Abstract
Acute kidney injury (AKI) is common (especially during critical illness), increasing in incidence, and is associated with considerable morbidity and mortality. The Risk, Injury, Failure, Loss, and End-stage renal disease (RIFLE) classification currently provides a standardized estimate of incidence and outcomes from AKI. Despite advances in the understanding of the pathogenesis of human AKI, our ability to assess kidney function is limited and functional impairment poorly correlates with structural injury to the kidneys. Emerging novel biomarkers are, however, likely to further enhance risk stratification, facilitate early diagnosis, enable early enrollment in therapeutic trials, and assess prognosis. Sepsis remains the leading cause of AKI among the critically ill and over the past few years insights into the pathogenesis of AKI in sepsis are beginning to shift attention from renal blood flow to inflammation-mediated organ injury. Emerging evidence suggests that survivors of AKI incur long-term risks for developing chronic kidney disease and end-stage renal disease compared with those without AKI. Despite decades of research, no specific therapy for AKI other than supportive care currently exists and further work is required to better understand the pathogenesis of AKI during critical illness and to develop novel treatments.
Introduction
Approximately 2 million people worldwide will die this year of acute kidney injury (AKI), a disease for which no effective treatment exists.1–3 Despite advances in the understanding of pathogenesis of acute kidney dysfunction in humans,4,5 we only have a vague notion as to why kidney function decreases so dramatically in many patients with acute illness or injury, or why, despite renal replacement therapy, mortality is so high. Although this ‘disease’ is in fact a manifestation of multiple different pathological processes and, to some extent, even normal physiology in the face of stress, common elements to this syndrome exist. We know, for example, that as kidney function declines mortality increases.2,3,6–9 Additionally, an imperfect relationship between functional impairment and histopathological evidence of damage to the kidney is known.10,11 How can we then hope to improve the prognosis for patients with AKI and how can we make progress for the field in general? In our view, the key to progress in the treatment of AKI is to begin with the epidemiology and apparent pathogenesis of the disease and develop therapies based on these facts. This Review discusses the epidemiology, pathogenesis, treatment, and prognosis of AKI as well as describes the role of biomarkers in the diagnosis and management of AKI.
What is acute kidney injury?
Severe and acute impairment in vital organ function is the hallmark of critical illness and indeed the purpose of intensive care is to provide support for, and protection of, vital organs. However, comprehensive kidney support would be difficult to achieve, as the kidneys perform many complex homeostatic functions.12,13 For instance, regulation of extracellular fluid volume, concentration of osmotically active substances, plasma pH, excretion of unwanted products of metabolism, and catabolism of hormones are all impaired during AKI. In addition, homeostasis of blood pressure, platelet function, and electrolytes are also dysregulated. Given the wide range of dysfunction that occurs during AKI, our ability to define and quantify impairment of the kidneys in a single definition that captures all of the functional domains is limited. Furthermore, damage to the kidney may occur before, during, or after loss of kidney function is manifested (Figure 1).
Figure 1.
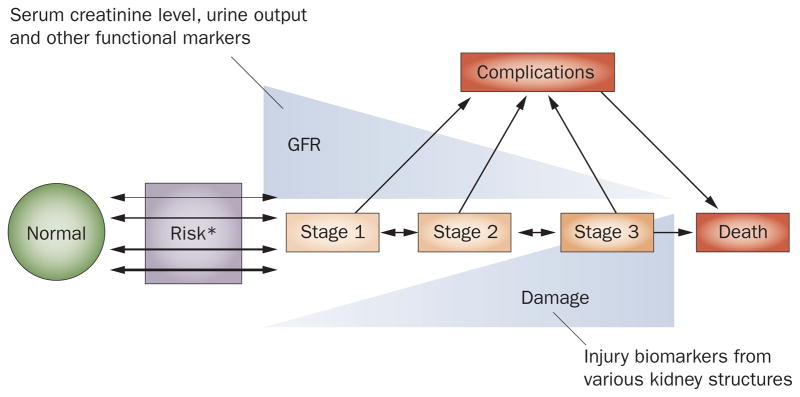
Conceptual model of AKI. The new conceptual model of AKI incorporates changes in renal function and structure. It also illustrates the potential inverse relationship that may exist between changes in renal function as well as renal structure as captured by injury biomarkers. *Risk incorporates both patient susceptibilities (for example, advanced age) as well as exposures (for example, sepsis). When susceptibilities are great, exposure may be limited but still result in AKI. Abbreviations: AKI, acute kidney injury; GFR, glomerular filtration rate.
Definition of AKI
The term AKI has now been adopted to describe the range of renal impairment from mild alteration, which presumably occurs without actual damage, to complete organ failure. Thus, the spectrum of AKI encompasses mild impairment in kidney function to overt organ failure; however, there exists an inconsistent relationship between ‘injury’ and ‘impairment’ of renal function such that injury may either precede or follow impairment. Furthermore, injury may exist with or without renal impairment.
Importantly, the concept of AKI should be flexible enough to include clinically meaningful changes in structure even when function is not impaired, a concept that raises some concerns. First, if there is no kidney damage why do we use the term ‘injury’? Second, how do we define clinically meaningful changes in kidney structure if function is not impaired? The first issue may seem purely semantic. If an organ or an organism is functionally impaired to the point of disability surely we can agree that this change is pathological and whether we call it injury or impairment is not important. However, some reduced function, particularly in the service of organ protection, may not be pathological at all. For instance, the kidneys avidly conserve salt and water when renal hypoperfusion occurs. When normal renal hemodynamics are restored, urine flow returns to normal. As this change is undoubtedly considered a ‘healthy’ response (a means of organ and even organism protection), decreased function, in this case, can be viewed as ‘acute renal success’. Furthermore, given that kidney impairment is often asymptomatic, one must question at what point this condition should be called a disease. For practical purposes, if functional impairment leads to reduced survival or other patient-centered outcomes, one can easily justify the label of disease, or in this case the somewhat awkward term ‘injury’. As we will discuss later, whether the current definition for AKI is too strict or too lenient in this regard is presently debated.
The second issue regarding the definition of clinically meaningful changes in kidney structure mentioned earlier could also be viewed as semantic. When, if ever, do changes in organ structure result in pathology in the absence of reduced function? This problem with disease definitions is, of course, not unique to AKI but it is important to recognize that even changes that are considered pathological in certain circumstances may not be clinically relevant. For example, if a condition were to result in a small number of renal tubular epithelial cells undergoing changes typical of ischemia, would this finding alone be sufficient to define a disease even if the changes were entirely reversible? Only when pathological changes are associated with subsequent clinical outcomes do we argue that they are relevant. Structural changes in cells and tissues are only considered important when they alter organ function or in other cases by producing pain, anxiety, or social stigmatism. Clearly, for AKI, we are only concerned when changes in structure are accompanied by changes in function.
Unfortunately, current evidence from human, transplant, and autopsy studies indicate that there is considerable dissociation between structure and function in human AKI. Sampling of renal tissue in critical illness is extremely rare owing to associated risks, but current evidence suggests that widespread overt tissue injury is rare.10,14 Although the finding of some degree of necrosis of tubular cells in the urine is suggestive of changes in kidney structure, the vast majority of patients with AKI during critical illness have bland histology, with the exception of a minority of patients who have overt hemorrhage and shock in whom widespread cellular injury occurs because of prolonged renal ischemia. Indeed, even in such cases, intact tubular cells in urine sediments are often present and have been shown to be viable.15
AKI criteria
Unsurprisingly, given the caveats on the meaning and assessment of changes in kidney structure, definitions of AKI are based on changes in function. Given the relative importance and ease of measurement, glomerular filtration rate (GFR) has been used as the primary metric to assess renal function. Two main limitations exist to this approach of using GFR as a measure of renal function. First, the relationship between functional and structural changes in the kidney is inconsistent. For instance, in sepsis, changes in kidney function are severe and yet histological findings are bland.16 Moreover, in the context of kidney donation, renal mass is effectively reduced by 50% and yet serum creatinine level is unchanged (though renal reserve is reduced). Second, measuring glomerular function can be difficult in AKI. Although serum creatinine level correlates well with GFR under steady-state conditions, AKI is, by definition, not a steady-state condition.17 Furthermore, given that a number of factors (age, sex, muscle mass, metabolism of creatinine, and volume status) markedly affect serum creatinine concentration, an isolated serum creatinine measurement has very limited utility. The diagnosis of AKI and determination of severity of AKI, therefore, depends on changes from baseline kidney function as measured by serum creatinine concentration.
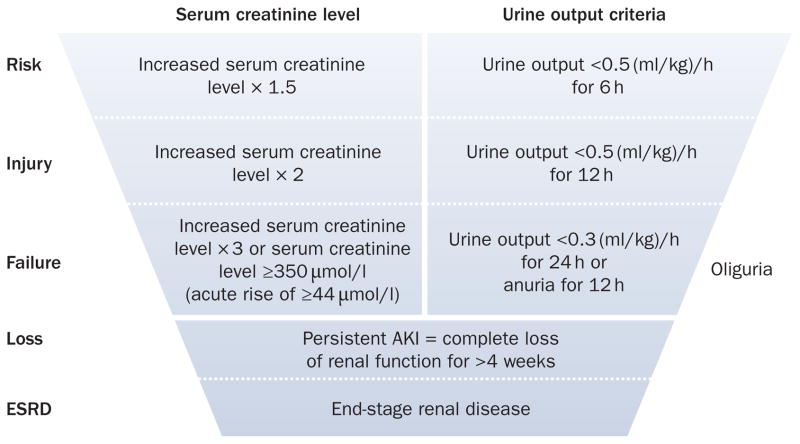
Two measures of glomerular function have been standardized for purposes of defining AKI—changes in serum creatinine level and urine output18—both of which have been codified in the Risk, Injury, Failure, Loss, and End-stage renal disease (RIFLE) criteria (Figure 2).19 Importantly, these criteria derive their strength from their ability to identify subgroups of individuals who sustain important adverse clinical outcomes (for example, death or requirement for renal replacement therapy) rather than from their ability to serve as accurate surrogates for changes in function or structure of the kidney. This distinction is critical because neither changes in function nor structure are necessarily relevant without a clinical context to anchor them. The RIFLE criteria were further refined in 2005 by the Acute Kidney Injury Network (AKIN), a worldwide collaboration of nephrology and critical care societies (Table 1).20 The purpose of the modifications proposed by AKIN were primarily to include a small but important group of patients with early and mild AKI who experience a change in renal function that is greater than physiological variation but less than the 50% increase required for the RIFLE criteria. In particular, patients with underlying chronic kidney disease (CKD) may be missed by the original RIFLE criteria even when these patients experience large changes in serum creatinine level. For example, a patient with a baseline creatinine level of 180 μmol/l would not be classified as having AKI according to RIFLE criteria until their serum creatinine level reached 270 μmol/l. By contrast, using the AKIN modification, the same patient would be classified as having AKI if they were to have a documented increase in serum creatinine level of 25 μmol/l during any 48 h period. Importantly, the AKIN modification was proposed as an addition to the original RIFLE criteria, not a substitution. The RIFLE criteria have been validated in over 500,000 patients in several multinational studies, and have become a standard way to classify patients with AKI.2,3,6,7,21–23
Figure 2.
RIFLE criteria for diagnosing AKI. The classification system includes separate criteria for creatinine and urine output. A patient can fulfill the criteria through changes in serum creatinine levels or changes in urine output, or both. The criteria that lead to the most severe stage should be used. Note that the F stage of RIFLE is present even if the increase in serum creatinine concentration is under threefold as long as the new serum creatinine level is >350 μmol/l in the setting of an acute increase of at least 44 μmol/l. The shape of the figure denotes the fact that more patients (high sensitivity) will be included in the mild category, including some without actually having kidney damage (less specificity). By contrast, at the bottom of the figure the criteria are strict and therefore specific, but some patients will be missed. Abbreviations: AKI, acute kidney injury; ESRD, end-stage renal disease; RIFLE, Risk, Injury, Failure, Loss, and End-stage renal disease. Permission obtained from BioMed Central © Bellomo, R. et al. Crit. Care 8, R204–R212 (2004).
Table 1.
AKIN staging system for AKI*
| Stage | Serum creatinine criteria | Urine output criteria |
|---|---|---|
| 1 | Increase in serum creatinine level of ≥25 μmol/l or ≥150–200% (1.5–2-fold) from baseline | Change in urine output <0.5 (ml/kg)/h for >6 h |
| 2 | Increase in serum creatinine level to >200–300% (>2–3-fold) from baseline | Change in urine output <0.5 (ml/kg)/h for >12 h |
| 3 | Increase in serum creatinine level to >300% (>threefold) from baseline (or serum; creatinine level of ≥354 μmol/l with an acute increase of at least 44 μmol/l | Change in urine output <0.3 (ml/kg)/h for 24 h or anuria for 12 h |
The AKIN modification of RIFLE criteria19 is a highly sensitive staging system based on data indicating that a small change in serum creatinine influences outcome.20 Only one criterion (serum creatinine level or urine output) has to be fulfilled to qualify for a stage. Given wide variation in indications and timing of initiation of renal replacement therapy, individuals who receive renal replacement therapy are considered to have met the criteria for stage 3 irrespective of the stage they are in at the time of initiation of renal replacement therapy. Abbreviations: AKI, acute kidney injury; AKIN, acute kidney injury network; RIFLE, Risk, Injury, Failure, Loss, and End-stage renal disease. Permission obtained from BioMed Central © Mehta, R.L. et al. Crit. Care 11, R31 (2007).
Epidemiology
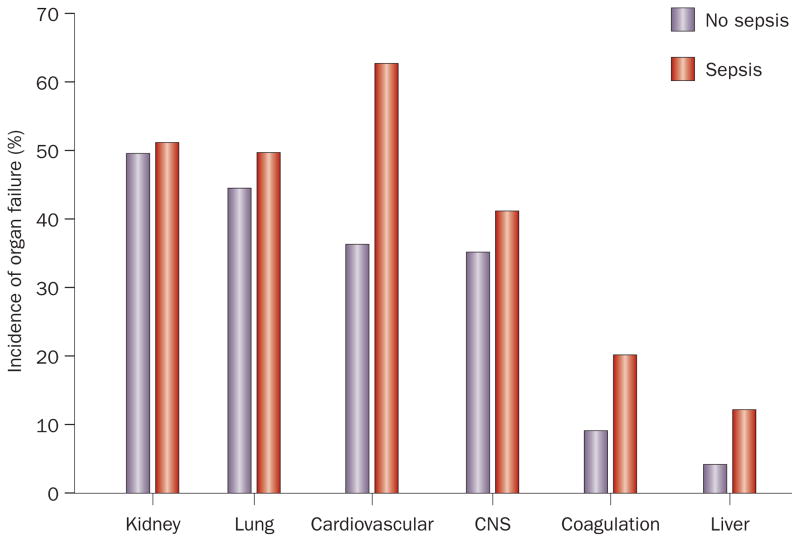
Results from a number of studies have indicated that AKI is common, increasing in incidence,3,24,25 and is associated with considerable morbidity and mortality.3,6,26 Rates of AKI in hospitalized patients have been reported to be between 3.2% and 20%,6,27,28 and AKI rates in intensive care units (ICUs) have been reported to be between 22% and as high as 67%.2,29 Indeed, rates of organ dysfunction for four vital organ systems (kidney, lung, cardiovascular, and central nervous system) are actually quite similar in critically ill patients, between one-third and one-half of patients have each type of organ system failure (Figure 3). Traditional measures of organ failure, however, likely underestimate the incidence of AKI, as does incomplete application of the available diagnostic criteria.
Figure 3.
Incidence of various organ failure among critically ill patients. Rates of organ dysfunction in 3,417 adults with or without sepsis treated in 198 intensive care units in 24 European countries. Figure constructed from data reported in Vincent, J.L. et al.74 Abbreviation: CNS, central nervous system.
Difficulties in assessing AKI incidence
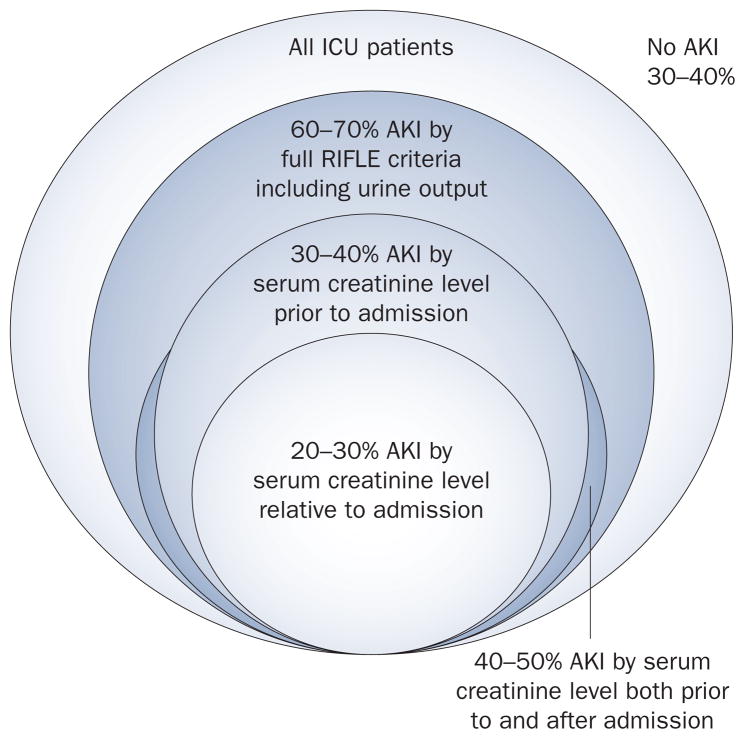
The relationship between application of RIFLE criteria and the apparent incidence of AKI varies according to the definition used and the time at which it is applied (Figure 4). First, approximately two-thirds of patients with AKI will manifest this condition before hospital admission.30 In other words, if one considers AKI as a change in renal function relative to admission, as was done in one 2009 study, the incidence of AKI will be dramatically underestimated.29 A similar problem occurs when AKI criteria is examined on day one of hospital or ICU admission.23 Varied estimates of incidence of AKI are, therefore, more a function of methodology rather than epidemiology. Another factor is whether urine output criteria are included. If urine output is included, some patients may be classified at a high stage of severity, and both diagnosis and staging may be more rapid by including measurements of urine output. However, approximately 20% of patients will exhibit changes in urine output sufficient for the diagnosis of AKI but never manifest a change in creatinine criteria. These groups of patients are almost exclusively RIFLE-R (AKIN Stage 1) and seem to have a low mortality (though often not quite as low as patients without any AKI criteria). Exclusion of these patients from a diagnosis of AKI is tempting. A low urine output is an entirely appropriate response of the kidney to a reduced intravascular volume and given that these patients never manifest an increase in serum creatinine levels it would seem inappropriate to diagnose them as having AKI. Whether these patients were prevented from developing more severe AKI because they received appropriate care triggered by the onset of oliguria is, however, unknown. Indeed, the majority of patients who fulfill urine output criteria will also, eventually, fulfill criteria for serum creatinine levels. For now, we believe that patients with sustained oliguria (<0.5 (ml/kg)/h for ≥6 h) should be classified as having AKI. Perhaps novel biomarkers will one day help differentiate these patients into those with and without injury (discussed later).
Figure 4.
Risk of AKI varies by definition used and timing of assessment. The relationship between application of RIFLE criteria and the apparent incidence of AKI varies according to the definition used and the time at which it is applied, which can lead to underestimations in the incidence of AKI. Abbreviations: AKI, acute kidney injury; RIFLE, Risk, Injury, Failure, Loss, and End-stage renal disease.
Incidence of AKI is increasing
Although the lack of standard definitions for AKI precludes accurate measures of incidence before 2005, longitudinal studies that applied consistent criteria in the diagnosis of AKI seem to show a dramatic increase in the incidence. Using the International Classification of Diseases (ICD)-9 codes to identify patients with AKI from their hospital discharge datasets, the US Centers for Disease Control and Prevention published data on trends in 1980–2005 hospitalizations for kidney disease.31 Although the sensitivity and positive predictive value for ICD-9 codes to detect AKI is low,25 during this 25-year period, >20-fold increase in the incidence of AKI was observed. Whether AKI was the primary reason for hospitalization or AKI occurred during the hospitalization is uncertain, but the age-adjusted rate of AKI increased from 18 cases per 100,000 population in 1980 to 365 cases per 100,000 population in 2005. Although it is unclear whether this profound increase in AKI is due to increased disease or increased awareness, these data suggest that AKI is emerging as a major public health problem. As the patient population ages in developed countries, the incidence of AKI is projected to increase correspondingly.32,33
In 2009, the US Renal Data System, a nationally representative monitoring system for end-stage renal disease (ESRD), reported incidence rates of AKI in the US using three different datasets from 1995 to 2007.24 The largest increase in AKI was seen among older individuals >85 years of age. Male sex and black race were also associated with an increased risk of AKI. The most common cause of hospitalization was AKI itself—accounting for 20–23% of all hospitalizations. Patients with CKD had nearly a sevenfold higher risk of developing AKI than those without, and patients with AKI had post-discharge mortality twice as great as those hospitalized without AKI.
Using RIFLE criteria to define AKI, Ali et al.3 studied the incidence of AKI in a geographical population of 523,390 in Northern Scotland. The researchers found that the attack rate of AKI was 2,147 cases per million population. RIFLE classification was useful for predicting recovery of renal function, requirement for renal replacement therapy, length of hospital stay, and hospital mortality.
Etiology of AKI
Many common causes of AKI in critically ill patients exist (Box 1). Sepsis is the major cause of AKI, accounting for nearly 50% of cases.1,3,34 Several studies have reported that sepsis-induced AKI is associated with short and long-term risk of death.8,21 In a 2010 study, we examined the risk of AKI in 1,836 hospitalized patients with community-acquired pneumonia, a common infectious cause of hospitalization in developed countries.21 Using RIFLE criteria, we found that one-third (34%) of all patients hospitalized with pneumonia and nearly 25% of patients with nonsevere pneumonia developed AKI.
Box 1. Causes of acute kidney injury in critically ill patients.
Sepsis (most common)
Major surgery
Cardiogenic shock
Hypovolemia
Complications with medications
Hepatorenal syndrome
Obstructive uropathy
Based on a multinational study of nearly 30,000 patients by Uchino et al.1
Pathogenesis
AKI rarely occurs in isolation in critically ill patients and is usually associated with other organ system dysfunction, and has a multifactorial etiology.35 Nevertheless, given that renal biopsies are extremely rare in critically ill patients with AKI, data regarding the pathogenesis of AKI is scarce and most of our current understanding of the pathogenesis of human AKI is based on animal models that employ warm ischemia–reperfusion injury with complete interruption of renal blood flow for varying periods of time by a renal artery occlusion. This AKI model is characterized by development of acute tubular necrosis that has underpinned our under standing of human AKI in critical illness for several decades. However, the fact that such models are extremely limited in their ability to inform on the most common forms of human AKI, especially sepsis, is increasingly recognized.36,37 In contrast to warm ischemia animal models, human AKI is characterized by limited histological changes and profound decreases in kidney function.38,39
Although lacking the ability to directly investigate pathogenesis, human studies of sepsis-induced AKI21 suggest that AKI is strongly correlated with other organ failures and both sepsis and AKI are correlated with cytokine activation. Although abnormalities in coagulation are also associated with AKI, the link between inflammation and AKI severity is strongest. Sustained systemic inflammation seems to be associated with development of AKI as well as other organ failures. Studies are now being conducted using RIFLE criteria to define AKI in animal models and these animal models are also being designed to better reflect the clinical conditions in which AKI occurs in humans.
Emerging evidence from laboratory and clinical studies suggests that inflammation and its associated molecules could be a key factor in AKI and cause dysfunction of renal cells.40 Cells in injured tissues release danger-associated molecular pattern molecules that can perpetuate the inflammatory response by acting as a signal to remote organs (including the kidney), leading to the activation of immune cells (such as dendritic cells and T cells) and thus the initiation of inflammation in these remote organs. After the initial insult has resolved, the surviving tubular epithelial cells are able to regenerate and ultimately redifferentiate into mature intrinsic cells.41 Persistent injury and de novo CKD can occur with continued dysfunction of cell responses in the kidney and a number of soluble mediators (for example, transforming growth factor β) initiate a variety of pathophysiological processes that occur when kidney injury is initiated42 and have a fundamental role in cell proliferation and interstitial fibrosis. Still, many of the mechanisms that occur during AKI are unknown and a better understanding of the pathogenesis is important for early diagnosis and to enable the design of improved interventions.
Treatment
Levy and colleagues43 examined outcomes of 1,036 patients with severe sepsis who were enrolled in the control arms of two, large sepsis trials.43 Early improvement (within 24 h) in cardiovascular, renal, or respiratory function was related to survival, and outcomes for patients with severe sepsis in the ICU are likely to be strongly associated with the resolution of organ dysfunction (including AKI). Rapid resolution of AKI might simply be a good prognostic indicator and therapies to hasten recovery from AKI may also improve outcomes in such patients.
Early intervention is important for the outcomes of patients with AKI but the ideal intervention is under debate. In the study by Hoste and co-workers,2 only 14% of patients in the ICU designated as class F in RIFLE criteria received renal replacement therapy and these same patients experienced higher hospital mortality than those in the ICU without AKI; a finding that raised the question of whether renal support is under-utilized or delayed. Notably, survival in patients with AKI who received more-intensive therapy was 55.3% by day 90 in the Randomized Evaluation of Normal versus Augmented Level Replacement Therapy (RENAL) trial44 versus 46.4% by day 60 in the Acute Renal Failure Trial Network (ATN) study.45 Although other differences existed between the two study cohorts,46 an important difference may have been the timing of renal replacement therapy with patients in the RENAL trial treated, on average, 4.6 days earlier relative to ICU admission than those in the ATN study.
Besides renal replacement therapy, no other supportive measures are available for patients with AKI. The best therapy for patients with AKI seems to be the avoidance of further injury to the kidney through titrated resuscitation and discontinuation or avoidance of any unnecessary nephrotoxic drugs. Titrated resuscitation is important because fluid overload is a substantial risk for patients with AKI and aggressive fluid therapy, particularly for patients who have already been resuscitated, is more likely to cause harm.47 Lastly, biomarker-guided early diagnosis of AKI may facilitate exploration of novel anti-inflammatory and antioxidant therapies in specific AKI syndromes, such as sepsis-induced AKI, and open new avenues to facilitate renal recovery and prevent short and long-term complications.
Prognosis
A number of studies indicate that onset of AKI is associated with a higher resource utilization and risk of death than in patients without AKI.1,2,21 Moreover, this increased risk of death rises incrementally with the severity of AKI.29,48
Mortality
In 2005, Uchino et al.1 found that mortality for patients with severe AKI requiring renal replacement therapy was 60.3%. In a study by Thakar et al.29 the risk of death increased with the increasing severity of AKI: AKIN stage 1, odds ratio (OR) 2.2; stage 2, OR 6.1 and stage 3, OR 8.6. Data from 2010 indicates that even transient perturbations in kidney function in hospitalized patients increases the risk of death.9 These findings suggest that even small changes in kidney function carry a notable attributable mortality.49
Importantly, the mortality associated with severe AKI (that is, RIFLE class F or AKIN stage 3) that requires renal replacement therapy is one of the highest observed. For example, in trials from the past 5 years, 60-day mortality was 28% for patients with adult respiratory distress syndrome50 and 28-day mortality was ~32% for individuals with septic shock.51 Of patients with severe AKI, however, the 90-day mortality was 44.7% in one study published in 200944 and 52.5% in a trial in 2008.45 Moreover, of survivors with severe AKI, 24.6% were still receiving renal replacement therapy on day 60.45
Why short-term outcomes for severe AKI are so poor is incompletely understood.52 One issue is that defining AKI severity by those that receive renal replacement therapy can be misleading. For example, if renal replacement therapy is reserved for the most severely ill patients, mortality will be very high. If, on the other hand, it is applied to those patients who have a low risk of death, mortality will be low. Of course, neither strategy is desirable; rather we should identify which patients with AKI will benefit from renal replacement therapy. Unfortunately, this issue remains an open question exemplified by highly variable practice patterns.53 Even if we define severe AKI as RIFLE class F (with or without renal replacement therapy), hospital mortality rates for individuals with this disease are typically 20–40%, as high as or higher than comparable organ failures.54 Hoste et al.2 found that patients classified as RIFLE class F were five times more likely to die than those without AKI, despite renal replacement therapy.
Although the pathophysiology of AKI is not completely understood,52 sustained AKI can profoundly alter fluid, electrolyte, acid–base and hormonal regulation and lead to abnormalities in the central nervous, immune and coagulation systems. Many patients with AKI already have multisystem organ failure but the incremental influence of AKI on remote organ function and how it affects outcome is unclear.
Risk of chronic kidney disease
Emerging evidence suggests that a substantial percentage of patients who suffer from AKI do not return to normal renal function (Table 2). Morgera and colleagues55 followed 979 critically ill patients after recovery from AKI who required continuous renal replacement therapy. In this cohort, only 69% of patients survived to discharge (of whom 10% required chronic renal replacement therapy). Liaño and co-workers56 followed 187 patients with AKI for 15 years and found that 19% of patients had abnormal renal function, and that age, comorbidity, and circumstances of the renal injury influenced patients’ outcomes. Similarly, Chertow et al.57 demon strated in a cohort of critically ill patients with AKI who required renal replacement therapy that 33% of the survivors were still on renal replacement therapy after 12 months. In a large study of 1,124 patients with severe AKI, nearly 25% of survivors were dependent on renal replacement therapy at day 60.45 However, in another study of 1,508 patients with severe AKI, only 5.4% of survivors (irrespective of study group) were still on renal replacement therapy by day 90.44 These studies indicate that AKI is associated with long-term risk of impaired renal function but also, perhaps, that this risk is at least partially modifiable.
Table 2.
Long-term consequences of AKI
| Study | Period studied | No. of patients studied | Hospital mortality (%) | Renal outcome in survivors |
|---|---|---|---|---|
| Turney et al.75 (1990) | 1956–1988 | 1,347 | 21 | 48% with increased serum creatinine level |
| Chertow et al.57 (1995) | 1991–1993 | 132 | 70 | 33% on chronic RRT |
| Brivet et al.76 (1996) | 1991 | 360 | 58 | 28% have serum creatinine level >129 μmol/l |
| McCarthy et al.77 (1996) | 1977–1979; 1991–1992 | 142 | 48 | 21% on chronic RRT |
| Korkeila et al.78 (2000) | 1989–1990 | 3,447 | 45 | 8% on chronic RRT |
| Morgera et al.55 (2002) | 1993–1998 | 979 | 69 | 10% on chronic RRT |
| Liaño et al.79 (1996) | 1977–1992 | 748 | 55 | 19% have abnormal renal function, 2% on chronic RRT |
| Palevsky et al.45 (2008) | 2003–2007 | 1,124 | 49.6 | 24.6% were on RRT at day 60 |
| Bellomo et al.44 (2009) | 2005–2008 | 1,508 | 44 | 5.4% were on RRT at day 90 |
| Van Berendoncks et al.80 (2010) | 2001–2004 | 595 | 50.7 | 10.3% on RRT at 2 years |
Abbreviations: AKI, acute kidney injury; RRT, renal replacement therapy.
AKI is now increasingly recognized as having a bidirectional relationship with CKD in hospitalized patients. Although pre-existing CKD increases susceptibility towards development of AKI, exposure to AKI has been found to accelerate the development of CKD compared with those without AKI.58,59 Moreover, AKI superimposed on CKD leads to ESRD at a higher frequency than AKI alone.3,33,60
Biomarkers
Given that AKI is defined by functional changes in the kidney and linked to clinical outcomes, the use of injury biomarkers to predict AKI can be questioned. In the past few years, an entire industry has emerged around the discovery and validation of biomarkers for AKI.61–69 These biomarkers show promise as a more-precise method for diagnosis of AKI and could conceivably improve the sensitivity, specificity, and time in which AKI is diagnosed. Biomarkers could also help risk stratification and/or provide prognostic information including prediction of recovery of renal function. If analogous biomarkers from other diseases, such as troponin for acute myocardial infarction, are considered the potential value of biomarkers of kidney damage is obvious. However, unlike the situation in myocardial infarction in which there is a strong relationship between serum troponin concentration and left ventricular function, the relationship between structural change (damage) and functional impairment for AKI is less direct.70
Several novel imaging techniques, such as blood- oxygen-level-dependent MRI (which assesses renal oxygen bioavailability), and multiphoton microscopy (which facilitates direct visualization and integration of structure and function of the kidneys) have emerged and may one day improve our ability to link kidney structure and function.71–73 However, the utility of these techniques to assess kidney structure and function in humans with AKI, at present, is unclear.16,73 Nevertheless, it seems virtually assured that some pattern of one or more biomarkers, perhaps in combination with imaging techniques, will provide new information about the state of structural integrity of the kidney. How we validate the clinical utility of these markers is, however, a subject of some debate.
Table 3 provides a complete list of potential scenarios in which a biomarker may provide new and possibly valuable information about the diagnosis of AKI. Similar tables can be composed for risk stratification, severity assessment, and prognosis. For example, Table 4 shows the potential utility of a biomarker for predicting renal recovery after AKI. The difficulty with sorting out these various scenarios is the lack of an accepted ‘gold standard’ for the diagnosis and prognosis of AKI. The standard that is currently accepted is one that uses RIFLE criteria. However, as RIFLE criteria are based on changes in serum creatinine level or urine output, we must accept that there may be cases when injury is subclinical (that is, missed by RIFLE) and only later becomes clinically apparent. Furthermore, some individuals without kidney injury might fulfill AKI criteria but nevertheless are determined in hindsight as not having had AKI (that is, falsely identified as AKI by RIFLE criteria). For instance, a patient may satisfy RIFLE criteria very transiently when AKI is not suspected clinically (such as when transient serum creatinine elevations are caused by medications, such as trimethoprim, or by increased protein intake). Such circumstances are common in clinical medicine whereby provisional diagnoses are dismissed with time and further evaluation.
Table 3.
Interpretation of AKI biomarkers for diagnosis
| Functional impairment | Kidney damage | Adverse kidney outcomes* | Possible interpretations |
|---|---|---|---|
| No | No | No | No AKI |
| No | No | Yes | No AKI, subclinical AKI with false-negative marker for damage |
| Yes | No | No | Normal response to altered renal perfusion, self-limited AKI with resolution |
| Yes | No | Yes | AKI with false-negative damage marker, occult CKD |
| No | Yes | No | False-positive marker of damage, self-limited AKI with resolution |
| No | Yes | Yes | Early or subclinical AKI |
| Yes | Yes | No | Self-limited AKI with resolution |
| Yes | Yes | Yes | AKI |
Adverse kidney outcomes may include death or need for renal replacement therapy that is attributable to AKI. They may also include long term outcomes such as development of CKD or cardiovascular events. At present there is no standard methodology for defining or capturing such events. Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease.
Table 4.
Interpretation of AKI biomarkers for prognosis*
| Functional recovery | Repair biomarker | Adverse long- term outcomes‡ | Possible interpretations |
|---|---|---|---|
| Yes | Yes | No | Complete recovery from AKI with no measurable sequelae |
| Yes | No | No | Functional recovery from AKI; false-negative for marker of repair |
| Yes | No | Yes | Functional recovery from AKI but unresolved damage leading to decreased renal reserve and ultimately long-term adverse outcome |
| Yes | Yes | Yes | Complete or partial recovery but possibly with occult CKD |
| No | No | No | No recovery without adverse clinical outcomes |
| No | Yes | No | No recovery of function but marker of recovery predicts good outcome |
| No | Yes | Yes | No functional recovery with false-positive recovery marker |
| No | No | Yes | Recovery is absent or incomplete |
In this scenario, a hypothetical marker of renal repair is tested in the setting of known AKI.
In this case, adverse outcomes include progression of CKD or de novo CKD, decreased functional renal reserve or morbidity or mortality attributable to decreased renal function. Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease.
Importantly, a valuable diagnostic biomarker will be one that can predict AKI by RIFLE criteria some hours before clinical manifestations, provide a means to distinguish between types of AKI and provide information on prognosis (Table 4), and/or identify patients with subclinical AKI who ultimately will manifest clinical outcomes of interest. Biomarkers that fail to do any of these functions will not be useful even if they enable changes in histopathology to be detected.
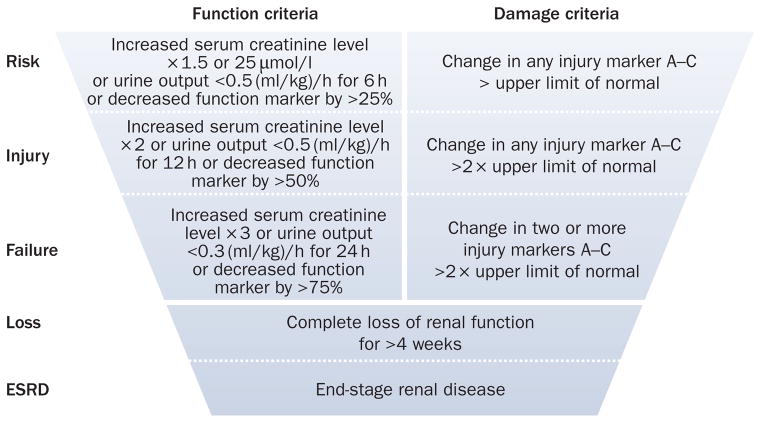
Finally, it must be remembered that serum creatinine level and urine output are also considered biomarkers and, although currently used to define AKI, future advances in AKI biomarker research will likely replace these markers with ones that are more sensitive, specific and timely. A possible classification system based on new injury markers is shown in Figure 5.
Figure 5.
Theoretical next generation in AKI diagnosis and classification. This future classification system would likely have two domains, one for measures of function and one for measures of damage. The functional domain, unlike the existing RIFLE criteria, might also require persistent change over a longer duration (for example, 48–72 h) to acknowledge that very transient functional impairment is unlikely to be as important as persistent changes. The damage domain would likely include biomarkers (listed as A–C) from different cell types (for example, tubular epithelial cells, mesangial cells, and vascular endothelium). Classification of ‘risk’ might only require small changes in one marker while ‘failure’ might require more than one changes in several markers or possibly large changes in a single marker. Importantly, analytical cutoffs for injury markers can be set to the corresponding functional impairment level so as to give improved construct validity to the system. Abbreviations: AKI, acute kidney injury; ESRD, end-stage renal disease; RIFLE, Risk, Injury, Failure, Loss, and End-stage renal disease.
Conclusions
AKI is common during critical illness, increasing in incidence, and is associated with increased resource utilization and mortality. The RIFLE classification provides a robust assessment of the risk of AKI and outcomes and has been widely validated. Current understanding of pathogenesis and pathophysiology of AKI is poor and considerable differences exist between AKI in animal models and in humans. Treatment of AKI is largely supportive and further research is urgently needed to improve AKI diagnosis and patient’s outcomes.
Review criteria.
PubMed was searched to identify articles for this Review using the terms “acute kidney injury”, “acute renal failure”, “epidemiology”, “prognosis”, and “biomarkers”, as Medical Subject Headings. The reference lists of identified papers were also searched for additional relevant papers and only full-text articles in English were included for review.
Key points.
Acute kidney injury (AKI) is common and is associated with higher resource utilization and mortality than that of other critical care syndromes
Risk, Injury, Failure, Loss, and End-stage renal disease classification is a validated definition of AKI and together with the AKI Network modification is widely accepted
Emerging biomarkers may further aid early diagnosis and risk stratification of AKI
Current understanding of the pathogenesis and pathophysiology of AKI is poor and treatment is largely supportive
AKI increases susceptibility to chronic kidney disease and end-stage renal disease
Acknowledgments
This publication was made possible in part by funding from grants R01DK070910 and R01DK083961 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the grant KL2RR024154 from the National Center for Research Resources (NCRR), and components of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this Review are solely the responsibility of the authors and do not necessarily represent the official view of NIDDK, NCRR or NIH.
Footnotes
Competing interests
R. Murugan and J. A. Kellum declare an association with the following company: Baxter. J. A. Kellum declares associations with the following companies: Alere, Abbott Laboratories, Astute Medical, Gambro. See the article online for full details of the relationships.
Author contributions
R. Murugan and J. A. Kellum contributed equally to the discussions, research, writing, editing, and reviewing of this manuscript.
References
- 1.Uchino S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 2.Hoste EA, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali T, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 4.Alejandro V, et al. Mechanisms of filtration failure during postischemic injury of the human kidney. A study of the reperfused renal allograft. J Clin Invest. 1995;95:820–831. doi: 10.1172/JCI117732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers BD, et al. Nature of the renal injury following total renal ischemia in man. J Clin Invest. 1984;73:329–341. doi: 10.1172/JCI111217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–1917. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 7.Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35:1837–1843. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- 8.Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12:R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchino S, Bellomo R, Bagshaw SM, Goldsmith D. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant. 2010;25:1833–1839. doi: 10.1093/ndt/gfp624. [DOI] [PubMed] [Google Scholar]
- 10.Rosen S, Heyman SN. Difficulties in understanding human “acute tubular necrosis”: limited data and flawed animal models. Kidney Int. 2001;60:1220–1224. doi: 10.1046/j.1523-1755.2001.00930.x. [DOI] [PubMed] [Google Scholar]
- 11.Brun C, Munck O. Lesions of the kidney in acute renal failure following shock. Lancet. 1957;272:603–607. doi: 10.1016/s0140-6736(57)91069-3. [DOI] [PubMed] [Google Scholar]
- 12.Smith HW. Lectures on the Kidney. University Extension Division, University of Kansas; Kansas City: 1943. pp. 3–23. [Google Scholar]
- 13.Natochin YV. Evolutionary aspects of renal function. Kidney Int. 1996;49:1539–1542. doi: 10.1038/ki.1996.220. [DOI] [PubMed] [Google Scholar]
- 14.Solez K, Morel-Maroger L, Sraer JD. The morphology of “acute tubular necrosis” in man: analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine (Baltimore) 1979;58:362–376. [PubMed] [Google Scholar]
- 15.Racusen LC, Fivush BA, Li YL, Slatnik I, Solez K. Dissociation of tubular cell detachment and tubular cell death in clinical and experimental “acute tubular necrosis”. Lab Invest. 1991;64:546–556. [PubMed] [Google Scholar]
- 16.Dear JW, et al. Dendrimer-enhanced MRI as a diagnostic and prognostic biomarker of sepsis-induced acute renal failure in aged mice. Kidney Int. 2005;67:2159–2167. doi: 10.1111/j.1523-1755.2005.00321.x. [DOI] [PubMed] [Google Scholar]
- 17.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20:672–679. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellum JA, Levin N, Bouman C, Lameire N. Developing a consensus classification system for acute renal failure. Curr Opin Crit Care. 2002;8:509–514. doi: 10.1097/00075198-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta RL, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murugan R, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77:527–535. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73:538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 23.Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1203–1210. doi: 10.1093/ndt/gfm744. [DOI] [PubMed] [Google Scholar]
- 24.Acute Kidney Injury—United States Renal Data System 2009 Annual Data Report. United States Renal Data System. 2009 [online], http://www.usrds.org/2009/pdf/V1_08_09.PDF.
- 25.Waikar SS, et al. Validity of international classification of diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol. 2006;17:1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 26.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 27.Fang Y, et al. Acute kidney injury in a Chinese hospitalized population. Blood Purif. 2010;30:120–126. doi: 10.1159/000319972. [DOI] [PubMed] [Google Scholar]
- 28.Lafrance JP, Miller DR. Defining acute kidney injury in database studies: the effects of varying the baseline kidney function assessment period and considering CKD status. Am J Kidney Dis. 2010;56:651–660. doi: 10.1053/j.ajkd.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009;37:2552–2558. doi: 10.1097/CCM.0b013e3181a5906f. [DOI] [PubMed] [Google Scholar]
- 30.Hackworth LA, Wen X, Clermont G, Murugan R, Kellum JA. Hospital versus community-acquired acute kidney injury in the critically Ill: differences in epidemiology. J Am Soc Nephrol. 2009;20:355A. [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC) Hospitalization discharge diagnoses for kidney disease–United States, 1980–2005. MMWR Morb Mortal Wkly. 2005;57:309–312. [PubMed] [Google Scholar]
- 32.Hsu CY, et al. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4:891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue JL, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 34.Bagshaw SM, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 35.de Mendonça A, et al. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000;26:915–921. doi: 10.1007/s001340051281. [DOI] [PubMed] [Google Scholar]
- 36.Wan L, et al. Pathophysiology of septic acute kidney injury: what do we really know? Crit Care Med. 2008;36(Suppl 4):S198–S203. doi: 10.1097/CCM.0b013e318168ccd5. [DOI] [PubMed] [Google Scholar]
- 37.Langenberg C, Wan L, Egi M, May CN, Bellomo R. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006;69:1996–2002. doi: 10.1038/sj.ki.5000440. [DOI] [PubMed] [Google Scholar]
- 38.Heyman SN, Rosenberger C, Rosen S. Experimental ischemia-reperfusion: biases and myths–the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int. 2010;77:9–16. doi: 10.1038/ki.2009.347. [DOI] [PubMed] [Google Scholar]
- 39.Rosen S, Heyman SN. Difficulties in understanding human “acute tubular necrosis”: limited data and flawed animal models. Kidney Int. 2001;60:1220–1224. doi: 10.1046/j.1523-1755.2001.00930.x. [DOI] [PubMed] [Google Scholar]
- 40.Wen X, Murugan R, Peng Z, Kellum JA. Pathophysiology of acute kidney injury: a new perspective. Contrib Nephrol. 2010;165:39–45. doi: 10.1159/000313743. [DOI] [PubMed] [Google Scholar]
- 41.Humphreys BD, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Zhang XL, Topley N, Ito T, Phillips A. Interleukin-6 regulation of transforming growth factor (TGF)-β receptor compartmentalization and turnover enhances TGF-β1 signaling. J Biol Chem. 2005;280:12239–12245. doi: 10.1074/jbc.M413284200. [DOI] [PubMed] [Google Scholar]
- 43.Levy MM, et al. Early changes in organ function predict eventual survival in severe sepsis. Crit Care Med. 2005;33:2194–2201. doi: 10.1097/01.ccm.0000182798.39709.84. [DOI] [PubMed] [Google Scholar]
- 44.Bellomo R, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 45.Palevsky PM, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kellum JA, Ronco C. Dialysis: results of RENAL—what is the optimal CRRT target dose? Nat Rev Nephrol. 2010;6:191–192. doi: 10.1038/nrneph.2010.15. [DOI] [PubMed] [Google Scholar]
- 47.Himmelfarb J, et al. Evaluation and initial management of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:962–967. doi: 10.2215/CJN.04971107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoste EA, Kellum JA. RIFLE criteria provide robust assessment of kidney dysfunction and correlate with hospital mortality. Crit Care Med. 2006;34:2016–2017. doi: 10.1097/01.CCM.0000219374.43963.B5. [DOI] [PubMed] [Google Scholar]
- 49.Kellum JA, Angus DC. Patients are dying of acute renal failure. Crit Care Med. 2002;30:2156–2157. doi: 10.1097/00003246-200209000-00041. [DOI] [PubMed] [Google Scholar]
- 50.Wiedemann HP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 51.Sprung CL, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 52.Elapavaluru S, Kellum JA. Why do patients die of acute kidney injury? Acta Clin Belg Suppl. 2007:326–331. doi: 10.1179/acb.2007.074. [DOI] [PubMed] [Google Scholar]
- 53.Uchino S, et al. Continuous renal replacement therapy: a worldwide practice survey. The beginning and ending supportive therapy for the kidney (B E S T kidney) investigators. Intensive Care Med. 2007;33:1563–1570. doi: 10.1007/s00134-007-0754-4. [DOI] [PubMed] [Google Scholar]
- 54.Vincent JL, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 55.Morgera S, Kraft AK, Siebert G, Luft FC, Neumayer HH. Long-term outcomes in acute renal failure patients treated with continuous renal replacement therapies. Am J Kidney Dis. 2002;40:275–279. doi: 10.1053/ajkd.2002.34505. [DOI] [PubMed] [Google Scholar]
- 56.Liaño F, et al. Long-term outcome of acute tubular necrosis: a contribution to its natural history. Kidney Int. 2007;71:679–686. doi: 10.1038/sj.ki.5002086. [DOI] [PubMed] [Google Scholar]
- 57.Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med. 1995;155:1505–1511. [PubMed] [Google Scholar]
- 58.Hoste EA, et al. Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol. 2003;14:1022–1030. doi: 10.1097/01.asn.0000059863.48590.e9. [DOI] [PubMed] [Google Scholar]
- 59.Ishani A, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in, U. S veterans: focus on acute tubular necrosis. Kidney Int. 2009;76:1089–1097. doi: 10.1038/ki.2009.332. [DOI] [PubMed] [Google Scholar]
- 61.Devarajan P. Review: neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–428. doi: 10.1111/j.1440-1797.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- 62.Soto K, et al. Cystatin C as a marker of acute kidney injury in the emergency department. Clin J Am Soc Nephrol. 2010;5:1745–1754. doi: 10.2215/CJN.00690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med. 2010;4:265–280. doi: 10.2217/bmm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu KD, et al. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care. 2009;13:R104. doi: 10.1186/cc7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bennett MR, et al. Using proteomics to identify preprocedural risk factors for contrast induced nephropathy. Proteomics Clin Appl. 2008;2:1058–1064. doi: 10.1002/prca.200780141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Portilla D, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73:465–472. doi: 10.1038/sj.ki.5002721. [DOI] [PubMed] [Google Scholar]
- 67.Parikh CR, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 68.Ramesh G, Krawczeski CD, Woo JG, Wang Y, Devarajan P. Urinary netrin-1 is an early predictive biomarker of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2010;5:395–401. doi: 10.2215/CJN.05140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24:3265–3268. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 70.Solomon R, Segal A. Defining acute kidney injury: what is the most appropriate metric? Nat Clin Pract Nephrol. 2008;4:208–215. doi: 10.1038/ncpneph0746. [DOI] [PubMed] [Google Scholar]
- 71.Xiao WB, Wang QD, Xu JJ, Han F, Zhang MM. Evaluation of kidney oxygen bioavailability in acute renal failure by blood oxygen level dependent magnetic resonance imaging [Chinese] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2010;39:157–162. doi: 10.3785/j.issn.1008-9292.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 72.Han F, et al. The significance of BOLD MRI in differentiation between renal transplant rejection and acute tubular necrosis. Nephrol Dial Transplant. 2008;23:2666–2672. doi: 10.1093/ndt/gfn064. [DOI] [PubMed] [Google Scholar]
- 73.Molitoris BA, Sandoval RM. Multiphoton imaging techniques in acute kidney injury. Contrib Nephrol. 2010;165:46–53. doi: 10.1159/000313744. [DOI] [PubMed] [Google Scholar]
- 74.Vincent JL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 75.Turney JH, Marshall DH, Brownjohn AM, Ellis CM, Parsons FM. The evolution of acute renal failure, 1956–1988. Q J Med. 1990;74:83–104. [PubMed] [Google Scholar]
- 76.Brivet FG, Kleinknecht DJ, Loirat P, Landais PJ. Acute renal failure in intensive care units--causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. French study group on acute renal failure. Crit Care Med. 1996;24:192–198. doi: 10.1097/00003246-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 77.McCarthy JT. Prognosis of patients with acute renal failure in the intensive-care unit: a tale of two eras. Mayo Clin Proc. 1996;71:117–126. doi: 10.4065/71.2.117. [DOI] [PubMed] [Google Scholar]
- 78.Korkeila M, Ruokonen E, Takala J. Costs of care, long-term prognosis and quality of life in patients requiring renal replacement therapy during intensive care. Intensive Care Med. 2000;26:1824–1831. doi: 10.1007/s001340000726. [DOI] [PubMed] [Google Scholar]
- 79.Liaño F, Pascual J The Madrid acute renal failure study group. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Kidney Int. 1996;50:811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 80.Van Berendoncks AM, Elseviers MM, Lins RL. Outcome of acute kidney injury with different treatment options: long-term follow-up. Clin J Am Soc Nephrol. 2010;5:1755–1762. doi: 10.2215/CJN.00770110. [DOI] [PMC free article] [PubMed] [Google Scholar]