Abstract
Objectives
To determine if biomarkers of subclinical myocardial injury and hemodynamic stress identify asymptomatic individuals with left ventricular hypertrophy (LVH) at higher risk for heart failure (HF) and death.
Background
The interaction between LVH, low but detectable cardiac troponin T (cTnT), and elevated NT-proBNP on cardiovascular (CV) outcomes in the general population is unknown.
Methods
Participants in the Dallas Heart Study without clinical HF, LV dysfunction, or chronic kidney disease underwent measurement of LV mass by MRI, cTnT by highly sensitive assay, and NT-proBNP (n=2413). Subjects were stratified by LVH and by detectable cTnT (≥3 pg/mL) and increased NT-proBNP (>75th age- and sex-specific percentile).
Results
9% of participants were LVH+, 25% cTnT+, and 24% NT-proBNP+. Those LVH+ and cTnT+ and/or NT-proBNP+ (n=144) were older, more likely to be male, with greater risk factor burden and more severe LVH compared with those LVH+ biomarker- (p<0.01 for each). The cumulative incidence of HF or CV death over 8 years among LVH+ cTnT+ was 21% vs. 1% (LVH- cTnT-), 4% (LVH- cTnT+), and 6% (LVH+ cTnT-), p<0.0001. The interactions between LVH and cTnT (pinteraction=0.0005) and LVH and NT-proBNP (pinteraction=0.014) were highly significant. Individuals LVH+ and either cTnT+ or NT-proBNP+ remained at >4-fold higher risk for HF or CV death after multivariable adjustment for CV risk factors, renal function, and LV mass compared with those LVH- biomarker-.
Conclusions
Minimal elevations in biomarkers of subclinical cardiac injury and hemodynamic stress modify the association of LVH with adverse outcomes, identifying a malignant sub-phenotype of LVH with high risk for progression to HF and CV death.
Keywords: heart failure, left ventricular hypertrophy, troponin, natriuretic peptides
Introduction
Left ventricular (LV) hypertrophy (LVH), most commonly due to chronic hypertensive heart disease, is associated with substantial morbidity and mortality, including the development of heart failure (HF) and death from cardiovascular (CV) disease (1-2). LVH develops in response to chronic pressure and volume overload and may ultimately progress to pathologic systolic or diastolic dysfunction and symptomatic heart failure (3). Maladaptive LV remodeling plays a central role in the transition from asymptomatic LVH to clinical HF and results from cardiomyocyte injury and tissue fibrosis (4), as well as increased diastolic wall stress and neurohormonal activation (5).
Although clearly a risk factor for HF and CV death, the natural history of LVH is heterogeneous, with a progressive course in some individuals but an uncomplicated course in many others. Identification of biological pathways that contribute to the transition from LVH to clinical HF, and biomarkers that accurately represent these pathways, may help to identify individuals at high risk for adverse outcomes and to develop therapeutic targets to prevent disease transition. Biomarkers of myocardial injury and neurohormonal activation due to hemodynamic stress may therefore play key roles in defining the transition from asymptomatic LVH to clinical HF (6-8).
Cardiac troponin T (cTnT) and the N-terminal fragment of the prohormone of B-type natriuretic peptide (NT-proBNP) are released from cardiac myocytes in response to a variety of pathologic stimuli including increased LV wall stress and hypertrophy, and are markers of cardiac injury and ventricular wall stress (9-10). Both biomarkers have been shown to associate strongly with incident HF (11-12) and mortality (13-14) in the general population; however, the impact of minimally elevated circulating levels of cTnT and NT-proBNP among individuals with LVH is unknown. We sought to test the hypothesis that biomarker evidence of subclinical myocardial injury and hemodynamic stress would identify asymptomatic individuals with LVH at higher risk for transition to HF and CV death.
Methods
Study population
The Dallas Heart Study (DHS) is a multi-ethnic, probability-based, population cohort study of Dallas County adults in which deliberate over-sampling of African-Americans was performed. Detailed methods of the DHS have been described previously (15). Briefly, between 2000 and 2002, 3072 subjects completed the three DHS visits, including a detailed in-home survey, laboratory testing, and imaging studies, as described below. Participants were then followed for the occurrence of pre-defined clinical events and death. For the present study, we excluded participants with a LV ejection fraction <40%, estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, and those with prevalent clinical HF (defined by self-report of “congestive heart failure, an enlarged heart, a weak heart, or cardiomyopathy”) at baseline, yielding a final sample size of 2413. Participants provided written informed consent, and the protocol was approved by the Institutional Review Board of UT Southwestern Medical Center.
Biomarker, imaging, and body composition measurements
Detailed methods describing measurements of cTnT using a highly sensitive assay (Elecsys-2010 Troponin T hs STAT, Roche Diagnostics, Indianapolis, IN) and NT-proBNP (Elecsys, Roche Diagnostics) in the DHS have been published previously (14, 16). The lowest concentrations within the analytical measurement range of the assays were 3 pg/mL and 5 pg/mL for cTnT and NT-proBNP, respectively. Cardiac MRI was performed using a 1.5-Tesla system (Intera, Philips Medical Systems, Best, The Netherlands). LV mass, wall thickness, end diastolic and systolic volumes, and ejection fraction (LVEF) were calculated from short-axis sequences. LV concentricity was defined as the ratio of LV mass to end-diastolic volume (17).
Fat-free mass was measured with dual-energy x-ray absorptiometry (Delphi W scanner, Hologic Inc., Bedford, MA and Discovery software [version 12.2]) (18). Body mass index (BMI) was calculated as weight (kilograms)/height (meters)2 based on weight and height measured at study entry. Body surface area (BSA) was calculated using the method of Tikuisis et al (19). 12-lead electrocardiograms (ECG) were recorded at 25mm/s and 1mV/cm standardization with a sampling rate of 0.5 kHz using the Marquette 12SL ECG analysis program version 229 (GE Marquette Medical Systems, Milwaukee, WI). Voltage measurements were obtained electronically using median voltages from an aligned group of all beats from each lead. Two DHS investigators blinded to demographic and clinical information reviewed each ECG to verify the computer identified parameters and provide a clinical interpretation.
Definitions
LVH was defined as LV mass/BSA ≥89 g/m2 in women and ≥112 g/m2 in men, based on a phenotypically normal subpopulation of the DHS cohort, as previously described (17). As a sensitivity analysis, LVH was also defined by indexing LV mass to height2.7 (LV mass/height2.7 ≥39 g/m2.7 [women] and ≥48 g/m2.7 [men]) and fat-free mass (LV mass/fat-free mass ≥3.7 g/kg [both men and women]). Analyses of LVH by the Sokolow-Lyon ECG criteria, defined as the sum of the S wave amplitude in lead V1 plus the maximum R wave amplitude in V5 or V6 ≥3.5 mV (35 mm) or aVL R wave amplitude ≥1.1 mV (11 mm) (20), were also performed.
cTnT was characterized as elevated if equal to or above the limit of blank of the assay (3 pg/mL). The limit of blank corresponds to the lowest cTnT concentration within the analytical measurement range of the assay. NT-proBNP was defined as increased if above the age- and sex-specific 75th percentile of the population (using 5 age categories with cut-offs of 35, 40, 50, and 60 years). The NT-proBNP threshold at the 75th percentile was selected to yield a similar proportion of individuals characterized with elevated NT-proBNP as with detectable cTnT. Both thresholds were prospectively defined based on prior studies (14, 21).
Race/ethnicity, history of cardiovascular diseases, and smoking status were self-reported. Detailed descriptions of variable definitions for hypertension, hypercholesterolemia, and low high-density lipoprotein (HDL) cholesterol have been previously described using conventional clinical definitions (22). Presence of the metabolic syndrome was defined and Framingham 10-year CVD risk estimates were calculated according to the National Cholesterol Education Program’s (NCEP) Adult Treatment Panel III report (23). GFR was estimated using the Modification of Diet in Renal Disease equation (24).
Outcomes
The primary outcome was the composite of incident HF or CV death. Incident HF was defined as first hospitalization for systolic or diastolic HF as determined through 1) a detailed health survey regarding interval cardiovascular events administered by the Data Coordinating Center during annual calls to study subjects and/or 2) for subjects providing informed consent (>90%), quarterly tracking for hospital admissions using the Dallas-Fort Worth Hospital Council Data Initiative Database that includes all hospital admission data for 70 out of 72 hospitals in the Dallas-Fort Worth area. Primary clinical source documents were collected and reviewed for all suspected non-fatal cardiovascular events (including myocardial infarction and HF) and were independently adjudicated by a blinded endpoint committee. Systolic HF was defined as a clinical diagnosis of symptomatic HF in the setting of a LVEF<50% or documentation of a “depressed or low” LVEF. Diastolic HF was defined as a clinical diagnosis of symptomatic HF in the setting of a LVEF ≥50% or documentation of a “preserved or normal” LVEF. Death events were ascertained through December 31, 2009 from the National Death Index and classified as cardiovascular if the primary cause was related to the cardiovascular system according to the International Statistical Classification of Diseases, 10th Revision codes I00-I99 (25).
Statistical analysis
For each analysis, participants were categorized into groups based on the presence (+) or absence (-) of LVH and biomarker levels above (+) or below (-) the pre-defined threshold. Baseline characteristics were compared between those without LVH, those with LVH but without elevated biomarkers, and those with LVH and elevated biomarkers using chi-square tests for dichotomous variables and Wilcoxon rank-sum tests for continuous variables. The cumulative incidence of the primary outcome among groups with LVH- biomarker-, LVH- biomarker+, LVH+ biomarker-, and LVH+ biomarker+ was estimated using time-to-event analysis and Kaplan-Meier curves were constructed and compared using the log-rank test. Cox proportional hazards models were used to calculate the hazard ratios and 95% confidence limits for the primary outcome among each group after conditions of proportionality were confirmed. Interaction terms were included in the unadjusted models to determine if qualitative interactions between LVH, cTnT, and NT-proBNP were present. Multivariable models were used to adjust for age, sex, African-American race, diabetes, hypertension, prior CV disease, smoking, body mass index, eGFR, and LV mass/BSA. Shrinkage coefficients were tested for each multivariable model to ensure against model overfitting. Sensitivity analyses were performed using a 5 pg/mL threshold to define detectable cTnT and defining LVH using LV mass indexed to height2.7 and fat-free mass, and also by Sokolow-Lyon ECG criteria. Exploratory analyses were performed comparing outcomes among those with LVH and 0, 1, or 2 elevated biomarkers. For all statistical testing, a 2-sided p-value <0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.2 software (SAS Corporation, Cary, NC).
Results
Prevalence and Univariable Associations of LVH Phenotypes
Among the 2413 participants meeting study criteria (mean age 44; 56% women; 48% African-Americans), 223 (9.2%) had LVH, 590 (24.5%) had detectable cTnT (cTnT+), and 584 (24.4%) had a NT-proBNP value >75th percentile (NT-proBNP+). The correlation between cTnT and NT-proBNP among all study participants was not significant (Spearman’s rho=0.03, p=0.14); however, among the sub-group with detectable cTnT, NT-proBNP was weakly correlated with cTnT (Spearman’s rho=0.14, p=0.001). Among those with LVH, 35.4% had no biomarker elevation, 20.2% were cTnT+ only, 18.8 % were NT-proBNP+ only, and 25.6% were both cTnT+ and NT-proBNP+. The frequency of LVH with cTnT+ was highest in African-American men (12%), with sequentially lower rates seen in African-American women (4%), Caucasian men (2%) and Caucasian women (1%), respectively.
Baseline characteristics are presented in Table 1. Compared with both those without LVH and those with LVH but without detectable cTnT, participants with LVH and detectable cTnT were older, more likely to be male and African-American, with more hypertension, diabetes, metabolic syndrome, prior CVD, and lower eGFR (p<0.05 for each). Additionally, compared with LVH+ cTnT- individuals, those LVH+ cTnT+ had greater LV mass and wall thickness, a higher LV concentricity index, and higher levels of NT-proBNP. Generally similar findings were seen when LVH+ NT-proBNP+ individuals were compared with those LVH+ NT-proBNP-, with the exception that larger LV end-diastolic and end-systolic volumes, rather than wall thickness and concentricity, associated with increased NT-proBNP.
Table 1.
Baseline Characteristics of the Study Population
| Variable | No LVH | LVH | LVH | ||
|---|---|---|---|---|---|
| (n=2190) | cTnT- (n=121) |
cTnT+ (n=102) |
NT-proBNP- (n=124) |
NT-proBNP+ (n=99) |
|
| Age (yrs) | 43 (36, 51) | 43 (37, 50) | 51 (45, 59)*† | 45 (38, 53) | 49 (42, 56)*‡ |
| Male (%) | 43.7 | 31.4 | 68.6*† | 45.2 | 52.5 |
| Race (%) | |||||
| Caucasian | 35.8 | 16.5 | 14.7* | 14.5 | 17.2* |
| African-American | 45.0 | 76.9 | 78.4* | 78.2 | 76.8* |
| Hispanic | 17.0 | 5.0 | 6.9* | 5.6 | 6.1* |
| Other | 2.1 | 1.7 | 0.0 | 0.0 | 0.0 |
| Hypertension (%) | 27.3 | 65.8 | 76.8* | 64.2 | 79.2* |
| Systolic BP (mmHg) | 120 (111, 130) | 138 (123, 156) | 151 (134, 167)*† | 139 (123, 153) | 154 (132, 170)*‡ |
| Diastolic BP (mmHg) | 76 (71, 83) | 84 (78, 95) | 87 (79, 94)* | 83 (77, 93) | 89 (80, 100)*‡ |
| Diabetes (%) | 8.5 | 14.0 | 30.4*† | 20.2 | 23.2* |
| Hypercholesterolemia (%) | 13.1 | 11.6 | 15.7 | 13.7 | 13.1 |
| Low HDL Cholesterol (%) | 39.4 | 38.0 | 38.2 | 38.7 | 37.4 |
| Metabolic Syndrome (%) | 31.8 | 42.1 | 58.8*† | 50.8 | 48.5* |
| Current Smoking (%) | 24.7 | 49.6 | 29.4† | 36.3 | 45.5* |
| Prior CHD (%) | 1.6 | 1.7 | 10.8*† | 3.2 | 9.1* |
| Prior CVD (%) | 2.9 | 5.0 | 15.7*† | 4.0 | 17.2*‡ |
| Framingham 10-year CVD Risk Estimate ≥6 (%) | 16.6 | 24.0 | 52.9*† | 31.5 | 44.4* |
| Estimated GFR (mL/min per 1.73 m2) | 97.1 (85.3, 111.4) | 103.9 (91.1, 116.3) | 93.5 (80.3, 108.6) *† | 98.3 (86.4, 115.1) | 97.6 (83.9, 112.2) |
| Body Mass Index (kg/m2) | 29.1 (25.2, 33.9) | 29.0 (25.5, 34.7) | 31.0 (26.3, 35.7)* | 29.7 (26.4, 34.9) | 29.5 (25.2, 35.4) |
| LV Mass/BSA (g/m2) | 77.5 (68.7, 88.0) | 103.7 (93.1, 116.9) | 119.4 (108.2, 129.0)*† | 108.7 (94.6, 117.7) | 119.8 (101.1, 130.0)*‡ |
| LV Wall Thickness (mm) | 11.2 (10.2, 12.3) | 13.5 (12.3, 14.5) | 14.6 (13.7, 16.4)*† | 13.9 (13.0, 15.1) | 14.3 (12.9, 15.4)* |
| LV Ejection Fraction (%) | 73.3 (68.4, 77.7) | 71.7 (65.6, 75.9) | 70.5 (61.9, 76.0)* | 71.8 (66.3, 75.6) | 70.5 (62.8, 76.3)* |
| LV End-Diastolic Volume/BSA (mL/m2) | 50.6 (44.7, 57.0) | 55.6 (49.0, 62.8) | 58.1 (48.6, 68.1)* | 54.9 (47.6, 62.1) | 58.4 (50.1, 68.7)*‡ |
| LV End-Systolic Volume/BSA (mL/m2) | 13.4 (10.6, 16.7) | 16.1 (12.5, 20.2) | 16.1 (12.9, 22.9)* | 15.1 (12.3, 20.2) | 17.3 (13.1, 23.0)*‡ |
| Concentricity (g/mL) | 1.5 (1.4, 1.7) | 1.9 (1.7, 2.1) | 2.0 (1.8, 2.5)*† | 1.9 (1.7, 2.3) | 1.9 (1.7, 2.3)* |
| cTnT (pg/mL) | <3.0 (<3.0, <3.0) | <3.0 (<3.0, <3.0) | 7.4 (5.1, 10.9)*† | <3.0 (<3.0, 4.8) | 4.1 (<3.0, 9.7)*‡ |
| NT-proBNP (pg/mL) | 26.4 (12.6, 52.9) | 35.5 (13.1, 85.5) | 71.0 (25.9, 194.0)*† | 20.1 (9.4, 39.4) | 127 (86.2, 257.7)*‡ |
Data are presented as median (25%, 75% percentile) or proportion (%) where indicated
Abbreviations: BP= blood pressure; BSA= body surface area; CHD= coronary heart disease; cTnT= cardiac troponin T; CVD= cardiovascular disease; GFR= glomerular filtration rate; HDL= high density lipoprotein; LV= left ventricular
p<0.05 vs. No LVH group
p<0.05 vs. LVH+ cTnT- group
p<0.05 vs. LVH+ NT-proBNP- group
Associations of LVH Phenotypes with Heart Failure and Cardiovascular Death
During a median follow-up period of 8.1 (interquartile range [IQR] 7.6 to 8.6) years, the primary outcome of HF or CV death occurred in 65 (2.7%) participants, including 28 HF events (1.36 per 1000 person-years) and 37 CV deaths (1.80 per 1000 person-years). Among those who developed HF or died of CV causes, 63.1% were men and 78.5% were African-American. Of those with incident HF, 65.2% had systolic and 34.8% had diastolic HF, with a median LVEF at the time of diagnosis of 30% (IQR 20-36) and 55% (IQR 55-70), respectively; 20% of those with systolic HF and 25% with diastolic HF had a myocardial infarction during the study interval.
The cumulative incidence of HF or CV death was 20.6% in the LVH+ cTnT+ group compared with 1.1% (LVH- cTnT-), 3.9% (LVH- cTnT+), and 5.8% (LVH+ cTnT-), log-rank p<0.0001 (Figure 1a). Among those who were LVH+ NT-proBNP+, the primary outcome occurred in 20.2% compared with 1.5% (LVH- NT-proBNP-), 2.5% (LVH- NT-proBNP+), and 6.5% (LVH+ NT-proBNP-), log-rank p<0.0001 (Figure 1b). Although only 6% of the study population had LVH with cTnT+ and/or NT-proBNP+, these individuals accounted for approximately 40% of all HF or CV death events. The crude hazard ratio (HR) with 95% confidence interval (CI) for the primary outcome was 22.6 (95% CI 12.1 to 42.5) for LVH+ cTnT+ and 15.5 (95% CI 8.6 to 28.0) for LVH+ NT-proBNP+ participants compared with those who were LVH- cTnT- and LVH- NT-proBNP-, respectively (Table 2). Highly significant statistical interactions were observed both between LVH and detectable cTnT (pinteraction=0.0005) and between LVH and elevated NT-proBNP (pinteraction=0.014) for the primary outcome. Interactions remained significant after including each biomarker as a continuous variable (pinteraction=0.026 for LVH-cTnT and pinteraction=0.044 for LVH-NT-proBNP). Findings were consistent across sub-groups defined by age, sex, race, ejection fraction, and co-morbidities (Supplemental Figure 1) and for both individual components of the composite outcome (Figure 2). Results were also insensitive to the use of height2.7 or fat-free mass as the indexing variable for LV mass and to use of a 5 pg/mL threshold to define detectable cTnT (data not shown).
Figure 1. Kaplan-Meier Curves for Incident Heart Failure or Cardiovascular Death.
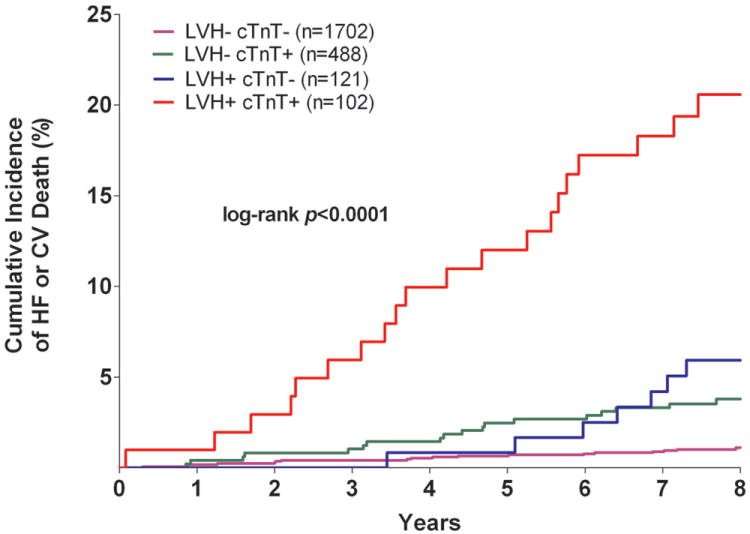
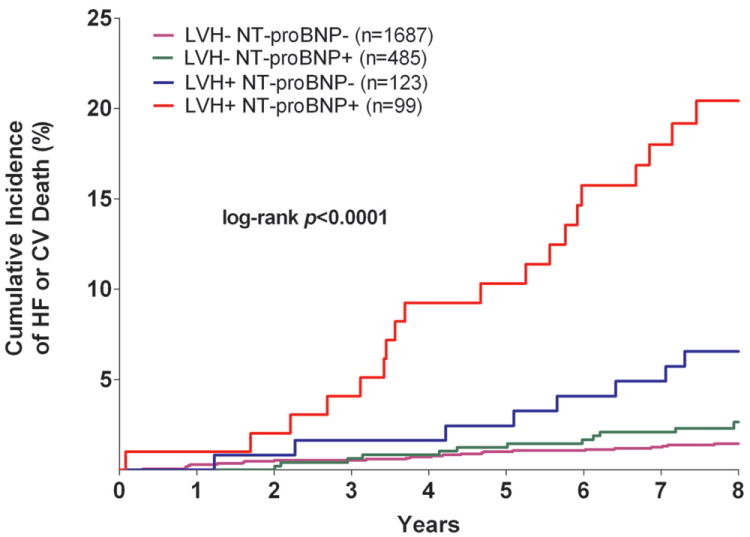
Unadjusted Kaplan-Meier Curves for Incident Heart Failure or Cardiovascular Death Stratified by the Presence (+) or Absence (-) of Left Ventricular Hypertrophy and Detectable Cardiac Troponin T (Panel A) or Increased NT-proBNP (Panel B)
Abbreviations: cTnT= cardiac troponin T; CV= cardiovascular; HF= heart failure; LVH= left ventricular hypertrophy
Table 2.
Unadjusted and Multivariable-Adjusted Associations of Left Ventricular Hypertrophy, Cardiac Troponin T, and NT-proBNP with Incident Heart Failure or Cardiovascular Death
| Model | Hazard Ratio (95% CI) | p-interaction | |||
|---|---|---|---|---|---|
| LVH- cTnT-* | LVH- cTnT+ | LVH+ cTnT- | LVH+ cTnT+ | ||
|
|
|||||
| Model 1 (unadjusted) | 1.00 | 3.7 (2.0, 7.1) | 5.5 (2.8, 13.1) | 22.6 (12.1, 42.5) | p=0.0005 |
| Model 2 | 1.00 | 1.8 (0.9, 3.9) | 2.8 (1.1, 7.0) | 6.2 (2.8, 13.7) | |
| Model 3 | 1.00 | 1.9 (0.9, 4.0) | 2.0 (0.7, 5.7) | 4.3 (1.7, 11.1) | |
| LVH- NT-proBNP-* | LVH- NT-proBNP+ | LVH+ NT-proBNP- | LVH+ NT-proBNP+ | ||
|
|
|||||
| Model 1 (unadjusted) | 1.00 | 1.7 (0.8, 3.3) | 4.5 (2.0, 9.9) | 15.5 (8.6, 28.0) | p=0.014 |
| Model 2 | 1.00 | 2.2 (1.0, 4.5) | 2.4 (1.1, 5.6) | 6.0 (3.0, 11.8) | |
| Model 3 | 1.00 | 2.1 (1.0, 4.4) | 2.0 (0.8, 5.3) | 4.5 (1.7, 11.8) | |
Model 1 is unadjusted. Model 2 is adjusted for age, sex, African-American race, diabetes, hypertension, prior cardiovascular disease, smoking, body mass index, and estimated glomerular filtration rate. Model 3 is adjusted for Model 2 plus left ventricular mass/body surface area.
Referent.
Figure 2. Incidence of Heart Failure and Cardiovascular Death Stratified by Biomarker Group.
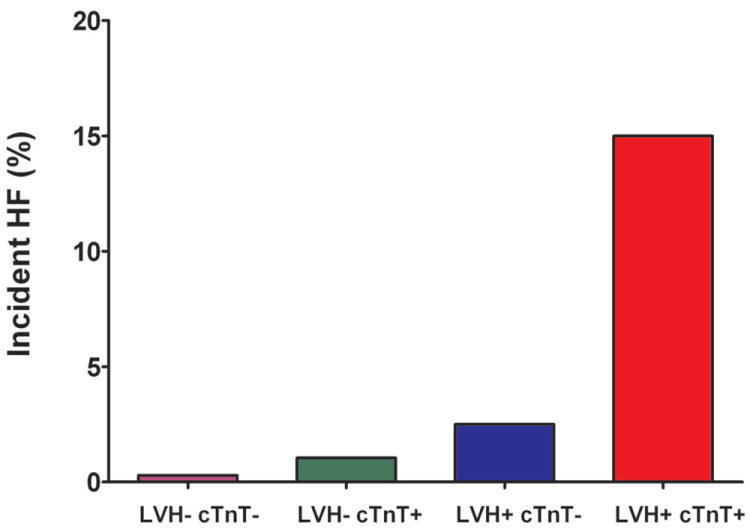
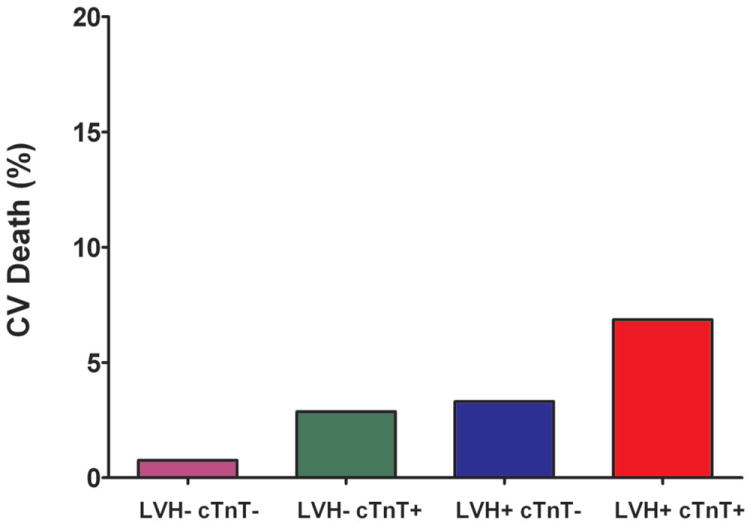
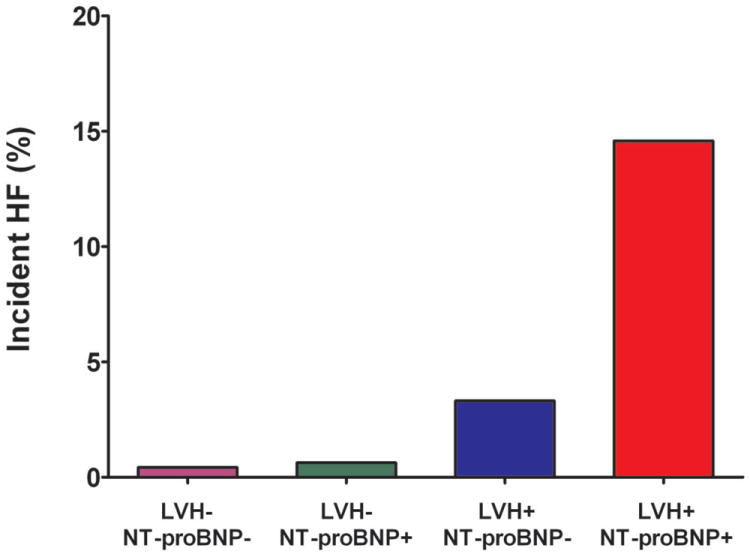
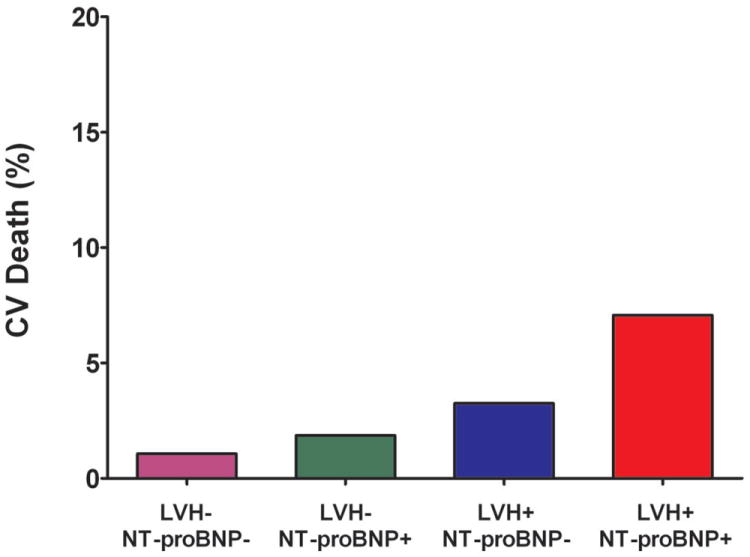
Incidence of Heart Failure and Cardiovascular Death Stratified by the Presence (+) or Absence (-) of Left Ventricular Hypertrophy and Detectable Cardiac Troponin T (Panels A and B) or Increased NT-proBNP (Panels C and D)
Abbreviations: same as in Figure 1
Detectable cTnT was associated with a higher risk for HF or CV death across sex-specific tertiles of LV mass, with the largest effect seen among those with the highest LV mass (p<0.0001 for cTnT+ vs. cTnT- in tertile 3, Figure 3a), consistent with the statistical interaction reported above. Additionally, the presence of LVH was associated with a marked increase in the risk for HF or CV death across the entire spectrum of cTnT levels (p<0.001 for each, Figure 3b), with the greatest effect seen at cTnT levels >14 pg/mL (the previously reported 99th percentile value for the assay in normal controls).
Figure 3. Incidence of Heart Failure or Cardiovascular Death.
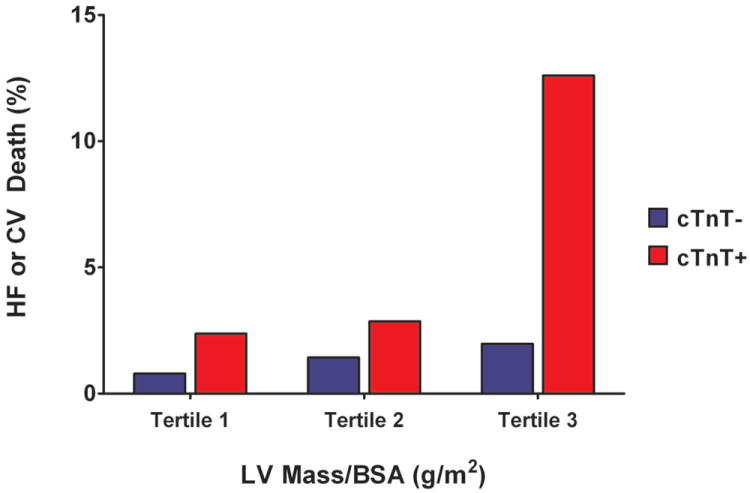
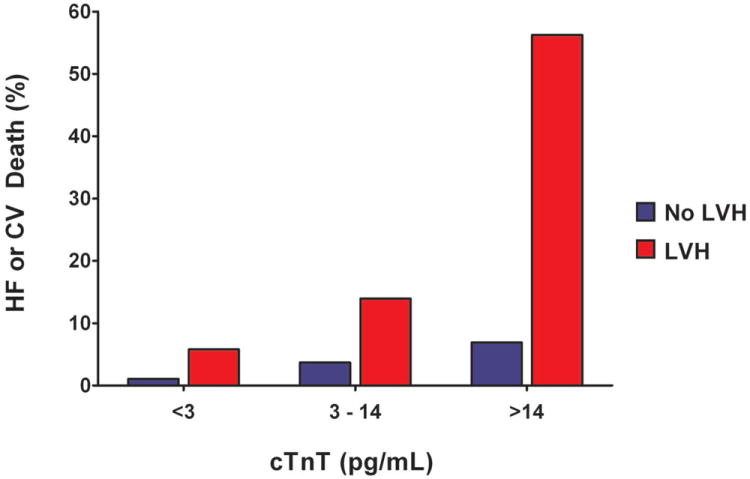
Panel A: Incidence of Heart Failure or Cardiovascular Death by Tertile of Left Ventricular Mass Stratified by Detectable or Undetectable Cardiac Troponin T. Panel B: Incidence of Heart Failure or Cardiovascular Death by Cardiac Troponin T Level Stratified by the Presence or Absence of Left Ventricular Hypertrophy
Abbreviations: same as in Figure 1
Among the sub-group of individuals with LVH, the presence of detectable cTnT was associated with a >4-fold increase in the risk of the composite outcome (crude HR 4.2 [95% CI 1.8 to 9.8]) compared with those who were LVH+ cTnT-. Similar findings were seen for the LVH+ NT-proBNP+ group compared with those who were LVH+ NT-proBNP- (crude HR 3.5 [95% CI 1.5 to 7.9]). In exploratory analyses restricted to those with LVH, graded associations were also seen between the number of elevated biomarkers and the incidence of HF or CV death. The primary outcome occurred in 5.1% of those with LVH and normal biomarkers, 8.1% of those with LVH and either cTnT+ or NT-proBNP+, and 29.8% of those with LVH and both cTnT+ and NT-proBNP+, log-rank p<0.0001 (Figure 4).
Figure 4. Unadjusted Kaplan-Meier Curves for Incident Heart Failure or Cardiovascular Death.
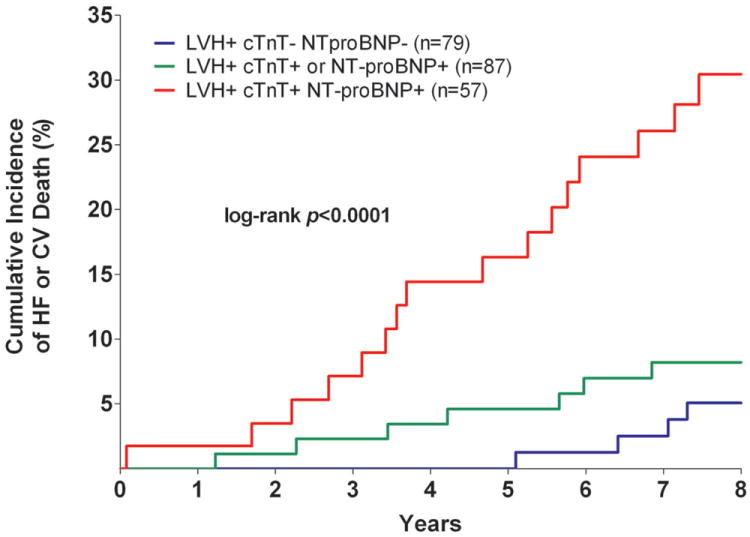
Among Individuals with Left Ventricular Hypertrophy Stratified by the Number of Elevated Biomarkers
Abbreviations: same as in Figure 1
In multivariable analyses adjusting for age, sex, African-American race, diabetes, hypertension, CV disease, smoking, body mass index, eGFR, and LV mass/BSA, LVH with cTnT+ or NT-proBNP+ remained strongly associated with incident HF or CV death compared with individuals LVH- and cTnT- or NT-proBNP-, respectively (adjusted HR 4.3 [95% CI 1.7 to 11.1] for LVH+ cTnT+ and adjusted HR 4.5 [95% CI 1.7 to 11.8] for LVH+ NT-proBNP+, Table 2). Results were insensitive to substitution of systolic blood pressure (as a continuous measure) for hypertension status in the multivariable model.
Replacing MRI Definitions of LVH with Electrocardiographic LVH Criteria
Using the Sokolow-Lyon ECG criteria, 215 (8.9%) participants had LVH, of whom 35.8% were cTnT+ and 32.9% NT-proBNP+. The primary outcome occurred in 16.9% who were ECG LVH+ cTnT+ compared with 1.3% (ECG LVH- cTnT-), 5.3% (ECG LVH- cTnT+), and 2.9% (ECG LVH+ cTnT-), log-rank p<0.0001 (Supplemental Figure 2a). Among those who were ECG LVH+ NT-proBNP+ the primary outcome occurred in 15.7% compared with 1.6% (ECG LVH- NT-proBNP-), 4.1% (ECG LVH- NT-proBNP+), and 4.2% (ECG LVH+ NT-proBNP-), log-rank p<0.0001 (Supplemental Figure 2b). Significant interactions were observed between ECG LVH and both cTnT (pinteraction=0.013) and NT-proBNP (pinteraction=0.017) for the primary outcome. Associations remained significant after multivariable adjustment, with an adjusted HR of 3.2 (95% CI 1.4 to 7.6) for ECG LVH+ cTnT+ and an adjusted HR 3.4 (95% CI 1.5 to 7.9) for ECG LVH+ NT-proBNP+ compared with ECG LVH-biomarker- groups (Supplemental Table 1).
Discussion
In a representative population-based sample of US adults without HF, we report substantial heterogeneity in the clinical phenotype of LVH, with a very high risk of HF or CV death observed among individuals who have LVH with concomitant biomarker evidence of subclinical myocardial injury or neurohormonal activation due to hemodynamic stress, and a more benign course among those with LVH but without elevated biomarkers. Although high risk phenotypes with LVH and biomarker elevation were observed in fewer than 6% of the population at baseline, such individuals represented ~40% of HF or CV death events during follow-up. Moreover, these associations were independent of traditional CV risk factors and renal function, and consistent across sub-groups defined by age, sex, race, and baseline LV ejection fraction. The findings were insensitive to indexing methods for LVH, and performed similarly when LVH was defined using ECG criteria, suggesting that simple and inexpensive strategies may be available to identify this high risk group. Importantly, the observations are not explained simply by higher LV mass among those with abnormal cTnT or NT-proBNP as the findings were also robust to further adjustment for precise MRI measurements of LV mass. Based on these findings, small elevations in cTnT and NT-proBNP may be pathophysiological indicators of adverse remodeling on the pathway from LVH to clinical HF, and not merely surrogate markers for more severe LVH.
Increasing evidence suggests that circulating biomarkers of cardiac injury and neurohormonal activation provide biological insight into chronic CV disease in the population. Studies have demonstrated that cTnT is detectable by highly sensitive assay in >90% of patients with chronic stable coronary artery disease (CAD) (26) or ambulatory HF (27) and in 25-67% of middle-aged adults in the general population (12, 14). The concentration of NT-proBNP also varies widely in the population, with the highest levels among those with older age and female sex (28). LVH has been shown to be an independent determinant of circulating cTnT and NT-proBNP levels in stable, ambulatory populations (29-31). In our study, the prevalence of cTnT+ and NT-proBNP+ was twice as high among those with LVH compared to those without, and higher levels of both markers were associated with more severe LVH. Notably, within the population with LVH, structural changes associated with cTnT included increased LV wall thickness and concentricity; in contrast, NT-proBNP associated with increased LV end-diastolic and end-systolic volumes. These findings, along with the observation that cTnT and NT-proBNP were weakly correlated with each other in our study, support the notion that each biomarker may reflect partially overlapping but non-redundant pathways through which LVH may transition to clinical HF.
LVH is independently associated with adverse CV outcomes including HF and death. Each 50 gram increment in echocardiographically assessed LV mass was associated with a 73% increased risk of CV death among men and a 112% increased risk among women in the Framingham Heart Study (2), and a 50% increased risk of developing systolic HF in the Cardiovascular Health Study (1). Although interval myocardial infarction is an important contributor to the transition from LVH with a normal LVEF to a reduced LVEF, our findings raise the possibility that chronic subclinical myocardial injury may mediate the progression from concentric LVH to LV systolic dysfunction in some individuals without myocardial infarction. Additionally, given that much of the progression to HF occurred among those with preserved LVEF, our findings also suggest that cardiac injury and hemodynamic stress may be important in the transition from LVH to diastolic HF.
The interaction between LVH and cTnT and NT-proBNP has not been previously described. Investigators from the PEACE (Prevention of Events with Angiotensin Converting Enzyme Inhibition) trial demonstrated that each unit increase in cTnT (measured by highly sensitive assay) was associated with a >2-fold risk of HF among patients with stable CAD and normal LVEF, independent of NT-proBNP levels (26). Similar associations have been observed in ambulatory cohorts representative of the general population, where very low concentrations of cTnT (measured with a highly sensitive assay) and NT-proBNP confer independent prognostic information with regard to HF, as well as CV and all-cause mortality (12, 14, 32). However, data on patient sub-groups with LVH are lacking. Our study provides robust evidence for effect modification of the association between LVH and HF and CV death by both cTnT and NT-proBNP, as highly significant interaction terms were seen. In addition, although only exploratory, we found an absolute 25% increase in the risk for HF or CV death among those with LVH and elevation in both biomarkers compared to those with LVH alone. These findings suggest that not only do cardiac injury and neurohormonal activation independently confer an adverse prognosis among individuals with LVH, but they likely reflect ongoing processes that act synergistically to contribute to the transition from asymptomatic LVH to clinical HF. Future studies evaluating associations of these biomarkers with imaging-based assessments of cardiac remodeling in individuals with LVH are needed.
Clinical and Therapeutic Implications
African-Americans have an increased prevalence of LVH and are at increased risk for HF and CV death compared with other race/ethnic groups (17). Although the associations of cTnT, NT-proBNP, and LVH on HF and CV death were consistent across race/ethnicity sub-groups in this study (see Supplemental Figure 1), it is important to note that African-American men had the highest proportion of LVH and detectable cTnT within the study cohort and that the majority of the events occurred among this sub-group. A particularly notable finding is that African-American women were more likely than Caucasian men to have the LVH+ cTnT+ phenotype. Given that African-Americans are 8 times as likely to have hypertension as an antecedent to clinical heart failure (33) and 2-3 times more likely to have LVH (17) compared with Caucasians, our findings may contribute to understanding the biological mechanisms underpinning the disproportionate burden of HF and CV death among African Americans.
Preliminary observations suggest that levels of both cTnT(34) and NT-proBNP (35), as well as the subsequent risk for death and HF associated with elevations in these biomarkers, may be modifiable. Given the extraordinarily high risk observed in the sub-groups with LVH and abnormal biomarkers, early identification and targeted treatments to modify this malignant phenotype represents an important clinical and research priority, with particular implications for African-Americans.
Currently, screening for LVH in the population is performed most extensively with ECG, although the prevalence of ECG LVH varies significantly by age, sex, race, and ECG criteria used. ECG criteria systematically underestimate the true prevalence of LVH by MRI, with ECG LVH prevalence ranging between 0.6% (2) to 4.9% (36), compared with MRI prevalence of 7.7% (37) in the Multi-Ethnic Study of Atherosclerosis and 9.2% in the current study. Despite systematic misclassification by ECG of a significant proportion participants as not having LVH, the finding of an interaction between LVH and cTnT and NT-proBNP was maintained, such that those with ECG-defined LVH and elevated cardiac biomarkers had an absolute increased risk for HF or CV death of >11% compared with those with ECG-defined LVH but without elevated biomarkers. These findings suggest that biomarkers may be used to sub-phenotype those with ECG-defined LVH, identifying individuals at particularly high risk for transition to cardiac failure and death. The clinical implications of this approach require further prospective study.
Strengths and Limitations
Strengths of the current study include use of both advanced cardiac MRI imaging and standard ECG criteria to define LVH, the assessment of cardiac injury using a novel highly sensitive troponin assay, and longitudinal follow-up in a well-validated prospective cohort. Additionally, the large proportion of African-Americans included in our study population allows robust examination of outcomes in this important sub-group. Several limitations also merit comment. First, the number of HF and CV death events was relatively small despite the large sample size, due to the low-risk general population sample studied. For this reason, our findings are preliminary and should be primarily considered in light of their pathophysiological, rather than clinical, implications. Further study is required to validate these observations in larger populations with LVH and long term follow-up. Second, extrapolation to older populations from our relatively young cohort should not be made since older populations have higher cTnT (32) and NT-proBNP levels and different thresholds may be needed to explore potential interactions with LVH. Third, the prognostic differences between LVH with elevation in a single biomarker vs. both biomarkers should be considered hypothesis-generating given the relatively low number of participants and events modeled in these groups.
Conclusions
Elevated circulating levels of cTnT and NT-proBNP identify a malignant LVH phenotype in the general population, reflecting chronic cardiac injury and hemodynamic stress that may contribute to the transition from asymptomatic LVH to clinical HF. Highly significant interactions were observed between LVH and both cTnT and NT-proBNP, as individuals with LVH and elevated biomarkers had an extremely high risk for HF or CV death over 8 years of follow-up. These findings suggest that circulating cTnT and NT-proBNP may identify a sub-population of those with LVH in need of aggressive prevention and treatment to improve CV outcomes.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by Award Number T32HL007360 from the National Heart, Lung, and Blood Institute to Dr. Neeland, the Donald W. Reynolds Foundation, and by United States Public Health Service General Clinical Research Center (USPHS GCRC) grant #M01-RR00633 from National Institutes of Health/National Center for Research Resources-Clinical Research (NCRRCR). Biomarker measurements were supported by investigator-initiated grants from Roche Diagnostics (Indianapolis IN).
Relationship with Industry
Dr. Nambi: Monitor for study sponsored by Anthera; former member of “virtual advisory board” for Roche; research collaboration without financial support with General Electric, Tomtec.
Dr. Seliger: Consulting income from Roche Diagnostics.
Dr. deFilippi: Consulting income from Roche Diagnostics; honoraria from Roche Diagnostics and Siemens Healthcare Diagnostics; research grant support from Siemens Healthcare Diagnostics
Dr. McGuire: Consulting income from Tethys Bioscience.
Dr. Omland: Speaker’s honoraria from Abbott Diagnostics, Siemens Healthcare Diagnostics and Roche Diagnostics; research grant support from Abbott Diagnostics and Roche Diagnostics through Akershus University Hospital.
Dr. de Lemos: Research grant support from Roche Diagnostics and Abbott Diagnostics; consulting income from Tethys Bioscience.
Abbreviations
- cTnT
cardiac troponin T
- CV
cardiovascular
- ECG
electrocardiogram
- HF
heart failure
- LV
left ventricular
- LVH
left ventricular hypertrophy
- NT-proBNP
N-terminal fragment of the prohormone of B-type natriuretic peptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drazner MH, Rame JE, Marino EK, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–15. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 3.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–34. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed SH, Clark LL, Pennington WR, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–96. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 5.Zile MR, Bennett TD, St John Sutton M, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–41. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation. 2006;113:2851–60. doi: 10.1161/CIRCULATIONAHA.105.600437. [DOI] [PubMed] [Google Scholar]
- 7.Milani RV, Drazner MH, Lavie CJ, Morin DP, Ventura HO. Progression from concentric left ventricular hypertrophy and normal ejection fraction to left ventricular dysfunction. Am J Cardiol. 2011;108:992–6. doi: 10.1016/j.amjcard.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 8.Krishnamoorthy A, Brown T, Ayers CR, et al. Progression from normal to reduced left ventricular ejection fraction in patients with concentric left ventricular hypertrophy after long-term follow-up. Am J Cardiol. 2011;108:997–1001. doi: 10.1016/j.amjcard.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 9.Wallace TW, Abdullah SM, Drazner MH, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006;113:1958–65. doi: 10.1161/CIRCULATIONAHA.105.609974. [DOI] [PubMed] [Google Scholar]
- 10.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316–22. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- 11.Smith JG, Newton-Cheh C, Almgren P, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;56:1712–9. doi: 10.1016/j.jacc.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. Jama. 2005;293:1609–16. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 14.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–80. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 16.Abdullah SM, Khera A, Das SR, et al. Relation of coronary atherosclerosis determined by electron beam computed tomography and plasma levels of n-terminal pro-brain natriuretic peptide in a multiethnic population-based sample (the Dallas Heart Study) Am J Cardiol. 2005;96:1284–9. doi: 10.1016/j.amjcard.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 17.Drazner MH, Dries DL, Peshock RM, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–9. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 18.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91:4459–66. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 19.Tikuisis P, Meunier P, Jubenville CE. Human body surface area: measurement and prediction using three dimensional body scans. Eur J Appl Physiol. 2001;85:264–71. doi: 10.1007/s004210100484. [DOI] [PubMed] [Google Scholar]
- 20.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–86. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 21.de Lemos JA, McGuire DK, Khera A, et al. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: results from the Dallas Heart Study. Am Heart J. 2009;157:746–53 e2. doi: 10.1016/j.ahj.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Deo R, Khera A, McGuire DK, et al. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol. 2004;44:1812–8. doi: 10.1016/j.jacc.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 23.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 24.Levey AS, Adler S, Caggiula AW, et al. Effects of dietary protein restriction on the progression of advanced renal disease in the Modification of Diet in Renal Disease Study. Am J Kidney Dis. 1996;27:652–63. doi: 10.1016/s0272-6386(96)90099-2. [DOI] [PubMed] [Google Scholar]
- 25.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omland T, de Lemos JA, Sabatine MS, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–47. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latini R, Masson S, Anand IS, et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116:1242–9. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 28.Fradley MG, Larson MG, Cheng S, et al. Reference limits for N-terminal-pro-B-type natriuretic peptide in healthy individuals (from the Framingham Heart Study) Am J Cardiol. 2011;108:1341–5. doi: 10.1016/j.amjcard.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otsuka T, Kawada T, Ibuki C, Seino Y. Association between high-sensitivity cardiac troponin T levels and the predicted cardiovascular risk in middle-aged men without overt cardiovascular disease. Am Heart J. 2010;159:972–8. doi: 10.1016/j.ahj.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 30.Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345–53. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheven L, de Jong PE, Hillege HL, et al. High-sensitive troponin T and N-terminal pro-B type natriuretic peptide are associated with cardiovascular events despite the cross-sectional association with albuminuria and glomerular filtration rate. Eur Heart J. 2012 Jun 27; doi: 10.1093/eurheartj/ehs163. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourassa MG, Gurne O, Bangdiwala SI, et al. Natural history and patterns of current practice in heart failure. The Studies of Left Ventricular Dysfunction (SOLVD) Investigators. J Am Coll Cardiol. 1993;22:14A–9A. doi: 10.1016/0735-1097(93)90456-b. [DOI] [PubMed] [Google Scholar]
- 34.Masson S, Anand I, Favero C, et al. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation. 2012;125:280–8. doi: 10.1161/CIRCULATIONAHA.111.044149. [DOI] [PubMed] [Google Scholar]
- 35.Hartmann F, Packer M, Coats AJ, et al. NT-proBNP in severe chronic heart failure: rationale, design and preliminary results of the COPERNICUS NT-proBNP substudy. Eur J Heart Fail. 2004;6:343–50. doi: 10.1016/j.ejheart.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Desai CS, Ning H, Lloyd-Jones DM. Competing cardiovascular outcomes associated with electrocardiographic left ventricular hypertrophy: the Atherosclerosis Risk in Communities Study. Heart. 2012;98:330–4. doi: 10.1136/heartjnl-2011-300819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain A, Tandri H, Dalal D, et al. Diagnostic and prognostic utility of electrocardiography for left ventricular hypertrophy defined by magnetic resonance imaging in relationship to ethnicity: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2010;159:652–8. doi: 10.1016/j.ahj.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


