Abstract
Matrix metalloproteinases (MMPs) are a family of endoproteases that break down extracellular matrix and whose upregulation contributes to several diseases. An LC/MS/MS method was developed to quantify MMP-1 and -9 substrates and their N-terminal peptide products in samples obtained from implanted microdialysis sampling probes. In vitro studies with purified human MMP-1 and MMP-9 were used to optimize the assay and determine the effectiveness of the local delivery of a broad spectrum MMP inhibitor, GM 6001. Localized delivery of GM 6001 at 10 μM was sufficient to completely inhibit product formation in vitro. In vivo studies in male Sprague-Dawley rats were performed with microdialysis probes implanted into the subcutaneous tissue. Directly after microdialysis probe implantation, infusions of the MMP-1 and MMP-9 substrates (50 μM each) resulted in recovered product concentrations of approximately 2 μM. During a 50 μM GM 6001 co-infusion with the substrates, a 30% and 25% reduction in product formation for the MMP-1 and MMP-9 substrates was obtained, respectively. Blank dialysates were negative for enzymatic activity that could cleave the MMP substrates. This method allowed for the activity of different MMPs surrounding the microdialysis probe to be observed during in vivo sampling.
Implantation of materials leads to a sequence of events known as the foreign body reaction (FBR), which includes the release of synergistic bioactive molecules including cytokines, eicosanoids, and matrix metalloproteinases (MMPs). 1–4 Knowing the concentrations of these modulators and their temporal sequence of release is of great importance for understanding the multifaceted series of reactions during the FBR. These modulators work in concert in an in vivo environment and are not easily mimicked in vitro,4, 5 suggesting that developing in vivo bioanalytical collection and detection methodologies for these molecules is crucial.
Of all these modulators, MMPs have long been underestimated since they have been simplistically viewed as proteolytic enzymes that serve to hydrolyze components of the extracellular matrix (ECM). It has become increasingly apparent that MMPs also release many bioactive cytokines and growth factors that are entrapped in the extracellular matrix.6,7 MMPs are a large family of zinc-dependent endoproteases that can cleave various protein components within the ECM or at the cell surface.8,9 In addition to their role in wound healing, these matrix remodeling enzymes play critical roles in numerous biological processes from embryonic development to cancer metastasis.
The presence of MMPs in biological samples is typically determined by 1) bioassays or activity assays using labeled substrates, 2) immunoassays and/or immunohistochemical staining, and 3) zymography.10–13 Bioassays using labeled substrates can be carried out by using either labeled natural protein substrates, e.g. radiolabeled proteins, fluorescently labeled proteins, biotinylated gelatin or succinylated gelatin, or initially quenched fluorogenic peptide substrates.14 Zymography, is an electrophoretic technique to analyze the activities of MMPs. The main limitation of zymography is that it is tedious, difficult to quantitate and limited to a few MMPs, such as MMP-1, -2, -7, -9, and -13.
Microdialysis sampling is a well-established diffusion-based sample collection method that has gained wide acceptance for collection of solutes in vivo.15,16 During microdialysis sampling, the exchange of solutes can occur in both directions across the membrane, both into and out of the probe, depending on the concentration gradient of the solute. Microdialysis sampling effectiveness is determined via the bi-directional extraction efficiency (EE), which can be calculated as shown in eq. 1, where Cinlet, Coutlet, Csample stand for the analyte concentration in perfusion fluid, dialysate, and external sample medium, respectively.17 This equation allows the calculation of extraction for a delivered substrate that is locally infused through the dialysis probe.
| Eq. 1 |
Microdialysis sampling can be performed to collect analytes from the sample medium in the recovery mode, or it can be used to deliver certain substances with a molecular weight smaller than the membrane molecular weight cutoff (MWCO) to the fluid outside the probe in the delivery mode. Coupled with appropriate analytical methods and the judicious choice of substrates, microdialysis sampling has been applied towards in situ detection of enzyme activities.18–20 The in vivo localized delivery of a substrate followed by collection of an enzymatic product during microdialysis sampling has been demonstrated by several researchers.21,22
Since microdialysis sampling has been so widely used in biomedicine, it is not surprising that several researchers have focused their attention on determining MMP activity in various in vivo settings. MMPs are known to be upregulated in tumors. These enzymes have been collected into 100 kDa MWCO microdialysis probes implanted into human breast tumors.23 Additionally, MMPs are believed to play important roles with respect to atherosclerotic plaque formation and remodeling. An on-line fluorescence based sensor has been described to monitor the colorimetric products formed from MMP substrates infused through microdialysis sampling probes.24,25 These substrates all produce a common fluorescent coumarin. Selectivity can only be gained via individual perfusion of each substrate. To test the presence of different MMPs with each individual substrate requires switching the perfusion fluid.
Microdialysis sampling combined with LC-ESI-MS has been applied to achieve an in situ and multi-substrate detection of elastase enzymatic activity external to the microdialysis sampling probes.26 With mass spectrometry, analyte detection is based on the m/z ratio requiring no modification to the substrate structure or synthesis of chromophore-containing compounds.27 Thus, the distinct advantage of the mass spectrometry approach over monitoring fluorescent products is that the activity of multiple MMPs could be simultaneously monitored.
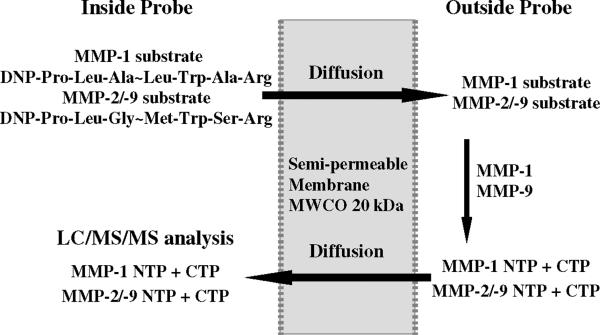
This work focuses on the in vitro and in vivo method development and validation of MMP activity using microdialysis sampling combined with LC/MS detection. MMP-1, -2/-9 were chosen as the initial targets since they are known to be involved in the initiation and progression of FBR associated with biomaterial implantation.28 The substrate specificities for MMP-2 and -9 overlap and the substrate used in this study can be cleaved by both enzymes. These substrates were infused through the microdialysis sampling probe as denoted in Figure 1. A broad-spectrum MMP inhibitor (GM 6001)29,30 was included in the perfusate to determine if MMPs surrounding the dialysis probe could be inhibited, providing validation that MMPs and not other proteases were cleaving the infused substrates. Additionally, zymography was performed to confirm the presence of MMPs around the implanted microdialysis probes.
Figure 1. Microdialysis sampling schematic for in situ MMPs detection.
MMP-1 substrate and MMP-2/-9 substrate diffuse to the surrounding medium and react with MMP-1 and MMP-9, forming the products that diffuse back into the probe.
Experimental Section
Chemicals
MMP-1 (pro-enzyme (55 kDa), from human rheumatoid synovial fibroblasts, ≥15 mU/mg protein); MMP-9 (pro-enzyme (92 kDa), monomer, from human neutrophils, ≥1000 mU/mg protein); MMP-1 substrate I, fluorogenic (DNP-Pro-Leu-Ala-Leu-Trp-Ala-Arg-OH, DNP denotes the 2,4-dinitrophenyl derivative to the terminal amine), MMP-2/MMP-9 substrate I, fluorogenic (DNP-Pro-Leu-Gly-Met-Trp-Ser-Arg-OH) and MMP inhibitor GM 6001 (N-[(2R)-2-(hydroxamidocarbonylmethyl)-4-methylpentanoyl]-L-tryptophan methylamide) were purchased from EMD Chemicals, Inc. (Gibbstown, NJ). Stock solutions of the MMP-1 substrate, MMP-2/-9 substrate and GM 6001 were prepared in DMSO and stored at −80 °C.
The MMP-1 N-terminal peptide product (NTP, DNP-Pro-Leu-Ala, >95% purity) and MMP-2/-9 NTP (DNP-Pro-Leu-Gly, >95% purity) were custom synthesized from Biomatik Corp. (Cambridge, Ontario, Canada). The sequence and molecular weight was confirmed by mass spectrometry. Stock solutions of MMP-1 NTP and MMP-2/-9 NTP were prepared in DMSO and stored at 4 °C.
Phosphate buffered saline (PBS, 1X, pH 7.4, sterile, Dulbecco's formula, consisting of CaCl2 0.90 mM, MgSO4 0.49 mM, KCl 2.68 mM, KH2PO4 1.47 mM, NaCl 136.89 mM, Na2HPO4 8.10 mM) was purchased from MP biomedicals (Irvine, CA). Trypsin (from bovine pancreas, TPCK treated, essentially salt-free, lyophilized powder, 13500 BAEE units/mg solid) and trypsin inhibitor SBTI (type I-S) were purchased from Sigma (St. Louis, MO). Stock solutions of trypsin (500 μg/mL) were prepared in 1 mM HCl and stored at −80 °C until use.31 The activation buffer for pro-MMP activation was prepared by dissolving 50 mM Tris-HCl, 0.1 M NaCl, 5 mM CaCl2 in water with the pH adjusted to 7.5. All other chemicals were reagent grade or better. HPLC grade water was purchased from Fisher Scientific.
Novex 10% zymogram (gelatin) gel (1.0 mm, 10 well), 12% zymogram (casein) gel (1.0 mm, 10 well), zymogram renaturing buffer (10X), zymogram developing buffer (10X), tris-glycine SDS sample buffer (2X), tris-glycine SDS running buffer (10X), XCell SureLock Mini-Cell system, SimplyBlue SafeStain, and DryEase Mini-Gel Drying system were all purchased from Invitrogen Corp. (Carlsbad, CA). Zymography was performed according to manufacturer's instructions.
Liquid Chromatography-Electrospray Ionization Mass Spectrometry (LC-MS) System
An Agilent 1100 series HPLC with an MSD 1100 ion trap mass analyzer (Agilent Technologies, Santa Clara, CA) operating with electrospray ionization (ESI) in positive ion mode was used for separation and detection. A binary pump was used to deliver the solvents. Solvent A contained 0.2% formic acid in water and solvent B contained 0.2% formic acid in acetonitrile. An Alltech Alltima 5 micron C18 column (150×4.6 mm ID) with guard column (Grace Company, Deerfield, IL) was employed for analyte separation. The mobile phase flow rate was 0.5 mL/min. An isocratic elution with solvent B (v/v 40%) was used to separate the analytes. Samples (5 μL) were injected onto the LC column using the system autosampler.
MS conditions were set as follows: capillary voltage was set to 3.5 kV; −143.5 V on the capillary exit and −40.0 V at the skimmer. Octopole 1 DC was set to −12.0 V and octopole 2 DC was set to −2.0 V. Octopole RF amplitude was set to 200 Vpp. Ion lens 1 and 2 was set to −5.0 V and −60.0 V, respectively. Further ion source parameters were nebulizer gas (nitrogen) 25.0 psi and drying gas (nitrogen) 10.0 L/min at a drying temperature of 350 °C.
Calibration Curves and Analyte Quantification
For substrates and products, standard solutions of MMP-1 substrate (0.5 to 50 μM), MMP-2/-9 substrate (0.5 to 50 μM), MMP-1 NTP (0.1 to 50 μM) and MMP-2/-9 NTP (0.1 to 50 μM) were prepared from the stock solutions (1 mM in DMSO) with PBS. Table 1 shows the peptide sequences and the m/z values for the [M+H]+ ions of the substrates and their N-terminal products. Standards were analyzed in triplicate by LC/MS/MS in product ion monitoring mode. The peak area from the extracted ion chromatograms (EIC) of MS/MS of m/z 992 for the MMP-1 substrate, MS/MS of m/z 1012 for the MMP-2/-9 substrate, MS/MS of m/z 466 for the MMP-1 NTP, MS/MS of m/z 452 for the MMP-2/-9 NTP versus concentration were used to generate calibration curves.
Table 1.
Structure and molecular ion of MMP-1, MMP-2/-9 substrate and products
| MMP-1 | MMP-2/-9 | |||
|---|---|---|---|---|
| Sequence | m/z of [M+H]+ | Sequence | m/z of [M+H]+ | |
| Substrate | DNP-Pro-Leu-Ala~Leu-Trp-Ala-Arg | 992.5 | DNP-Pro-Leu-Gly~Met-Trp-Ser-Arg | 1012.5 |
| N-terminal product (NTP) | DNP-Pro-Leu-Ala | 466.2 | DNP-Pro-Leu-Gly | 452.2 |
| C-terminal product (CTP) | Leu-Trp-Ala-Arg | 545.3 | Met-Trp-Ser-Arg | 579.3 |
DNP denotes - the 2,4-dinitrophenyl derivative to the terminal amine.
“~” denotes the enzyme cleavage site.
Standard solutions of MMP inhibitor GM 6001 (0.1 to 50 μM) were prepared by diluting the stock solution (2.5 mM in DMSO) with PBS. Standards were analyzed by LC/MS in triplicate. Peak area of EIC of molecular ion (MS of m/z 389) versus concentration was used to generate the calibration curve.
Dialysates were analyzed in duplicate using LC/MS/MS. MS of m/z 389 for GM 6001, MS/MS of m/z 992 for MMP-1 substrate, MS/MS of m/z 1012 for MMP-2/-9 substrate, MS/MS of m/z 466 for MMP-1 NTP, and MS/MS of m/z 452 for MMP-2/-9 NTP were monitored on each segment. Peak areas from the above extracted ion chromatogram were used for quantitation using the appropriate calibration curves.
Analyte Stability and Intra-/Inter-day variation
The stability of the MMP substrates, MMP-1 and -9 NTP, and GM 6001 was tested by storing each as a 50 μM solution in PBS in the dark at 4 °C or at room temperature for 1, 3 and 7 days. The intra-day variances were tested on four runs of 50 μM standards using LC/MS/MS method. The inter-day difference was tested on 50 μM standards on three different days using LC/MS/MS method.
Pro-MMPs Activation
The MMPs are obtained in their pro-enzyme form and require activation using trypsin.32 Upon activation, human MMP-1 is converted from a 55 kDa pro-enzyme to a 45 kDa active enzyme. Similarly, human MMP-9 is converted from a 92 kDa pro-enzyme to an 86 kDa active enzyme. One volume (5 μL) of pro-MMP-1 or pro-MMP-9 at a concentration of approximately 100 μg/mL was incubated with two volumes of trypsin at a concentration of 15 μg/mL in the activation buffer, giving a final trypsin concentration of 10 μg/mL. After incubation at 37 °C for 2 hrs, the reaction was terminated by the addition of one volume of trypsin inhibitor SBTI at a concentration of 200 μg/mL (diluted form stock with PBS). The two reaction mixtures were combined together and diluted 12.5 times in PBS to get enough volume for microdialysis experiments with a final solution volume of 500 μL. This reaction mixture contained activated MMPs and was used for further microdialysis experiments.
Microdialysis Sampling – in vitro
Microdialysis sampling was performed with a 1 mL Hamilton syringe (Hamilton Company, Reno, NV, USA) with plastic tip and a BAS baby bee syringe pump with controller (BASi, W. Lafayette, IN). CMA 20/04 microdialysis probes with a 10 mm 20 kDa MWCO polyarylethersulphone (PAES) membrane (CMA/Microdialysis, North Chelmsford, MA) were used. All in vitro experiments were performed at 1 μL/min flowrate, in a 37 °C water bath.
EE% of Substrates: Quiescent versus Well-Stirred
Microdialysis probes were used to locally deliver mixtures of MMP-1 substrate (50 μM) and MMP-2/-9 substrate (50 μM) to 2 mL solutions of PBS at 37°C. The PBS sample medium was kept quiescent in one set of experiment and well-stirred in the other set of experiment. The dialysates were collected 10 min after the probe was immersed in the sample medium to allow for equilibration. Two probes were employed for each set of experiments and dialysates were collected from both probes.
EE% of Inhibitor GM 6001
The loss (EE) of inhibitor GM 6001 from the microdialysis probes was measured in vitro. Solutions of GM 6001 (10, 20, or 50 μM) were prepared in PBS buffer with or without the mixture of MMP-1 substrate (50 μM) and MMP-2/-9 substrate (50 μM). These solutions were perfused (1 μL/min) through microdialysis probes placed in a quiescent PBS sample medium (2 mL). The dialysate collection started 10 min after the probe was immersed in the sample medium to allow for equilibration. Three probes were employed and dialysates were collected for 30 min.
Microdialysis Sampling for in situ MMPs Detection
The inhibitor was first included into the sample medium containing activated MMPs to see whether their enzymatic activity could be effectively inhibited by GM 6001. GM 6001 stock solution was added to the activated MMPs solution to achieve a final GM 6001 concentration of 10, 20, or 50 μM. This solution was kept at 37 °C and used as sample medium outside the probe. In separate vials, the same volume of activated MMPs solution with no inhibitor added was used as a control. The substrate mixture solution with MMP-1 substrate 50 μM and MMP-2/-9 substrate 50 μM in PBS was perfused through microdialysis sampling probes immersed in either the sample medium containing GM 6001 or the control. Dialysates were collected every 30 min for 3.5 hrs.
In a separate set of experiments, the GM 6001 inhibitor was included in the perfusate at concentrations of 10, 20 or 50 μM to determine if a local inhibitor infusion could be used to completely inhibit the MMPs. The same enzyme and substrate concentrations were used as described above. Dialysates were collected every 30 min for 3.5 hrs. Dialysates were analyzed using LC/MS/MS and the analytes were quantified using the calibration curves.
In vitro Collection of MMPs
Two microdialysis sampling probes were immersed into the activated MMPs solution with PBS perfused at 1 μL/min to determine if any of the active MMPs could pass through the probe membrane. The dialysate collection started 10 min after the probe was immersed in the sample medium to allow for equilibration. Dialysates were collected from both probes and were subsequently spiked with MMP-1 substrate (50 μM) and MMP-2/-9 substrate (50 μM) to achieve an initial concentration of 20 μM for each substrate. The reaction mixtures were kept dark at 25 °C for 12 hrs and then analyzed using LC/MS/MS.
In vivo Microdialysis Sampling with Freely-Moving Rats
Male Sprague-Dawley rats (210 to 250 g, purchased from Taconic, NY) were used and had free access to food and water as well as a 12-hr on/off light cycle. All surgical procedures were performed using aseptic technique. The Albany Medical College IACUC committee approved the protocols and these protocols also met the guidelines set forth by the NIH for the care and use of laboratory animals.
Before probe implantation, the microdialysis probes were perfused with 70% ethanol then sterile water in a biosafety cabinet. Under isoflurane (Abbott Laboratories, North Chicago, IL) anesthesia, two identical PAES probes (CMA 20/04, 10 mm, 20 kDa MWCO membrane) were implanted in the dorsal subcutaneous space using an incision of less than one inch with one probe implanted on each side of the dorsal spine. The microdialysis probe was inserted into the subcutaneous space, and the incision was closed using surgical staples. The inlet and outlet tubing was cleaned with 70% ethanol and tunneled out from the neck of the rat. Immediately after surgery, the rats were connected to a CMA 120 freely moving animals system (CMA Microdialysis, North Chelmsford, MA). Microdialysis sampling was performed with two 1-mL CMA glass syringes with plastic tip controlled by a CMA 102 microdialysis dual channel pump (CMA Microdialysis, North Chelmsford, MA, USA). Food and water were provided to the rats during sample collection.
After connecting the fluid lines in the freely moving animals system, both probes were perfused with sterile PBS buffer with the following sequence 5 μL/min for 10 min, 1 μL/min for 10 min, and then a blank PBS sample (no substrate included) was collected at 1 μL/min for 30 min. The 5 μL/min flush for the first 10 minutes was performed ensure that fluid lines were open after the implantation of the probe. Additionally, as microdialysis sampling is an invasive procedure, any solutes released due to the surgery or probe insertion into the tissue will be flushed out with a higher flux as mass removal during microdialysis sampling is directly correlated with perfusion flow rate. The perfusate was then switched to a substrate mixture (MMP-1 substrate (50 μM) and MMP-2/-9 substrate (50 μM) in PBS) that was infused in one probe. In the other probe, the substrate mixture combined with GM 6001 (50 μM) was perfused. After initiating the switch in perfusate from collection of blanks to infusion of substrates, the probes were again perfused at 5 μL/min for 10 min, 1 μL/min for 10 min, and then the dialysates sample were collected every 30 min for 3.5 hrs.
After the perfusion of the substrates through the probes was complete, the tubing lines were placed in a surgical pocket that was created at the nape of the neck of the rat. This pocket was closed using surgical staples. The microdialysis probes were also infused on days 3 and 7 post-implantation. After completion of the dialysate collection on Day 7, the probes and surrounding tissue was surgically explanted. Tissue far away from the probe was also explanted as a control.
MMP Activity of Blank PBS Dialysates
The MMP activities in the collected blank PBS samples were tested to see whether activated MMPs were collected into the microdialysis sampling probe. After collection, the MMP-1 and-9 substrate mixture solution (50 μM each) was added to the blank PBS samples to achieve an initial substrate concentration of 20 μM for each substrate. The reaction mixtures were kept dark at 25 °C for 12 hrs and the production of product was determined using LC/MS/MS.
Zymography
Tissue lysates were prepared by homogenization in RIPA (radio immunoprecipitation assay) buffer (50mM Tris, pH 7.4, 150 mM NaCl, 12.7 mM deoxycholate, 25 mM glycerophosphate, 1% SDS, and 1% Triton X-100 containing protease inhibitors); tissue was sonicated briefly to decrease viscosity and cleared of insoluble material by ultracentrifugation (1 hr×100,000 ×g, 4°C).
Protein concentration in the tissue lysates was determined by BCA Assay using a bovine serum albumin (BSA, Sigma, St. Louis, MO) standard curve (0– 300 μg/mL). Lysates were diluted to obtain readings on the standard curve. Samples were incubated at 60 °C for 30 min in a water bath. Absorbance was read at 540 nm using a plate reader. The protein concentration was determined using the standard curve.
Protein (10 μg per well) was loaded onto the gelatin zymography gels; 20 μg per well was used for casein zymography; 20 μg of HT15 media containing MMP-1, -2 and -9 was used as positive control. Electrophoresis was performed according to manufacture's directions (Invitrogen, Carlsbad, CA, USA) using XCell Surelock Mini-Cell system at 125V constant voltage for about 90 min or until the tracking dye reached the bottom of the gel. After the electrophoresis, MMPs were renatured in-gel using the zymogram renaturing buffer (30 min, room temperature with gentle agitation). Following renaturation, the gels were equilibrated in zymogram developing buffer and incubated overnight at 37°C. During this time the renatured MMPs degraded either the gelatin or casein, clearing the region of the gel containing the MMP of protein. By staining the gels for protein with SimplyBlue SafeStain (1 hr, room temperature), the presence of MMPs could be detected as clear bands against the blue background. The gels were scanned and dried using the DryEase Mini-Gel Drying system.
Results and Discussion
Analytical Method Performance
At 4 °C, the MMP-1 substrate, MMP-2/-9 substrate and their N-terminal products (NTPs) at 50 μM concentrations were found to be stable for at least 7 days with less than 10% variance in analyte concentration. At room temperature, this set of analytes (50 μM initial concentrations) was found to be stable for at least 24 hrs with less than 10% RSD.
LC/MS/MS in product ion monitoring mode was used for the detection scheme. The peak area of the extracted ion chromatogram (EIC) of all the product ions was used to construct the calibration curves and to quantify the analytes in the dialysates. Generally for peptide quantitation, MS/MS in SRM (selective reaction monitoring) mode is preferred due to its high sensitivity and selectivity, by fragmenting the parent ion and quantify a specific product ion. However, peptides undergo fragmentation in MS/MS and form a series of product ions from the N- or C- terminal loss of amino acids. Several product ions may show comparable signal intensity and a specific product ion signal is much lower comparing to the sum of product ion signals. In this case, the sum of the product ion signals is sometimes used for quantitation.33,34
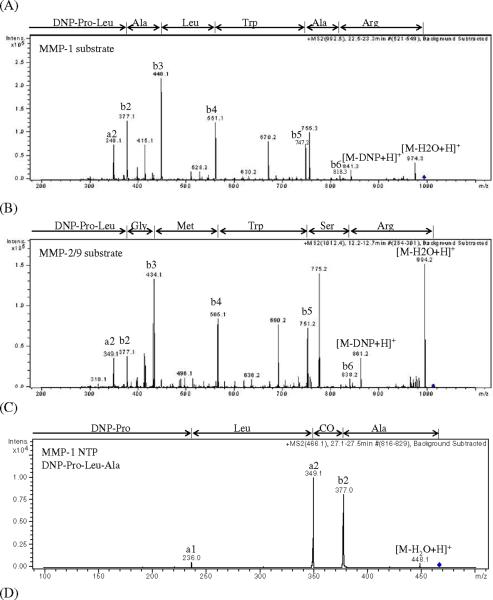
Upon enzymatic cleavage, the substrates will produce both N- and C-terminal peptides. Both of these peptides could be used for quantitation purposes. However, the C-terminal peptides for both substrates were poorly retained on the chromatographic column compared to the N-terminal peptides as well as the substrates. Additionally, the C-terminal peptides produced approximately ten-fold lower signal intensities as compared to the N-terminal peptides at equimolar concentrations. For these reasons, the N-terminal peptides were chosen as the products to quantify in the dialysate samples. Figure 2 shows the MS/MS spectra obtained for the protonated ions for each of the different analytes. The corresponding fragment ions are labeled noting that in Figure 2A for the MMP-1 substrate, m/z 755.3 and 670.2 and in Figure 2B for the MMP-2/-9 substrate, m/z 775.2 and 690.2 are unassigned.
Figure 2. Full scan product ion spectra for analyte characterization.
(A) MS/MS of m/z 992 for MMP-1 substrate, (B) MS/MS of m/z 1012 for MMP-2/-9 substrate, (C) MS/MS of m/z 466 for MMP-1 NTP, (D) MS/MS of m/z 452 for MMP-2/-9 NTP, Peaks are labeled with the corresponding fragment ions.
Within the calibration range of (5 to 50 μM) the calibration curves were linear for both the MMP-1 substrate and MMP-2/-9 substrate (5 to 50 μM) based on the EIC of MS/MS of m/z 992 and MS/MS of m/z 1012 versus concentration, respectively. The limit of quantitation (LOQ) was used as the lowest concentration standard for the calibration curve and was achieved at the concentration that yields a signal to noise ratio (S/N) of 10:1; and the limit of detection (LOD) was achieved at the concentration that yields a S/N of 3:1. For both substrates, LOD was 1 μM and LOQ was 5 μM. For MMP-1 NTP and MMP-2/-9 NTP, linear calibration curves (1 to 50 μM) were obtained based on the EIC of MS/MS of m/z 466 and MS/MS of m/z 452 versus concentration, respectively. For both peptide products, LOD was 0.2 μM and LOQ was 1 μM.
The intra-day and inter-day relative standard deviation (RSD%) were determined and were found to be 4.9% (intra-day) and 10.8% (inter-day) for GM 6001, 1.4% (intra-day) and 9.5% (inter-day) for the MMP-1 substrate, 3.1% (intra-day) and 4.2% (inter-day) for the MMP-2/-9 substrate, 4.7 %(intra-day) and 8.8% (inter-day) for the MMP-1 NTP and 4.1% (intra-day) and 7.3% (inter-day) for the MMP-2/-9 NTP.
In vitro Microdialysis Sampling
The EE% values for the different substrates were determined under quiescent as well as well-stirred conditions at 37°C. For the MMP-1 substrate the EE% was 58.0±8.1 (quiescent) and 69.1±2.1 (well-stirred), n=4. For the MMP-2/-9 substrate the EE% values were 64.5±7.6 (quiescent) and 73.5±1.6 (well-stirred), n=4. For GM 6001, its EE% was determined for different concentrations (10, 20 and 50 μM) and was approximately 70% with no variation among the different concentrations that were perfused through the microdialysis sampling probe. Additionally, there was no change in either the substrate EE% values or the GM 6001 EE% values when compounds were co-perfused.
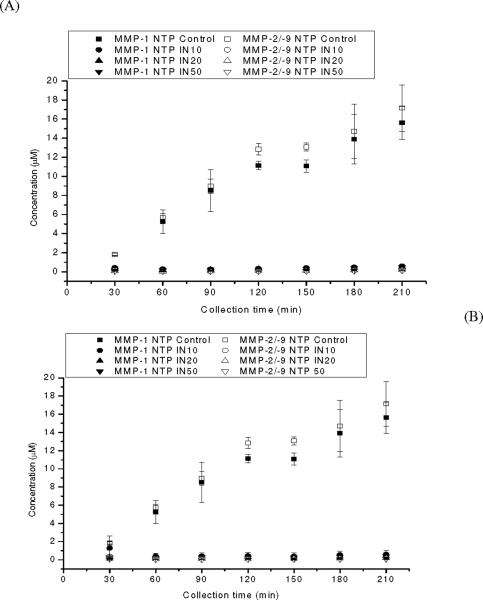
The time-dependent formation of MMP-1 NTP and MMP-2/-9 NTP during the localized infusion of the MMP substrates under control and inhibited in vitro conditions is shown in Figure 3. The formation of the N-terminal peptide products increased during the entire collection period. To test the effectiveness of the broad spectrum MMP inhibitor, GM 6001, it was added at concentrations of 10, 20, and 50 μM to the vial containing the enzyme solution. In separate experiments, GM 6001 was included in the perfusion fluid at concentrations of 10, 20 and 50 μM. For all concentrations of GM 6001 and under both experimental conditions, i.e., delivered through the dialysis probe or included in the external solution, the formation of products was completely inhibited at all time points as shown in Figure 3. Table 2 shows the calculated inhibition at the 210 minute collection point. Nearly 100% inhibition was obtained for all the inhibiter concentrations. Additionally, the EE% for the substrates was not altered when the inhibitor was added to the perfusion fluid (data not shown).
Figure 3. Time-dependent MMP-1 NTP and MMP-2/-9 NTP formation.
(A) Microdialysis with inhibitor included in the sample medium with activated MMPs, and (B) microdialysis with inhibitor included in the perfusate with substrates. Control denotes MMP-1 substrate (50 μM) and MMP-2/-9 substrate (50 M) being perfused to the sample medium containing activated MMP-1 and MMP-9, with no inhibitor present. IN10, IN20, IN50 denotes inhibitor concentration of 10, 20, 50 μM present in the sample medium or perfusate. Data represent mean±S.D., n=4.
Table 2.
Percentage inhibition of MMP-1 and MMP-9 (in vitro) with different concentrations of inhibitor in the system
| Inhibitor concentration (μM) | Microdialysis with inhibitor in the sample medium | Microdialysis with inhibitor in the perfusate | ||
|---|---|---|---|---|
| MMP-1 | MMP-2/-9 | MMP-1 | MMP-2/-9 | |
| 10 | 96.1±1.0 | 99.1±0.3 | 95.2±1.8 | 97.9±0.7 |
| 20 | 96.7±0.5 | 99.0±0.2 | 96.7±0.9 | 98.9±0.3 |
| 50 | 96.7±0.7 | 99.3±0.2 | 97.1±0.9 | 99.6±0.1 |
The percentage inhibition was calculated the difference of product concentration in control and inhibition experiment divided by the control. Data represent mean±S.D., n=4.
Microdialysis sampling was performed to determine if MMP enzymes in the sample medium would be excluded by the PAES 20 kDa MWCO membrane. The MMP substrate mixture was spiked into the dialysate. The results indicated no significant difference between the substrate concentration in the dialysate sample and control substrate mixture sample (less than 5% variance). In addition, no product peaks were obtained in the dialysate sample. These results suggest that, under these experimental conditions, the activated MMPs cannot pass through PAES probes having a 20 kDa MWCO membrane.
LC/MS/MS Method Validation and Analyte Quantitation
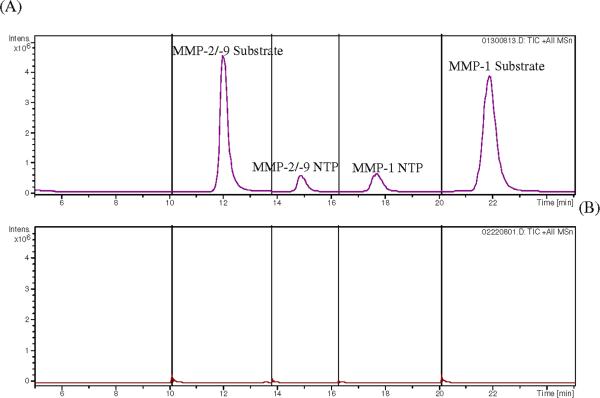
The method used for the analysis of the dialysates collected in vitro was applied to analysis of in vivo dialysates with a few changes. The eluate from the first 5 min of the chromatographic run was directed to waste and the entire run time was shortened to 25 min. Figure 4 shows a typical LC chromatogram for the in vivo dialysates. Dialysates obtained during perfusion with the MMP substrates as well as blanks (no substrates) were compared (Figure 4). For the dialysate obtained when the MMP substrates were perfused, the peaks corresponding to both substrates and products are shown, indicating the presence of active MMPs outside the probe, thus converting the substrates to products that were recovered in the dialysates. For the blank dialysates(those collected that had no substrates included), no peaks that co-eluted with either the MMP substrates or their products were obtained, indicating that no peptide or other endogenous component with the same retention time, structure or fragmentation pattern similar to the MMP substrates or their products was present in the blanks.
Figure 4. LC/MS/MS chromatograms of in vivo dialysates.
(A) Dialysate with substrate perfusion, and (B) PBS blank sample. Peaks were labeled with corresponding analyte. Full scan product ion spectra of the [M+H]+ ions were monitored in different segments including MS/MS of m/z 1012 for MMP-2/-9 substrate (10 to 13.8 min), MS/MS of m/z 452 for MMP-2/-9 NTP (13.8 to 16.3 min), MS/MS of m/z 466 for MMP-1 NTP (16.3 to 20 min), and MS/MS of m/z 992 for MMP-1 substrate (20 to 25 min).
Blank dialysates were also tested for MMP activity to determine whether active MMPs or other proteolytic enzymes would diffuse through the probe membrane. Spikes of the MMP substrates into these blank dialysates obtained on the implantation day resulted in substrate concentrations that were 103.8 ± 2.6% (n=4) in the blanks vs. controls for the MMP-1 substrate and 106.1 ± 2.7% (n=4) for the MMP-2/-9 substrate. This indicates that the blank dialysates after implantation did not contain additional proteases, which has been recently reported as a potential problem with microdialysis of peptides.35 Similar results were observed for dialysates collected on Day 3 (Table 3). However, on Day 7, significant protease activity was observed in the collected blanks. The source of these proteases is unknown at this time and being further investigated.
Table 3.
Protease activity found in vivo in blank dialysates
| MMP-1 | MMP-2/-9 | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 7 | Day 0 | Day 3 | Day 7 | |
| Substrate ratio | 103.8±2.6 | 97.7±2.1 | 46.2±0.4 | 106.1±2.7 | 100.0±6.4 | 40.6±6.5 |
| NTP ratio | 0.7±0.2 | 2.8±1.6 | 15.4±5.9 | 0.7±0.2 | 2.7±1.0 | 27.0±3.4 |
Substrate ratio denotes substrate concentration formed in blank dialysates divided by substrate concentration in a control solution. NTP ratio denoted NTP concentration formed in blank dialysate divided by corresponding substrate concentration in a control solution. Data represent mean±S.D., n=4.
In vivo Microdialysis Sampling Through Long-Term Implanted Microdialysis Probes: Control
The time-dependent formation of products for the control experiment is illustrated in Figure 5. The concentrations of both the MMP-1 NTP and MMP-2/-9 NTP increase and stabilize around 90 min. The maximum concentrations obtained for the MMP-1 NTP was 2.6 μM and for the MMP-2/-9 NTP was 3.1 μM. These concentrations were significantly lower than those obtained in vitro (15.6±1.7 M MMP-1 NTP and 17.1±2.4 M for MMP-2/-9 NTP, n=4) and may be caused by lower activity of MMPs in vivo since the concentration as well as the distribution of MMPs within the tissue site surrounding the dialysis probe are not known. Additionally, there are mass transport differences caused by the tissue space as compared to the in vitro setting. Since the substrate EE% values were between both the in vivo and in vitro experiments (nearly 70%), it is unlikely that differences in the product concentrations obtained are due to small differences in EE% between the two experiments.
Figure 5. MMP-1 NTP and MMP-2/-9 NTP formation in the control dialysate versus inhibition dialysate on day 0 of implantation.
The control dialysates were obtained by perfusing the MMPs substrate mixture (MMP-1 substrate 50 μM and MMP-2/-9 substrate 50 μM in PBS) through the implanted probes at 1 μL/min. The inhibition dialysates were obtained by perfusing MMP inhibitor GM 6001 50 μM in the above substrates mixture through the implanted probes. Data represents mean value. n=4 rats.
Figure 5 shows the time-dependent MMP-1 NTP and MMP-2/9 NTP formation in both the control and GM 6001-containning dialysate on different collection days. Similar to the control dialysates, MMP-1 NTP and MMP-2/9 NTP formation in the GM 6001-containing dialysate showed an increase in product formation that then stabilized. Lower amounts of products were obtained in the GM 6001-containing dialysate, indicating the MMP activities outside the probe can be partially inhibited by the inhibitor co-perfusion with the substrates. The average concentration of the individual products in control and inhibition experiments were obtained from the dialysate data collected between 90 and 210 min, and the percentage inhibition can be calculated by the difference between the concentrations obtained from the control and GM 6001-containing dialysate divided by the control concentration.
The broad spectrum MMP inhibitor GM 6001 was added included to the microdialysis perfusion fluid to determine if MMP activity can be effectively inhibited by this assay scheme. GM 6001 is a potent, cell-permeable, broad-spectrum hydroxamic acid inhibitor of MMPs (Ki=400 pM for MMP-1; Ki =500 pM for MMP-2; Ki =27 nM for MMP-3; Ki =100 pM for MMP-8; and Ki =200 pM for MMP-9) that can be used to indicate the presence of active MMPs.29,30,36 The use of the inhibitor is important as it allows us to rule out the activity of other proteases that might cleave the peptide substrates.
The percentage inhibition for MMP-1 was approximately 30% and for MMP-2/-9 was approximately 25%, which is much lower compared to the complete inhibition observed with the in vitro experiment. There are several possible explanations for this apparent disparity. The first could be that not enough GM 6001 reached the tissue space to inhibit the enzymes. Another is that GM 6001 was tested using human MMPs; rat MMPs may be slightly different in terms of their Ki values. This is unlikely as GM 6001 is used as an inhibitor for MMPs across different species including rat. 37 (ref, anything about GM6001 and rat?). Additionally, endogenous inhibitors of MMPs known as TIMPs from drosophila can inhibit mammalian MMPs suggesting conservation of activity across many species.38 Finally, the most likely explanation is that other GM6001-insensitive proteases are activated in vivo that cleave the substrates. These different possibilities are being addressed in our laboratory.
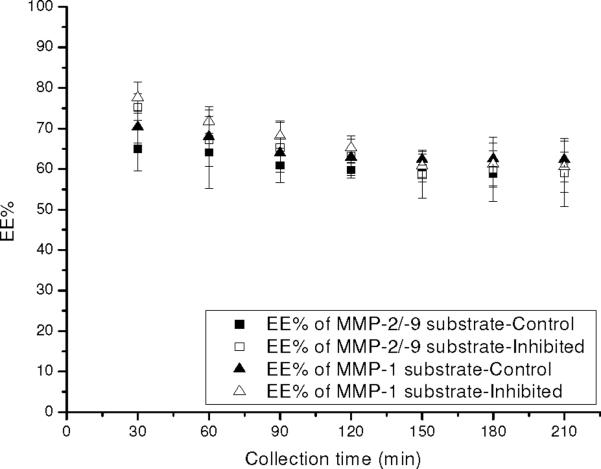
Figure 6 illustrates the EE% for substrates from the probes implanted with and without the inclusion of the GM 6001 inhibitor. There was no significant difference for the EE% of substrates between the controls and the inhibited infusions. These results indicated that the EE% of substrates and inhibitor are not sensitive enough to reflect the inhibition of MMPs external to the probe. This indicates that other removal processes in the tissue contribute more to the EE% than enzymatic activity. However, decreased product formation was observed with inhibited enzyme activities outside the probe despite the small or no change in EE% of substrate and inhibitor. While at this point this methodology is not able to give quantitative information regarding the enzymatic activities surrounding the dialysis probes, the difference in the amount of product formed under control and inhibited conditions provides information regarding the presence of MMPs external to the microdialysis sampling probes.
Figure 6. EE% of MMP-1 substrate and MMP-2/-9 substrate in the control dialysate versus inhibition dialysate on day 0 of implantation.
The control dialysates were obtained by perfusing the MMPs substrate mixture (MMP-1 substrate 50 μM and MMP-2/-9 substrate 50 μM in PBS) through the implanted probes at 1 μL/min. The inhibition dialysates were obtained by perfusing MMP inhibitor GM 6001 50 μM in the above substrates mixture through the implanted probes. Data represents mean±S.D., n=4 rats.
Table 4 shows the product concentrations obtained for the infused substrates on Days 3 and 7. As compared to the initial implantation day, there is no significant difference among the concentrations obtained and the percentage of inhibition with the infusion of the GM 6001 MMP inhibitor. Again, the use of the inhibitor partially blocked product formation, but did not completely inhibit it. Additional experiments beyond the scope of this initial work are necessary to determine the MMP activities as well as their localization external to the dialysis probes.
Table 4.
MMP-1 NTP and MMP-2/-9 NTP formation and percentage inhibition on different collection days.
| MMP-1 NTP | MMP-2/-9 NTP | |||||
|---|---|---|---|---|---|---|
| Conc.-control (μM) | Conc.-inhibition (μM) | Percentage inhibition (%) | Conc.-control (μM) | Conc.-inhibition (μM) | Percentage inhibition (%) | |
| Day 0 | 2.6±0.5 | 1.8±0.4 | 28.5 | 3.1±0.8 | 2.4±0.6 | 22.3 |
| Day 3 | 1.7±0.2 | 1.2±0.1 | 31.0 | 2.1±0.9 | 1.5±0.2 | 27.5 |
| Day 7 | 1.5±0.3 | 1.0±0.2 | 31.7 | 1.8±0.5 | 1.4±0.3 | 24.2 |
The control dialysates were obtained by perfusing the MMPs substrates mixture (MMP-1 substrate 50 μM and MMP-2/-9 substrate 50 μM in PBS) through the implanted probes. The inhibition dialysates were obtained by perfusing MMP inhibitor GM 6001 50 μM in the above substrates mixture through the implanted probes. Data represents mean±S.D., n=4 rats.
Confirmation of MMP activity via Zymography
Figure 7 shows the zymograms of normal tissue vs. that of encapsulated tissue around the probes that have been implanted for a week. In the gelatin gel, the pro-MMP-9 (92 kDa), pro-MMP-2 (72 kDa), active MMP-2 (62 kDa) were shown as white bands against the blue background, indicating the presence of these enzymes in the lysates obtained from the tissue that encapsulated the probe. Another band at around 220 kDa was also present, and it may be a complex of MMP-9.39 Additional bands can be seen which correspond to active MMP-9 (86 kD) and active MMP-2 (67 kDa). It should be noted that MMP-1, MMP-8 and MMP-13 can also cleave the gelatin substrate, but typically these bands are weak as gelatin is not their preferred substrate. For the normal tissue, only very light band corresponding to the pro-MMP-2 (72 kDa), active MMP-2 (62 kDa) were present, indicating a very low concentration of MMP-2 in the normal tissue; no MMP-9 was detected. In the 10% casein gel, a very light band corresponding to active MMP-1 (45 kDa) was present. Bands corresponding to pro-MMP-9 (92 kD) were also present as MMP-9 can also cleave the casein substrate. All the bands are very light comparing to the gelatin gel even with double the protein amount loaded, as the casein zymograms are much less sensitive than their gelatin counterparts. For the normal tissue, no MMP bands were detected. The above zymogram results clearly indicated that the encapsulation tissue around the probe showed higher MMPs activity comparing to the normal tissue, suggesting induction of MMP-1, MMP-2, and MMP-9 during the long-term microdialysis probe implantation.
Figure 7. Zymogram of capsulation tissue around implanted microdialysis probes vs. normal tissue.
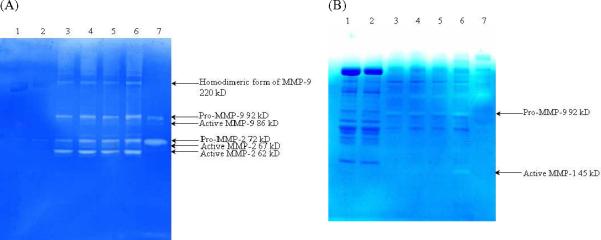
(A) 12% gelatin gel; (B) 10% casein gel. Lane 1 and 2, normal tissue; lane 3 to 6, capsulation tissue around the implanted microdialysis probe; lane 7, HT-15 medium containing MMP-2 and MMP-9.
Conclusions
An in vitro and in vivo assay has been developed to simultaneously detect MMP-1 and MMP-9 activity using microdialysis sampling and LC/MS/MS. The perfusion of appropriate MMP substrates, combined with recovery of products, allows in situ detection of MMPs. A broad spectrum MMP inhibitor was also included into the system, either in the sample medium containing activated enzyme, or in the perfusate along with substrate co-perfusion. Regardless of the method of delivery, complete inhibition was shown from the product formation, suggesting the product formation can provide valuable information regarding changes in the enzymatic reaction outside the probe. In addition, the assay scheme for inclusion of inhibitor in the perfusate has the potential to be applied in the further in vivo MMPs activities detection as a validation for the presence of MMPs activities around the probe.
Acknowledgements
NIH EB 001441 supported this work. The Agilent MSD Trap Instrument was purchased under grant NSF CHE-0091892
References
- 1.Anderson JM, Rodriguez A, Chang DT. Seminars in Immunology. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones JA, McNally AK, Chang DT, Qin LA, Meyerson H, Colton E, Kwon ILK, Matsuda T, Anderson JM. J. Biomed. Mater. Res. 2008;84A:158–166. doi: 10.1002/jbm.a.31220. [DOI] [PubMed] [Google Scholar]
- 3.Luttikhuizen DT, Amerongen MJ, Feijter PC, Petersen AH, Harmsen MC, Luyn MJA. Biomaterials. 2006;27:5763–5770. doi: 10.1016/j.biomaterials.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Vaday GG, Lider O. J. Leukoc. Biol. 2000;67:149–159. doi: 10.1002/jlb.67.2.149. [DOI] [PubMed] [Google Scholar]
- 5.Sternlicht MD, Werb Z. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somerville RPT, Oblander SA, Apte SS. Genome Biol. 2003;4(216):1–11. doi: 10.1186/gb-2003-4-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCawley LJ, Matrisian LM. Curr. Opin. Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 8.Clark IM, editor. Matrix metalloproteinase protocols, methods in molecular biology. Vol. 151. Humana Press, Inc.; Totowa, NJ: 2001. [Google Scholar]
- 9.Egeblad M, Werb Z. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 10.Catterall JB, Cawston TE. Methods Mol. Biol. 2003;225:353–364. doi: 10.1385/1-59259-374-7:353. [DOI] [PubMed] [Google Scholar]
- 11.Iked K, Tate G, Suzuki T, Mitsuya T. Gynecol. Oncol. 2005;97:323–329. doi: 10.1016/j.ygyno.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 12.Kleiner DE, Stetler-Stevenson WG. Anal. Biochem. 1994;218:325–329. doi: 10.1006/abio.1994.1186. [DOI] [PubMed] [Google Scholar]
- 13.Gogly B, Groult N, Hornebeck W, Godeau G, Pellat B. Anal. Biochem. 1998;255:211–216. doi: 10.1006/abio.1997.2318. [DOI] [PubMed] [Google Scholar]
- 14.Lombard C, Saulnier J, Wallach J. Biochimie. 2005;87:265–272. doi: 10.1016/j.biochi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Stenken JA. Microdialysis Sampling. In: Webster JG, editor. Encyclopedia of Medical Devices and Instrumentation. 2nd edition Vol 4. John Wiley & Sons, Inc.; Hoboken, NJ: 2006. pp. 400–420. [Google Scholar]
- 16.Bourne JA. Clin. Exp. Pharmacol. Physiol. 2003;30:16–24. [Google Scholar]
- 17.Bungay PM, Morrison PF, Dedrick RL. Life Sci. 1990;46:105–119. doi: 10.1016/0024-3205(90)90043-q. [DOI] [PubMed] [Google Scholar]
- 18.Bergström SK, Goiny M, Danielsson R, Ungerstedt U, Andersson M, Markides KE. J. Chromatogr. A. 2006;1120:21–26. doi: 10.1016/j.chroma.2006.01.110. [DOI] [PubMed] [Google Scholar]
- 19.Modi SJ, LaCourse WR. J. Chromatogr. A. 2006;1118:125–133. doi: 10.1016/j.chroma.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson C, Nilsson F, Turner P, Sixtensson M, Nordberg Karlsson E, Holst O, Cohen A, Gorton L. Anal. Bioanal. Chem. 2006;385:1421–1429. doi: 10.1007/s00216-006-0570-7. [DOI] [PubMed] [Google Scholar]
- 21.Scott DO, Lunte CE. Pharm. Res. 1993;10:335–342. doi: 10.1023/a:1018971818689. [DOI] [PubMed] [Google Scholar]
- 22.Stenken JA, Holunga DM, Decker SA, Sun L. Anal. Biochem. 2001;290:314–323. doi: 10.1006/abio.2000.4985. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson UW, Dabrosin C. Cancer. Res. 2006;66:4789–4794. doi: 10.1158/0008-5472.CAN-05-4012. [DOI] [PubMed] [Google Scholar]
- 24.Etoh T, Joffs C, Deschamps AM, Davis J, Dowdy K, Hendrick J, Baicu S, Mukherjee R, Manhaini M, Spinale FG. Am. J. Physiol. 2001;281:H987–H994. doi: 10.1152/ajpheart.2001.281.3.H987. [DOI] [PubMed] [Google Scholar]
- 25.Spinale FG, Koval CN, Deschamps AM, Stroud RE, Ikonomidis JS. Circulation. 2008;118:S16–S23. doi: 10.1161/CIRCULATIONAHA.108.786640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Zagorevski DV, Stenken JA. Anal. Chem. 2008;80:2050–2057. doi: 10.1021/ac702047w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saghatelian A, Jessani N, Joseph A, Himphrey M, Cravatt BF. Proc. Natl. Acad. Sci. USA. 2004;27:10000–10005. doi: 10.1073/pnas.0402784101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu P, Sefton MV. J. Biomed. Mater. Res. 2004;71:226–232. doi: 10.1002/jbm.a.30139. [DOI] [PubMed] [Google Scholar]
- 29.Galardy RE, Cassabonne ME, Giese C, Gilbert JH, Lapierre F, Lopez H, Schaefer ME, Stack R, Sullivan M, Summers B. Ann. N. Y. Acad. Sci. 1994;732:315–323. doi: 10.1111/j.1749-6632.1994.tb24746.x. [DOI] [PubMed] [Google Scholar]
- 30.Galardy RE, Grobelny D, Foellmer HG, Fernandez LA. Cancer Res. 1994;54:4715–4718. [PubMed] [Google Scholar]
- 31.Walsh KA. Meth. Enzymol. 1970;19:41–63. [Google Scholar]
- 32.Duncan ME, Richardson JP, Murray GI, Melvin WT, Fothergill JE. Eur. J. Biochem. 1998;258:37–43. doi: 10.1046/j.1432-1327.1998.2580037.x. [DOI] [PubMed] [Google Scholar]
- 33.John H, Walden M, Schäfer S, Genz S, Forssmann W. Anal. Bioanal. Chem. 2004;378:883–897. doi: 10.1007/s00216-003-2298-y. [DOI] [PubMed] [Google Scholar]
- 34.Prokai L, Zharikova AD, Janáky T, Prokai-Tatrai K. Rapid Commun. Mass Spectrom. 2000;14:2412–2418. doi: 10.1002/1097-0231(20001230)14:24<2412::AID-RCM180>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Zubieta J-K, Kennedy RT. Anal. Chem. 2009;81:2242–2250. doi: 10.1021/ac802391b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grobelny D, Poncz L, Galardy RE. Biochemistry. 1992;31:7152–7154. doi: 10.1021/bi00146a017. [DOI] [PubMed] [Google Scholar]
- 37.Amantea D, Russo R, Gliozzi M, Fratto V, Berliocchi L, Bagetta G, Bernardi G, Corasaniti MT. Int. Rev. Neurobiol. 2007;82:149–169. doi: 10.1016/S0074-7742(07)82008-3. [DOI] [PubMed] [Google Scholar]
- 38.Wei S, Xie Z, Filenova E, Brew K. Biochemistry. 2003;42:12200–12207. doi: 10.1021/bi035358x. [DOI] [PubMed] [Google Scholar]
- 39.Pucci-Minafra I, Minafra S, La Roccaa G, Barrancaa M, Fontanaa S, Alaimoa G, Okadac Y. Matrix Biol. 2001;20:419–427. doi: 10.1016/s0945-053x(01)00146-9. [DOI] [PubMed] [Google Scholar]