Abstract
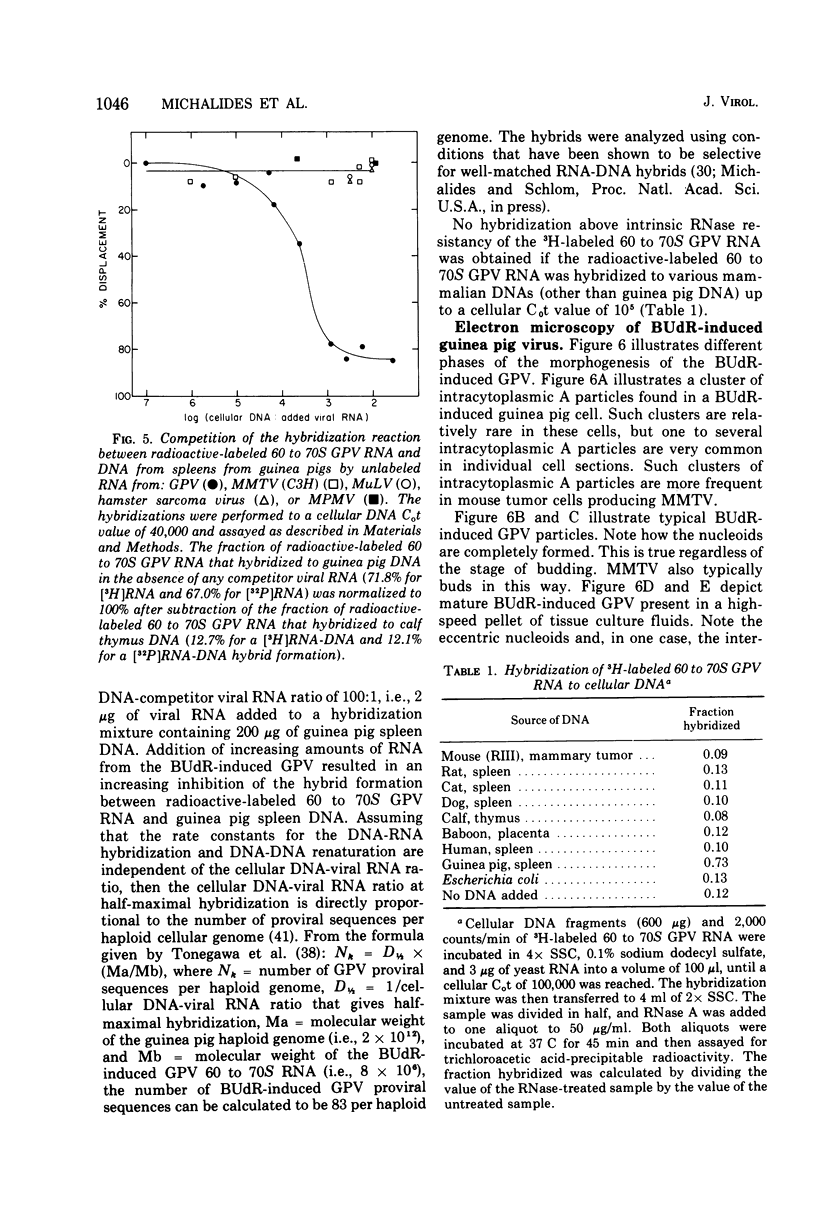
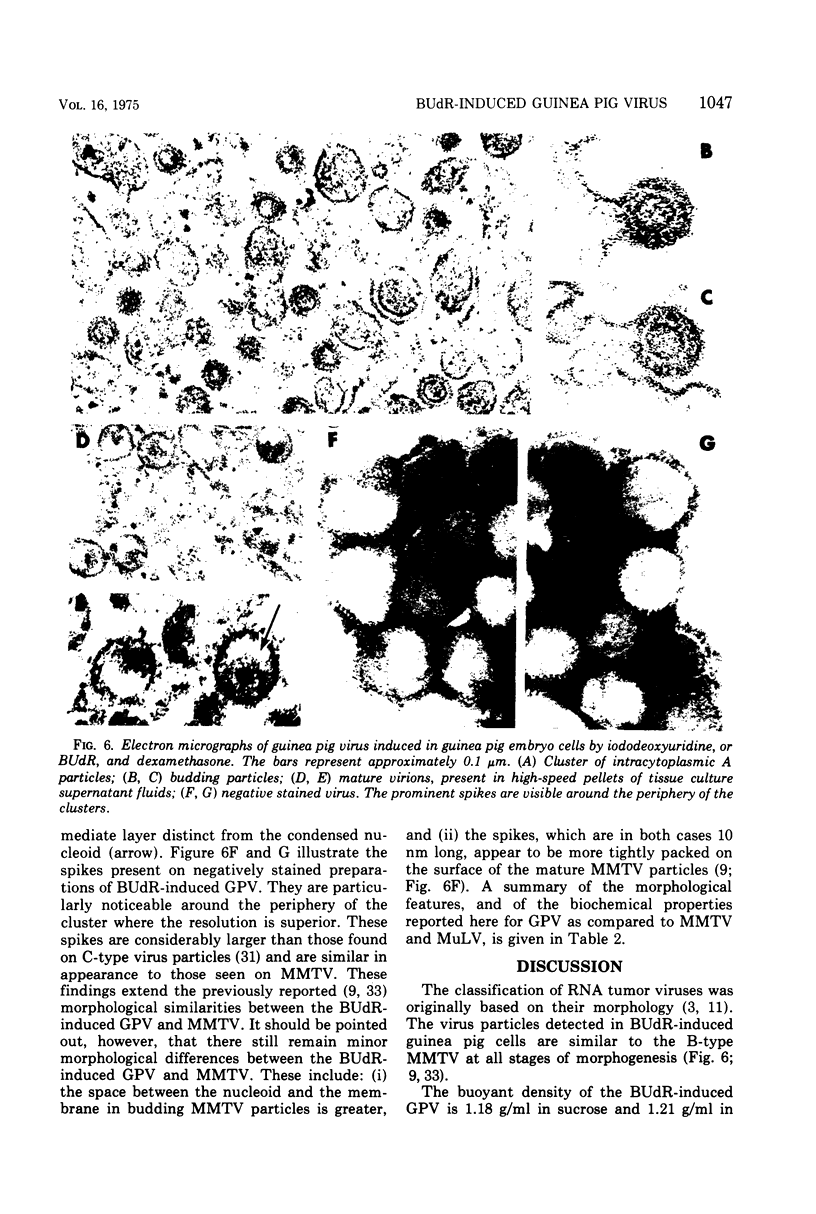
The biophysical and biochemical properties of the virus particles released by guinea pig embryo cells treated with 5-bromo-2'-deoxyuridine (BUdR) have been compared to those of the B-type mouse mammary tumor virus (MMTV) and the C-type Rauscher murine leukemia virus. The high-molecular-weight (60 to 70S) RNA of the BUdR-induced guinea pig virus (GPV) has a molecular weight of 8 X 106 when measred by mixed agarose polyacylamide gel electrophoresis. The virus particles isolated from the tissue culture medium of BUdR-induced guniea pig cells have the following properties in common with MMTV: (i) a buoyant density of 1.18 g/ml in sucrose and 1.21 g/ml in CsCl, and (ii) a DNA polymerase that prefers Mg2+ over Mn2+ in an assay using the synthetic template poly(rC):oligo(dG). No nucleic acid sequence homology between GPV RNA and the viral RNAs of the MMTV, murine leukemia virus, hamster sarcoma virus, or Mason-Pfizer monkey virus could be observed in a competition hybridization assay using the radioactive-labeled GPV 60 to 70S RNA. By this same competition by hybridization assay the frequency of GPV proviral sequences was estimated to be at least 83 per haploid cellular genome of guniea pig cells. No nucleic acid sequences related to be GPV RNA were detected in the DNA of normal tissues of mice, rats, cats, dogs, baboons, or humans by direct RNA-DNA hybridization using radioactive GPV60 to 70S RNA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHARD W. The detection and study of tumor viruses with the electron microscope. Cancer Res. 1960 Jun;20:712–727. [PubMed] [Google Scholar]
- Baltimore D., Smoler D. Primer requirement and template specificity of the DNA polymerase of RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1507–1511. doi: 10.1073/pnas.68.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M. A., Parsons J. T., Coffin J. M. The nucleotide sequence complexity of avian tumor virus RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3560–3564. doi: 10.1073/pnas.71.9.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Callahan R., Benveniste R. E., Lieber M. M., Todaro G. J. Nucleic acid homology of murine type-C viral genes. J Virol. 1974 Dec;14(6):1394–1403. doi: 10.1128/jvi.14.6.1394-1403.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra H. C., Zelljadt I., Jensen E. M., Mason M. M., Woodside N. J. Infectivity of cell cultures by a virus isolated from a mammary carcinoma of a rhesus monkey. J Natl Cancer Inst. 1971 Jan;46(1):127–137. [PubMed] [Google Scholar]
- Chopra H. C., Zelljadt I., Woodside N., Walling M. J. Studies on virus particles resembling oncogenic RNA viruses in monkey breast carcinoma. Cancer. 1971 Dec;28(6):1406–1414. doi: 10.1002/1097-0142(197112)28:6<1406::aid-cncr2820280613>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E., Perk K., Dalton A. J. Virus-like particles induced in guinea pig cells by 5-bromo-2'-deoxyuridine are morphologically similar to murine B-type virus. Nature. 1974 Jun 28;249(460):828–830. doi: 10.1038/249828a0. [DOI] [PubMed] [Google Scholar]
- Dalton A. J. RNA tumor viruses. Terminology and ultrastructural aspects of virion morphology and replication. J Natl Cancer Inst. 1972 Aug;49(2):323–327. [PubMed] [Google Scholar]
- De-Thé G., O'Connor T. E. Structure of a murine leukemia virus after disruption with tween-ether and comparison with two myxoviruses. Virology. 1966 Apr;28(4):713–728. doi: 10.1016/0042-6822(66)90256-x. [DOI] [PubMed] [Google Scholar]
- Dion A. S., Vaidya A. B., Fout G. S. Cation preferences for poly(rC)-oligo(dG)-directed DNA synthesis by RNA tumor viruses and human milk particulates. Cancer Res. 1974 Dec;34(12):3509–3515. [PubMed] [Google Scholar]
- Duesberg P., Helm K. V., Canaani E. Comparative properties of RNA and DNA templates for the DNA polymerase of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2505–2509. doi: 10.1073/pnas.68.10.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman N. C., Spiegelman S. Distinguishing reverse transcriptase of an RNA tumor virus from other known DNA polymerases. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2203–2206. doi: 10.1073/pnas.68.9.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Gerard G. F. RNA-directed DNA polymerase--properties and functions in oncogenic RNA viruses and cells. Prog Nucleic Acid Res Mol Biol. 1974;14(0):187–334. [PubMed] [Google Scholar]
- Gross P. A., Fong C. K., Hsiung C. D. Characterization of guinea pig C-type virus. Proc Soc Exp Biol Med. 1973 Jun;143(2):367–370. doi: 10.3181/00379727-143-37322. [DOI] [PubMed] [Google Scholar]
- Howk R. S., Rye L. A., Killeen L. A., Scolnick E. M., Parks W. P. Characterization and separation of viral DNA polymerase in mouse milk. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2117–2121. doi: 10.1073/pnas.70.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung G. D. Activation of guinea pig C-type virus in cultured spleen cells by 5-bromo-2'-deoxyuridine. J Natl Cancer Inst. 1972 Aug;49(2):567–570. [PubMed] [Google Scholar]
- Klement V., Nicolson M. O., Huebner R. J. Rescue of the genome of focus forming virus from rat non-productive lines by 5'-bromodeoxyruidine. Nat New Biol. 1971 Nov 3;234(44):12–14. doi: 10.1038/newbio234012a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Livingston D. M., Todaro G. J. Endogenous type C virus from a cat cell clone with properties distinct from previously described feline type C virus. Virology. 1973 May;53(1):142–151. doi: 10.1016/0042-6822(73)90473-x. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Manning J. S., Hackett A. J., Cardiff R. D., Mel H. C., Blair P. B. Isopycnic zonal centrifugation and characterization of the mouse mammary tumor virus (MTV) in different gradient solutions. Virology. 1970 Apr;40(4):912–919. doi: 10.1016/0042-6822(70)90137-6. [DOI] [PubMed] [Google Scholar]
- Murray P. R., Nayak D. P. Characterization of bromodeoxyuridine-induced endogenous guinea pig virus. J Virol. 1974 Sep;14(3):679–688. doi: 10.1128/jvi.14.3.679-688.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P. Endogenous guinea pig virus: equability of virus-specific DNA in normal, leukemic, and virus-producing cells. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1164–1168. doi: 10.1073/pnas.71.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P., Murray P. R. Induction of type C viruses in cultured guinea pig cells. J Virol. 1973 Jul;12(1):177–187. doi: 10.1128/jvi.12.1.177-187.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman P. E. Measurement of endogenous leukosis virus nucleotide sequences in the DNA of normal avian embryos by RNA-DNA hybridization. Virology. 1973 May;53(1):196–203. doi: 10.1016/0042-6822(73)90478-9. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Frank H., Schäfer W. Properties of mouse leukemia viruses. 3. Electron microscopic appearance as revealed after conventional preparation techniques as well as freeze-drying and freeze-etching. Virology. 1972 Aug;49(2):345–358. doi: 10.1016/0042-6822(72)90487-4. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Wuu K. D., Ro H. S., Vernon M. L., Huebner R. J. Induction of guinea pig leukemia-like virus from cultured guinea pigs cells. Proc Soc Exp Biol Med. 1974 Oct;147(1):323–330. doi: 10.3181/00379727-147-38335. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Moore D. H. Separation of B and C type virions by centrifugation in gentle density gradients. J Virol. 1974 May;13(5):1143–1147. doi: 10.1128/jvi.13.5.1143-1147.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochetman G., Schlom J. RNA subunit structure of Mason-Pfizer monkey virus. J Virol. 1975 Feb;15(2):423–427. doi: 10.1128/jvi.15.2.423-427.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E., Rands E., Aaronson S. A., Todaro G. J. RNA-dependent DNA polymerase activity in five RNA viruses: divalent cation requirements. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1789–1796. doi: 10.1073/pnas.67.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Faras A. J., Varmus H. E., Goodman H. M., Levinson W. E., Bishop J. M. Transcription of ribonucleic acid by the ribonucleic acid directed deoxyribonucleic acid polymerase of Rous sarcoma virus and deoxyribonucleic acid polymerase I of Escherichia coli. Biochemistry. 1973 Jan 30;12(3):460–467. doi: 10.1021/bi00727a016. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Steinberg C., Dube S., Bernardini A. Evidence for somatic generation of antibody diversity. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4027–4031. doi: 10.1073/pnas.71.10.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trávnícek M., Ríman J. Subunits of oncornavirus high-molecular-weight RNA. I. Stepwise conversion of 60 S AMV (avian myeloblastosis virus) RNA to subunits. Biochem Biophys Res Commun. 1973 Jul 2;53(1):217–223. doi: 10.1016/0006-291x(73)91422-8. [DOI] [PubMed] [Google Scholar]
- Waters L. C., Yang W. K. Comparative biochemical properties of RNA-directed DNA polymerases from Rauscher murine leukemia virus and avian myeloblastosis virus. Cancer Res. 1974 Oct;34(10):2585–2593. [PubMed] [Google Scholar]
- Wright S. E., Neiman P. E. Base-sequence relationships between avian ribonucleic acid endogenous and sarcoma viruses assayed by competitive ribonucleic acid-deoxyribonucleic acid hybridization. Biochemistry. 1974 Mar 26;13(7):1549–1554. doi: 10.1021/bi00704a035. [DOI] [PubMed] [Google Scholar]
- Zavada J., Macpherson I. Transformation of hamster cell lines in vitro by a hamster sarcoma virus. Nature. 1970 Jan 3;225(5227):24–26. doi: 10.1038/225024a0. [DOI] [PubMed] [Google Scholar]