Abstract
Introduction
The B-cell lymphoma-2 (Bcl-2) family of proteins is central to the regulation of apoptosis, which is vital for proper tissue development and cellular homeostasis. Anti-apoptotic proteins, members of the Bcl-2 family, are an important survival factor for many cancers and their overexpression has been associated with tumor initiation, progression and resistance to current anticancer therapies. Therefore, strategies seeking to antagonize the function of Bcl-2 anti-apoptotic proteins have been extensively studied for developing novel cancer therapy.
Areas covered
This review covers research and patent literature of the last 15 years dealing with the discovery and development of inhibitors of the Bcl-2 protein family.
Expert opinion
The feasibility of disrupting protein-protein interactions between anti-apoptotic and pro-apoptotic proteins, members of the Bcl-2 family, using peptidomimetics and small-molecule inhibitors has been successfully established. Three small-molecule inhibitors have entered human clinical trials, which will allow the evaluation of this potential therapeutic approach in cancer patients. It will be important to gain a better understanding of pan and selective Bcl-2 inhibitors in order to facilitate future drug design efforts.
Keywords: apoptosis, protein-protein interactions, inhibitors of anti-apoptotic Bcl-2 proteins, BH3 mimetics, cancer
1. Introduction
Protein-protein interactions (PPIs) control many biological processes, such as cell proliferation, growth, differentiation, signal transduction, and programmed cell death (apoptosis) [1]. In the genomic era, the studies of protein networks have provided many insights about how proteins interact with each other leading to elucidation of the molecular basis of a number of different diseases, including cancer [2]. Thus, PPIs represent an important class of molecular targets for novel human therapeutics [3]. Developing small-molecule inhibitors (SMIs) to disrupt PPIs is a challenging task mainly because of typical flatness, largeness, non-contiguity of the interface between the proteins that interact and flexibility of the protein surfaces. Despite the difficulties, successfully discovered small molecules that inhibit several PPIs have been reported [3, 4].
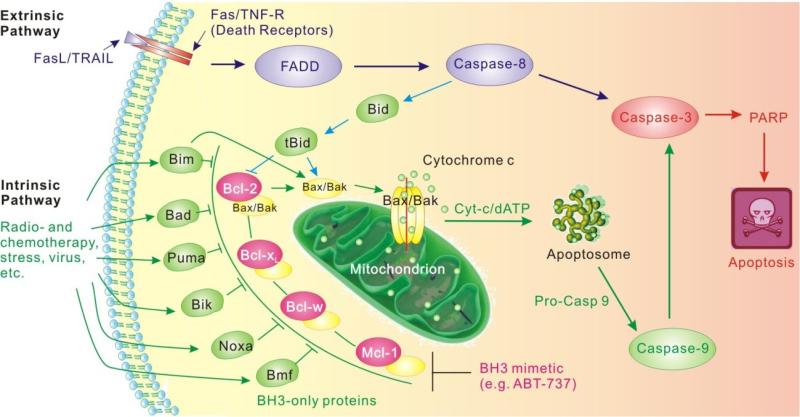
The B-cell lymphoma-2 (Bcl-2) family of proteins is central to the regulation of apoptosis, which is vital for proper tissue development and cellular homeostasis. Altered responses to normal apoptotic signals are one of the hallmarks of cancer and they are connected to defects in the apoptotic machinery in cancer cells. Apoptosis occurs via activation of two different pathways (Figure 1), the extrinsic pathway, triggered by the activation of the cell surface death receptors, and the intrinsic pathway, followed by the perturbation of mitochondrial membrane integrity [5]. Structural and functional studies have shown that the intrinsic pathway is tightly controlled by the PPIs between the pro- and anti-apoptotic Bcl-2 family proteins which control the integrity of the outer mitochondrial membrane [6]. Therefore, strategies seeking to antagonize the function of Bcl-2 anti-apoptotic proteins have been extensively studied for developing novel cancer therapy [7, 8].
Figure 1.
Extrinsic and intrinsic pathways of apoptosis. The onset of apoptosis is controlled by numerous interrelating processes. The extrinsic pathway is activated by external signals such as Fas ligand (FasL) or TRAIL, which act through death receptors. Subsequently Caspase-8 is activated through Fas-associated death domain (FADD). The intrinsic pathway is initiated by different signals, primarily extracellular stimuli. Mitochondria are an essential component of the intrinsic pathway and harbor an array of apoptotic factors. The Bcl-2 family is a primary regulator of the intrinsic pathway.
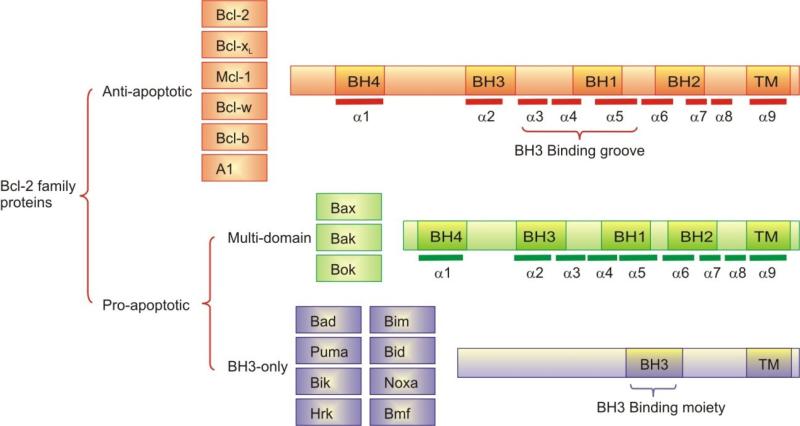
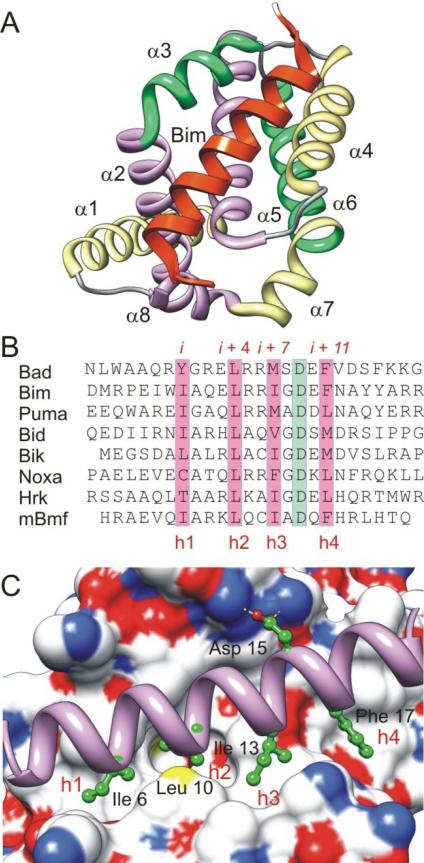
Twenty five known members of the Bcl-2 protein family can be grouped functionally according to their pro- and anti-apoptotic effects, as well as structurally according to the Bcl-2 homology (BH) regions they contain (Figure 2) [9]. The six known anti-apoptotic family members, Bcl-2, Bcl-xL, Mcl-1, Bcl-w, Bcl-b and A1, contain four BH domains (BH1-4) and a transmembrane domain (TM). Each of these proteins has 8 to 9 α-helices and a 20 Å hydrophobic cleft, the “BH3 binding groove” (Figure 3A). Pro-apoptotic proteins are subdivided into two classes: multi-domain members, such as Bax, Bak and Bok, which contain and share homology in the BH1, BH2, BH3 and BH4 domains and BH3-only proteins, including Bad, Bim, Puma, Bid, Bik, Noxa, Hrk and Bmf, which show homology only in the BH3 domain. BH3 domains possess four conserved hydrophobic residues 3 to 4 residues apart (Figure 3B), which project into four hydrophobic pockets within the ligand binding groove of the anti-apoptotic proteins. Additionally, a conserved aspartic acid forms a salt bridge to a conserved arginine on the Bcl-2 family proteins (Figure 3C). Mutation of any of these residues significantly reduces the affinity of BH3 proteins to their targets, compromising their pro-apoptotic activity [10].
Figure 2.
Members of the Bcl-2 family proteins; anti-apoptotic and pro-apoptotic. Conserved Bcl-2 homology domains (BH1-4) are denoted as is the carboxy-terminal hydrophobic (TM) domain.
Figure 3.
(A) An eight-helix bundle creates a hydrophobic groove into which a BH3 peptide binds. The binding groove is formed largely by the helices α3 – 5. The example shown here is Bcl-xL bound to Bim (PDB code: 3FDL). (B) The alignment of several BH3-only proteins. The conserved four hydrophobic residues and the Asp residue are highlighted by pink and light green respectively. (C) Structure of Mcl-1 (surface representation) bound to Bim (purple helix), which binds in a long, hydrophobic groove that has four non-contiguous hydrophobic pockets labeled h1 – h4 in the surface of Mcl-1 (PDB code: 2PQK). The five conserved residues are shown.
Different models, including the “direct activation model” [11] and the “derepression model” [12], have been proposed about the mechanism of apoptosis regulation by the PPIs between the Bcl-2 family members. The common feature of these models is that the PPIs between the different classes of the Bcl-2 family occur through the BH3 “ligand” domain of pro-apoptotic proteins which bind to a “receptor” BH3 binding groove formed by BH1-3 regions on the anti-apoptotic proteins. This rational was successfully used for development of new anti-cancer therapies, in which peptidomimetics or SMIs bind in the BH3 binding groove of Bcl-2 proteins and behave as BH3-mimetics. Such compounds hold promise for the development of new anticancer therapies.
2. Strategies for targeting the anti-apoptotic Bcl-2 protein family
2.1 Targeting the protein expression level
One of the strategies to target the anti-apoptotic proteins is to inhibit their expression level with antisense oligonucleotides. Oblimersen sodium (G3139, Genasense), a Bcl-2-specific antisense phosphorothioate oligodeoxynucleotide designed to be complementary to the first six codons of the human Bcl-2 mRNA sequence, has been evaluated as a single agent or in combination therapy in several clinical studies [13]. Its clinical effect has been modest and it is not clear how effectively such an antisense oligonucleotide can down-regulate the Bcl-2 levels in cells in vivo.
2.2 Targeting the BH3 binding groove
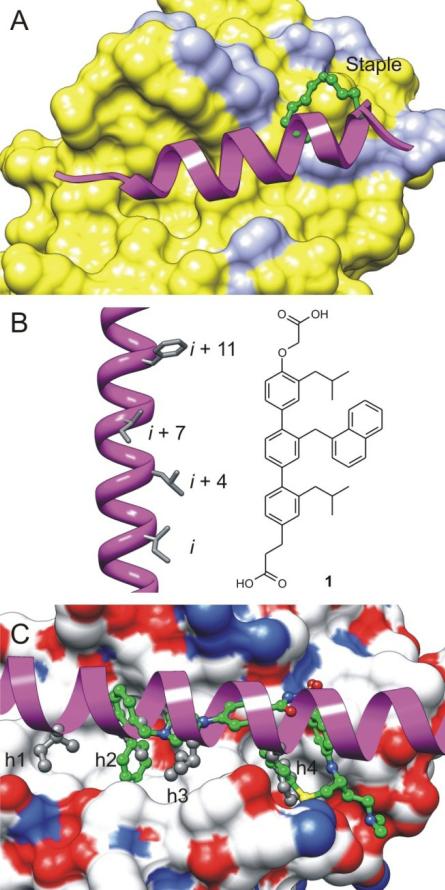
The binding profiles of different BH3 peptides and their x-ray crystal structures complexed with anti-apoptotic Bcl-2 proteins provide valuable information for understanding the PPIs of Bcl-2 protein family. Therefore, the second strategy that is extensively studied is targeting the BH3 binding groove with BH3 mimetics thus antagonizing the function of Bcl-2. Peptide-based inhibitors have shown to attenuate Bcl-2 activity [14, 15], but the use of peptides as a therapeutic strategy is hindered by their lack of stability and effective delivery. To overcome this issue, a chemical strategy has been pursued to maintain the α helical structure by introducing a chemical staple (Figure 4A) [14, 16, 17]. Other approaches include the design of peptidomimetics based on α-helix mimicry to the BH3-only proteins such as compound 1, a terphenyl-based Bak BH3 α-helical peptidomimetic (Figure 4B) [18-20].
Figure 4.
Peptidomimetics and small-molecule inhibitors (SMIs) targeting the canonical BH3-binding groove of anti-apoptotic proteins and antagonizing their function. (A) Crystal structure of the stapled peptide SAHBD–in complex with Mcl-1 protein (PDB code: 3MK8). (B) Terphenyl-based Bak BH3 α-helical peptidomimetic as antagonist of Bcl-xL (C) Superimposition of the x-ray structures of SMI ABT-737 (green, PDB code: 2YXJ) and Bim BH3 peptide (magenta, PDB code: 1PQ1) with Bcl-xL protein.
SMIs, however, have considerable advantages over peptides and in the last decade, such compounds have emerged as effective and specific inhibitors of Bcl-2 protein family. Discovery of SMIs of Bcl-2 proteins involved high throughput screening [21], fragment-based NMR screening [22-24], virtual screening [25, 26], structure-based design [27-29], and analysis of the mechanism of action of a known compound [30]. For example, ABT-737 (Figure 4C) was developed using a fragment-based NMR method in which two identified fragments bound to the h2 and h4 pockets and mimicking residues Leu 94 and Phe 101 of Bim were linked [22]. Further modification led to the development of ABT-737 and other analogues with sub-nanomolar affinities against Bcl-2, Bcl-xL and Bcl-w [24].
3. Evaluation of patents
Patent literature in this review is divided into two areas: (1) peptides and (2) SMIs which can be further classified into two categories i) pan SMIs and ii) selective SMIs of Bcl-2 proteins. In this review the International Patent Publications have been evaluated and representative examples, together with information of issued patents, are presented in order to illustrate the diversity of the disclosed structures. This review specifically focuses on patents and patent applications for compounds in clinical trials: the pan inhibitors, (-)-gossypol and obatoclax, and the selective inhibitor ABT-263.
3.1 Peptides as Bcl-2 inhibitors
Several groups have published patent applications covering BH3 peptides as Bcl-2 protein inhibitors [31, 32]. Conformationally constrained peptides that mimic BH3-only proteins were disclosed in a 2004 patent application [33], which claimed a series of constrained peptides with amino acid sequence
where Haa1-4 = residues with hydrophobic side chains; Saa = a residue with a small side chain; Naa = a residue with a negatively charged side chain; Xaa1-4 = independent residues; and L = a linker tether between two non-adjacent amino acids in an i(i+7) relationship and R and R’ = N-terminal and C-terminal capping groups. The most potent of these peptides exhibited improved affinities for Bcl-2 and Bcl-w, 290 nM and 65 nM respectively, over the unconstrained 12-mer peptide (240,000 nM and 870 nM against Bcl-2 and Bcl-w) which correlates with increased helicity of the conformationally constrained peptides. In a subsequent patent application, conjugation of the constrained peptides to a cell targeting compound allowing direct delivery to unwanted or damaged cells was claimed [34], but no evidence was provided for the anti-tumor efficacy of the conjugates in vivo.
Stapled peptides, or “stabilized α-helix of Bcl-2 domains” (SAHBs), a promising class of peptide drugs with improved pharmacokinetic properties, were disclosed together with the methods for the preparation of BH3 SAHBs [35]. SAHBs are generated using a ring-closing metathesis reaction to construct an all hydrocarbon macrocyclic cross-link, thereby stabilizing peptide α-helices and significantly increasing the helicity and potency of α-helical peptides by transforming unfolded Bid, Bad and Bim BH3 peptides into protease-resistant and cell-permeable α-helices that bind with increased affinities [16].
An SAHB obtained from the BH3 domain of Bid specifically activates the apoptotic pathway to kill leukemia cells and inhibits the growth of human leukemia xenograft in vivo [14]. Stewart et al. also described the development and synthesis of SAHBs to identify potent and selective Mcl-1 inhibitors [16]. In vitro fluorescence polarization (FP) assays revealed that stapling the α-helix from Mcl-1 itself led to a selective inhibitor for Mcl-1 (Kd = 43 nM). By scanning a series of mutated peptides, the authors discovered the Mcl-1 SAHBD, a staple peptide with ~90% α-helical content and the strongest binding activity (Kd = 10 nM), retaining the Mcl-1 selectivity. Using X-ray crystallography and mutagenesis studies, the authors defined key binding and specificity determinants, and demonstrated that the staple has additional favorable hydrophobic contacts with the Mcl-1 protein.
3.2 Small-molecule pan Bcl-2 inhibitors
3.2.1 Gossypol and its analogues
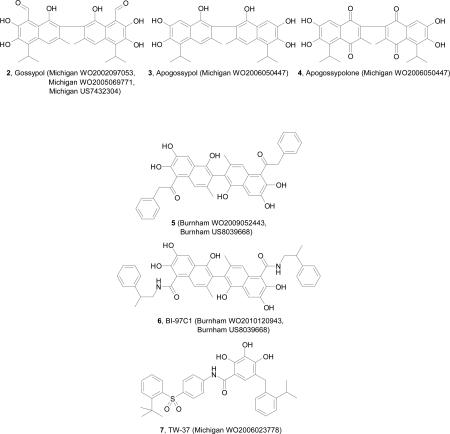
Gossypol (2) is a natural polyphenol, isolated from the cotton plant, Gossypium sp., and has been well studied in clinical trials as a contraceptive for human males, demonstrating the safety of long-term administration [36]. In 2002, the University of Michigan published a patent application relating to gossypol and its derivatives as SMIs of Bcl-2 family proteins granted in United States of America, Australia, New Zealand and by the European Patent Office [37]. Evidence was provided that gossypol and its derivatives bind to and inhibit the anti-apoptotic functions of Bcl-2 and Bcl-xL proteins especially in cancer cells that overexpress Bcl-2 family proteins, such as breast, leukemia and colon cancer cell lines. The therapeutic potential of gossypol was further evaluated in a human breast cancer MDA-MB-231 (subclone 2LMP) xenograft model in nude mice in which it was shown that it significantly inhibits tumor growth in breast cell lines and when used in combination with docetaxel it considerably improves inhibition of tumor growth. A following patent application claimed further validation of gossypol, and its enantiomers ((-)-gossypol and (+)-gossypol), as well as gossypolone, as inhibitors of the Bcl-2 proteins [38]. It was shown that gossypol induces intrinsic apoptotic pathway through release of cytochrome c and caspase activity in breast cancer cell lines, MDA-231 and T47D, HT-29 colon cancer, DU-145 prostate cancer cells and panel of squamous head-neck cancer cell lines. In this invention it was also demonstrated that (-)-gossypol is very effective in potentiating radiation in combination treatment regimens to induce apoptosis and to inhibit angiogenesis even with doses at which it was not very effective as a single agent, using a mouse PC-3 xenograft model. An additional patent related to compositions comprising co-crystals of (-)-gossypol with a C1-8 carboxylic or sulfonic acid and their use as inhibitors of anti-apoptotic Bcl-2 family proteins was disclosed by the University of Michigan [39].
It has been shown that gossypol congeners exhibit inhibitory activity and induce mitochondrial-mediated apoptosis in a wide range of human carcinoma cell lines [40-43] and that (-)-gossypol has significant in vivo antitumor activity either as a single agent or in combination with chemotherapy and radiotherapy [41, 44-49]. The anti-tumor activity of gossypol was shown to be due, at least in part, to inhibition of anti-apoptotic proteins Bcl-2, Bcl-xL and the subsequent induction of apoptosis in cancer cells. However, other mechanisms of action have also been proposed. It has been shown that in the presence of metal ions, gossypol can induce oxidative DNA breakage in vitro [50]. In a recent report it has been shown that gossypol induces apoptosis in chronic lymphocytic leukemia (CLL) through the generation of reactive oxygen species which in turn mediate the release of cytochrome c causing apoptosis [51]. Furthermore, it was shown that (-)-gossypol significantly suppresses the growth of human prostate PC-3 xenografts, which was largely dependent on the suppression of angiogenesis in the solid tumors [52]. Furthermore, (-)-gossypol can also interrupt the interactions between Beclin1 and Bcl-2/Bcl-xL at the endoplasmic reticulum, thus releasing the BH3-only pro-autophagic protein Beclin1 and activating the autophagic pathway [53]. These studies validate the clinical potential of (-)-gossypol and provide new insights into the mode of cell death.
Ascenta Therapeutics Inc. published two patent applications [54, 55] disclosing the pulsed dose administration of gossypol and its enantiomers, which provides clinical efficacy coupled with a reduction in adverse events. The (-) enantiomer is associated with higher activity in most bioassays and these two patents provide a method for preparation of (-)-gossypol enantiomer and its acetic acid co-crystal with high purity for clinical usage. The orally available (-)-gossypol enantiomer AT-101 has been tested for its safety and efficacy in several clinical trials [56, 57].
A phase I/II study was conducted combining AT-101 with topotecan in patients with relapsed and refractory small cell lung cancer (SCLC). The observed response rates did not meet the criteria for additional enrollment, but patients with stable disease showed the best response and the median time to progression was favorable [56]. In a multi-institution phase I/II trial, evaluation of AT-101 as a single agent in men with prostate cancer showed some evidence of decline of prostate-specific antigen and a clinical trial combining AT-101 with androgen deprivation is in progress [57]. The maximum tolerated dosage of AT-101 is 40 mg/day and it is currently being assessed in phase II clinical trials in combination with lenalidomide for CLL, and in combination with docetaxel is being tested in patients with recurrent, locally advanced or metastatic squamous cell carcinoma of the head and neck. AT-101 is also undergoing phase II clinical trials as a single agent in patients with recurrent, metastatic, or primary unresectable adrenocortical carcinoma.
A 2006 patent application from University of Michigan [58] claims four new gossypol analogs, gossypolic acid, gossypolonic acid, apogossypol (3) and apogossypolone (4), and in vitro activity using panel of breast cancer cell lines and in vivo efficacy of apogossypolone in a prostate PC-3 xenograft model. Although, gossypolic acid and gossypolonic acid were found to be more potent than (-)-gossypol with Ki values of 120 and 280 nM respectively against Bcl-2, in the cell growth inhibition assays using prostate cancer PC-3 cells IC50 values were >10 μM for both of the compounds. One possible explanation for this is that the two acid groups are negatively charged at physiological condition and are thus prevented from entering cells. Indeed, apogossypol and apogossypolone, analogs lacking the carboxylic group, are 2-9 fold more potent than (-)-gossypol in cell growth inhibition assay using breast cancer cell lines. The binding affinity of apogossypolone was determined to be Ki = 76 nM, 51 nM and 1,270 nM against Bcl-2, Mcl-1 and Bcl-xL respectively. In addition, as was predicted that removal of the aldehydes will significantly reduce the toxicity, apogossypolone showed 8 fold higher maximum tolerated dose than (-)-gossypol in oral and intravenous routes of administrations.
Currently apogossypolone is in the preclinical phase of testing. Studies have shown that apogossypolone induces apoptosis and effectively inhibits growth of follicular small cleaved cell lymphoma, diffuse large-cell lymphoma (DLCL2) cells, nasopharyngeal carcinoma, and hepatocellular carcinoma, in vitro and in vivo as a single agent or in combination with chemotherapy [59-61]. It blocks the heterodimerization of Mcl-1/Bax and Bcl-2/Bim in BxPC-3 cells and in combination with gemcitabine leads to a statistically higher antitumor activity compared to either apogossypolone or gemcitabine alone [62].
Preclinical in vivo data show that apogossypol has better efficacy, reduced toxicity and pharmacokinetic characteristics than gossypol [63, 64]. Two patent applications from Burnham Institute for Medical Research [65, 66] claim a series of designed derivatives of apogossypol and their use for treating cancer, autoimmune diseases and/or inflammation. These applications report synthesis and evaluation of 5,5’-alkyl, ketone and amide substituted apogossypol derivatives. Compounds 5 and 6 are claimed as the best compounds, displaying improved in vitro and in vivo efficacy compared to apogossypol [67, 68]. The most potent diastereo-isomer of compound 6, BI-97C1, also called sabutoclax, inhibits binding of BH3 peptides to Bcl-xL, Bcl-2, Mcl-1, and A1 with IC50 values of 0.31, 0.32, 0.20 and 0.62 μM, respectively. This compound potently inhibits cell growth of human prostate cancer, lung cancer, and lymphoma cell lines with little cytotoxicity against Bax-/-Bak-/- cells [69]. Preclinical studies have shown that BI-97C1 shows in vivo efficacy in transgenic mice models and in a prostate cancer mouse xenograft model [69]. BI-97C1 was tested in combination with adenovirus (Ad)-based gene therapy, melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24), demonstrating significant objective responses in a Phase I clinical trial for advanced solid tumors. A combination treatment of mda-7/IL-24 and BI-97C1 significantly inhibits the growth of human prostate cancer (PC) xenografts in nude mice and a transgenic mouse model of PC [70]. This combination was also tested in colorectal cancer and a combination regimen of suboptimal doses of Ad.5/3-mda-7 and BI-97C1 profoundly enhanced cytotoxicity in RKO cells both in vitro and in vivo [71]. It is expected that BI-97C1 will enter the clinical trials soon.
The University of Michigan published a patent application claiming a series of compounds that mimic the interactions between (-)-gossypol and Bcl-2 exemplified by compound 7, known as TW-37 [72] which binds to Bcl-2, Bcl-xL and Mcl-1 with Ki values of 290, 1110 and 260 nM respectively, representing a pan-inhibitor of Bcl-2 proteins. TW-37 effectively and dose-dependently inhibits cell growth and induces apoptosis in PC-3 prostate cancer cells. Inhibition of tumor growth in xenograft model of prostate cancer was observed with TW-37 alone or in combination therapy with taxotere. TW-37 has both pro-apoptotic [73] and anti-angiogenic effects [74] and has been tested by many different groups who have demonstrated in vitro and in vivo growth inhibition of Kaposi's sarcoma (SLK), breast cancer (MCF-7), prostate cancer (LNCap), diffuse large cell lymphoma (DLCL), pancreatic cancer cell lines (BxPC-3, Colo-357, L3.6pl, and HPAC), and head and neck squamous cell carcinoma (HNSCC) [73-77]. Jointly administered with the mitogen-activated protein kinase inhibitors U0126 or CL-1040, it was found to be in vitro and in vivo effective against melanoma-derived tumors [76]. TW-37 significantly enhanced the killing of lymphoma cells when used in combination therapy with cyclophosphamide-doxorubicin-vincristine-prednisone (CHOP) regimen in WSU-DLCL2-SCID mouse xenograft model in comparison with either CHOP or TW-37 treatment alone [78]. This compound is still in the preclinical testing.
3.2.2 Obatoclax
In two international patent applications, Gemin X Biotechnologies described a series of substituted triheterocyclic compounds represented by obatoclax (GX 15-070, compound 8) and their use for treatment or prevention of neoplastic disease and viral infections, granted in New Zealand and United States of America [79, 80]. Obatoclax is a synthetic compound based on cycloprodigiosin, a tripyrrole pigment from Serratia marcescens, with poor solubility in water. In order to improve its solubility, a mesylate, a tartrate salt and two phosphate pro-drugs were also disclosed. Obatoclax showed potent inhibition of all tested cell lines, but less effect in HMEC normal mammary epithelial cells, demonstrating selectivity as an anti-cancer agent. Obatoclax mesylate salt and phosphate pro-drug statistically significantly reduce the tumor growth in xenograft models of prostate adenocarcinoma cancer (PC3 cells) and human cervical cancer (C33A cells), compared to animals treated with vehicle only. A subsequent patent application disclosed 44 new analogues of obatoclax exemplified by compound 9 [81]. Inhibition of cell growth of C33A cervical carcinoma cells and H1299 human non-small cell lung cancer cells was reported. Furthermore, compound 9 was tested in a prostate xenograft model and showed significant dose dependent reduction of the tumor growth in vivo.
Obatoclax is a pan Bcl-2 inhibitor [82] with IC50 from 1 to 7 μM to six members of Bcl-2 family in a FP-based assay [83]. It shows in vitro promising preclinical efficacy against non-small cell lung carcinoma (NSCLC), mantle cell lymphoma, and multiple myeloma cells both as a single agent and in combination with clinically relevant cytotoxics [84-86], through blocking the binding of Bak to Mcl-1 and inducing intrinsic apoptosis [86]. Obatoclax has also demonstrated enhanced apoptosis in combination with Apo2L/TRAIL in cholangiocarcinoma cells [87] and pancreatic cancer cells [88] and with tyrosine kinase inhibitors in breast cancer [89] and NSCLC [85]. Although many studies demonstrated that the mechanism of action of obatoclax is through intrinsic apoptotic pathway, some data strongly suggest the existence of mechanisms of obatoclax-induced cell death alternative to the established BH3 sensitizer or effector models that modulate Bcl-2 family interactions to drive apoptosis [90, 91]. It is believed that these Bcl-2–independent targets of this agent may have clinical applicability, which has to be studied further.
Currently obatoclax is in multiple phase I/II clinical trials for solid and hematological malignancies. In phase I trials, obatoclax was well tolerated and it has displayed single agent antitumor activity in patients with advanced hematological malignancies [92-94]. The combination with topotecan in patients with solid tumors was well tolerated [95]. Obatoclax is also undergoing evaluation in phase I trial in combination with vincristine, doxorubicin, and dexrazoxane to study the side effects and best dose of obatoclax mesylate in treatment of young patients with relapsed or refractory solid tumors, lymphoma, or leukemia. Another phase I/II trial is studying the side effects and the best dose of obatoclax mesylate when given together with rituximab and bendamustine in patients with relapsed or refractory non-Hodgkin lymphoma.
3.3 Small-molecule selective Bcl-2 family inhibitors
3.3.1 ABT-737, ABT-263 and their analogues
Abbott Laboratories are very active in this field and from 2002 have published a series of patents and patent applications describing potent selective Bcl-2 family inhibitors bearing N-acylsulfonamide and N-sulfonyl carboximidamide as core scaffolds. ABT-737 (10) and its orally active analog, ABT-263 (11), are the best-characterized small-molecule Bad-like BH3 mimetics and with related compounds were disclosed in several patents [96]. Using NMR fragment based approach, a 10,000 fragment library was screened and linking two identified fragments yielded the fluoro biaryl compound 12 with high binding affinity to Bcl-xL (Ki = 36 ± 1.6 nM). 12 was further modified by incorporating a basic 2-dimethylaminoethyl group at 1-amino position of the thioethyl amino linkage group and fluorophenyl group was replaced with a substituted piperazine thus yielding ABT-737. It selectively binds Bcl-2, Bcl-xL, and Bcl-w with very high affinity (Ki ≤ 1 nM) [22] and has significantly lower affinity for Mcl-1, Bcl-b, and A1, showing the binding affinity pattern of Bad.
Several international patent applications (granted by European Patent Office and New Zealand) and two issued US patents to Abbott described a series of analogs based on N-acylsulfonamide genus 13 [97, 98], in which A1 = N or C bearing H, F, CN, acid, amide or ester group, D1 = H, F, Cl or CN and E1 = H, F, or Cl. The Z1 group is defined as phenyl or pyridinyl, substituted with cyclohexyl, heteroalkyl or heteroaromatic moieties and B1 and Y1 = imidazole or triazole. These patents contained more than 950 examples with N-acylsulfonamide core structure, with Ki values to both Bcl-2 and BclxL proteins ranging from < 1 nM to 13.5 μM.
An international patent application from Abbott Laboratories in 2006 and issued in USA 2010, disclosed 18 novel chemical entities as inhibitors of Bcl-2 family proteins represented by the core 14 bearing the same N-acylsulfonamide core [99]. A representative structure from this work is compound 15. As in earlier applications, Z1 group = phenyl or pyridinyl, substituted with cyclohexyl, heteroalkyl or heteroaromatic moieties. A1 = CN, NO2, CF3, OCF3, halo, acid, amide, ester etc. B1 and X1 = substituted or unsubstituted alkylene and D1 = H, alkyl or phenyl. These analogues exhibited binding affinities to Bcl-xL with Ki values in the range of < 1 nM to 227 nM and to Bcl-2 in the range of <1 nM to 893 nM.
In a subsequent application, granted in USA 2009, Abbott Laboratories reported novel chemical entities as apoptosis promoters bearing a second core scaffold, N-sulfonyl carboximidamide, illustrated by 16 and exemplified by compound 17, were disclosed in patent application from 2005 and issued patent to Abbott Laboratories [100]. In genus 16, A1 = heteroalkyl/heteroaromatic moiety; R1 = alkyl, cyano, halo, haloalkyl or nitro group; R2, R3, R4 and R5 groups = H, alkyl, alkoxy, amino, heterocyclyl, halo etc. No biological activity data were reported for these analogues.
ABT-737 has been extensively evaluated as a trigger of apoptosis in cancer cells. It was effective in delaying growth of tumors overexpressing Bcl-2 and its response is selectively stronger in tumor cells than in cells from normal tissue [101, 102]. ABT-737 as a single agent activity induces apoptosis in leukemia, lymphoma, multiple myeloma, glioma and small-cell lung cancer cell lines, but is not very effective in killing ovarian or pancreatic carcinoma cells [103, 104]. Primary cells from patients with acute lymphoblastic leukemia (ALL) [101], AML [105], CLL [106], follicular lymphoma and marginal zone lymphoma [107], are sensitive to ABT-737 treatment. In vivo, using mouse xenografts derived from patients with ALL at diagnosis (ALL-7) or at relapse (ALL-19), ABT-737 potentiated the effect of a three-drug regimen, vincristine, dexamethasone, and L-asparaginase (VXL) [108], and in combination with L-asparaginase, topotecan, vincristine, and etoposide, delayed leukemia progression in drug-resistant xenografts [109].
A patent application describing synthesis and pharmaceutical formulations developed for clinical investigations of orally available ABT-263 (navitoclax) was published by Abbott Laboratories [110]. Preclinical studies confirmed that navitoclax, like ABT-737, has a high affinity for Bcl-2, Bcl-xL and Bcl-w, and induction of apoptosis depends on Bax/Bak. ABT-236 shows single-agent efficacy on CLL, SCLC, and lymphoma cell lines and is synergistic with γ-irradiation and other anti-cancer agents [111]. In xenograft models of H889 (SCLC) and RS4;11 (ALL) tumors, ABT-263 treatment led to rapid and complete tumor regression [111, 112]. The clinical activity of navitoclax is in good agreement with the BH3 profiling proposed model, which can explain the differential sensitivity of lymphoma cells to Bcl-2 inhibition [101]. Consistent with this, the gene expression microarray analysis showed that cells overexpressing Mcl-1 are resistant to ABT-263, and siRNA knockdown of Mcl-1 in the resistant SCLC cell line H196 restores sensitivity to ABT-263 [113, 114].
Phase I studies in patients with SCLC and other solid tumors [115], as well as in lymphoid malignancies, showed that navitoclax is safe and well tolerated, with dose-dependent, reversible thrombocytopenia as the major adverse effect. There are currently several different phase I and II clinical trials testing navitoclax as a single agent or in combination in patients with solid tumors or CLL.
3.3.2 Bcl-2 selective inhibitors
The major side effect of navitoclax is dose-dependent thrombocytopenia which is mediated by inhibition of Bcl-xL rather than Bcl-2. A patent application from Abbott Laboratories recently reported additional analogues of ABT-737 and ABT-263 with modifications on the N-acylsulfonamide core structure [116]. This application disclosed 48 novel analogues with Ki values = 0.04 nM - 0.45 μM against Bcl-2 as determined by TR-FRET assay; the most potent compounds are compounds 18 and 19 with Ki = 0.06 and 0.04 nM respectively against Bcl-2. Another Abbott Laboratories patent application disclosed an additional 481 analogues of ABT-737 with activity against Bcl-2 and Bcl-xL [117]. Compound 20 is the most selective Bcl-2 inhibitor disclosed in this patent and has a very high selectivity ratio (Bcl-xL, Ki/Bcl-2, Ki > 263263). The most potent Bcl-2 inhibitor in this patent is compound 21 with Ki = 0.001 nM and selectivity ratio of 388.
Recently Abbott Laboratories has described the discovery of new class of potent, selective Bcl-2 inhibitors using NMR and structure-based drug design. Based on the NMR-derived structure, the diphenylmethane and biaryl acid ligands were linked and modified giving compound 22 as the most potent Bcl-2 protein inhibitor with Ki = 40 nM [23]. This compound showed > 1000-fold specificity for Bcl-2 versus Bcl-w, >100-fold versus Bcl-B, 58-fold versus A1, and 28-fold versus Mcl-1. This is in contrast to the specificity profile of ABT-737 that binds with sub-nanomolar affinity to Bcl-2, Bcl-xL, and Bcl-w, but with micromolar affinity to Mcl-1, Bcl-B, and A1.
3.3.3 Selective Mcl-1 inhibitors
Many SMIs, including ABT-737 and ABT-263, bind to Bcl-xL and Bcl-2 but not to Mcl-1. This is significant, because upregulation of Mcl-1 seems to be a major source of resistance to ABT-737 and ABT-263 [105]. Development of selective Mcl-1 inhibitors has become a high priority. The development of selective inhibitors targeting anti-apoptotic proteins is feasible but challenging. Mcl-1 has a very similar structure to other Bcl-2 proteins, but it shares only ~25% sequence identity with other members and has a noteworthy different BH3 binding profile and distinct BH3 interactions [118].
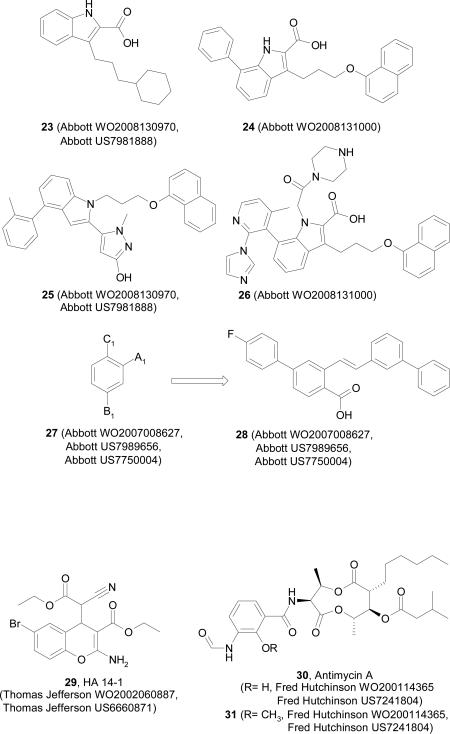
In two international patent applications from Abbott Laboratories [119, 120], compounds such as 23 – 26 that selectively inhibit the activity of Mcl-1 were disclosed. These applications revealed several hundred examples of 7-nonsubstituted (issued US patent in 2011) and 7-substituted indole derivatives with selective inhibition of Mcl-1 with IC50 = <0.03 μM - 10.14 μM. These compounds are expected to have utility in the treatment of tumors overexpressing Mcl-1 but no biological validation data was published on these compounds.
A patent application published by Abbott Laboratories in 2007 and two issued US patents disclosed 229 novel chemical entities as inhibitors of anti-apoptotic Mcl-1 protein represented by genus 27 bearing tri-substituted benzene core structure [121]. A1 group was broadly defined as substituted alkyl, alkenyl, alkynyl, alkoxy, amino, sulfonylamino, carboxylic or amide group. B1 was defined as phenyl substituted with one or two of independently selected F, Br, Cl or I and C1 group was broadly defined as CN, NO2, CF3, alkoxy, carbonyl, carboxylic acid or its derivatives, halo, amino or sulfonylalkyl group. A representative structure from this work is shown in compound 28. The analogues disclosed in this patent exhibited binding affinities to Mcl-1 with IC50 values ranging from 0.1 nM to >10 nM.
3.4 Others Compounds
HA 14-1 (29), discovered by virtual screening, is a synthetic chromene molecule, the first SMI of Bcl-2 to be described [25] and has been shown to bind to Bcl-2 with Ki = 9 μM. A patent describing HA 14-1 and its analogs was issued to Thomas Jefferson University in 2003 [122].
Antimycin A (30) and its derivatives were disclosed in the international application in 2001 and granted by the European Patent Office, Australia, and United States of America [123]. It is an antifungal Streptomyces-derived compound which can mimic pro-apoptotic BH3 peptides, discovered during a screen of known inhibitors of mitochondrial respiratory complexes I, II, and III, and ATP synthase [26]. It has been shown to bind to BH3 binding domain of Bcl-2 and Bcl-xL by competing with Bak BH3 peptide. 2-methoxy antimycin A (31) fails to inhibit the mitochondrial respiration chain, but was still able to kill Bcl-xL overexpressing cells [30].
In 2008, a patent application from Infinity Pharmaceuticals claimed isoxazolidine analogues as inhibitors of Bcl-2 and Bcl-xL [124]. Compounds 32 and 33 are two typical isoxazolidines with Ki values to Bcl-2 and Bcl-xL < 1 nM and < 1 μM respectively. These compounds showed a dose-dependent killing of lymphoma and pancreatic cancer cell lines as single agents and in combination with camptothecin.
In a 2006 international application granted in New Zealand and United States of America, researchers at the Walter and Eliza Hall Institute of Medical Research reported a series of benzoyl urea derivatives as inhibitors of pro-survival Bcl-2 family proteins [125]. This application disclosed 146 novel chemical entities bearing benzoyl urea core structure which mimics α helical peptide of BH3-only proteins. A representative structure from this work is shown in compound 34. These analogues exhibited weak binding affinities to Bcl-w, Bcl-xL, and Mcl-1 with IC50 values in micromolar range.
4. Expert opinions
Much progress has been made in the last decade on the detailed knowledge of regulation of apoptosis at the molecular level. Specific components of the apoptosis machinery are targeted for anticancer therapy, in particular the mechanism by which the Bcl-2 family functions through selective PPIs to control mitochondrial apoptosis. Recently, SMIs capable of inhibiting the interactions of the antiapoptotic Bcl-2 protein family have been developed and three SMIs, (-)-gossypol, obatoclax and ABT-236, have progressed into clinical studies.
To evaluate how a BH3 mimetic might best be used, the mechanism of action of ABT-737 and several other putative BH3 mimetics, including gossypol and obatoclax, has been explored [126]. Of all tested compounds, only ABT-737 induced apoptosis was completely inhibited in cells deficient for Bax/Bak or caspase-9, demonstrating that only ABT-737 is a specific Bcl-2 inhibitor and behaved as an authentic BH3 mimetic. In this review, we already discussed that for gossypol and obatoclax additional mechanism of actions was reported. For example, the ability of gossypol and obatoclax to elicit Bax/Bak independent cell death by autophagy [53, 90] might explain the apparent nonselective cytotoxicity reported for these two compounds [126]. It is believed that the Bcl-2–independent targets of these two agents may have clinical applicability, which has to be studied further.
Preclinical studies have shown that SMIs of Bcl-2 family of proteins are effective in physiologically relevant systems such as primary patient samples or mouse xenograft models, either as monotherapy or in combination with other drugs. They provide important clinical insights and demonstrate the role of Bcl-2 inhibitors in blocking the Bcl-2 mediated intrinsic and acquired resistance, facilitating killing by conventional chemotherapy. There are many examples of an enhanced apoptotic response when the BH3 mimetics are combined with traditional therapies to treat cancers such as melanoma [76], pancreatic [127], glioma [128], breast [129], multiple myeloma [130] and B-cell malignant models [111]. Other important findings included a distinct gradient of sensitivity of cells, depending on their Bcl-2 status, to the cytotoxic effect of ABT-737. Resistance to ABT-737 has been linked to high expression levels of Mcl-1, which can be overcome by treatment with agent(s) that down-regulate Mcl-1 [104, 114] but obatoclax, as a pan Bcl-2 inhibitor, overcomes Mcl-1 mediated resistance to apoptosis by interfering with Mcl-1–Bak interactions [82]. These results suggest that it is necessary to neutralize both arms of the anti-apoptotic Bcl-2 family and raise important questions concerning the specificity of Bcl-2 inhibitors. It is unclear whether pan inhibitors of Bcl-2 are superior to specific inhibitors. From the efficacy point of view, it is important to utilize the BH3 profiling tool which will facilitate identification of the apoptotic block used by cancer cells or of the block acquired on resistance to chemotherapy. Such a tool could identify cancers that are susceptible to either pan- or selective BH3 mimetics, enabling a personalized and effective approach to treatment [131]. It is known that ABT-263 causes reversible, dose-dependent, mechanism-based thrombocytopenia because of Bcl-xL inhibition in platelets [132] and it is expected that more specific inhibitors will be less toxic. The possible clinical use of these compounds will depend on efficacy and on acceptable toxicities. Targeting of Mcl-1 may provide a therapeutic window and a broad clinical utility for developing agents that target tumors over-expressing Mcl-1. The roles of Mcl-1 is attracting attention as a critical survival factor in a broad range of human cancers and in the near future, more patents about selective Mcl-1 inhibitors, as well as pan Bcl-2 family inhibitors, are expected.
Evasion of apoptosis has been established as a hallmark of cancer and the impaired apoptotic signaling characteristic of cancer cells is frequently linked to tumor development and progression as well as resistance to treatment. Therefore, the intrinsic apoptotic pathway is a promising cancer eradication pathway and intensive research and drug development is ongoing at both pharmaceutical and academic research laboratories. Targeting Bcl-2 family proteins using SMI strategy is gaining momentum with several classes of inhibitors emerged in clinical trials, discussed in this review. Important questions that need to be investigated in the future include the role of these drugs as monotherapy versus combination therapy with other anticancer drugs and the related issue of the relative toxicity to cancerous versus normal cells. In addition, there is still a need of potent and selective SMIs of individual members of this family (for example, selective Mcl-1 inhibitors) for further understanding the mechanistic basis of their activity and to exploit advantages and disadvantages of this selectivity for therapy. In addition, as for most targeted therapies, the success of ABT-263, obatoclax, gossypol and new developed inhibitors, will be strongly dependent on the innovation of biomarkers that can direct these exciting potential therapeutics where they can be used for maximum efficacy.
Other strategies of targeting Bcl-2 family member interactions include designing a Bax/Bak activator that acts as an agonist for this interaction. Walensky et al. have already shown the ability of a stapled Bid BH3 peptide to activate Bax in vitro, raising the possibility that Bax (or Bak) could be targeted [17]. However, this approach needs more detailed preclinical studies, particularly assessing the toxicity of these compounds in vivo to determine if more apoptosis will be promote in normal cells than a compound which engages selected anti-apoptotic proteins.
After many years of development, it now seems likely that the design of compounds inhibiting specific PPIs may lead to significant therapeutic advances. With the increasing interest in small-molecule Bcl-2 inhibitors as a therapeutic approach to the treatment of human cancer it should now be possible to determine whether these drugs represent a true breakthrough in cancer treatment.
ACKNOWLEDGMENT
We are grateful for financial support from the National Cancer Institute, National Institutes of Health grants R01CA149442 and R21CA158976 (Z. Nikolovska-Coleska). The authors thank Dr. G.W.A. Milne for his critical reading of the manuscript and useful suggestions.
Footnotes
Declaration of Interest:
The authors have received funding from the NIH, grant numbers R01CA149442 and R21CA158976.
References
- 1.Toogood PL. Inhibition of protein-protein association by small molecules: approaches and progress. J Med Chem. 2002;45:1543–58. doi: 10.1021/jm010468s. [DOI] [PubMed] [Google Scholar]
- 2.Ideker T, Sharan R. Protein networks in disease. Genome Res. 2008;18:644–52. doi: 10.1101/gr.071852.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Cummings CG, Hamilton AD. Disrupting protein-protein interactions with non-peptidic, small molecule alpha-helix mimetics. Curr Opin Chem Biol. 2010;14:341–6. doi: 10.1016/j.cbpa.2010.04.001. [This review describes applications of several chemical scaffolds, as non-peptidic, small molecule α-helix mimetics, to disrupt PPIs.] [DOI] [PubMed] [Google Scholar]
- 4.Arkin MR, Wells JA. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat Rev Drug Discov. 2004;3:301–17. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- 5.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 6.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- 8.Vogler M, Dinsdale D, Dyer MJ, Cohen GM. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 2009;16:360–7. doi: 10.1038/cdd.2008.137. [DOI] [PubMed] [Google Scholar]
- 9.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 10.Petros AM, Nettesheim DG, Wang Y, et al. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci. 2000;9:2528–34. doi: 10.1110/ps.9.12.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–92. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Willis SN, Wei A, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien SM, Cunningham CC, Golenkov AK, Turkina AG, Novick SC, Rai KR. Phase I to II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in patients with advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23:7697–702. doi: 10.1200/JCO.2005.02.4364. [DOI] [PubMed] [Google Scholar]
- 14**.Walensky LD, Kung AL, Escher I, et al. Activation of apoptosis in vivo by a hydrocarbonstapled BH3 helix. Science. 2004;305:1466–70. doi: 10.1126/science.1099191. [This paper for the first time describes the stapled peptides, called “stabilized alpha-helix of BCL-2 domains” (SAHBs), their in vitro and in vivo characterization, and potential application of this approach for targeting different protein-protein interactions.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang JL, Zhang ZJ, Choksi S, et al. Cell permeable Bcl-2 binding peptides: a chemical approach to apoptosis induction in tumor cells. Cancer Res. 2000;60:1498–502. [PubMed] [Google Scholar]
- 16.Stewart ML, Fire E, Keating AE, Walensky LD. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat Chem Biol. 2010;6:595–601. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walensky LD, Pitter K, Morash J, et al. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Kutzki O, Park HS, Ernst JT, Orner BP, Yin H, Hamilton AD. Development of a potent Bclx(L) antagonist based on alpha-helix mimicry. J Am Chem Soc. 2002;124:11838–9. doi: 10.1021/ja026861k. [DOI] [PubMed] [Google Scholar]
- 19.Davis JM, Truong A, Hamilton AD. Synthesis of a 2,3';6',3”-terpyridine scaffold as an alpha-helix mimetic. Org Lett. 2005;7:5405–8. doi: 10.1021/ol0521228. [DOI] [PubMed] [Google Scholar]
- 20.Yin H, Hamilton AD. Terephthalamide derivatives as mimetics of the helical region of Bak peptide target Bcl-xL protein. Bioorg Med Chem Lett. 2004;14:1375–9. doi: 10.1016/j.bmcl.2003.09.096. [DOI] [PubMed] [Google Scholar]
- 21.Degterev A, Lugovskoy A, Cardone M, et al. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat Cell Biol. 2001;3:173–82. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]
- 22**.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [This is the first report of ABT-737, discovered by NMR fragment based screening, its mechanistic studies and in vivo activity as a single agent in small-cell lung carcinoma animal model.] [DOI] [PubMed] [Google Scholar]
- 23.Petros AM, Huth JR, Oost T, et al. Discovery of a potent and selective Bcl-2 inhibitor using SAR by NMR. Bioorg Med Chem Lett. 2010;20:6587–91. doi: 10.1016/j.bmcl.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 24.Petros AM, Dinges J, Augeri DJ, et al. Discovery of a potent inhibitor of the antiapoptotic protein Bcl-xL from NMR and parallel synthesis. J Med Chem. 2006;49:656–63. doi: 10.1021/jm0507532. [DOI] [PubMed] [Google Scholar]
- 25.Wang JL, Liu D, Zhang ZJ, et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2000;97:7124–9. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enyedy IJ, Ling Y, Nacro K, et al. Discovery of small-molecule inhibitors of Bcl-2 through structure-based computer screening. J Med Chem. 2001;44:4313–24. doi: 10.1021/jm010016f. [DOI] [PubMed] [Google Scholar]
- 27.Bruncko M, Oost TK, Belli BA, et al. Studies leading to potent, dual inhibitors of Bcl-2 and Bcl-xL. J Med Chem. 2007;50:641–62. doi: 10.1021/jm061152t. [DOI] [PubMed] [Google Scholar]
- 28.Wang G, Nikolovska-Coleska Z, Yang CY, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49:6139–42. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 29.Wei J, Kitada S, Stebbins JL, et al. Synthesis and biological evaluation of Apogossypolone derivatives as pan-active inhibitors of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2010;53:8000–11. doi: 10.1021/jm100746q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzung SP, Kim KM, Basanez G, et al. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nat Cell Biol. 2001;3:183–91. doi: 10.1038/35055095. [DOI] [PubMed] [Google Scholar]
- 31.Friedhelm Herrmann Substance for use against tumor growth and viral infections. 1997 WO9704006.
- 32.Washington University Cell death agonist. 1999 WO 9916787.
- 33.Walter and Eliza Hall Institute of Medical Research Peptides and their therapeutic uses thereof. 2004 WO2004058804 A1.
- 34.Walter and Eliza Hall Institute of Medical Research Conjugates and therapeutic uses thereof. 2006 WO2006000034 A1.
- 35.Dana-Farber Cancer Institute Stabilized alpha helical peptides and uses thereof. 20052010 WO2005044839 US7723469.
- 36.Coutinho EM, Athayde C, Atta G, et al. Gossypol blood levels and inhibition of spermatogenesis in men taking gossypol as a contraceptive. A multicenter, international, dose-finding study. Contraception. 2000;61:61–7. doi: 10.1016/s0010-7824(99)00117-1. [DOI] [PubMed] [Google Scholar]
- 37.University of Michigan Small molecule antagonists of Bcl-2 family proteins. 20022008 WO2002097053 A2 US7432304.
- 38.University of Michigan Small molecule antagonists of Bcl-2 family proteins. 2005 WO2005069771 A2.
- 39.University of Michigan Gossypol co-crystals and the use thereof. 200520082008 WO2005094804 A1 US7342046 US7432300.
- 40.Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem. 2003;46:4259–64. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 41.Xu L, Yang D, Wang S, et al. (-)-Gossypol enhances response to radiation therapy and results in tumor regression of human prostate cancer. Mol Cancer Ther. 2005;4:197–205. [PubMed] [Google Scholar]
- 42.Zhang M, Liu H, Guo R, et al. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochem Pharmacol. 2003;66:93–103. doi: 10.1016/s0006-2952(03)00248-x. [DOI] [PubMed] [Google Scholar]
- 43.Oliver CL, Miranda MB, Shangary S, Land S, Wang S, Johnson DE. (-)-Gossypol acts directly on the mitochondria to overcome Bcl-2- and Bcl-X(L)-mediated apoptosis resistance. Mol Cancer Ther. 2005;4:23–31. [PubMed] [Google Scholar]
- 44.Mohammad RM, Wang S, Aboukameel A, et al. Preclinical studies of a nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-X(L) [(-)-gossypol] against diffuse large cell lymphoma. Mol Cancer Ther. 2005;4:13–21. [PubMed] [Google Scholar]
- 45.Atmaca H, Gorumlu G, Karaca B, et al. Combined gossypol and zoledronic acid treatment results in synergistic induction of cell death and regulates angiogenic molecules in ovarian cancer cells. Eur Cytokine Netw. 2009;20:121–30. doi: 10.1684/ecn.2009.0159. [DOI] [PubMed] [Google Scholar]
- 46.Meng Y, Tang W, Dai Y, et al. Natural BH3 mimetic (-)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol Cancer Ther. 2008;7:2192–202. doi: 10.1158/1535-7163.MCT-08-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolter KG, Wang SJ, Henson BS, et al. (-)-gossypol inhibits growth and promotes apoptosis of human head and neck squamous cell carcinoma in vivo. Neoplasia. 2006;8:163–72. doi: 10.1593/neo.05691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliver CL, Bauer JA, Wolter KG, et al. In vitro effects of the BH3 mimetic, (-)-gossypol, on head and neck squamous cell carcinoma cells. Clin Cancer Res. 2004;10:7757–63. doi: 10.1158/1078-0432.CCR-04-0551. [DOI] [PubMed] [Google Scholar]
- 49.Bauer JA, Trask DK, Kumar B, et al. Reversal of cisplatin resistance with a BH3 mimetic, (-)-gossypol, in head and neck cancer cells: role of wild-type p53 and Bcl-xL. Mol Cancer Ther. 2005;4:1096–104. doi: 10.1158/1535-7163.MCT-05-0081. [DOI] [PubMed] [Google Scholar]
- 50.Zaidi R, Hadi SM. Complexes involving gossypol, DNA and Cu(II). Biochem Int. 1992;28:1135–43. [PubMed] [Google Scholar]
- 51.Balakrishnan K, Wierda WG, Keating MJ, Gandhi V. Gossypol, a BH3 mimetic, induces apoptosis in chronic lymphocytic leukemia cells. Blood. 2008;112:1971–80. doi: 10.1182/blood-2007-12-126946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pang X, Wu Y, Lu B, et al. (-)-Gossypol suppresses the growth of human prostate cancer xenografts via modulating VEGF signaling-mediated angiogenesis. Mol Cancer Ther. 2011;10:795–805. doi: 10.1158/1535-7163.MCT-10-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lian J, Karnak D, Xu L. The Bcl-2-Beclin 1 interaction in (-)-gossypol-induced autophagy versus apoptosis in prostate cancer cells. Autophagy. 2010;6:1201–3. doi: 10.4161/auto.6.8.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ascenta Therapeutics Inc. Pulsatile dosing of gossypol for treatment of disease. 2008 WO2008150506 A1.
- 55.Ascenta Therapeutics Inc. Process for preparing R-gossypol L-phenyl alaninol dienamine. 2009 WO2009045410 A1.
- 56.Heist RS, Fain J, Chinnasami B, et al. Phase I/II study of AT-101 with topotecan in relapsed and refractory small cell lung cancer. J Thorac Oncol. 2010;5:1637–43. doi: 10.1097/JTO.0b013e3181e8f4dc. [DOI] [PubMed] [Google Scholar]
- 57*.Liu G, Kelly WK, Wilding G, Leopold L, Brill K, Somer B. An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin Cancer Res. 2009;15:3172–6. doi: 10.1158/1078-0432.CCR-08-2985. [This manuscript describes the report on the efficacy of novel oral Bcl-2 inhibitor, AT-101, in patients with prostate cancer.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.University of Michigan Apogossypolone and the uses thereof. 2006 WO2006050447 A2.
- 59.Sun Y, Wu J, Aboukameel A, et al. Apogossypolone, a nonpeptidic small molecule inhibitor targeting Bcl-2 family proteins, effectively inhibits growth of diffuse large cell lymphoma cells in vitro and in vivo. Cancer Biol Ther. 2008;7:1418–26. doi: 10.4161/cbt.7.9.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu ZY, Zhu XF, Zhong ZD, et al. ApoG2, a novel inhibitor of antiapoptotic Bcl-2 family proteins, induces apoptosis and suppresses tumor growth in nasopharyngeal carcinoma xenografts. Int J Cancer. 2008;123:2418–29. doi: 10.1002/ijc.23752. [DOI] [PubMed] [Google Scholar]
- 61.Mi JX, Wang GF, Wang HB, et al. Synergistic antitumoral activity and induction of apoptosis by novel pan Bcl-2 proteins inhibitor apogossypolone with adriamycin in human hepatocellular carcinoma. Acta Pharmacol Sin. 2008;29:1467–77. doi: 10.1111/j.1745-7254.2008.00901.x. [DOI] [PubMed] [Google Scholar]
- 62.Banerjee S, Choi M, Aboukameel A, et al. Preclinical studies of apogossypolone, a novel pan inhibitor of bcl-2 and mcl-1, synergistically potentiates cytotoxic effect of gemcitabine in pancreatic cancer cells. Pancreas. 2010;39:323–31. doi: 10.1097/MPA.0b013e3181bb95e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kitada S, Kress CL, Krajewska M, Jia L, Pellecchia M, Reed JC. Bcl-2 antagonist apogossypol (NSC736630) displays single-agent activity in Bcl-2-transgenic mice and has superior efficacy with less toxicity compared with gossypol (NSC19048). Blood. 2008;111:3211–9. doi: 10.1182/blood-2007-09-113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coward L, Gorman G, Noker P, et al. Quantitative determination of apogossypol, a proapoptotic analog of gossypol, in mouse plasma using LC/MS/MS. J Pharm Biomed Anal. 2006;42:581–6. doi: 10.1016/j.jpba.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 65.Burnham Institute for Medical Research Naphthale-based inhibitors of anti-apoptotic proteins. 20092011 WO2009052443 A1 US8039668.
- 66.Burnham Institute for Medical Research Naphthale-based inhibitors of anti-apoptotic proteins. 2010 WO2010120943 A1.
- 67.Wei J, Kitada S, Rega MF, et al. Apogossypol derivatives as antagonists of antiapoptotic Bcl-2 family proteins. Mol Cancer Ther. 2009;8:904–13. doi: 10.1158/1535-7163.MCT-08-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei J, Kitada S, Rega MF, et al. Apogossypol derivatives as pan-active inhibitors of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2009;52:4511–23. doi: 10.1021/jm900472s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei J, Stebbins JL, Kitada S, et al. BI-97C1, an optically pure Apogossypol derivative as pan-active inhibitor of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2010;53:4166–76. doi: 10.1021/jm1001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dash R, Azab B, Quinn BA, et al. Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proc Natl Acad Sci U S A. 2011;108:8785–90. doi: 10.1073/pnas.1100769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Azab B, Dash R, Das SK, et al. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) in combination with the Apogossypol derivative BI-97C1 (Sabutoclax) improves therapeutic efficacy in low CAR colorectal cancer cells. J Cell Physiol. 2011 doi: 10.1002/jcp.22947. DOI 10.1002/jcp.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.University of Michigan Small molecule inhibitors of anti-apoptotic Bcl-2 family members and the uses thereof. 2006 WO2006023778 A2.
- 73.Mohammad RM, Goustin AS, Aboukameel A, et al. Preclinical studies of TW-37, a new nonpeptidic small-molecule inhibitor of Bcl-2, in diffuse large cell lymphoma xenograft model reveal drug action on both Bcl-2 and Mcl-1. Clin Cancer Res. 2007;13:2226–35. doi: 10.1158/1078-0432.CCR-06-1574. [DOI] [PubMed] [Google Scholar]
- 74.Zeitlin BD, Joo E, Dong Z, et al. Antiangiogenic effect of TW37, a small-molecule inhibitor of Bcl-2. Cancer Res. 2006;66:8698–706. doi: 10.1158/0008-5472.CAN-05-3691. [DOI] [PubMed] [Google Scholar]
- 75.Ashimori N, Zeitlin BD, Zhang Z, et al. TW-37, a small-molecule inhibitor of Bcl-2, mediates S-phase cell cycle arrest and suppresses head and neck tumor angiogenesis. Mol Cancer Ther. 2009;8:893–903. doi: 10.1158/1535-7163.MCT-08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Verhaegen M, Bauer JA, Martin de la Vega C, et al. A novel BH3 mimetic reveals a mitogen-activated protein kinase-dependent mechanism of melanoma cell death controlled by p53 and reactive oxygen species. Cancer Res. 2006;66:11348–59. doi: 10.1158/0008-5472.CAN-06-1748. [DOI] [PubMed] [Google Scholar]
- 77.Wang Z, Song W, Aboukameel A, et al. TW-37, a small-molecule inhibitor of Bcl-2, inhibits cell growth and invasion in pancreatic cancer. Int J Cancer. 2008;123:958–66. doi: 10.1002/ijc.23610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Mohammad RM, Goustin AS, Aboukameel A, et al. Preclinical studies of TW-37, a new nonpeptidic small-molecule inhibitor of Bcl-2, in diffuse large cell lymphoma xenograft model reveal drug action on both Bcl-2 and Mcl-1. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:2226–35. doi: 10.1158/1078-0432.CCR-06-1574. [DOI] [PubMed] [Google Scholar]
- 79.Gemin X Biotechnologies Inc. Triheterocyclic compounds, compositions, and methods for treating cancer or viral diseases. 2004 WO2004106328 A1.
- 80.Gemin X Biotechnologies Inc. Triheterocyclic compounds compositions, and methods for treating cancer or viral diseases. 20052008 WO2005117908 A2 US7425553.
- 81.Gemin X Biotechnologies Inc. Dipyrrole compounds, compositions, and methods for treating cancer or viral diseases. 2006 WO2006069441 A1.
- 82**.Nguyen M, Marcellus RC, Roulston A, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–7. doi: 10.1073/pnas.0709443104. [First report on small molecule obatoclax as pan-Bcl-2 inhibitor and demonstrates rational clinical development opportunity for this compound in cancer indications or treatments where MCL-1 contributes to resistance to cell killing.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006;13:1419–21. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- 84**.Perez-Galan P, Roue G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX15-070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007;109:4441–9. doi: 10.1182/blood-2006-07-034173. [This paper focuses on preclinical evaluation of rational combination of Bcl-2 inhibitors with other novel agents such as a proteasome inhibitor in mantle cell lymphoma.] [DOI] [PubMed] [Google Scholar]
- 85.Li J, Viallet J, Haura EB. A small molecule pan-Bcl-2 family inhibitor, GX15-070, induces apoptosis and enhances cisplatin-induced apoptosis in non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2008;61:525–34. doi: 10.1007/s00280-007-0499-3. [DOI] [PubMed] [Google Scholar]
- 86.Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109:5430–8. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 87.Mott JL, Bronk SF, Mesa RA, Kaufmann SH, Gores GJ. BH3-only protein mimetic obatoclax sensitizes cholangiocarcinoma cells to Apo2L/TRAIL-induced apoptosis. Mol Cancer Ther. 2008;7:2339–47. doi: 10.1158/1535-7163.MCT-08-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang S, Okumura K, Sinicrope FA. BH3 mimetic obatoclax enhances TRAIL-mediated apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2009;15:150–9. doi: 10.1158/1078-0432.CCR-08-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Witters LM, Witkoski A, Planas-Silva MD, Berger M, Viallet J, Lipton A. Synergistic inhibition of breast cancer cell lines with a dual inhibitor of EGFR-HER-2/neu and a Bcl-2 inhibitor. Oncol Rep. 2007;17:465–9. [PubMed] [Google Scholar]
- 90.Bonapace L, Bornhauser BC, Schmitz M, et al. Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest. 2010;120:1310–23. doi: 10.1172/JCI39987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Konopleva M, Watt J, Contractor R, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax). Cancer Res. 2008;68:3413–20. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schimmer AD, O'Brien S, Kantarjian H, et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:8295–301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
- 93*.O'Brien SM, Claxton DF, Crump M, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113:299–305. doi: 10.1182/blood-2008-02-137943. [This is the first report of the safety and clinical efficacy of a new Bcl-2 inhibitor in patients with CLL.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hwang JJ, Kuruvilla J, Mendelson D, et al. Phase I dose finding studies of obatoclax (GX15-070), a small molecule pan-BCL-2 family antagonist, in patients with advanced solid tumors or lymphoma. Clin Cancer Res. 2010;16:4038–45. doi: 10.1158/1078-0432.CCR-10-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paik PK, Rudin CM, Brown A, et al. A phase I study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in solid tumor malignancies. Cancer Chemother Pharmacol. 2010;66:1079–85. doi: 10.1007/s00280-010-1265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abbott Laboratories Apoptosis Promoters. 200720102011 US20070072860 US7642260 US7973161.
- 97.Abbott Laboratories N-acylsulfonamide apoptosis promoters. 200220052005 WO2002024636 WO2005049593 WO2005049594.
- 98.Abbott Laboratories Apoptosis Promoters. 20102011 US7767684 US7906505.
- 99.Abbott Laboratories Apoptosis Promoters. 2006 WO2006127364 A1.; Macrocyclic Inhibitors of Bcl proteins. 2010 US7777076.
- 100.Abbott Laboratories N-Sulfonyl carboximidamide apoptosis promoters. 20052009 WO2005117543 A2 US7585858.
- 101.Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood. 2008;111:2300–9. doi: 10.1182/blood-2007-06-098012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buron N, Porceddu M, Brabant M, et al. Use of human cancer cell lines mitochondria to explore the mechanisms of BH3 peptides and ABT-737-induced mitochondrial membrane permeabilization. PLoS One. 2010;5:e9924. doi: 10.1371/journal.pone.0009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kline MP, Rajkumar SV, Timm MM, et al. ABT-737, an inhibitor of Bcl-2 family proteins, is a potent inducer of apoptosis in multiple myeloma cells. Leukemia. 2007;21:1549–60. doi: 10.1038/sj.leu.2404719. [DOI] [PubMed] [Google Scholar]
- 104.Chauhan D, Velankar M, Brahmandam M, et al. A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene. 2007;26:2374–80. doi: 10.1038/sj.onc.1210028. [DOI] [PubMed] [Google Scholar]
- 105**.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–88. doi: 10.1016/j.ccr.2006.10.006. [This paper focuses on mechanisms of action of the Bcl-2 (BH3) inhibitor ABT-737 and for the first time demonstrates that the activity of this potent inhibitor is largely diminished in cells overexpressing Mcl-1, establishing Mcl-1 as an important therapeutic target.] [DOI] [PubMed] [Google Scholar]
- 106.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–21. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vogler M, Dinsdale D, Sun XM, et al. A novel paradigm for rapid ABT-737-induced apoptosis involving outer mitochondrial membrane rupture in primary leukemia and lymphoma cells. Cell Death Differ. 2008;15:820–30. doi: 10.1038/cdd.2008.25. [DOI] [PubMed] [Google Scholar]
- 108.Kang MH, Kang YH, Szymanska B, et al. Activity of vincristine, L-ASP, and dexamethasone against acute lymphoblastic leukemia is enhanced by the BH3-mimetic ABT-737 in vitro and in vivo. Blood. 2007;110:2057–66. doi: 10.1182/blood-2007-03-080325. [DOI] [PubMed] [Google Scholar]
- 109.High LM, Szymanska B, Wilczynska-Kalak U, et al. The Bcl-2 homology domain 3 mimetic ABT-737 targets the apoptotic machinery in acute lymphoblastic leukemia resulting in synergistic in vitro and in vivo interactions with established drugs. Mol Pharmacol. 2010;77:483–94. doi: 10.1124/mol.109.060780. [DOI] [PubMed] [Google Scholar]
- 110.Abbott Laboratories A process for the preparation of the apoptosis promoter ABT-263. 2009 WO2009155386 A1.
- 111*.Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [This paper summarizes the preclinical studies demonstrating the biologic activity of the Bcl-2 inhibitor ABT-236, which has shown clinical activity in initial phase 1/2 studies.] [DOI] [PubMed] [Google Scholar]
- 112.Shoemaker AR, Mitten MJ, Adickes J, et al. Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin Cancer Res. 2008;14:3268–77. doi: 10.1158/1078-0432.CCR-07-4622. [DOI] [PubMed] [Google Scholar]
- 113.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–91. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 115**.Gandhi L, Camidge DR, Ribeiro de Oliveira M, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–16. doi: 10.1200/JCO.2010.31.6208. [This paper summarizes the encouraging preliminary efficacy data of navitoclax (ABT-263) in patients with SCLC and identification of pro-gastrin releasing peptide (pro-GRP) as a marker of treatment response.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abbott Laboratories Apoptosis-inducing agents for the treatment of cancer and immune and autoimmune diseases. 2010 WO2010083442 A1.
- 117.Abbott Laboratories Bcl-2 selective apoptosis-inducing agents for the treatment of cancer and immune diseases. 2010 WO2010065865 A2.
- 118.Czabotar PE, Lee EF, van Delft MF, et al. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad Sci U S A. 2007;104:6217–22. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abbott Laboratories 7-Nonsubstituted indole Mcl-1 inhibitors. 20082011 WO2008130970 A1 US7981888 B2.
- 120.Abbott Laboratories 7-Substituted indole Mcl-1 inhibitors. 2008 WO2008131000 A2.
- 121.Abbott Laboratories Apoptosis promoters. 200720102011 WO2007008627 US7750004 B2 US7989656 B2.
- 122.Thomas Jefferson University Synthesis of 4H-chromene derivatives. 20022003 WO2002060887 A2 US6660871 B2.
- 123.Fred Hutchinson Cancer Research Center Compositions and methods for modulating apoptosis in cells over-expressing Bcl-2 family member proteins. 20012007 WO200114365 A1 US7241804 B1.
- 124.Infinity Pharmaceuticals Inc. Compounds and methods for inhibiting the interaction of Bcl proteins with binding partners. 200820102010 WO2008060569 A1 US7842815 US7851637.
- 125.Walter and Eliza Hall Institute of Medical Research Alpha-helical mimetics. 20062011 WO2006002474 A1 US7956216 B2.
- 126.Vogler M, Weber K, Dinsdale D, et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 2009;16:1030–1039. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- 127.Huang S, Sinicrope FA. BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res. 2008;68:2944–51. doi: 10.1158/0008-5472.CAN-07-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tagscherer KE, Fassl A, Campos B, et al. Apoptosis-based treatment of glioblastomas with ABT-737, a novel small molecule inhibitor of Bcl-2 family proteins. Oncogene. 2008;27:6646–56. doi: 10.1038/onc.2008.259. [DOI] [PubMed] [Google Scholar]
- 129.Kutuk O, Letai A. Alteration of the mitochondrial apoptotic pathway is key to acquired paclitaxel resistance and can be reversed by ABT-737. Cancer Res. 2008;68:7985–94. doi: 10.1158/0008-5472.CAN-08-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Trudel S, Stewart AK, Li Z, et al. The Bcl-2 family protein inhibitor, ABT-737, has substantial antimyeloma activity and shows synergistic effect with dexamethasone and melphalan. Clin Cancer Res. 2007;13:621–9. doi: 10.1158/1078-0432.CCR-06-1526. [DOI] [PubMed] [Google Scholar]
- 131**.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–85. doi: 10.1016/j.ccr.2007.07.001. [This paper describes novel strategy, BH3 profiling, in order to predict the sensitivity to the Bcl-2 inhibitors, like ABT-737. Importantly, this assay can also predict sensitivity to conventional chemotherapeutic agents.] [DOI] [PubMed] [Google Scholar]
- 132.Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–86. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]