Introduction
Trauma, hemorrhagic shock (T/HS), and subsequent multiple organ dysfunction syndrome (MODS) remain a current challenge in modern medicine. During trauma-hemorrhagic shock, intestinal injury and increased permeability leads to the generation of tissue injurious factors that are carried to the systemic circulation via the mesenteric lymphatics (1, 2). The gut has been shown to be a source of inflammatory factors with the capability of priming neutrophils and driving multiple organ failure after injury (3, 4), while gut protective strategies, involving both intraluminal and extraluminal modulators have demonstrated significant protection against the development of toxic post-shock mesenteric lymph and distant organ dysfunction (5–7). However, neural regulation via the vagus nerve, has been shown to be a critical component regulating normal intestinal function and intestinal defenses, the potentially protective effect of neuromodulation via stimulation of the vagus nerve on T/HS-induced gut injury and the production of biologically active mesenteric lymph has not been tested.
While neural regulation of gut injury has not been extensively studied, the vagus nerve represents the longest parasympathetic nerve connecting the central nervous system with the principal visceral organs. The classical function of the vagus nerve is to control heart rate, hormone secretion, as well as digestion and peristalsis in the gastrointestinal tract. Furthermore, the vagus nerve allows bidirectional communication between the brain and the immune system as well as the organs it innervates. By activating the sensory fibers of the vagus nerve, the immune and organ systems can send signals to the brain that in turn stimulate the efferent fibers of the vagus nerve to control the peripheral immune system as well as organ function. For example, the effector neurons in the vagus nerve can inhibit the production of pro-inflammatory cytokines from tissue macrophages (8). Likewise, the vagus nerve is thought to be involved in maintenance of the intestinal mucosal barrier function as reflected in intrinsic neural and central neural modulation of host defense activity in infectious colitis and other inflammatory diseases (9, 10).
In studies of endotoxemia, vagus nerve stimulation (VNS) was shown to decrease the systemic inflammatory response syndrome (SIRS) by attenuating the systemic inflammatory response to endotoxin (11). The pathway by which vagus nerve stimulation attenuates systemic inflammation after endotoxemia involved the spleen, because vagus nerve stimulation did not inhibits systemic inflammation in splenectomized mice (12). This pathway is termed the ‘cholinergic anti-inflammatory pathway’ because acetylcholine, the principle vagal neurotransmitter, inhibits the production of proinflammatory cytokines via the α7 nicotinic acetylcholine receptor subunit (α7nAChR) (8) as does nicotinic cholinergic agonists (13). Thus, from a clinical perspective, nicotine and other cholinergic agonists can potentially provide an alternative pharmacological strategy to mimic vagus nerve stimulation and to control systemic inflammation.
Based on studies showing VNS is protective in burn injury (14) as well as endotoxemia and bacterial infective models (8, 11, 12), we hypothesize that neuromodulation via vagal stimulation would be protective in our trauma-hemorrhagic shock model. Specifically, we hypothesize that vagus nerve stimulation will prevent gut barrier injury after trauma and hemorrhagic shock and therefore decrease the production of toxic mesenteric lymph thereby protecting the lung from injury. We examine whether the spleen is a necessary organ conferring protection in the vagus nerve stimulation model and, finally, whether a nicotinic agonist can mimic the protective effects of the vagus nerve.
Materials and Methods
Animals
Specific Pathogen-free male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 330–380 g and male C57BL/6 mice (Jackson Laboratories, Bar Harbor, Maine) weighing 25–28g were housed under barrier-sustained conditions and kept at 25°C with 12-hour light/dark cycles. The animals had free access to water and food. All animals were maintained in accordance with the recommendations of the "Guide for the Care and Use of Laboratory Animals," and the experiments were approved by the New Jersey Medical School Animal Care Committee.
Experimental Design
The goal of this study was to investigate the ability of vagal stimulation to limit trauma-hemorrhagic shock (T/HS)-induced gut and subsequent lung injury and the development of a systemic inflammatory state as well as investigate potential mechanisms underlying this protective effect. Thus, the first set of experiments tested the hypothesis that vagal stimulation would decrease T/HS-induced increases in gut and lung permeability and systemic neutrophil priming. In this experiment, rats were subjected to T/HS or trauma-sham shock (T/SS) with or without vagal stimulation. At 3 hrs after the end of the shock or sham-shock period, gut permeability was quantified using a 4 KDa fluorescent dextran (FD4) permeability probe, lung permeability was assessed using Evans blue dye and the response of circulating neutrophils to in vitro stimulation with PMA was determined. The second set of experiments tested the hypothesis that vagal stimulation prevented T/HS-induced lung injury and neutrophil priming by limiting gut injury and the subsequent production of biologically-active mesenteric lymph. To test this hypothesis mesenteric lymph was collected from rats subjected to T/HS or T/SS with or without vagal stimulation and subsequently infused into naïve mice. The in vivo effects of the mesenteric lymph samples on lung injury was tested by measuring lung permeability to Evans blue dye. Neutrophils from these lymph-injected mice were harvested and tested for priming by challenging them in vitro with PMA. Additionally, the effects of the injected lymph on RBC deformability was measured. Since the spleen has been shown to actively participate in the vagal response in animals challenged with endotoxin (12), in the third set of experiments we tested the role of the spleen in the vagal stimulation response to T/HS. This was accomplished by measuring gut and lung permeability in splenectomized rats subjected to T/HS or T/SS, with or without vagal stimulation. Lastly, in experiment four, we tested the hypothesis that pre-treatment with nicotine could be as protective as vagal stimulation. This hypothesis is based on work showing that the vagal anti-inflammatory response involves cholinergic activation through the nicotinic acetylcholine receptor(13). Thus, in this experiment, rats had nicotine patches placed on their skin 24 hrs prior to being subjected to T/HS or T/SS. At 3 hrs after the end of the T/HS or T/SS period, gut permeability was measured using FD4.
Trauma-hemorrhagic shock
As previously described (5), male Sprague Dawley rats were anesthetized with pentobarbital (50mg/kg) injected intraperitoneally (ip). Using an aseptic technique, a midline laparotomy is performed and the intestine is eviscerated from the abdominal cavity. The intestines are then protected by gauze moistened with 0.9% saline for 15 minutes. The intestines are then returned to the abdominal cavity and the laparotomy incision is closed using 4-0 suture. The femoral artery is dissected, cannulated with PE-50 tubing connected to a syringe flushed with the anticoagulant arixtra (5µL/mL) in saline, and then attached to a BP-2 blood pressure monitor (Columbus Instruments, Columbus, Ohio). The right internal jugular vein is dissected and cannulated for blood withdrawal.
Blood is withdrawn through the jugular vein cannula into an arixtra anticoagulated syringe (3mg/kg) until the MAP reaches 30–35mmHg (at a rate of 1mL/min) and maintained for 90 minutes. At the end of the shock period rats are resuscitated with their shed blood at a rate of 1mL/min and observed for 3 hrs. The Trauma-sham shock (T/SS) rats were subjected to anesthesia, a laparotomy and instrumentation, but no blood was withdrawn or administered.
Vagus Nerve Stimulation
The vagus nerve was stimulated as previously described (12). Briefly, once all cannulas were in place, the animal’s left neck was subjected to dissection and the carotid artery was identified. The vagus nerve was carefully dissected from the left carotid artery and a platinum electrode was placed across the nerve. The platinum electrode was attached to the stimulation device (STM 150) controlled by AcqKnowledge software (Biopac Systems). Vagus nerve stimulation was applied for 10 minutes at 5V. Sham animals underwent left neck dissection and isolation of the left vagus nerve and placement of electrode without stimulation of the electrode. Both groups were then subjected to trauma and then either hemorrhagic shock or sham shock.
Lymph collection
As previously described (15) the mesenteric lymph duct was exposed and the efferent mesenteric lymphatic was cannulated with a silastic catheter secured with a 6-0 silk suture and brought out through an incision in the right flank. Lymph was collected in a graduated cylinder on ice. Once the procedure was completed, the lymph was centrifuged at 2000RPM, 4°C, for 20 minutes. The cell-free supernatant was removed and stored in −80°C until completion of assays. The pellet was discarded.
Lung permeability with Evans Blue Dye
Lung permeability was assessed by permeation of Evans Blue dye as previously described (15). After completion of the shock-resuscitation period, animals were injected with 10mg Evans blue dye through the internal jugular catheter. After 5 minutes, a 1 mL blood sample was drawn from the femoral artery catheter and centrifuged at 1500 rpm at 4°C for 20 minutes. The plasma was serially diluted to form a standard curve. After twenty minutes after dye injection, the animals were sacrificed and a cardiopulmonectomy performed. Modified bronchial lavage was performed on the excised lungs using two small cuts to facilitate fluid removal and the BALF was centrifuged at 1500 rpm as above to remove any cellular debris. The supernatant fluid was assayed with a spectrophotometer at 620 nm for dye concentration and compared to a standard curve.
Gut Permeability FD4
Gut permeability was measured by an in-vivo method as previously described (16).At the end of shock-resuscitation period, the midline laparotomy incision was released and the cecum identified and protected with moist gauze. A ten centimeter segment of terminal ileum was identified, incised distally and ligated proximally and flushed with 5mL of 0.9% sodium chloride saline to remove feces. Once flushed, the enterotomy was closed and the distal end of the segment was ligated as well. Fluorescein isothiocyanate dextran (average molecular weight 4 KDa: FD4: Sigma, St. Louis) in concentration of 25mg/ml was prepared. A total of 1mL FD4 tracer was injected into the 10 cm segment, paying careful attention to avoid any spillage onto the external bowel, until the bowel segment was slightly dilated. The segment was protected from light and let to rest allowing for circulation for 30 minutes. Once the time was completed, 1mL of venous blood was removed from the internal jugular catheter into a heparinized syringe, protected from light and placed on ice. The blood specimen was centrifuged at 3000 RPFs for 10 minutes and the plasma was compared to a standard curve measured on FLX800 MicroplateFlourescence reader (Biotek, Winooski, VT) at excitation 485/20, emission 528/20 and a sensitivity of 40.
Nicotine application
Nicotine patches (7mg nicoderm CQ, Rite Aid, NJ) were cut into 6 equal pieces for a dosage of 3mg/kg or the equivalent to the serum level of 1pack per day of cigarette smoking (17). Male Sprague Dawley rats are shaved on their neck with a 1cmx1cm area. Nair hair removal cream is applied for 3 minutes then wiped off with moist gauze to ensure removal of stubble and facilitate adherence of the patch to the skin. The patch is placed on the denuded area and secured with two coats of liquid bandage (Johnson and Johnson liquid bandaid, Rite Aid, NJ). The patch was applied for 24 hours prior to shock.
Lymph Infusion Protocol – in vivo
Lymph samples previously collected from rats were injected into male C57BL/6 black mice as previously described (18). Mice underwent laparotomy and internal jugular vein cannulation with heparinized (0.1U/mL) silastic tubing. Lymph collected from each rat from 1–3hours post shock and pooled. The pooled lymph was infused into the mice via the jugular vein catheter at a rate of 1mL/kg/h for 3 hours. On completion of infusion, 50 uL blood was withdrawn from the jugular vein for the respiratory burst assay. Lung permeability to injected Evans Blue Dye (EBD) was determined.
Respiratory Burst
At the end of resuscitation, blood is drawn into a heparinized syringe and aliquoted into 5mL flow cytometry tubes and neutrophils are assayed as previously described (19). Lyse Buffer, 1% PharmM Lyse (BD Pharmingen, San Diego), 3mL, is added to each tube and incubated for 15 minutes. Tubes are spun at 1135 RPM for 5 minutes at 25°C. Neutrophil tubes are washed twice with 1mL of Hanks Balanced Salt Solution (HBSS). The supernatant is discarded and the pellet resuspended with 400uL of HBSS. Plasma from animals is added at 5% into the tubes and incubated for 15 minutes at 37°C and protected from light. Dihydrorhodamine (10mg,Molecular Probes, Inc) at 15ng/mL is added to tubes and incubated for 15 min at 37°C. Phorbol 12-myristate 13 acetate (PMA, Sigma) is added at 90ng/mL to the samples and incubated for 15 min at 37°C. Respiratory burst is measured by flow cytometry.
Myeloperoxidase (MPO) assay in pulmonary tissue
Frozen lung tissue was homogenized and processed for MPO level measurement as previously described(1). One unit of MPO activity represents the amount of enzyme that will reduce 1 µmol/min of peroxide.
Red Blood Cell (RBC) deformability
Red blood cell deformability was determined by laser diffraction analysis using an ektacytometer (LORCA, RR Mechatronics; Hoorn, the Netherlands) as previously described (20). Briefly, rotational shear stress was applied to RBC samples, and the degree of RBC deformability measured. In this system, a laser beam was projected through the sample, and the RBC diffraction pattern produced was analyzed by a microcomputer. Red blood cell deformability was assessed by calculating the elongation index (EI) at shear stresses ranging from 0.3 to 30 Pa. From the shear stress elongation curve created above, the data were further analyzed using a double-reciprocal plot to determine the overall degree of deformability changes as previously described(21). The calculated Kei is the level of shear stress (in Pascal) that is required for the erythrocytes to reach 50 percent of their maximal elongation. Thus, RBC deformability alterations were assessed by either the EI measured at low shear stress similar to that occurring during low flow conditions in the microcirculation (0.3 Pa) as well as by calculating Kei, which is an indicator of the overall status of RBC deformability. Because EI is a direct measure of RBC deformability, the smaller the number, the less deformable are the cells. In contrast, because the Kei is a measure of the amount of stress needed to deform the RBC to half-maximal deformation, an increase in Kei indicates a decrease in RBC deformability.
Statistical Analysis
Results were expressed as mean and standard error of the mean (SEM). Continuous data were analyzed by unpaired t test and one-way analysis of variance using the post hoc Tukey-Kramer multiple comparison tests. P values less than 0.05 were considered statistically significant.
Results
Vagus nerve stimulation decreases organ injury after trauma-hemorrhage shock
T/HS caused approximately a 10-fold increase in gut permeability to the permeability probe FD4 (Table 1). Consistent with our hypothesis, vagus nerve stimulation prevented this T/HS-induced loss of barrier function (Table 1). VNS was also associated with abrogation of lung injury as reflected in decreased lung permeability to Evans blue dye (EBD) (Table 1). In a similar fashion, the increase in neutrophil priming observed after T/HS was prevented by vagal stimulation. This protective effect of vagal stimulation was not due to a less severe shock state, since the base deficit was equally decreased in the two T/HS shock groups at the end of the 90 minute shock period (Table 2), while no changes were observed in the T/SS groups. Similar decreases in serum biocarbonate and anion gap values were observed in the T/HS groups as was observed for the base deficit (data not shown). No significant decrease in blood pH values or other electrolyte values (Na, K, Cl) were observed in any of the groups (data not shown). Lastly, although vagus nerve stimulation reduced organ injury after T/HS, the maximal volume of blood required to be removed during the 90 minute shock period was less in the vagal-stimulated rats (2.6 ± 0.4 ml/100 gm body weight) than the sham vagal-stimulated T/HS rats (3.1 ± 0.2 ml/100 gm body weight) (p < 0.05).
Table 1. VNS protects organs from T/HS-induced injury.
VNS protects organs from T/HS-induced injury: A) VNS significantly decreases gut permeability as compared to T/HS (0.53 ± 0.4 vs 4.3± 2.8 µg/mL of plasma FD4). B) VNS decreases lung injury as measured by Evans Blue dye (EBD) permeated through lung parenchyma as compared to T/HS (4.9 ± 0.8 vs 8.5±0.4 % EBD permeated). C) Neutrophil priming is reduced with VNS in T/HS as compared with T/HS controls (282 ± 38 vs 396± 69 MFU). There was no statistical significance between T/HS + VNS and the sham-shock groups. *represents p < 0.05.
| GUT INJURY (FD4 ng/mL) |
LUNG INJURY (%EBD) | PMN PRIMING (MFU) | |
|---|---|---|---|
| T/HS | *4.3±2.8 (n=4) | *8.5±0.4 (n=4) | *396±69 (n=4) |
| T/HS + VNS | 0.53±.4 (n=6) | 4.9±0.8 (n=4) | 282±38 (n=4) |
| T/SS | 0.33±0.2 (n=6) | 2.8±0.8 (n=4) | 210±31 (n=4) |
| T/SS + VNS | 0.3±0.1 (n=6) | 2.2±0.6 (n=4) | 221±13 (n=4) |
p<0.05 T/HS vs all groups,
p<0.05 T/HS vs T/SS, T/SS+VNS. Data presented as mean +/− SD
Table 2. Base Deficit is unchanged in both T/HS groups.
Protective changes in VNS were not a result of different degrees of shock as represented by the base deficit obtained from the animals during shock. There were no statistical differences between the T/HS and the T/HS+VNS groups.
| Pre-shock | After shock | Post 3 hours | |
|---|---|---|---|
| T/HS | 5.0±4.1 | −10.5±4.4* | 2.0±0.8 |
| T/HS + VNS | 1.25±0.1 | −9.25±2.6* | 3.0±1.6 |
| T/SS | 2.25±1.3 | 3.75±2.6 | 4.0±1.8 |
| T/SS + VNS | 3.5±1.3 | 2.25±2.1 | 1.75±1.0 |
N-4 in all groups, Data shown as mean±SEM;
p< 0.01 vs T/SS groups
Mesenteric lymph from vagus nerve-stimulated T/HS rats has reduced biologic activity
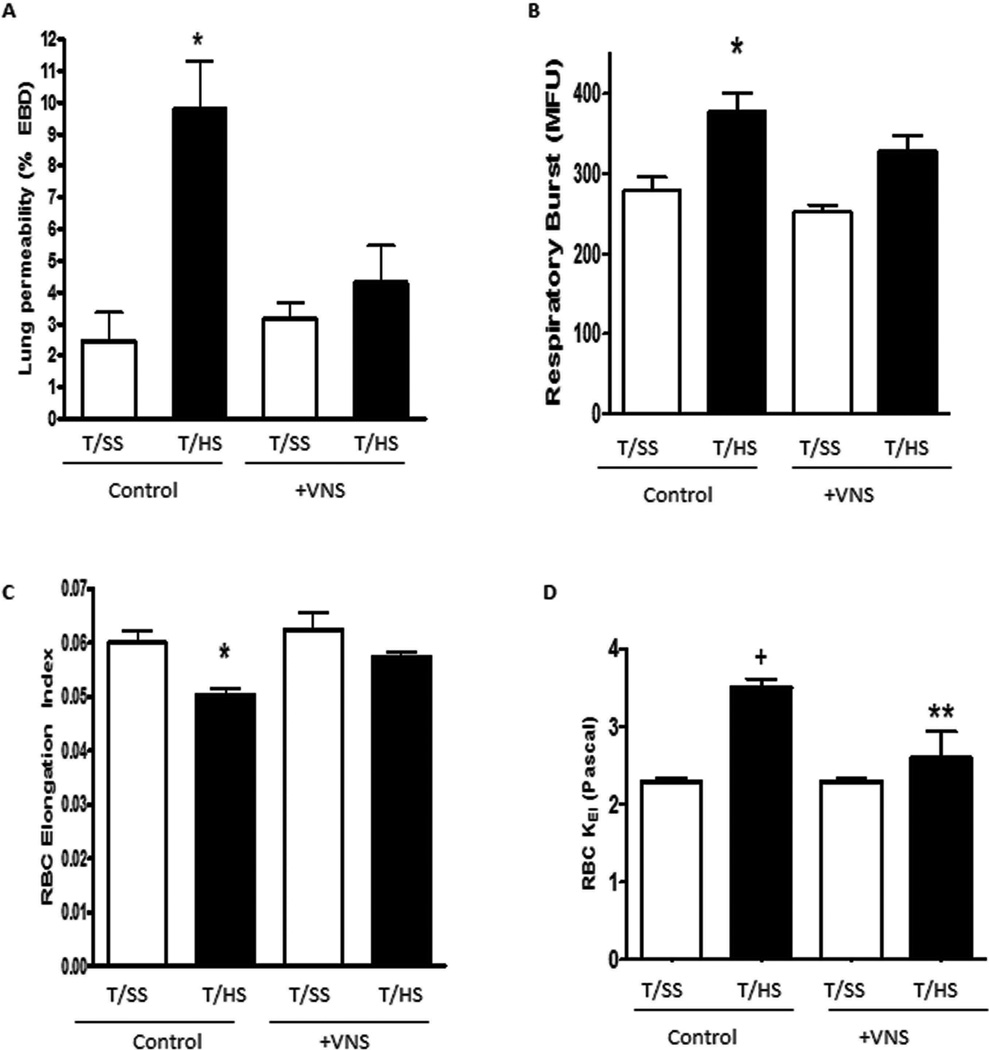
Infusion of mesenteric lymph from rats subjected to T/HS, but not T/SS, into naïve mice caused lung injury, increased neutrophil priming and loss of RBC deformability (Figure 1). Thus the in vivo injection of T/HS lymph recreates the changes observed in lung injury, neutrophil priming and RBC dysfunction observed after actual T/HS. However, the mesenteric lymph from the vagus nerve-stimulated T/HS rats did not cause an increase in lung permeability (Figure 1A). Additionally, the neutrophils collected from the mice injected with vagus nerve-stimulated mesenteric lymph were not primed for an augmented in vitro respiratory burst (Figure 1B) nor had the RBCs from these mice become less deformable (Figure 1C). The changes in RBC correlate with the measured stress required to deform the RBCs (Figure 1D). These in vivo lymph injection results support the second hypothesis of this study, which is that vagus-nerve stimulation limits T/HS-induced lung injury and neutrophil priming, at least in part, by preventing gut injury and hence limiting the generation of biologically active mesenteric lymph.
FIG 1.
In vivo effect of the injection of mesenteric lymph from rats subjected to T/HS or T/SS with or without VNS on (A) lung permeability to EBD, (B) neutrophil respiratory burst, and (C) RBC deformability as measured by the elongation index and (D) stress forces required to deform RBC as measured by Kei. Data expressed as mean (SEM). *P G 0.05 T/HS versus all other groups; +P G 0.01 versus all other groups; **P G 0.05 versus T/SS. n = 6Y7 in T/HS groups, n = 4Y7 in T/SS groups.
Role of the spleen and stimulation of the nicotinic acetylcholine receptor in the pathogenesis of the protective effects of vagus nerve stimulation
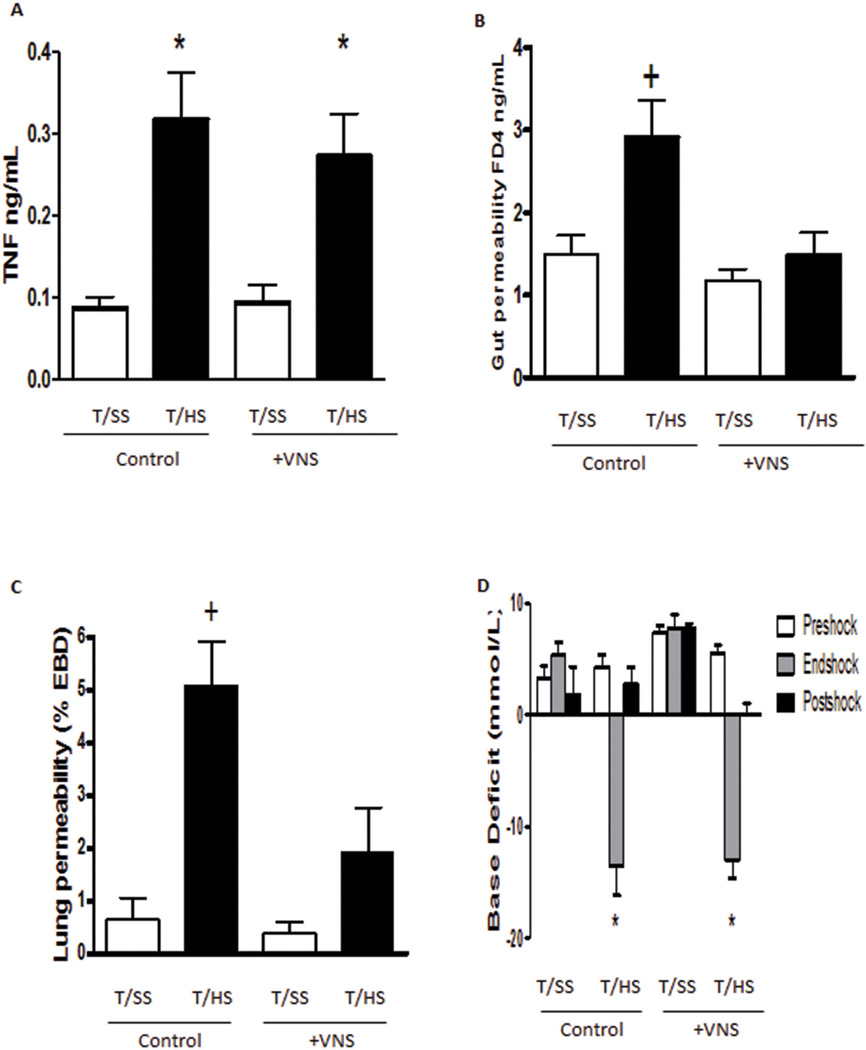
Since the previous studies indicate that the anti-inflammatory potential of the vagus nerve in endotoxemia is mediated by the spleen, we also analyzed whether its protective effect in trauma-hemorrhagic shock was also mediated by the spleen. Similar to endotoxemia, trauma-hemorrhagic shock also caused systemic inflammation and the increases of serum TNFα levels in the rats subjected to T/HS but not T/SS (Figure 2A). However, this increase in T/HS-induced TNF was not reduced by vagus nerve stimulation (Figure 2A). To determine whether the presence of the spleen was required for the protective effects of vagus nerve stimulation, splenectomized rats were subjected to T/HS or T/SS with or without vagus nerve stimulation. Vagus nerve stimulation abrogated T/HS-induced increases in gut and lung permeability in the splenectomized rats (Figure 2B, C) similar to the effects observed in non-splenectomized rats. T/HS resulted in a significant base deficit in both the vagus nerve stimulated and sham stimulated splenectomized rats (Figure 2D). Thus, in the current T/HS model, vagus nerve protection does not reduce serum TNF levels nor is it mediated by the spleen.
FIG 2.
A, T/HS-induced plasma TNF is not prevented by VNS; (B) VNS remains protective after splenectomy, because VNS protects against T/Hsinduced gut injury and (C) lung injury after splenectomy (SPX). D, VNS does not reduce the increased base deficit observed after T/HS. Data expressed as mean (SEM). n = 6 in T/HS groups, n = 4 in the T/SS groups. *P G 0.01 versus T/SS groups; +P G 0.05 versus all other groups.
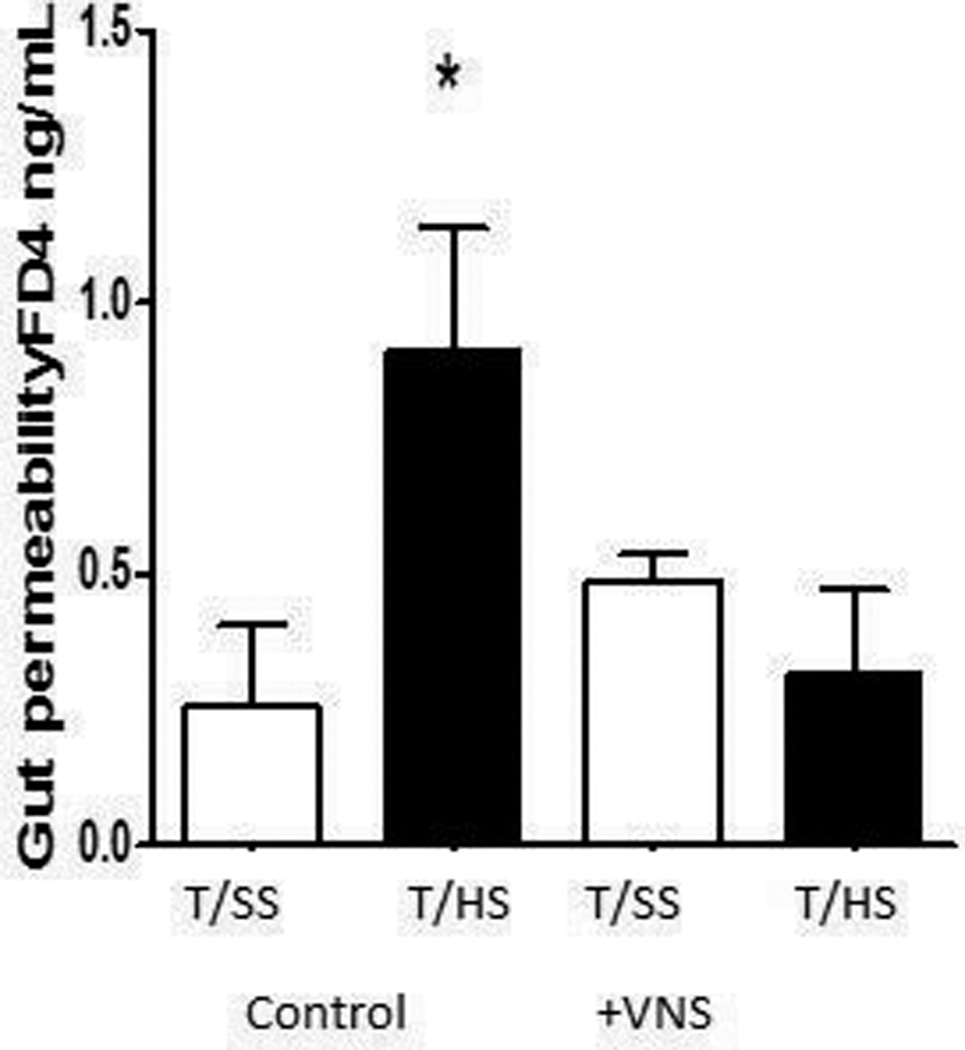
Since vagus nerve stimulation was gut protective in rats subjected to T/HS, we tested and found that this vagal protection could be replicated by the topical administration of nicotine (Figure 3). This observation supports previous studies in non-T/HS models indicating that protective effect of vagal stimulation involves activation of the nicotinic acetylcholine receptor (22–24)
FIG 3.
Nicotine protects against T/HS-induced gut injury. n = 5 in T/HS groups, n = 3 in T/SS groups. Data expressed as mean (SEM). *P G 0.05 versus all other groups.
Discussion
The key observation of this study was that VNS was sufficient to prevent T/HS-induced gut injury, the production of biologically active mesenteric lymph and the subsequent development of PMN priming and lung injury. These results are consistent with studies in other model systems showing that VNS can be gut protective (14, 25, 26) and extends our studies on the gut lymph hypothesis of MODS, where T/HS-induced gut injury is transduced into a systemic inflammatory state which leads to distant organ dysfunction (27, 28). Although VNS has been shown to limit gut injury in other models (14, 25, 26), to our knowledge, this is the first study directly examining the effects of VNS on the biologic activity of mesenteric lymph. Because of the fact that in vitro observations do not always correlate with in vivo responses, we chose to test the ability of VNS to abrogate or limit the biologic activity of T/HS mesenteric lymph using an in vivo model system where lymph samples are infused into naïve mice and their systemic effects are measured. Using this stringent and more clinically relevant in vivo system, we previously documented that naïve rats or mice challenged with T/HS but not T/SS lymph manifest lung injury, PMN priming and RBC dysfunction (18, 28, 29). Abnormalities in red blood cell deformability are a marker of systemic inflammation and contribute to microvascular dysfunction. Thus, in essence, the injection of T/HS lymph was sufficient to recreate several of the adverse consequences of T/HS in otherwise unstressed rodents. Using this in vivo model system, the current work clearly documented that VNS, not only preserved gut barrier function after T/HS, but also abrogated the biologic activity of T/HS lymph, since the injection of T/HS lymph from VNS rats into the mice did not cause cellular or organ dysfunction. This ability of VNS to abrogate the biologic activity of T/HS lymph is of potential biologic and clinical significance, since it is through the production of biologically active mesenteric lymph that T/HS induces SIRS response and organ injury (28). Additionally, our observation that VNS prevents the production of biologically active mesenteric lymph is also of potential significance in interpreting the results of earlier studies showing that VNS protected against burn-induced acute lung injury (30), since we have documented that it is the proinflammatory and tissue injurious factors released from the stressed gut and present in mesenteric lymph that are primarily responsible for acute lung injury and innate immune activation that occurs after burn injury as well as T/HS (1, 7, 28).
The notion that systemic hemodynamic insults, such as hemorrhagic, cardiac or septic shock, can be modulated by the nervous system is not new, since the role of the sympathetic and parasympathetic nervous systems in regulating cardiac function and vasomotor tone is well established (31). However, the recent discovery of the parasympathetic anti-inflammatory pathway, where VNS through activation of the α7 nicotinic acetylcholine receptor subunit (α7nAChR), has been shown to limit the inflammatory response in infectious and inflammatory states has expanded our recognition of the importance of neuromodulation of non-vascular tissues in these conditions (8, 10, 23, 24). This organism-wide modulatory effect of VNS stimulation is not surprising since the vagus nerve innervates most of the peripheral organs as well as the cardiovascular system. The now classical studies establishing the nictonic anti-inflammatory response and investigating the mechanisms underlying this anti-inflammatory response were largely carried out in models of endotoxemia (8, 10, 11, 23, 24). These and subsequent studies indicate that VNS protection requires an intact spleen and is associated with a reduction in several pro-inflammatory cytokines, especially TNF (8, 10–13, 23, 24, 32). Thus, we examined the role of the spleen and TNF in the protective effects of VNS after T/HS. Since VNS did not reduce the systemic TNF response to T/HS in our studies, it does not appear likely that VNS is exerting its gut-protective effects by inhibiting the TNF response to T/HS. In contrast to what is observed in studies examining the ability of VNS to inhibit the immuno-inflammatory response to endotoxin in splenectomized animals (12, 32), removal of the spleen did not abrogate the protective effects of VNS after T/HS. Thus, the pathway and mechanisms by which VNS exerted its protective effects after T/HS appears to be independent of the spleen. However, consistent with the vagus nerve-mediated anti-inflammatory pathway hypothesis, nicotine was able to recreate the gut-protective effects of VNS in rats subjected to T/HS. This protective effect of nicotine in T/HS complements studies showing that treatment with nicotine attenuates systemic inflammation and improves survival in experimental sepsis (8, 13). This work also highlights the importance of the nicotinic receptor in the protective effects of VNS. Although the results show very clearly that Vagus nerve stimulation produces consistent results, at this time clinical strategy is not well established. Although the findings highlight an important potential intervention, work in this field requires more established clinical implementation. As we better understand the molecular mechanisms behind the Vagus nerve stimulation, we strive to develop a pharmacologic strategy for potential treatment.
In conclusion, we find that vagus nerve stimulation protects against trauma-hemorrhagic shock-induced gut injury and the formation of toxic lymph thereby limiting a SIRS state and avoiding lung injury. This is a splenic-independent pathway giving rise to the question of whether this is primarily a local effect of the vagus nerve on the gut or if other systemic systems are involved.
Acknowledgments
Sources of support: RO1 GM059841 (EAD) and T32-GM069330 (VA)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest to report
Contributor Information
Gal Levy, Email: levyga@umdnj.edu.
Jordan E Fishman, Email: fishmajo@umdnj.edu.
Dazhong Xu, Email: dxu@umdnj.edu.
Benjamin TJ Chandler, Email: benjichandler@gmail.com.
Eleonora Feketova, Email: feketeel@umdnj.edu.
Wei Dong, Email: dongw1@umdnj.edu.
Yong Qin, Email: qinmdyo@umdnj.edu.
Vamsi Alli, Email: vamsi.alli@gmail.com.
Luis Ulloa, Email: mail@luisulloa.com.
Edwin A Deitch, Email: edeitch@umdnj.edu.
References
- 1.Magnotti L, Upperman J, Xu D, et al. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228(4):518–527. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deitch E, Morrison J, Berg R, Specian R. Effect of hemorrhagic shock on bacterial translocation, intestinal morphology, and intestinal permeability in conventional and antibiotic-decontaminated rats. Crit Care Med. 1990;18(5):529–536. doi: 10.1097/00003246-199005000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Deitch E, Xu D, Franko L, et al. Evidence favoring the role of the gut as a cytokine-generating organ in rats subjected to hemorrhagic shock. Shock. 1994;1(2):141–145. doi: 10.1097/00024382-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Upperman J, Deitch E, Guo W, et al. Post-hemorrhagic shock mesenteric lymph is cytotoxic to endothelial cells and activates neutrophils. Shock. 1998;10(6):407–414. doi: 10.1097/00024382-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Cohen D, Magnotti L, Lu Q, et al. Pancreatic duct ligation reduces lung injury following trauma and hemorrhagic shock. Ann Surg. 2004;240(5):885–891. doi: 10.1097/01.sla.0000143809.44221.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin X, Sheth SU, Sharpe SM, et al. The mucus layer is critical in protecting against ischemia-reperfusion-mediated gut injury and in the restitution of gut barrier function. Shock. 2011;35(3):275–281. doi: 10.1097/SHK.0b013e3181f6aaf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnotti LJ, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph: a link between burn and lung injury. Arch Surg. 1999;134(12):1333–1340. discussion 40-1. [PubMed] [Google Scholar]
- 8.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4(8):673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 9.Downing JE, Miyan JA. Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today. 2000;21(6):281–289. doi: 10.1016/s0167-5699(00)01635-2. [DOI] [PubMed] [Google Scholar]
- 10.Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 11.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 12.Huston JM, Ochani M, Rosas-Ballina M, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203(7):1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10(11):1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 14.Costantini TW, Bansal V, Peterson CY, et al. Efferent vagal nerve stimulation attenuates gut barrier injury after burn: modulation of intestinal occludin expression. J Trauma. 2010;68(6):1349–1354. doi: 10.1097/TA.0b013e3181dccea0. discussion 54-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deitch E, Adams C, Lu Q, Xu D. A time course study of the protective effect of mesenteric lymph duct ligation on hemorrhagic shock-induced pulmonary injury and the toxic effects of lymph from shocked rats on endothelial cell monolayer permeability. Surgery. 2001;129(1):39–47. doi: 10.1067/msy.2001.109119. [DOI] [PubMed] [Google Scholar]
- 16.Caputo F, Rupani B, Watkins A, et al. Pancreatic duct ligation abrogates the trauma hemorrhage-induced gut barrier failure and the subsequent production of biologically active intestinal lymph. Shock. 2007;28(4):441–446. doi: 10.1097/shk.0b013e31804858f2. [DOI] [PubMed] [Google Scholar]
- 17.Kalra R, Singh SP, Pena-Philippides JC, et al. Immunosuppressive and anti-inflammatory effects of nicotine administered by patch in an animal model. Clin Diagn Lab Immunol. 2004;11(3):563–568. doi: 10.1128/CDLI.11.3.563-568.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senthil M, Watkins A, Barlos D, et al. Intravenous injection of trauma-hemorrhagic shock mesenteric lymph causes lung injury that is dependent upon activation of the inducible nitric oxide synthase pathway. Ann Surg. 2007;246(5):822–830. doi: 10.1097/SLA.0b013e3180caa3af. [DOI] [PubMed] [Google Scholar]
- 19.Deitch E, Ananthakrishnan P, Cohen D, et al. Neutrophil activation is modulated by sex hormones after trauma-hemorrhagic shock and burn injuries. Am J Physiol Heart Circ Physiol. 2006;291(3):H1456–H1465. doi: 10.1152/ajpheart.00694.2005. [DOI] [PubMed] [Google Scholar]
- 20.Zaets S, Berezina T, Caruso J, et al. Mesenteric lymph duct ligation prevents shock-induced RBC deformability and shape changes. J Surg Res. 2003;109(1):51–56. doi: 10.1016/s0022-4804(02)00024-0. [DOI] [PubMed] [Google Scholar]
- 21.Condon MR, Kim JE, Deitch EA, et al. Appearance of an erythrocyte population with decreased deformability and hemoglobin content following sepsis. Am J Physiol Heart Circ Physiol. 2003;284(6):H2177–H2184. doi: 10.1152/ajpheart.01069.2002. [DOI] [PubMed] [Google Scholar]
- 22.Cai B, Chen F, Ji Y, et al. Alpha7 cholinergic-agonist prevents systemic inflammation and improves survival during resuscitation. J Cell Mol Med. 2009;13(9B):3774–3785. doi: 10.1111/j.1582-4934.2008.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151(7):915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 25.Costantini TW, Bansal V, Krzyzaniak M, et al. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am J Physiol Gastrointest Liver Physiol. 2010;299(6):G1308–G1318. doi: 10.1152/ajpgi.00156.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bansal V, Costantini T, Ryu SY, et al. Stimulating the central nervous system to prevent intestinal dysfunction after traumatic brain injury. J Trauma. 2010;68(5):1059–1064. doi: 10.1097/TA.0b013e3181d87373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deitch E, Bridges W, Berg R, et al. Hemorrhagic shock-induced bacterial translocation: the role of neutrophils and hydroxyl radicals. J Trauma. 1990;30(8):942–951. doi: 10.1097/00005373-199008000-00002. discussion 51-2. [DOI] [PubMed] [Google Scholar]
- 28.Deitch E, Xu D, Kaise V. Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci. 2006;11:520–528. doi: 10.2741/1816. [DOI] [PubMed] [Google Scholar]
- 29.Condon M, Senthil M, Xu DZ, et al. Intravenous injection of mesenteric lymph produced during hemorrhagic shock decreases RBC deformability in the rat. J Trauma. 2011;70(2):489–495. doi: 10.1097/TA.0b013e31820329d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krzyzaniak MJ, Peterson CY, Cheadle G, et al. Efferent vagal nerve stimulation attenuates acute lung injury following burn: The importance of the gut-lung axis. Surgery. 2011;150(3):379–389. doi: 10.1016/j.surg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grigorean V, Sandu A, Popescu M, et al. Cardiac dysfunctions following spinal cord injury. J Med Life. 2009;2(2):133–145. [PMC free article] [PubMed] [Google Scholar]
- 32.Vida G, Peña G, Deitch EA, Ulloa L. α7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J Immunol. 2011;186(7):4340–4346. doi: 10.4049/jimmunol.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]