolated spinal cord preparation, L-AP-4 application on the spinal cord dose-dependently inhibited CAP-induced ventral root potentials (Ault and Hildebrand, 1993). However, the present study demonstrates a local anti-hyperalgesic effect can also be evoked following peripheral administration. Our findings are consistent with a previous report that peripheral group III mGluR activation attenuates bee-venom induced hyperalgesia (Chen et al., 2010). These findings suggest that a regimen that includes both a central and peripheral administration of a group III mGluR agonist could be advantageous in the treatment of pain.
The direct inhibition of TRPV1, through administration of TRPV1 antagonists, has been shown to produce hyperthermia in rats (Swanson et al., 2005; Gavva et al., 2007b; Wong and Gavva, 2009) and humans (Gavva et al., 2007a; Honore et al., 2009). In the human studies, onset of hyperthermia occurred following one or more doses of a TRPV1 antagonist but attenuated with repeated dosing. While there has been some progress in the development of TRPV1 antagonists that do not induce hyperthermia (Watabiki et al., 2011), other issues remain. For example, in a randomized healthy volunteer trial, the thermosensory profile of the subjects was shifted, causing patients to perceive hotter temperatures as optimal temperatures (Rowbotham et al., 2011). Thus targeting the TRPV1 receptor directly leads to unwanted side effects. However, modulating downstream effectors of TRPV1 such as cAMP/PKA, that are negatively coupled to group III mGluR activity, could possibly avoid these adverse effects.
These two receptor families, TRPV1 and group III mGluRs, most likely interact at the level of intracellular downstream effectors and of particular interest is PKA. TRPV1 activity (Bhave et al., 2002; Jeske et al., 2008) and its membrane insertion (Rathee et al., 2002) appear to be modulated through phosphorylation by PKA and other kinases (Cesare et al., 1999; Numazaki et al., 2002; Bhave et al., 2003; Zhang et al., 2005). In contrast, group III mGluRs are negatively coupled to AC, thus inhibiting cAMP formation and cAMP-dependent PKA activity (Neugebauer and Carlton, 2002). This group III mGluR-induced inhibition may decrease the phosphorylation of TRPV1 thereby inhibiting the enhancement of TRPV1 activity and/or its insertion into the plasma membrane.
Alternatively, group III mGluR activation may modulate TRPV1 through other mechanisms. First, basal vesicular cycling (Chavis et al., 1998) and glutamate release (Kumar et al., 2010) have been shown to decrease following activation of group III mGluRs. This could counteract the membrane depolarization caused by TRPV1-induced glutamate release (Ueda et al., 1993; Jin et al., 2009). Second, in a related fashion, this reduced extracellular glutamate may curtail activation of group I mGluRs (Bhave et al., 2001) and ionotropic glutamate receptors (iGluRs) N-Methyl-D-aspartate (NMDA) (Du et al., 2003), alpha-amino-3-hydroxy-5-methylisoxazolone-4-propionic acid (AMPA) (Carlton et al., 1995; Coggeshall and Carlton, 1998) and kainate receptors (Du et al., 2006) known to be present on peripheral nociceptors. Third, group III mGluR activation may inhibit voltage-gated Ca2+ channels, reducing neuronal excitability (Takahashi et al., 1996; Perroy et al., 2000; Capogna, 2004; Guo and Ikeda, 2005). Fourth, activation of group III mGluRs may lead to opening of K+ channels, both background K+ channels and G-protein coupled inwardly rectifying K+ (GIRK) channels (Saugstad et al., 1997; Cain et al., 2008; Niswender et al., 2008). Potassium efflux from the cell results in a more negative membrane potential, thus the opening of K+ channels could decrease excitability enough to prevent depolarization. However, based on our results in the present study, the interaction between TRPV1 and group III mGluRs most likely includes down regulation of the cAMP/PKA pathway.
It is now apparent that TRPV1 activity can be modulated indirectly through a variety of cell surface receptors expressed on nociceptors, resulting in inhibition (via mGluRs as shown here), or in sensitization. For example, activation of the bradykinin B2 receptor or nerve growth factor (NGF) TrkA receptor will sensitize TRPV1 channels, enhancing heat hyperalgesia and heat-evoked currents (Chuang et al., 2001; Ferreira et al., 2004). Furthermore, activation of prostaglandin E2 (PGE2) and PGI2 receptors (EP1 and IP, respectively), will indirectly enhance/sensitize TRPV1 responses (Moriyama et al., 2005). These actions occur through stimulation of PLC and protein kinase C (PKC) signaling pathways (Chuang et al., 2001; Julius and Basbaum, 2001). It is attractive to pursue Group III mGluRs for drug development because TRPV1 is the ultimate target for so many algogenic substances and second messenger pathways.
Conclusion
In conclusion, the present study identifies several important characteristics of the group III mGluRs. Their activation has an anti-hyperalgesic effect; this effect can be evoked locally at cutaneous nociceptors, and most likely involves down regulation of the AC/cAMP/PKA pathway. An ideal candidate for pain therapy is one that has few side effects, which might be attainable with a peripherally applied group III mGluR agonist. Additionally, the use of anti-hyperalgesics is favorable over analgesics since administration of the former inhibits enhanced nociceptive responses while allowing for normal responses to potentially noxious stimuli. Due to the fact that mGluR8 is highly co-localized with TRPV1 and significantly attenuates TRPV1-induced nociceptive behaviors, Group III mGluRs are attractive targets for novel therapies in peripheral pain treatment.
Highlights.
Group III mGluRs are localized on peripheral unmyelinated axons
Group III mGluRs and TRPV1 co-localize on dorsal root ganglion cells
Activation of group III mGluRs is anti-hyperalgesic on CAP-induced nociception
Group III mGluR activation inhibits FSK-induced heat sensitization
Acknowledgments
We thank Zhixia Ding for her excellent assistance with the electron microscopy. This work was supported by NIH grants GM069285 (R.M. Govea), and NS054765 and NS027910 (S.M. Carlton).
Abbreviations
- AC
adenyl cyclase
- AMPA
alpha-amino-3-hydroxy-5-methylisoxazolone-4-propionic acid
- cAMP
cyclic AMP
- DCPG
(S)-3,4-Dicarboxyphenylglycine
- DRG
dorsal root ganglia
- EPSC
excitatory postsynaptic current
- FSK
forskolin
- iGluR
ionotropic glutamate receptor
- L-AP-4
L-(+)-2-Amino-4-phosphonobutyric acid
- mGluR
metabotropic glutamate receptor
- NDS
normal donkey serum
- NGF
nerve growth factor
- NGS
normal goat serum
- NMDA
N-Methyl-D-aspartate
- PB
phosphate buffer
- PBS
phosphate buffered saline
- PGE2
prostaglandin E2
- PGI2
prostaglandin I2
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
- PWL
paw withdrawal latency
- PWT
paw withdrawal threshold
- RT
room temperature
- SCG
satellite glial cells
- TRPV1
transient receptor potential vanilloid 1
- TSA
tyramide signal amplification
- TrkA
tyrosine kinase A
- UBP
α-methyl-3-methyl-4-phosphonophenylglycine (UBP1112)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anjaneyulu M, Berent-Spillson A, Russell JW. Metabotropic glutamate receptors (mGluRs) and diabetic neuropathy. Curr Drug Targets. 2008;9:85–93. doi: 10.2174/138945008783431772. [DOI] [PubMed] [Google Scholar]
- Ault B, Hildebrand LM. Effects of excitatory amino acid receptor antagonists on a capsaicin-evoked nociceptive reflex: a comparison with morphine, clonidine and baclofen. Pain. 1993;52:341–349. doi: 10.1016/0304-3959(93)90168-O. [DOI] [PubMed] [Google Scholar]
- Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci U S A. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Karim F, Carlton SM, Gereau RW. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4:417–423. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Bölcskei K, Helyes Z, Szabó A, Sándor K, Elekes K, Németh J, Almási R, Pintér E, Petho G, Szolcsányi J. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain. 2005;117:368–376. doi: 10.1016/j.pain.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Cain SM, Meadows HJ, Dunlop J, Bushell TJ. mGlu4 potentiation of K(2P)2.1 is dependant on C-terminal dephosphorylation. Mol Cell Neurosci. 2008;37:32–39. doi: 10.1016/j.mcn.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Capogna M. Distinct properties of presynaptic group II and III metabotropic glutamate receptor-mediated inhibition of perforant pathway-CA1 EPSCs. Eur J Neurosci. 2004;19:2847–2858. doi: 10.1111/j.1460-9568.2004.03378.x. [DOI] [PubMed] [Google Scholar]
- Carlton SM. Peripheral excitatory amino acids. Curr Opin Pharmacol. 2001;1:52–56. doi: 10.1016/s1471-4892(01)00002-9. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Coggeshall RE. Peripheral capsaicin receptors increase in the inflamed rat hindpaw: a possible mechanism for peripheral sensitization. Neurosci Lett. 2001;310:53–56. doi: 10.1016/s0304-3940(01)02093-6. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Coggeshall RE. Inflammation-induced up-regulation of neurokinin 1 receptors in rat glabrous skin. Neurosci Lett. 2002;326:29–32. doi: 10.1016/s0304-3940(02)00299-9. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Du J, Zhou S. Group II metabotropic glutamate receptor activation on peripheral nociceptors modulates TRPV1 function. Brain Res. 2009;1248:86–95. doi: 10.1016/j.brainres.2008.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton SM, Du J, Zhou S, Coggeshall RE. Tonic control of peripheral cutaneous nociceptors by somatostatin receptors. J Neurosci. 2001;21:4042–4049. doi: 10.1523/JNEUROSCI.21-11-04042.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL. Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J Comp Neurol. 2007;501:780–789. doi: 10.1002/cne.21285. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci Lett. 1995;197:25–28. doi: 10.1016/0304-3940(95)11889-5. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Zhou S, Du J, Hargett GL, Ji G, Coggeshall RE. Somatostatin modulates the transient receptor potential vanilloid 1 (TRPV1) ion channel. Pain. 2004;110:616–627. doi: 10.1016/j.pain.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- Chavis P, Mollard P, Bockaert J, Manzoni O. Visualization of cyclic AMP-regulated presynaptic activity at cerebellar granule cells. Neuron. 1998;20:773–781. doi: 10.1016/s0896-6273(00)81015-6. [DOI] [PubMed] [Google Scholar]
- Chen HS, Qu F, He X, Kang SM, Liao D, Lu SJ. Differential roles of peripheral metabotropic glutamate receptors in bee venom-induced nociception and inflammation in conscious rats. J Pain. 2010;11:321–329. doi: 10.1016/j.jpain.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Carlton SM. Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. J Comp Neurol. 1998;391:78–86. doi: 10.1002/(sici)1096-9861(19980202)391:1<78::aid-cne7>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Costa SK, de Nucci G, Antunes E, Brain SD. Phoneutria nigriventer spider venom induces oedema in rat skin by activation of capsaicin sensitive sensory nerves. Eur J Pharmacol. 1997;339:223–226. doi: 10.1016/s0014-2999(97)01387-3. [DOI] [PubMed] [Google Scholar]
- D'Antoni S, Berretta A, Bonaccorso CM, Bruno V, Aronica E, Nicoletti F, Catania MV. Metabotropic glutamate receptors in glial cells. Neurochem Res. 2008;33:2436–2443. doi: 10.1007/s11064-008-9694-9. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Du J, Zhou S, Carlton SM. Kainate-induced excitation and sensitization of nociceptors in normal and inflamed rat glabrous skin. Neuroscience. 2006;137:999–1013. doi: 10.1016/j.neuroscience.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Du J, Zhou S, Carlton SM. Group II metabotropic glutamate receptor activation attenuates peripheral sensitization in inflammatory states. Neuroscience. 2008;154:754–766. doi: 10.1016/j.neuroscience.2008.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Zhou S, Coggeshall RE, Carlton SM. N-methyl-D-aspartate-induced excitation and sensitization of normal and inflamed nociceptors. Neuroscience. 2003;118:547–562. doi: 10.1016/s0306-4522(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Ferreira J, da Silva GL, Calixto JB. Contribution of vanilloid receptors to the overt nociception induced by B2 kinin receptor activation in mice. Br J Pharmacol. 2004;141:787–794. doi: 10.1038/sj.bjp.0705546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Hovland DN, Lehto SG, Klionsky L, Surapaneni S, Immke DC, Henley C, Arik L, Bak A, Davis J, Ernst N, Hever G, Kuang R, Shi L, Tamir R, Wang J, Wang W, Zajic G, Zhu D, Norman MH, Louis JC, Magal E, Treanor JJ. Repeated administration of vanilloid receptor TRPV1 antagonists attenuates hyperthermia elicited by TRPV1 blockade. J Pharmacol Exp Ther. 2007a;323:128–137. doi: 10.1124/jpet.107.125674. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, Kuang R, Le A, Tamir R, Wang J, Youngblood B, Zhu D, Norman MH, Magal E, Treanor JJ, Louis JC. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007b;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genever PG, Maxfield SJ, Kennovin GD, Maltman J, Bowgen CJ, Raxworthy MJ, Skerry TM. Evidence for a novel glutamate-mediated signaling pathway in keratinocytes. J Invest Dermatol. 1999;112:337–342. doi: 10.1046/j.1523-1747.1999.00509.x. [DOI] [PubMed] [Google Scholar]
- Goudet C, Magnaghi V, Landry M, Nagy F, Gereau RW, Pin JP. Metabotropic receptors for glutamate and GABA in pain. Brain Res Rev. 2009;60:43–56. doi: 10.1016/j.brainresrev.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Guo J, Ikeda SR. Coupling of metabotropic glutamate receptor 8 to N-type Ca2+ channels in rat sympathetic neurons. Mol Pharmacol. 2005;67:1840–1851. doi: 10.1124/mol.105.010975. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hoffman EM, Schechter R, Miller KE. Fixative composition alters distributions of immunoreactivity for glutaminase and two markers of nociceptive neurons, Nav1.8 and TRPV1, in the rat dorsal root ganglion. J Histochem Cytochem. 2010;58:329–344. doi: 10.1369/jhc.2009.954008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Chandran P, Hernandez G, Gauvin DM, Mikusa JP, Zhong C, Joshi SK, Ghilardi JR, Sevcik MA, Fryer RM, Segreti JA, Banfor PN, Marsh K, Neelands T, Bayburt E, Daanen JF, Gomtsyan A, Lee CH, Kort ME, Reilly RM, Surowy CS, Kym PR, Mantyh PW, Sullivan JP, Jarvis MF, Faltynek CR. Repeated dosing of ABT-102, a potent and selective TRPV1 antagonist, enhances TRPV1-mediated analgesic activity in rodents, but attenuates antagonist-induced hyperthermia. Pain. 2009;142:27–35. doi: 10.1016/j.pain.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain. 2008;138:604–616. doi: 10.1016/j.pain.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, Yamaki F, Takemura M, Koike Y, Furuyama A, Yonehara N. Capsaicin-induced glutamate release is implicated in nociceptive processing through activation of ionotropic glutamate receptors and group I metabotropic glutamate receptor in primary afferent fibers. J Pharmacol Sci. 2009;109:233–241. doi: 10.1254/jphs.08262fp. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kumar N, Laferriere A, Yu JS, Poon T, Coderre TJ. Metabotropic glutamate receptors (mGluRs) regulate noxious stimulus-induced glutamate release in the spinal cord dorsal horn of rats with neuropathic and inflammatory pain. J Neurochem. 2010;114:281–290. doi: 10.1111/j.1471-4159.2010.06761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabese I, de Novellis V, Palazzo E, Scafuro MA, Vita D, Rossi F, Maione S. Effects of (S)-3,4-DCPG, an mGlu8 receptor agonist, on inflammatory and neuropathic pain in mice. Neuropharmacology. 2007;52:253–262. doi: 10.1016/j.neuropharm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- McDougall JJ. Peripheral analgesia: Hitting pain where it hurts. Biochim Biophys Acta. 2011;1812:459–467. doi: 10.1016/j.bbadis.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem. 1993;268:11868–11873. [PubMed] [Google Scholar]
- Neugebauer V, Carlton SM. Peripheral metabotropic glutamate receptors as drug targets for pain relief. Expert Opin Ther Targets. 2002;6:349–361. doi: 10.1517/14728222.6.3.349. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Chen PS, Willis WD. Groups II and III metabotropic glutamate receptors differentially modulate brief and prolonged nociception in primate STT cells. J Neurophysiol. 2000;84:2998–3009. doi: 10.1152/jn.2000.84.6.2998. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Johnson KA, Luo Q, Ayala JE, Kim C, Conn PJ, Weaver CD. A novel assay of Gi/o-linked G protein-coupled receptor coupling to potassium channels provides new insights into the pharmacology of the group III metabotropic glutamate receptors. Mol Pharmacol. 2008;73:1213–1224. doi: 10.1124/mol.107.041053. [DOI] [PubMed] [Google Scholar]
- Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;77:361–369. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroy J, Prezeau L, De Waard M, Shigemoto R, Bockaert J, Fagni L. Selective blockade of P/Q-type calcium channels by the metabotropic glutamate receptor type 7 involves a phospholipase C pathway in neurons. J Neurosci. 2000;20:7896–7904. doi: 10.1523/JNEUROSCI.20-21-07896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M. PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci. 2002;22:4740–4745. doi: 10.1523/JNEUROSCI.22-11-04740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham MC, Nothaft W, Duan WR, Wang Y, Faltynek C, McGaraughty S, Chu KL, Svensson P. Oral and cutaneous thermosensory profile of selective TRPV1 inhibition by ABT-102 in a randomized healthy volunteer trial. Pain. 2011;152:1192–1200. doi: 10.1016/j.pain.2011.01.051. [DOI] [PubMed] [Google Scholar]
- Saugstad JA, Kinzie JM, Shinohara MM, Segerson TP, Westbrook GL. Cloning and expression of rat metabotropic glutamate receptor 8 reveals a distinct pharmacological profile. Mol Pharmacol. 1997;51:119–125. doi: 10.1124/mol.51.1.119. [DOI] [PubMed] [Google Scholar]
- Selvam C, Goudet C, Oueslati N, Pin JP, Acher FC. L-(+)-2-Amino-4-thiophosphonobutyric acid (L-thioAP4), a new potent agonist of group III metabotropic glutamate receptors: increased distal acidity affords enhanced potency. J Med Chem. 2007;50:4656–4664. doi: 10.1021/jm070400y. [DOI] [PubMed] [Google Scholar]
- Siemens J, Zhou S, Piskorowski R, Nikai T, Lumpkin EA, Basbaum AI, King D, Julius D. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature. 2006;444:208–212. doi: 10.1038/nature05285. [DOI] [PubMed] [Google Scholar]
- Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ständer S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, Brzoska T, Lippert U, Henz BM, Luger TA, Metze D, Steinhoff M. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- Swanson DM, Dubin AE, Shah C, Nasser N, Chang L, Dax SL, Jetter M, Breitenbucher JG, Liu C, Mazur C, Lord B, Gonzales L, Hoey K, Rizzolio M, Bogenstaetter M, Codd EE, Lee DH, Zhang SP, Chaplan SR, Carruthers NI. Identification and biological evaluation of 4-(3-trifluoromethylpyridin-2-yl)piperazine-1-carboxylic acid (5-trifluoromethylpyridin-2-yl)amide, a high affinity TRPV1 (VR1) vanilloid receptor antagonist. J Med Chem. 2005;48:1857–1872. doi: 10.1021/jm0495071. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, Onodera K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science. 1996;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Ueda M, Kuraishi Y, Satoh M. Detection of capsaicin-evoked release of glutamate from spinal dorsal horn slices of rat with on-line monitoring system. Neurosci Lett. 1993;155:179–182. doi: 10.1016/0304-3940(93)90702-m. [DOI] [PubMed] [Google Scholar]
- Walker K, Reeve A, Bowes M, Winter J, Wotherspoon G, Davis A, Schmid P, Gasparini F, Kuhn R, Urban L. mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology. 2001;40:10–19. doi: 10.1016/s0028-3908(00)00114-3. [DOI] [PubMed] [Google Scholar]
- Watabiki T, Kiso T, Kuramochi T, Yonezawa K, Tsuji N, Kohara A, Kakimoto S, Aoki T, Matsuoka N. Amelioration of neuropathic pain by novel transient receptor potential vanilloid 1 antagonist AS1928370 in rats without hyperthermic effect. J Pharmacol Exp Ther. 2011;336:743–750. doi: 10.1124/jpet.110.175570. [DOI] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res Rev. 2009;60:267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Wu S, Wright RA, Rockey PK, Burgett SG, Arnold JS, Rosteck PR, Johnson BG, Schoepp DD, Belagaje RM. Group III human metabotropic glutamate receptors 4, 7 and 8: molecular cloning, functional expression, and comparison of pharmacological properties in RGT cells. Brain Res Mol Brain Res. 1998;53:88–97. doi: 10.1016/s0169-328x(97)00277-5. [DOI] [PubMed] [Google Scholar]
- Yang ZQ. Agonists and antagonists for group III metabotropic glutamate receptors 6, 7 and 8. Curr Top Med Chem. 2005;5:913–918. doi: 10.2174/1568026054750272. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Chen SR, Pan HL. Effects of activation of group III metabotropic glutamate receptors on spinal synaptic transmission in a rat model of neuropathic pain. Neuroscience. 2009;158:875–884. doi: 10.1016/j.neuroscience.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]