Abstract
Background and Aims
The APC tumor suppressor is a multifunctional protein involved in cell migration, proliferation, differentiation and apoptosis. Cleavage of APC and the subsequent release of an amino-terminal segment are necessary for a transcription-independent mechanism of APC-mediated apoptosis. The aim of the current study is to elucidate the mechanism by which the amino-terminus of APC contributes to the enhancement of apoptosis.
Methods
Previous yeast-two-hybrid screens, using the ARM-repeat domain of APC as bait, identified hTID-1 as a potential binding partner. Co-immunoprecipitations, co-immunofluorescence and binding assays confirm a direct interaction between caspase-cleaved APC and hTID1 in vivo at the mitochondria. Overexpression and siRNA knockdown studies were designed to determine the significance of this interaction.
Results
These experiments have identified hTID-1 as a directly interacting protein partner of caspase-cleaved APC. hTID-1 is an apoptosis modulator: two of its known mitochondrial protein isoforms, 43-kDa and 40-kDa, have opposing effects in apoptosis. We demonstrate that the amino-terminal segment of APC interacts with both hTID-1 isoforms directly, although there is a stronger association with the apoptotic suppressor 40-kDa isoform in vitro. This interaction localizes to amino acids 202-512 of APC, a region including two of the seven ARM-repeats. Over-expression of the 40-kDa hTID-1 isoform partially rescues cells from apoptosis mediated by APC 1-777, while siRNA knock-down of this hTID-1 isoform enhances apoptosis.
Conclusions
These data suggest that the amino-terminal segment of APC promotes cell sensitivity to apoptosis modulated through its binding to 40- and 43-kDa hTID-1 isoforms.
Introduction
The APC tumor suppressor gene encodes a large 310-kDa protein normally expressed in non-proliferating colorectal epithelium1. It is essential for maintaining normal growth control and differentiation. Mutation of APC is a rate-limiting event in the development of most colorectal tumors, both inherited and sporadic2, and is associated with dysregulation of several physiological processes that govern intestinal epithelial cell homeostasis. Our recent work shows that APC also plays a role in apoptosis through both transcription-dependent and transcription-independent pathways via caspase-cleavage of APC3.
Apoptosis or programmed cell death is a normal, physiological program necessary for proper development and cell turnover. It maintains a constant cell number in continuously renewing cell populations. Improper regulation can facilitate tumor formation even if normal cell cycle control is maintained4, 5. There are generally two major pathways for apoptosis--the death receptor pathway and the mitochondrial pathway6, 7. Activation of cell surface death receptors of the Fas/tumor necrosis factor receptor family triggers initiator caspases activation, which in turn cleaves and activates an executioner caspase, procaspase-38, 9. In the mitochondrial pathway, death stimuli induce mitochondrial outer membrane permeabilization, leading to the release of mitochondrial pro-apoptotic proteins that either induce caspase activation, such as cytochrome c and Smac/Diablo, or trigger caspase-independent effectors such as the apoptosis-inducing factor (AIF) and HtrA27, 10-12. The death receptor pathway in some, if not all, types of cells requires the help of the mitochondrial pathway to amplify the downstream caspase cascade10, 11, 13-15. Therefore, the mitochondria can be considered the central integrator and regulator of cell life and death.
The colorectal epithelium is a dynamic cell population. Mitotically active stem cells reside at the base of the colonic crypts; post-mitotic daughter cells differentiate and migrate along the crypt axis within the lumen. A limited amount of apoptosis occurs along the crypt axis, although the main population of apoptotic cells in the epithelium is observed toward the luminal aspect of the crypts16-18. Understanding the mechanism by which APC contributes to apoptosis in normal colorectal epithelial cells will improve our understanding of tumor initiation following loss of APC in colon cancer.
To elucidate the molecular mechanism of APC-mediated transcription-independent apoptosis, we set out to find potential protein binding partners that specifically interact with the caspase-cleaved amino-terminal segment of APC. Using co-immunoprecipitation, in vitro binding assays and immunofluorescent colocalization assays, we demonstrate that the amino-terminal segment of APC (1-777) interacts directly with two isoforms of the mitochondrial protein hTID-1 in vitro and in vivo. Overexpression of the 40-kDa hTID-1 protein, a negative modulator of apoptosis, in cultured cells partially rescue cells from apoptosis induced by the amino-terminal segment of APC. Conversely, loss of this hTID-1 isoform in cells over-expressing APC 1-777 enhances apoptosis suggesting that the anti-apoptotic 40-kDa hTID-1 isoform suppresses the pro-apoptotic function of APC. Our data suggest that the caspase-cleaved segment of APC migrates to the mitochondria where it can promote cell sensitivity to apoptosis that is then modulated through its binding to 40- and 43-kDa hTID-1 isoforms.
Material and Methods
Cell culture
Human colorectal cancer cell lines, HCT 116, SW480 and DLD1, were obtained from the American Type Tissue Collection and grown in McCoy's 5A Medium, Leibovitz's L-15 Medium and RPMI Medium (Life Technologies), respectively, supplemented with 10% FBS (Hyclone) at 37°C and 5% CO2.
Plasmid constructs
Mammalian expression vectors encoding the Flag-tagged APC amino terminal segment (1-777) were generated by cloning PCR-generated APC cDNA into pcDNA3 (Invitrogen) using BamHI, NotI, the high fidelity polymerase Pwo (Roche Applied Sciences) and pBS(SK)APC as template. pEGFP APC was constructed as described19. Primers to generate pEGFP APC 1-777 were: (forward) 5′- GCC AGT GGA TCC ACC ATG GCT GCA GCT TCA TAT GAT CA -3′ and (reverse) 5′- AT GAC CGC GGC CGC GAG CTC TTA GTC TAT ATT GTC AAA AGT TTC TGA -3′. All constructs were verified by sequencing.
pGEX-TID1 to encode GST-fusion hTID-1 43-kDa protein was provided by Dr. I.R. Lehman, Stanford University; vector encoding a GST-fusion hTID-1 40-kDa protein was generated by cloning hTID-1 40-kDa cDNA provided by Dr. K. Munger, Harvard University into pGEX (Invitrogen) using SmaI.
Transfections, immunoprecipitations and western analyses
Cells were transiently transfected with a plasmid encoding Flag-tagged APC 1-777 using LipofectAMINE 2000 Reagent (Invitrogen). At 48 hrs, cells were washed and lysed in standard buffer with 1% NP-40 and complete protease inhibitors (Sigma). Supernatants were incubated with anti-FLAG M2 affinity gel (Sigma). Immunoprecipitates were washed and eluted with Laemmli sample buffer. Samples were resolved by SDS/10% PAGE, transferred to PVDF membrane (Millipore) and analyzed by western blotting with a 1:2500 dilution of anti-Flag monoclonal antibody (Sigma) or a 1:2000 dilution of anti-hTID-1 antibody (Santa Cruz Biotechnology). Blots were probed with a 1:5000 dilution of peroxidase-conjugated goat anti-mouse secondary antibody (Pierce) and visualized with chemiluminescence.
Pre-designed ON-TARGETplus SMART pool siRNAs targeting hTID-1 were used (Dharmacon). Transfection mixes included 100 nM siRNA (either hTid-1-specific or non-specific siRNA control) and Dharmafect 1 transfection reagent in Opti-MEM medium, which were incubated with cells for 96 hrs. Transfection of pEGFP-APC 1-777 or pEGFP occurred 48 hrs following siRNA transfection using LipofectAMINE 2000 (Invitrogen). Cells were harvested 48 hrs later.
Protein purification
GST-fused hTID-1 proteins were purified as described 20. Protein was >95% pure as determined by SDS/PAGE and Coomassie brilliant blue R-250 staining. Protein concentrations were determined by Bradford method using BSA standards.
In vitro binding assays
GST and GST-hTID-1 were immobilized on glutathione-sepharose beads by incubating purified GST-protein with glutathione-sepharose beads (Pharmacia). Unbound proteins were removed by washing; immobilized GST or GST-hTID-1 was mixed with His-tagged APC 1-777 in 0.5 ml of buffer A (20 mM HEPES, pH 7.4, 250 mM sucrose, 10 mM KCl, 1.5 mM MgCl2 1 mM EGTA, 1 mM DTT and complete protease inhibitor cocktail). Beads were collected by centrifugation and washed. Pellets were resuspended in SDS lysis buffer (20 mM Tris·HCl, pH7.5/50 mM NaCl/0.5% SDS/1 mM DTT), separated by SDS/8% PAGE, and transferred to PVDF membrane. Blots were probed with an anti-His antibody (Sigma) and visualized with chemiluminescence.
Amino-terminal APC segments were in vitro transcribed and translated using the TnT Quick for PCR DNA kit (Promega). GST hTID-43kDa and hTID-40kDa proteins were expressed and purified using glutathione-S-transferase beads. Proteins were visualized and quantitated using Coomassie-stained SDS-PAGE.
35S-methionine-labeled APC proteins were incubated with hTID-1 43-kDa, hTID-1 40-kDa, or GST in binding buffer (20mM Tris pH 7.5, 10 % glycerol, 150 mM NaCl, 5 mM EDTA, 0.1 % Tween-20 and protease inhibitor cocktail). hTID antibody was added and incubated overnight. Protein A/G beads were added and samples washed, visualized using SDS-PAGE, fixed for 30 minutes, dried and exposed to film.
Microscopy
DLD1 and HCT 116 cells were transiently co-transfected with pGFP-APC1-777, pGFP-APC 761-1339 or pGFP vector and hTID-1 using LipofectAMINE 2000 Reagent. After 48 hrs, cells were fixed in 3.7% (vol/vol) formaldehyde and permeabilized with 0.3% Triton X-100. Coverslips were probed with a 1:200 dilution of anti-hTID-1 antibody in blocking buffer (5 mg/ml BSA, 0.5% Nonidet P-40 in PBS). Slides were probed with a 1:200 dilution of Cy5.5-conjugated donkey anti-mouse antibody (Jackson ImmunoResearch). MitoTracker Red CMXRos was used (Molecular Probes) for mitochondrial staining. Slides were mounted with Fluoromount G (Electron Microscopy Sciences) and evaluated with an LSM510 laser confocal scanning confocal microscope (Zeiss); images were captured with a digital camera (Hamamatsu, Middlesex, NJ) and QED imaging software (QED Imaging).
Subcellular fractionation, immunoprecipitation and immunoblotting
HCT116 cells were treated with 4 mM sodium butyrate for 72 hrs; adherent and floating cells were harvested separately. Mitochondrial and cytosolic fractions were prepared and cell fractionation was performed as described21. Proteins from each fraction were used for immunoblotting with monoclonal antibodies against APC (Ab-1, Calbiochem), hTID-1 and cytochrome oxidase subunit 1 (1D6, Molecular Probes).
Lysates were precleared with normal mouse IgG antibody and protein A/G sepharose, and incubated with antibodies against APC (Ab 5, Calbiochem) or hTID-1. Immunocomplexes were adsorbed to protein G–Sepharose beads, washed, mixed with sample buffer and western blot analysis was performed.
Apoptosis assays
Apoptosis was assessed by staining nuclear chromatin of cells with 1 μM Hoechst 33342 (Sigma-Aldrich). Adherent cells were evaluated with an Axioplan2 microscope (Zeiss) and scored for apoptotic nuclei within GFP-positive cell populations (n>300/experiment). Cells were stained with annexin V and propidium iodide to determine dead cell percentage using FACS Calibur. Triplicate samples of 105 cells each were analyzed and percent death calculated by totaling annexin-positive cells with annexin plus PI-positive cells. Two independent experiments were performed for each. Remaining protein lysates were analyzed by western blot to confirm hTID-1 and APC expression, and to measure apoptosis by PARP cleavage (Promega).
Results
hTID-1 co-immunoprecipitates with the amino-terminus of APC (APC 1-777)
The hTID-1 protein was first identified as a potential protein partner of APC using a yeast two-hybrid screen with the armadillo repeat domain of APC as bait22. The interaction of full-length APC and cytosolic hTID50/TID48 protein was later reported by Kurzik-Dumke and Czaja in 200723, who also noted that the interaction was mediated through the amino-terminus of APC. To explore the contribution of hTID interactions to the role of APC in apoptosis, we first confirmed that the amino-terminus of APC interacted with the mitochondrial hTID protein isoforms with defined roles in apoptotic modulation24. Lysates from HCT116 cells transfected with a vector encoding Flag-tagged APC 1-777 or a pcDNA3 empty vector were immunoprecipitated with an anti-TID antibody, that recognizes the 43 and 40 kDa isoforms, and immunoblotted with anti-Flag and anti-APC antibodies. The Flag-tagged APC amino-terminus co-immunoprecipitated with the endogenous 40-kDa and 43-kDa hTID-1 (Figure 1). These results demonstrate that the amino-terminus of APC and hTID-1 interact in human cells.
Figure 1. The amino-terminus of APC co-immunoprecipitates with hTID-1.


A. Schematic diagram of Flag-tagged APC N-terminus. B. Proteins from HCT116 cells transfected with Flag-tagged APC 1-777 or the pcDNA3 empty vector, immunoprecipitated with an anti-TID antibody (RS-11) and resulting complexes analyzed by immunoblot with antibodies specific for Flag, APC or TID-1, as indicated.
Armadillo repeats within the amino-terminus of APC directly interact with hTID-1 in vitro
In order to define the TID-1 interaction domain within the amino-terminus of APC, we first examined the ability of a recombinant His-tagged APC amino-terminal segment to bind with purified GST-tagged hTID-1 proteins. In vitro binding was assayed by incubating purified His-tagged APC segments with GST, GST- hTID-1 43-kDa and GST-hTID-1 40-kDa immobilized on glutathione-sepharose beads. Figure 2A shows that the GST and GST-tagged hTID-1 proteins bind to beads. Figure 2B shows that the amino-terminus of APC interacts with GST-tagged 43- or 40-kDa hTID-1 proteins, but not with GST alone.
Figure 2. The amino-terminus of APC directly interacts with hTID-1 in vitro.

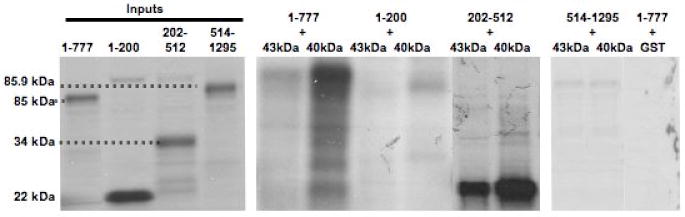
A. Recombinant GST (lane 1), GST-tagged hTID-1 43-kDa (lane 2), and GST-tagged hTID-1 40-kDa (lane 3) were immobilized on glutathione-sepharose beads and separated by 4-20% SDS/PAGE and the gel was stained with Coomassie. B. His-tagged APC 1-777 was mixed with immobilized GST or GST-hTID-1. After washing thoroughly, bound proteins were eluted and separated by 8% SDS/PAGE and immunoblotted with an anti-His antibody. C. Immobilized GST or GST-hTID-1 (43- and 40-kDa) proteins were incubated with 35S-labeled APC protein fragments overnight. Beads were washed and bound proteins were eluted and separated by 4-12% SDS/PAGE. APC 1-777 and APC 202-512 bind hTID-1, while the other APC fragments and GST-alone do not. Although both the 43- and 40-kDa hTID-1 isoforms bound to APC within the same region, we observed preferential binding of APC to the 40-kDa hTID-1 protein.
Non-overlapping deletion constructs of the APC amino-terminus were then designed using gene-specific primers to amplify APC segments by PCR. The gene-specific forward primers contained sequences encoding a T7 promoter, Kozak, and start codon allowing for in vitro transcription and translation of the PCR-generated APC segments. Expressed APC proteins were subsequently combined with the 43- or 40-kDa hTID-1 proteins or GST-only proteins. As shown in Figure 2C, both hTID-1 proteins interact directly with the region of APC containing amino acids 202-512, a region containing two of the seven armadillo repeats important for maintaining protein-protein interactions25. Although both hTID-1 isoforms bind to APC within the same region, APC preferentially binds to the 40-kDa hTID-1 isoform as shown in Figure 2C. Interestingly, this hTID-1 isoform is a negative modulator of apoptosis24. These data clearly demonstrate that hTID-1 interacts directly with the amino-terminus of APC through a domain of APC important for protein-protein interactions.
Caspase-3 cleavage of endogenous APC results in its translocation to the mitochondria
During apoptosis, full length APC (∼310-kD) is cleaved by group II caspases to form a stable 90-kD amino terminal segment found in floating cells3, 26-28 (Figure 3C). We have validated some of these earlier findings in vivo by pre-treating cells with a caspase 3 inhibitor and subsequently with an apoptosis inducing drug, 5-fluorouracil (5-FU) (Figure 3A). These studies demonstrate that endogenous full-length APC is cleaved upon treatment with 5-FU to release a 90-kDa protein, an effect that is at least partially blocked by pre-treatment with caspase 3 inhibitor. Additionally, truncated (mutant) APC expressed in DLD-1 (Figure 3B) and SW480 (data not shown) colon cancer cells was not cleaved as efficiently as wild type protein in vivo.
Figure 3. The amino-terminus of APC is cleaved by caspases following DNA damage and moves to mitochondria.


A. HCT116 cells were treated with 5-FU alone or first pretreated, 2h prior to 5-FU, with a caspase-3 inhibitor (CI). Lysates were separated by 4-12 % SDS/PAGE and immunoblotted for APC or actin. B. HCT116 and DLD-1 cells were treated with either 2 or 5 nM sodium butyrate (NaBt) for 48 h to induce apoptosis. Lysates were separated using 4-12% SDS/PAGE and immunoblotted for APC, PARP and actin. Wild-type APC is caspase-cleabed following NaBt treatment in HCT116 cells as denoted by the arrow indicating the 90 kDa APC fragment. The same treatment in DLD-1 cells did not lead to caspase-cleavage of mutant APC fragment. C. HCT116 cells were treated with 4 mM NaBt for three days to induce apoptosis. Control cells were left untreated. Adherent (A) and floating (F) cells were fractionated into mitochondria-enriched (M) and cytosolic (C) fractions by centrifugation. 30 μg of each fraction were separated using 12% SDS/PAGE and evaluated with antibodies specific for the APC amino-terminus (AB-1), hTID-1 (RS11) and the mitochondrial cytochrome oxidase subunit 1(1D6).
To study the physiological function of this protein released during apoptosis, we evaluated its localization in cells undergoing apoptosis. HCT116 cells, expressing full-length APC, were treated with 4 mM sodium butyrate for 72 hours to induce apoptosis. Treatment results in nearly 80% of normal healthy adherent cells detaching from the plate and floating in the medium due to apoptotic death. Protein lysates from both the adherent and floating cell populations were analyzed. The 90-kDa amino-terminal segment of APC can be identified in lysates from floating HCT116 cells in the same subcellular fraction as the mitochondrial proteins hTID-1 and cytochrome oxidase subunit 1 (Figure 3C). This segment of APC was not identified in attached cells. These results complement recent data from Brocardo et al. that demonstrate mutant truncated APC proteins targeted to the mitochondria via the amino-terminus of APC29. Our data show that APC, once caspase-cleaved following DNA damage, translocates to the mitochondria and suggest that this cleavage is critical to its role as a pro-apoptotic protein. Additionally, truncated APC is not as efficiently cleaved as wild-type protein by caspases in vitro3; truncated APC does not enhance apoptosis, even though detected at the mitochondria. These findings suggest that caspase cleavage of wild-type APC results in a cleaved protein with a new conformation permitting its binding to novel protein partners that contribute to the apoptotic cascade.
The amino-terminus of APC colocalizes with hTID-1 at the mitochondria in vivo
The resulting caspase-cleaved APC protein is substantially smaller in size than full-length APC and has a known role in apoptosis. Our findings that cleaved APC translocates to the mitochondria was intriguing. These findings were validated using co-localization studies. HCT116 cells were transfected with a plasmid encoding a GFP-tagged amino-terminal APC (1-777) or a plasmid encoding a downstream region of APC not required for apoptotic function, GFP-tagged APC (761-1339). Mitochondria were stained with a mitochondria-specific stain, Mitotrack Red CMXRos. As shown in Figure 4, GFP-tagged APC 1-777 displays a bright punctate perinuclear presence that partially overlaps with the MitoTrack staining. In contrast, expression of GFP-tagged APC 761-1339 in HCT116 cells led to a diffuse distribution throughout the entire cell, in both the cytoplasm and nucleus. These data suggest that following caspase cleavage, the amino-terminus of APC translocates to the mitochondria, a central site in the apoptotic process.
Figure 4. The amino-terminus of APC co-localizes with hTID-1 in human cells.
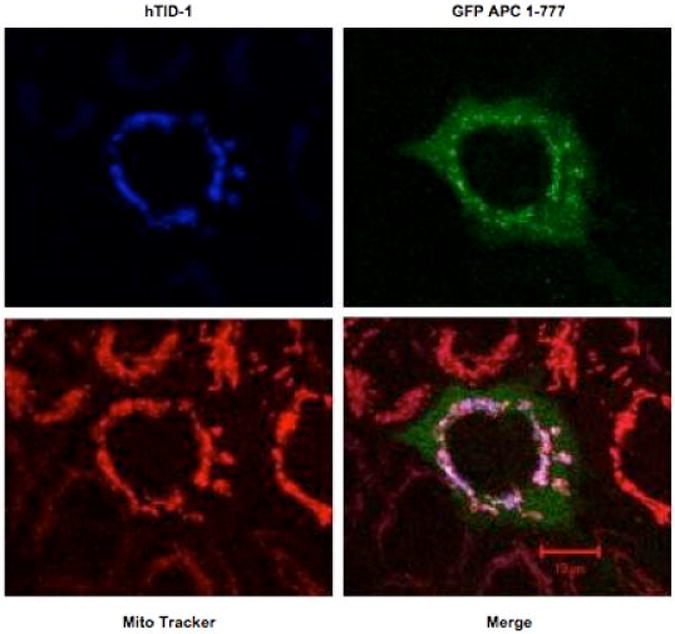
A vector encoding a GFP-tagged APC segment (1-777) was cotransfected with a vector encoding hTID-1 40-kDa into the colorectal cancer cell line HCT116. Mitochondria were stained with Mito Tracker Red CMXRox and images captured with LSM510 laser scanning confocal microscopy at 400× magnification.
Given the direct interaction between APC 1-777 and hTID-1, we asked whether these two proteins co-localize at the mitochondria. Colorectal carcinoma cell lines, DLD1 and HCT116, were co-transfected with vectors encoding GFP-tagged segments of APC 1-777 or APC 761-1339 and hTID-1. As shown in Figure 5 (A and B), GFP-tagged APC 761-1339 protein was distributed diffusely throughout the whole cell as expected. In contrast, co-expression of hTID-1 and GFP-tagged APC 1-777 display a bright punctate perinuclear presence consistent with previous co-localization studies of hTID-130. Importantly, co-localization of hTID-1 and APC 1-777 partially overlaps with MitoTrack staining. These data suggest that the amino-terminus of APC interacts with hTID-1 at the mitochondria in vivo.
Figure 5. The amino-terminus of APC colocalizes with 40-kDa hTID-1 at the mitochondria.
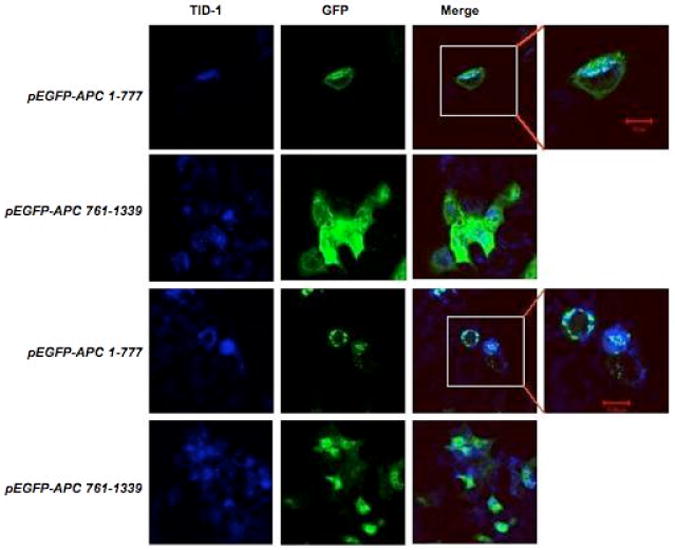
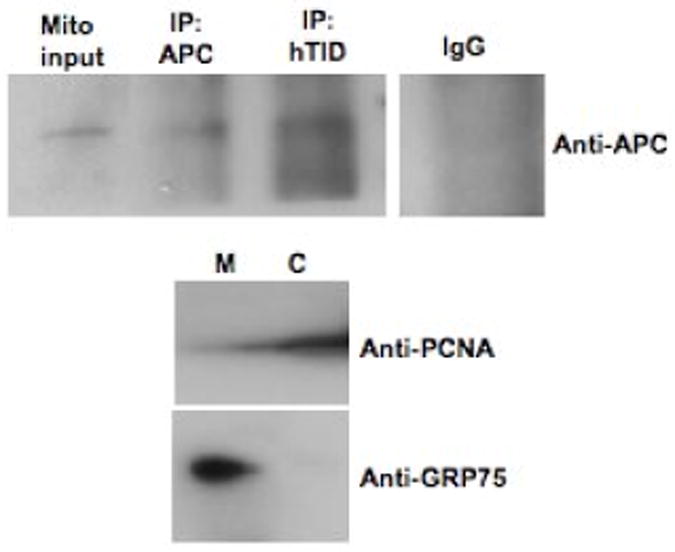
Vectors encoding GFP-tagged APC segments APC 1-777 or APC 761-1339 were cotransfected with a vector encoding hTID-1 in colorectal cancer cell lines DLD1 (A) and HCT116 (B). hTID-1 proteins were identified using Cy5.5-conjugated donkey anti-mouse antibody. Images were captured with LSM510 laser scanning confocal microscopy at 400× magnification. C. Mitochondria were isolated from apoptotic (floating) HCT116 cells treated with 5-FU for 48 h. Lysates were immunoprecipitated with an anti-hTID antibody, separated by 4-12 % SDS/PAGE and immunoblotted for hTID or APC. Anti-PCNA (cytosolic marker, Santa Cruz sc-56) and Anti-GRP75 (mitochondrial marker, Santa Cruz sc-1058) antibodies were used as controls for isolation procedures.
Colocalization studies were then validated with immunoprecipitation studies using mitochondrial-enriched cell fractions. HCT116 cells were treated with 5-FU to induce apoptosis and following drug treatment, floating cells harvested and mitochondria isolated. hTID-1 immunoprecipitated with endogenous caspase-cleaved APC from extracts prepared from these apoptotic cells, supporting co-localization studies (Figure 5C).
Expression of the 40-kDa hTID-1 isoform inhibits the pro-apoptotic function of the APC amino-terminus
Previous work from Syken et al. suggested that the 40-kDa hTID-1 isoform suppresses cytochrome c release and consequent caspase 3 activation in response to apoptotic stimuli, and that the 43-kDa hTID-1 isoform enhances the response to apoptotic stimuli24. We asked what effect the interaction between hTID-1 and APC 1-777 proteins might have given that both proteins independently have an effect on apoptosis. To test this, the 40-kDa hTID-1 isoform was over-expressed with and without APC 1-777 in HCT116 cells treated with 80 ng/ml of TRAIL (TNFα-related apoptosis-inducing ligand). Over-expression of the 40-kDa isoform (co-transfected with GFP-encoding vector only) reduced the number of apoptotic cells by 25-30% compared to cells transfected with GFP-encoding vector only in cells treated with 80 ng/ml of TRAIL (Figure 6). Cells transfected with both hTID-1 (40-kDa) and APC 1-777 reduced the percentage of apoptotic cells compared to the induction of apoptosis detected following transfection of APC 1-777 alone. Figure 6 shows that overexpression of the 40-kDa hTID-1 isoform partially rescues cells from apoptosis accelerated by the amino-terminus of APC in the absence or presence of TRAIL. These results suggest that the 40-kDa hTID-1 protein antagonizes the apoptotic function of APC 1-777.
Figure 6. Overexpression of hTID-1 40-kDa partially rescues cells from apoptosis mediated by the amino-terminus of APC (aa 1-777).
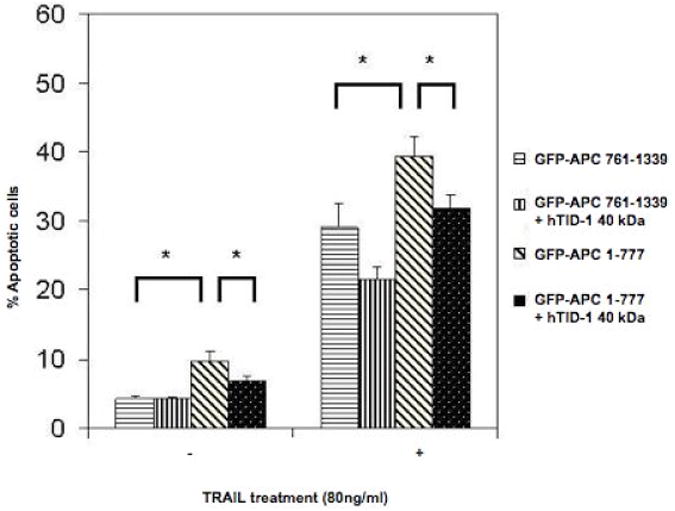
HCT116 cells were co-transfected with vectors encoding hTID-1 40-kDa and GFP-tagged APC segments, APC 1-777 or APC 761-1339. After 48 hours, cells were left untreated or treated with 80ng/ml TRAIL for 5.5 hours and stained with Hoechst 33324. Apoptotic nuclei within adherent GFP-positive cell populations were counted and the numbers compared. Results are expressed as the mean +/− S.D. from three separate experiments. Asterisks denote significant differences (P<0.05) between groups as indicated.
To complement these findings, DLD-1 cells were transiently transfected with a siRNA mixture targeting both hTID-1 isoforms (Figure 7) while concomitantly over-expressing APC 1-777. Cells were then evaluated by annexin and propidium iodide (PI) staining to determine the percentage of apoptotic cells. A significant increase in apoptosis was detected in co-transfected cells compared to cells transfected with APC 1-777 alone. Western blot analysis in parallel to the annexin/PI staining confirmed the selective knockdown of hTID-1 and over-expression of APC 1-777. Additionally, increased apoptosis was detected in cells with decreased hTID-1 expression and overexpression of APC 1-777 as indicated by a significant increase in PARP cleavage, a known protein indicator of apoptosis (Figure 7C). A slight reduction in overall apoptosis was detected following decreased expression of both hTID-1 isoforms alone. Given the previous finding that the hTID isoforms have opposing effects on apoptosis, the slight reduction in apoptosis detected following hTID knock-down may result from a disproportionate ratio of negative apoptotic modulator (40-kDa isoform) to positive apoptotic modulator (43-kDa isoform) (Figure 7C and D) in DLD-1 cells. Similar results were observed in HCT116 cells, which express higher levels of the 40-kDa hTID isoform. Finally, to distinguish between isoform-specific effects, we selectively targeted the 43-kDa isoform by siRNA, which did not disrupt the apoptotic response of cells to APC 1-777 transfection, with or without 5-FU. Our data from these complementary experiments suggest that the interaction between the 40-kDa hTID-1 protein and APC leads to a suppression of the pro-apoptotic function of APC. A significant increase in apoptosis occurs when this interaction is disrupted.
Figure 7. siRNA-mediated knockdown of hTID-1 and concomitant overexpression of APC 1-777 leads to enhanced apoptosis.

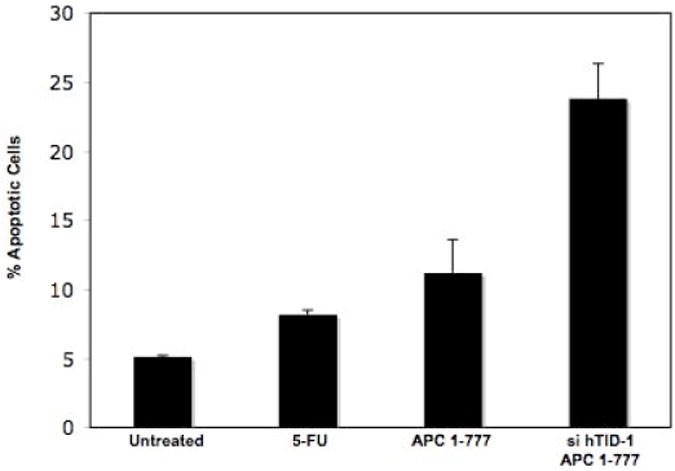
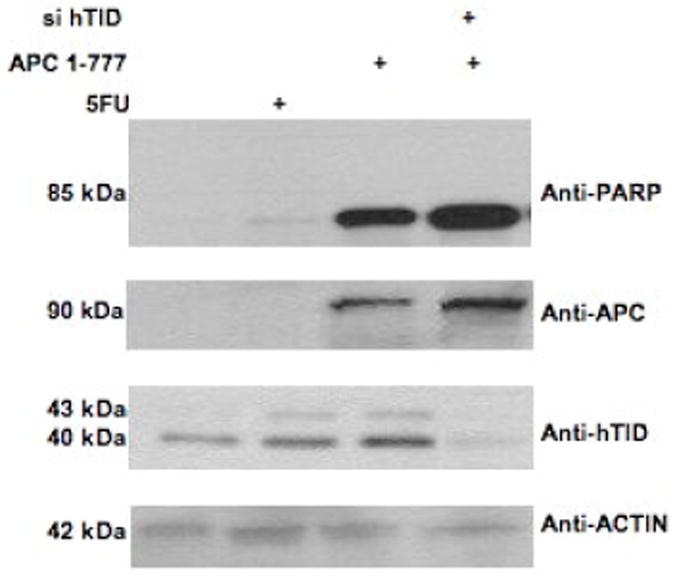
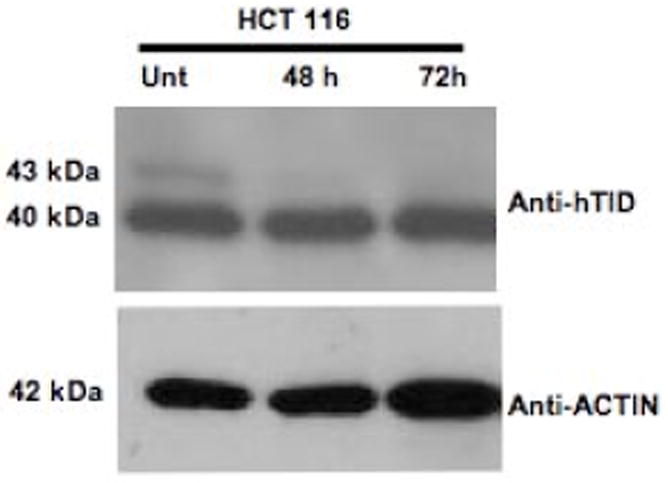
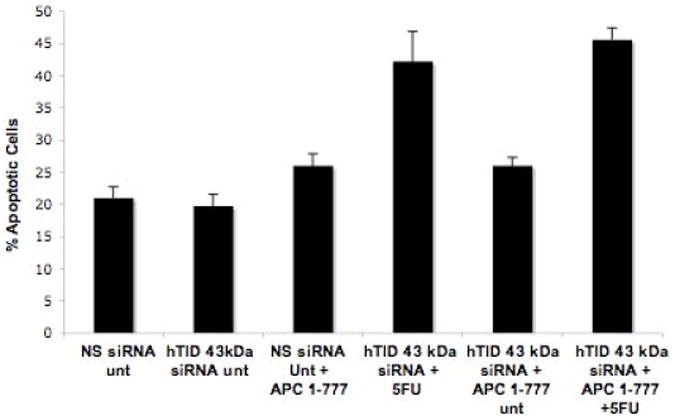
A. HCT116 cells transfected with a siRNA pool targeting hTID-1 for 96 h and treated with 5-FU for 24 h were assayed for apoptosis by both annexin/PI staining and western blot analysis. A slight decrease in apoptosis was detected compared to non-specific siRNA-transfected control cells. B. Overexpression of APC 1-777 in the absence of hTID-1 led to increased levels of apoptosis by annexin/PI analysis compared to cells overexpressing APC 1-777 alone. C. Western blot analysis of the same samples showed an increase in the cleavage of an apoptotic indicator protein, PARP, in samples overexpressing APC 1-777 and lacking hTID-1 compared to those overexpressing APC 1-777 alone. D. HCT116 cells transfected with siRNA targeting hTID-1 43 kDa specifically after 48h and 72 h. E. Overexpression of APC 1-777 in the absence of hTID-1 43 kDa alone did not result in significant increase in apoptosis as determined by annexin/pi staining.
Discussion
Work from our laboratory and others have shown that APC, an intestinal tumor suppressor protein, contributes to apoptosis through both transcription-dependent and -independent mechanisms. A caspase-cleaved amino-terminal segment of APC (1-777), released following apoptotic stimuli, facilitates an accelerated apoptotic time course in vitro and is required for the transcription-independent mechanism of APC-mediated apoptosis3, 26. We report that a caspase-cleaved APC protein segment binds directly to a mitochondrial tumor suppressor protein, hTID-1, in vivo. This interaction maps specifically to a portion of the ARM-repeat domain of APC important for mediating protein-protein interactions. Additionally, we report that the interaction of caspase-cleaved APC and the apoptotic-suppressor isoform of hTID-1 40-kDa, leads to a decrease in apoptosis in vivo. Conversely, when the interaction between caspase-cleaved APC and hTID-1 40-kDa is disrupted by siRNA knock-down of hTID-1, the suppression of apoptosis is lost. Although mutant truncated forms of APC can also be detected at the mitochondria, we have shown that these mutant proteins are not efficiently cleaved by caspases in vitro or in vivo29. We suggest that wild-type caspase-cleaved APC acquires a particular conformation that in turn permits interaction with specific pro-apoptotic protein binding partners at the mitochondria, and resulting in enhanced apoptosis. Presumably, the loss of the 90-kDa APC protein in tumors leads to the loss of apoptosis-promoting signals.
The lethal(2) tumorous imaginal discs (l(2)tid) gene was originally identified in Drosophila31,32. Homozygous mutation of the gene leads to neoplastic growth of the larval imaginal discs 31. hTID-1, the human homologue of dtid, was isolated by yeast two-hybrid screening as a molecular partner to a diverse set of tumor-related proteins19. hTID-1 encodes three splice-forms encoding three cytosolic (hTID50, hTID48 and hTID46) and three mitochondrial (hTID43, hTID40 and hTID38) proteins23, 33. Syken et al. reported that hTID1 encoded two mitochondrial splice variants, 43- and 40-kDa, that had opposing effects on the ability of a cell to respond to an exogenous apoptotic stimulus24. The 43-kDa hTID1 isoform increases apoptosis triggered by either TNFα or DNA-damage following mitomycin C treatment. Conversely, the 40-kDa hTID1 isoform suppresses apoptosis. The role of the 38-kDa hTID-1 isoform in apoptosis is unknown, although it is expressed at a low level in colon epithelium and therefore was not evaluated in the current study34.
The human homolog of the Drosophila tumor suppressor gene tid 56, was first mentioned as a putative APC partner in 1997 as a yeast two-hybrid screen using APC armadillo domains as bait identified hTID22. A more recent report by Kurzik-Dumke et al. demonstrated that full-length APC interacts with cytosolic hTID-1, both the 50- and 48-kDa protein isoforms, in a complex with Hsp70, Hsc70, Actin, Dvl and Axin23. A subsequent study demonstrated that alteration of hTID-1 isoform expression is accompanied by APC redistribution within the cell, loss of polarity and tumor progression34. Our data indicate that caspase-cleaved APC partners with hTID-1, specifically the 43- and 40-kDa mitochondrial isoforms, defining a novel role for this protein-protein interaction in apoptotic signaling. Consistent with the original finding by Syken et al. that the 40-kDa hTID-1 isoform suppresses apoptosis, we find that the 40-kDa isoform preferentially binds APC and negatively regulates the pro-apoptotic function of caspase-cleaved APC. It is possible that as the ratio of hTID-1 isoforms change in response to DNA damage that APC may be released from its negative suppression and binds other pro-apoptotic proteins. It is also reasonable to speculate that APC and hTID-1 interact with other protein partners in a complex, as described previously for hTID-1 that may fluctuate in response to different cellular stress signals23.
The ARM-repeat domain of APC, which partially mediates the interaction of hTID-1 and APC, consists of seven iterations of the characteristic 42 amino acid motif spanning amino acids 453-766 of APC35, 36. This APC segment is the most highly conserved region of the protein25 and mediates its interactions with several proteins that regulate growth and signaling. The armadillo repeat region of β-catenin, a well-known partner of APC, is essential for the induction of apoptosis by overexpressed β-catenin37. Given that the hTID1/APC interaction domain contains two of the seven ARM repeats, it is reasonable to speculate that the transcription-independent apoptotic effects of APC depend on its protein-protein interactions with these or other pro- or anti-apoptotic protein(s) via this conserved armadillo repeat domain. It is also likely that if the expression of either protein were altered, as in colorectal carcinoma, protein interactions that occur via binding to the APC ARM domain would be disrupted. Alternatively, increased expression of the hTID-1 40-kDa isoform could interfere with the ability of APC to bind other pro-apoptotic proteins and indirectly provide a mechanism by which hTID-1 suppresses apoptosis.
HTID-1 is a member of the DnaJ protein family and is a molecular co-chaperone of the heat shock protein 70 (Hsp70)24, 38. hTID-1 interacts with HSP70 chaperones and serves to regulate interactions with specific substrates. HSP70 proteins and their associated co-chaperones mediate a variety of cellular activities in intercellular signaling linked to cell survival and growth regulation39. Heat shock proteins provide a conserved mechanism to adapt and survive under conditions that threaten the folding integrity of cellular proteins by environmental insult, disease or aging40. An emerging body of literature demonstrates the antagonistic role of HSP70 during apoptosis (41, 42, 43, 44). Therefore, the inhibitory effect of hTID-1 on APC may be mediated partially through HSP70 interactions, given its role as a co-chaperone of HSP70. We tested the possibility that hTID may act as a chaperone in assisting the translocation of casapse-cleaved APC to the mitochondria. These unpublished data suggest that hTID is not required for APC localization to the mitochondria.
The contribution of caspase cleavage to the role of APC in apoptosis is of great interest. Given the large size of full-length APC protein (∼310 kDa), we initially considered that caspase-cleavage allowed the release of a smaller 90-kDa protein, free from protein-interactions mediated through the carboxyl terminus. This clipped form of APC then migrates to alternative subcellular localizations. Although still plausible, this view is complicated by data showing mutant forms of APC, not efficiently cleaved, at the mitochondria29. It is reasonable to speculate that caspase-cleavage results in a novel structural conformation of cleaved APC that permits a subset of protein partners to bind specifically. Since mutant APC proteins are not cleaved, the three dimensional structures of the proteins most likely differ and result in altered protein binding partners. This hypothesis would also explain why mutant APC does not enhance apoptosis despite its mitochondrial localization.
In summary, we report that the amino-terminal segment of APC interacts with the apoptosis modulator hTID-1 to promote cell sensitivity to apoptosis. The endogenous APC amino-terminus consisting of 777 amino acids released by caspase cleavage during apoptosis relocates to the mitochondria and acquires novel protein partners such as hTID-1. The release of APC 1-777 may provide positive feedback to accelerate the apoptotic process through modifying the mitochondrial changes that occur during apoptosis.
Acknowledgments
We thank Dr. K. Munger, (Harvard University), Dr. I.R. Lehman (Stanford University) and Dr. S. Pestka (University of Medicine and Dentistry of New Jersey) for providing hTID-1 plasmids.
This work was supported by NIH CA 63517 (JG) and by the NIH under Ruth L. Kirschstein National Research Service Award CA 009338 (EMP).
Abbreviations
- PCR
polymerase chain reaction
- GST
glutathione S-transferase
- GFP
green fluorescent protein
- PI
propidium iodide
- 5-FU
5-fluorouracil
Footnotes
Author Contributions: Jiang Qian: Study concept and design, acquisition of data, analysis and interpretation of data
Erin M. Perchiniak: Study concept and design, acquisition of data, analysis and interpretation of data, drafting and revision of manuscript
Kristine Sun: Technical support
Joanna Groden: Study concept and design, analysis and interpretation of data, drafting and revision of manuscript, and obtained funding
There were no conflicts of interest with any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goss KH, Groden J. Biology of the adenomatous polyposis coli tumor suppressor. J Clin Oncol. 2000;18:1967–79. doi: 10.1200/JCO.2000.18.9.1967. [DOI] [PubMed] [Google Scholar]
- 2.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 3.Qian J, Steigerwald K, Combs KA, Barton MC, Groden J. Caspase cleavage of the APC tumor suppressor and release of an amino-terminal domain is required for the transcription-independent function of APC in apoptosis. Oncogene. 2007;26:4872–6. doi: 10.1038/sj.onc.1210265. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–54. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 5.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann SH, Hengartner MO. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 2001;11:526–34. doi: 10.1016/s0962-8924(01)02173-0. [DOI] [PubMed] [Google Scholar]
- 7.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 8.Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Acad Sci U S A. 1999;96:10964–7. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–90. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 10.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–33. [PubMed] [Google Scholar]
- 11.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–9. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 12.Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 13.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 14.Green DR, Chipuk JE. Apoptosis: Stabbed in the BAX. Nature. 2008;455:1047–9. doi: 10.1038/4551047a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chipuk JE, Bouchier-Hayes L, Green DR. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 2006;13:1396–402. doi: 10.1038/sj.cdd.4401963. [DOI] [PubMed] [Google Scholar]
- 16.Bedi A, Pasricha PJ, Akhtar AJ, Barber JP, Bedi GC, Giardiello FM, Zehnbauer BA, Hamilton SR, Jones RJ. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995;55:1811–6. [PubMed] [Google Scholar]
- 17.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107(Pt 12):3569–77. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 18.Merritt AJ, Potten CS, Watson AJ, Loh DY, Nakayama K, Hickman JA. Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci. 1995;108(Pt 6):2261–71. doi: 10.1242/jcs.108.6.2261. [DOI] [PubMed] [Google Scholar]
- 19.Heppner Goss K, Trzepacz C, Tuohy TM, Groden J. Attenuated APC alleles produce functional protein from internal translation initiation. Proc Natl Acad Sci U S A. 2002;99:8161–6. doi: 10.1073/pnas.112072199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eom CY, Lehman IR. The human DnaJ protein, hTid-1, enhances binding of a multimer of the herpes simplex virus type 1 UL9 protein to oris, an origin of viral DNA replication. Proc Natl Acad Sci U S A. 2002;99:1894–8. doi: 10.1073/pnas.042689499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–37. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 22.Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim Biophys Acta. 1997;1332:F127–47. doi: 10.1016/s0304-419x(97)00008-5. [DOI] [PubMed] [Google Scholar]
- 23.Kurzik-Dumke U, Czaja J. Htid-1, the human homolog of the Drosophila melanogaster l(2)tid tumor suppressor, defines a novel physiological role of APC. Cell Signal. 2007;19:1973–85. doi: 10.1016/j.cellsig.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Syken J, De-Medina T, Munger K. TID1, a human homolog of the Drosophila tumor suppressor l(2)tid, encodes two mitochondrial modulators of apoptosis with opposing functions. Proc Natl Acad Sci U S A. 1999;96:8499–504. doi: 10.1073/pnas.96.15.8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi S, Rubinfeld B, Souza B, Polakis P, Wieschaus E, Levine AJ. A Drosophila homolog of the tumor suppressor gene adenomatous polyposis coli down-regulates beta-catenin but its zygotic expression is not essential for the regulation of Armadillo. Proc Natl Acad Sci U S A. 1997;94:242–7. doi: 10.1073/pnas.94.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steigerwald K, Behbehani GK, Combs KA, Barton MC, Groden J. The APC tumor suppressor promotes transcription-independent apoptosis in vitro. Mol Cancer Res. 2005;3:78–89. doi: 10.1158/1541-7786.MCR-03-0189. [DOI] [PubMed] [Google Scholar]
- 27.Webb SJ, Nicholson D, Bubb VJ, Wyllie AH. Caspase-mediated cleavage of APC results in an amino-terminal fragment with an intact armadillo repeat domain. FASEB J. 1999;13:339–46. doi: 10.1096/fasebj.13.2.339. [DOI] [PubMed] [Google Scholar]
- 28.Browne SJ, MacFarlane M, Cohen GM, Paraskeva C. The adenomatous polyposis coli protein and retinoblastoma protein are cleaved early in apoptosis and are potential substrates for caspases. Cell Death Differ. 1998;5:206–13. doi: 10.1038/sj.cdd.4400331. [DOI] [PubMed] [Google Scholar]
- 29.Brocardo M, Lei Y, Tighe A, Taylor SS, Mok MT, Henderson BR. Mitochondrial targeting of adenomatous polyposis coli protein is stimulated by truncating cancer mutations: regulation of Bcl-2 and implications for cell survival. J Biol Chem. 2008;283:5950–9. doi: 10.1074/jbc.M708775200. [DOI] [PubMed] [Google Scholar]
- 30.Cheng H, Cenciarelli C, Shao Z, Vidal M, Parks WP, Pagano M, Cheng-Mayer C. Human T cell leukemia virus type 1 Tax associates with a molecular chaperone complex containing hTid-1 and Hsp70. Curr Biol. 2001;11:1771–5. doi: 10.1016/s0960-9822(01)00540-1. [DOI] [PubMed] [Google Scholar]
- 31.Kurzik-Dumke U, Gundacker D, Renthrop M, Gateff E. Tumor suppression in Drosophila is causally related to the function of the lethal(2) tumorous imaginal discs gene, a dnaJ homolog. Dev Genet. 1995;16:64–76. doi: 10.1002/dvg.1020160110. [DOI] [PubMed] [Google Scholar]
- 32.Kurzik-Dumke U, Phannavong B, Gundacker D, Gateff E. Genetic, cytogenetic and developmental analysis of the Drosophila melanogaster tumor suppressor gene lethal(2)tumorous imaginal discs (1(2)tid) Differentiation. 1992;51:91–104. doi: 10.1111/j.1432-0436.1992.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 33.Yin X, Rozakis-Adcock M. Genomic organization and expression of the human tumorous imaginal disc (TID1) gene. Gene. 2001;278:201–10. doi: 10.1016/s0378-1119(01)00720-x. [DOI] [PubMed] [Google Scholar]
- 34.Kurzik-Dumke U, Horner M, Czaja J, Nicotra MR, Simiantonaki N, Koslowski M, Natali PG. Progression of colorectal cancers correlates with overexpression and loss of polarization of expression of the htid-1 tumor suppressor. Int J Mol Med. 2008;21:19–31. [PubMed] [Google Scholar]
- 35.Peifer M, Pai LM, Casey M. Phosphorylation of the Drosophila adherens junction protein Armadillo: roles for wingless signal and zeste-white 3 kinase. Dev Biol. 1994;166:543–56. doi: 10.1006/dbio.1994.1336. [DOI] [PubMed] [Google Scholar]
- 36.Su LK, Johnson KA, Smith KJ, Hill DE, Vogelstein B, Kinzler KW. Association between wild type and mutant APC gene products. Cancer Res. 1993;53:2728–31. [PubMed] [Google Scholar]
- 37.Kim K, Pang KM, Evans M, Hay ED. Overexpression of beta-catenin induces apoptosis independent of its transactivation function with LEF-1 or the involvement of major G1 cell cycle regulators. Mol Biol Cell. 2000;11:3509–23. doi: 10.1091/mbc.11.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schilling B, De-Medina T, Syken J, Vidal M, Munger K. A novel human DnaJ protein, hTid-1, a homolog of the Drosophila tumor suppressor protein Tid56, can interact with the human papillomavirus type 16 E7 oncoprotein. Virology. 1998;247:74–85. doi: 10.1006/viro.1998.9220. [DOI] [PubMed] [Google Scholar]
- 39.Trentin GA, Yin X, Tahir S, Lhotak S, Farhang-Fallah J, Li Y, Rozakis-Adcock M. A mouse homologue of the Drosophila tumor suppressor l(2)tid gene defines a novel Ras GTPase-activating protein (RasGAP)-binding protein. J Biol Chem. 2001;276:13087–95. doi: 10.1074/jbc.M009267200. [DOI] [PubMed] [Google Scholar]
- 40.Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–38. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]


