Abstract
Purpose
Epithelial junctions between tumor cells inhibit the penetration of anti-cancer drugs into tumors. We previously reported on recombinant adenovirus serotype 3 derived protein (JO-1), which triggers transient opening of intercellular junctions in epithelial tumors through binding to desmoglein 2 (DSG2), and enhances the anti-tumor effects of several therapeutic monoclonal antibodies. The goal of this study was to evaluate whether JO-1 co-therapy can also improve the efficacy of chemotherapeutic drugs.
Experimental Design
The effect of intravenous application of JO-1 in combination with several chemotherapy drugs including paclitaxel/Taxol™, nanoparticle albumin bound paclitaxel/Abraxane™, liposomal doxorubicin/Doxil™ and irinotecan/Camptosar™, was tested in xenograft models for breast, colon, ovarian, gastric and lung cancer. Because JO-1 does not bind to mouse cells, for safety studies with JO-1, we also used human DSG2 (hDSG2) transgenic mice with tumors that overexpressed human DSG2.
Results
JO-1 increased the efficacy of chemotherapeutic drugs, and in several models overcame drug resistance. JO-1 treatment also allowed for the reduction of drug doses required to achieve anti-tumor effects. Importantly, JO-1 co-admininstration protected normal tissues, including bone marrow and intestinal epithelium, against toxic effects that are normally associated with chemotherapeutic agents. Using the hDSG2 transgenic mouse model, we demonstrated that JO-1 predominantly accumulates in tumors. Except for a mild, transient diarrhea, intravenous injection of JO-1 (2mg/kg) had no critical side effects on other tissues or hematological parameters in hDSG2-transgenic mice.
Conclusions
Our preliminary data suggest that JO-1 co-therapy has the potential to improve the therapeutic outcome of cancer chemotherapy.
Introduction
One of the key features of epithelial tumors is the presence of intercellular junctions, which link cells to one another, and act as barriers to the penetration of molecules with a molecular weight (MW) of 500 dalton (Da) (1–3). Given that many chemotherapy drugs are larger than 500 Da, intercellular junctions represent a barrier to the penetration of these therapeutic agents into tumor. Several studies have shown that upregulation of epithelial junction proteins correlated with increased resistance to therapy, including therapy with monoclonal antibodies and chemotherapeutics (4, 5). One of these junction proteins is desmoglein 2 (DSG2). DSG2 is upregulated in malignant cells (6, 7). We found higher DSG2 immunoreactivity in breast cancer cells than in the surrounding normal epithelial tissue or tumor stroma cells (Suppl. Fig. 1).
Recently, we developed a recombinant protein (JO-1) that transiently triggers the opening of intercellular junctions in epithelial tumors. This work is based on our finding that DSG2 is a high-affinity receptor for a number of human adenoviruses (Ad), including Ad serotype 3 (8, 9). JO-1 is a self-dimerizing recombinant protein derived from the Ad3 fiber (10). JO-1 has a MW of ~60 kDa and binds with picomolar avidity to DSG2. It can be readily produced in E.coli and purified by affinity chromatography.
In mouse xenograft tumor models, we have shown that intravenous administration of JO-1 mediated cleavage of DSG2 dimers (between epithelial tumor cells) and activated intracellular signaling pathways, which reduced the expression of epithelial junction proteins in tumors (11). The morphological changes triggered by JO-1 occurred within one hour after intravenous JO-1 injection and allowed for increased intratumoral penetration of the anti-Her2/neu monoclonal antibody trastuzumab (Herceptin™) as well as for improved access to its target receptor, which is partly trapped in epithelial junctions (11). The effects of JO-1 on epithelial junctions translated into increased therapeutic efficacy of monoclonal antibodies (e.g. trastuzumab, cetuximab/Erbitux™) against several xenograft tumor models, including breast, colon, ovarian, gastric and lung carcinoma models (11).
Ad3 and its derivative JO-1 do not bind to mouse cells, implying that mouse DSG2 is not recognized (12). For safety studies with JO-1, we therefore used human DSG2 (hDSG2) transgenic mice that we recently generated. These mice express hDSG2 in a pattern and at a level similar to humans (12). For JO-1 efficacy studies we also created a mouse epithelial breast cancer line that expressed hDSG2 and formed tumors in hDSG2 transgenic mice.
Using human xenograft and mouse tumor models, we demonstrated that JO-1 increases the efficacy of a number of chemotherapy drugs that are widely used in the treatment of cancer patients.
Material and Methods
JO-1
The production of JO-1 in E.coli and its purification have been described previously (10).
Cell lines
Breast cancer BT474-M1 cells and MDA-MB-231 (ATCC, HTB-26) were cultured in DMEM/F12 with 10% FBS, 1% Pen/Strep and 2mM L-Glutamine. Breast cancer HCC1954 (ATTC, CRL-2338), lung cancer A549 (ATCC, CCL-185), prostate cancer 22Rv1 (ATCC CRL-2505), and mouse mammary carcinoma (MMC) and MMC-hDSG2 cells (12) were cultured in RMPI with 10% FBS and 1% Pen/Strep. To achieve cell polarization, 1.4×105 T84 cells (ATCC, CCL-248) were cultured in collagen-coated 6.5 mm Transwell inserts (0.4 μm pore size) (Costar Transwell Clears) for a period of 14 to 20 days until transepithelial resistance was stable (8).
Tissues
The preparation of OCT and paraffin sections has been described previously (12). Bones were decalcified before embedding in paraffin. A list of antibodies used for immunohistochemistry and flow cytometry is available in the SI. Images were taken with a Leica DMLB Microscope (Wetzlar, Germany), using Leica DFC300FX Digital camera and Leica Application Suite Version 2.4.1 R1 software (Heerbrugg, Germany).
Animal studies
All experiments involving animals were conducted in accordance with the institutional guidelines set forth by the University of Washington. Breast cancer xenografts were established by injecting tumor cells into the mammary fat pad of CB17 SCID-beige mice. Prostate and lung cancer models were created by subcutaneous injection. Human DSG2 transgenic mice were generated by knock-in of ~90kb of human DNA containing the desmoglein 2 gene together with its regulatory regions. The mice contain 2 copies of the human DSG2 gene and express hDSG2 in a pattern similar to humans (12). MMC-hDSG2 tumors were generated by subcutaneous injection of MMC-hDSG2 cells mixed with matrigel. JO-1 was intravenously injected one hour before the application of chemotherapy drugs. Tumor volumes were measured three times a week. Each treatment group consisted of a minimum of 5 mice. Animals were sacrificed and the experiment terminated when tumors in one of the groups reached a volume of 800 mm3 or tumors displayed ulceration. To produce anti-JO-1 antibodies, hDSG2 transgenic mice received three subcutaneous injections of 5 μg JO-1 at days 0, 3 and 14. Serum was collected 4 weeks later and analyzed for JO-1-specific antibodies by Western blot.
ELISA
To measure liposomal doxorubicin/Doxil concentrations, mice were sacrificed and blood was flushed from the circulation with 10ml PBS. Tissues were homogenized in PBS/0.1% Tween 20/Protease inhibitors. Anti-PEG antibody AGP4 (13) was used as a capture antibody. Binding was detected with anti-PEG antibody 3.3.-biotin (13) followed by a steptavidin-HRP conjugate. To measure JO-1 in tissue lysates, an anti-Ad3 fiber knob antibody (8) was used as capture antibody. JO-1 binding was detected using a mouse monoclonal antibody against the 6xHis tag of JO-1, followed by anti-mouse IgG-HRP.
Statistical analysis
All results are expressed as mean +/− SD. 2-Way ANOVA for multiple testing was applied. Animal numbers and P values are indicated in the figure legends.
Results
The goals of this study were to show that: i) JO-1 co-therapy specifically increases the anti-tumor efficacy of chemotherapeutic drugs in epithelial tumors and ii) JO-1 co-therapy allows dose sparing of chemotherapeutics, which in turn reduces adverse side effects associated with chemotherapy. Our study included the following chemotherapy drugs: i) Paclitaxel/Taxol™ (MW 856.9 Da). Paclitaxel is used to treat patients with lung, ovarian, breast, head and neck cancer and advanced forms of Kaposi’s sarcoma. ii) Nanoparticle albumin bound paclitaxel nab-paclitaxel/Abraxane™ (effective size of the particle: ~ 130 nm). nab-paclitaxel has recently been approved for the treatment of recurrent breast cancer. iii) Irinotecan/Camptosar™ (MW 586.7 Da). Irinotecan is used to treat colon and lung cancer. The most significant adverse effects of irinotecan are severe diarrhea and immunosuppression. iv) Liposomal doxorubicin/Doxil™ (effective size of the particle: ~ 90nm) is a polyethylene glycol coated, liposome-encapsulated form of doxorubicin. Liposomal doxorubicin is used in the treatment of several epithelial tumors, including ovarian and breast cancers. To test the effect of JO-1 on chemotherapy, we studied five xenograft models using immunodeficient CB17-SCID-beige mice carrying tumors derived from human tumor cell lines. The epithelial phenoptype and existence of tight junctions in the xenograft tumors was comfirmed by immunohistochemical detection of the junction proteins DSG2, E-cadherin, ZO-1 and claudin 7 (8, 14) (data not shown). Furthermore, we employed a hDSG2-transgenic mouse model with orthotopic breast tumors expressing hDSG2. The doses of chemotherapy drugs used in our studies were based on information reported in previous publications (15). These doses reflected doses used in patients converted through allometric scaling to the weigth of mice. Mice with pre-established tumors were intravenously injected with either JO-1 (2 mg/kg) or PBS one hour prior to intravenous/intraperitoneal administration of the chemotherapeutic agents. This regime had been found to be optimal in the combination therapy of JO-1 with monoclonal antibodies (14).
JO-1 improves the efficacy of chemotherapeutic drugs and can overcome drug resistance
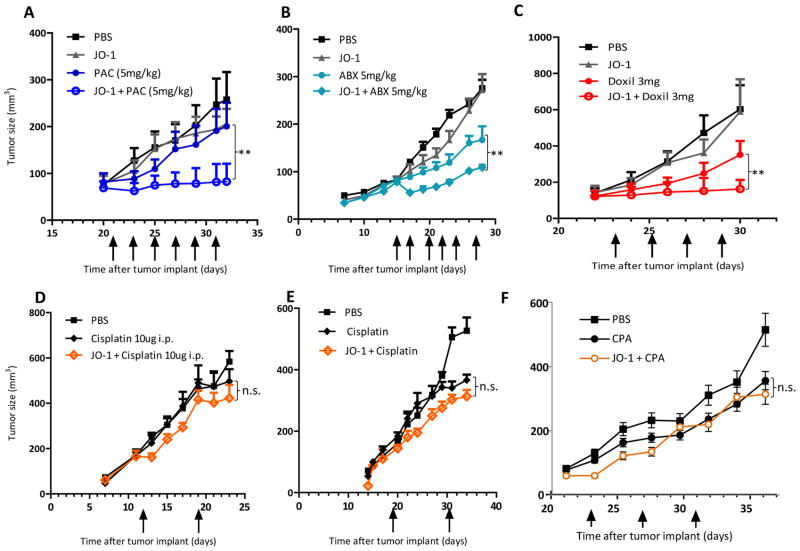
Co-therapy of JO-1 and paclitaxel was first tested in the BT474-M1 breast cancer model (Fig. 1A). These tumors are resistant to paclitaxel. JO-1 co-therapy was able to halt tumor growth and thus overcame resistance to paclitaxel treatment. JO-1/paclitaxel co-therapy was then evlatuated in the MDA-MB-231 breast cancer model (Suppl. Fig. 2). JO-1 injection alone had no significant therapeutic effect. Anti-tumor effect of paclitaxel alone was found, which was significantly enhanced by pre-injection of JO-1. The MDA-MB-231 tumor model was also used to study the effect of JO-1 on nab-paclitaxel therapy (Fig. 1B). Nab-paclitaxel was not significantly more effective than paclitaxel in decreasing tumor growth in this tumor model. Similar to paclitaxel, combining JO-1 with nab-paclitaxel therapy increased its therapeutic effects, whereby the enhancing effect was significantly more pronounced for nab-paclitaxel. This suggests that the enhancement in therapeutic efficacy through JO-1 is stronger for larger, particle-based drugs. Similarly, JO-1 was shown to enhance the anti-tumor effects of liposomal doxorubicin in the prostate cancer model (Fig. 1C) and the model with subcutaneous tumors derived from primary ovarian cancer cells (16) (Suppl. Fig. 3A). The residual tumor mass seen in JO-1 plus chemotherapy treated mice in Figs.1A and C only contained tumor stroma and infiltrating leukocytes upon histological inspection. Furthermore, in these studies, no tumor growth relapse was observed when mice were followed for a total of 60 days after the start of the experiment.
Figure 1. JO-1 improves efficacy of chemotherapy agents in several cancer models.
A) A total of 4×106 BT474-M1 cells, were injected into the mammary fat pad of CB17-SCID/beige mice. Twenty-one days later when tumors reached a volume of ~80 mm3, mice received an intravenous injection of 50 μg of JO-1 (2 mg/kg) or PBS, followed by an intravenous injection of paclitaxel (PAC) (5 mg/kg) or PBS one hour later. The treatment was given every other day until day 31. Animals received a total of 6 doses of co-therapy (marked by arrows). n=5. **p<0.05
B) MDA-MB-231 tumors were established as described in A). Nab-paclitaxel (Abraxane-ABX) at a dose of 5 mg/kg was injected intravenously 1 hour after JO-1 injection. The treatment was repeated on days 17, 20, 22, 24 and 27. n = 5. ABX vs. JO-1+ABX: **P<0.01 for all time points after day 22.
C) A similar study was performed with a prostate cancer cell line. A total of 2×106 22RvI cells, were injected subcutaneously into the flank of CB17-SCID/beige mice. When tumors reached a volume of ~ 130 mm3, mice received an intravenous injection of 50 μg JO-1 (2 mg/kg) or PBS, followed by an intravenous injection of liposomal doxorubicin (Doxil) (3mg/kg) or PBS one hour later (day 21). The treatment was repeated every other day until day 29. n = 5. Doxil vs JO-1+Doxil: **P<0.01 for day 30.
D) In a Her2/neu-positive breast cancer model, we tested whether JO-1 increased the therapeutic effects of cisplatin. In this experiment, 4×106 HCC1954 cells were injected into the mammary fat pad. The treatment was started on day 12, when tumors had reached a volume of 160 mm3. Mice were injected with JO-1 (2 mg/kg) or PBS intravenously and followed by intravenous injection of cisplatin (2 mg/kg*) or PBS one hour later. The injections was repeated on day 19. n = 5. The differences of tumor growth between animal treatment groups were not significant. *This dose of cisplatin is routinely used in preclinical studies (25).
E) Mice were injected subcutaneously with 4×106 lung cancer A549 cells. The treatment was started on day 12, when tumors had reached a volume of ~150 mm3. Mice were injected with JO-1 (2 mg/kg) or PBS intravenously and followed by intravenous injection of cisplatin (2mg/kg) or PBS one hour later. The injections was repeated on day 30. n = 5. The differences of tumor growth between “Cisplatin” and “JO-1+Cisplatin” were not significant.
F) Mice carrying tumors derived from breast cancer cells MDA-MB-231 cells (see A) were intraperitoneally injected once a week with cyclophosphamide (CPA) (100mg/kg) and with and without JO-1. Note that MDA-MB-231 cells are sensitive to CPA in vitro (17). N=5. The difference between “CPA” and “JO-1+CPA” was not significant.
Paclitaxel, nab-paclitaxel, and liposomal doxorubicin all have molecular weights of >500 Da; therefore, intercellular junctions could represent physical barriers to the penetration of these drugs into tumors. If our hypothesis is correct, JO-1-mediated junction opening should have less effects on drugs smaller than 500 Da. In order to test this hypothesis, we evaluated the effects of JO-1 co-therapy on cisplatin, which has a MW of 300 Da in models of breast cancer (Fig. 1D), lung cancer (Fig. 1E), and ovarian cancer (Suppl. Fig. 3B). Notably, it has been shown before that the cell lines used in this study were sensitive to cisplatin in culture (16, 17). Further support for the hypothesis that larger drugs benefit more from JO-1 co-therapy comes from a study with cyclophosphamide (MW 260), in which JO-1 also did not significantly enhance the therapeutic effect (Fig. 1F).
JO-1 allows for lowering chemotherapy doses and provides protective effects to normal tissues
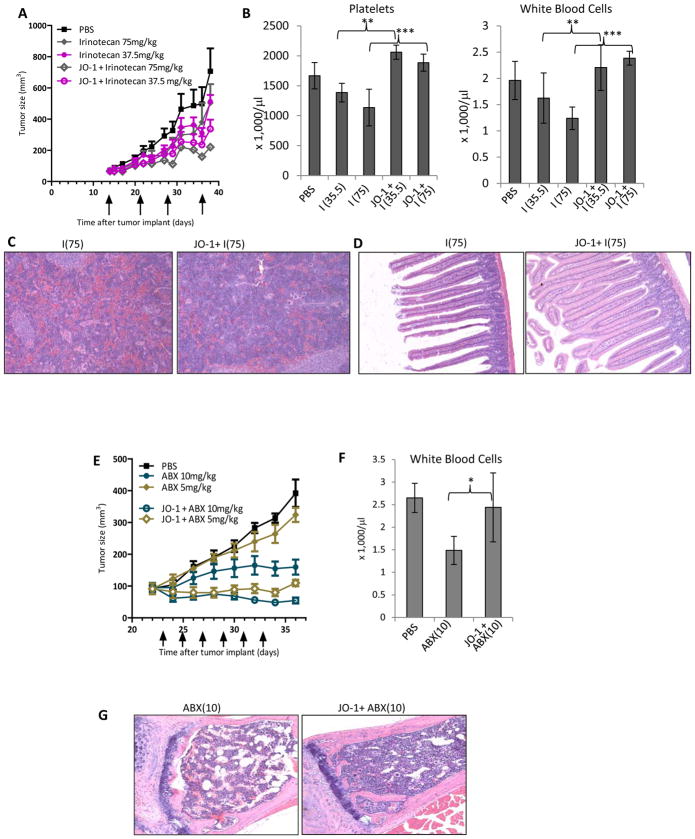
Our second hypothesis is that JO-1 co-therapy would allow decreasing the doses of chemotherapeutics required to achieve therapeutic efficacy. To test this, we injected chemotherapeutic agents at different dose levels into tumor-bearing mice and monitored tumor volumes as well as toxicity parameters, including blood cell counts, blood chemistry, and tissue histology. First, we tested JO-1 in combination with the topoisomerase inhibitor irinotecan in the A549 lung cancer model described above (Figs. 2A–D). As observed for the other drugs, JO-1 significantly increased the therapeutic efficacy of irinotecan. A combination of a low dose of irinotecan (37.5mg/kg) and JO-1 was significantly more effective than “standard” dose (75 mg/kg) irinotecan alone, indicating that JO-1 allows for lowering the effective drug dose (Fig. 2A). Irinotecan treatment caused thrombocytopenia and leukopenia as a result of myelotoxicity, although there was no significant difference in the severity of leukopenia or thrombocytopenia observed in animals treated at the the two different doses (Fig. 2B). Co-administration of JO-1 had a myelo-protective effect. It prevented the decrease in platelet and white blood cell counts, which was especially noteworthy at the higher drug dose. Compared to control animals, spleens were significantly smaller in irinotecan treated animals but not in animals that received irinotecan in combination with JO-1. Splenic atrophy in irinotecan treated animals is also visible in microscopic analysis of spleen sections (Fig. 2C). JO-1 also reduced irinotecan-mediated damage to the intestinal epithelium (Fig. 2D).
Figure 2. JO-1 allows for lowering of chemotherapeutic doses and mitigates toxic side effects.
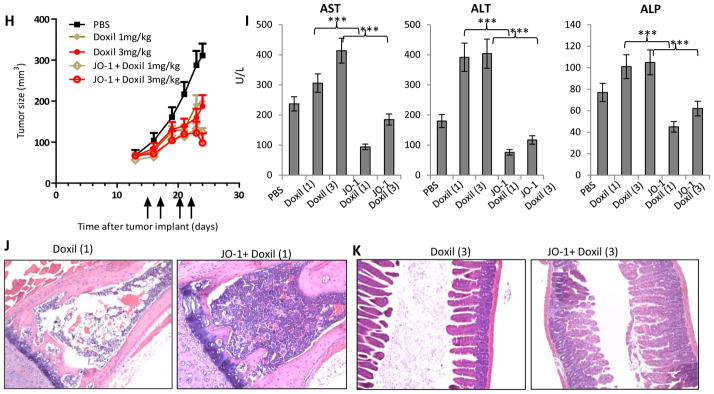
A-D) Irinotecan: Immunodeficient CB17-SCID/beige mice were injected subcutaneously with 4×106 A549 cells. JO-1 (2 mg/kg) was injected at day 14 intravenously (when tumors had a volume of 65 mm3) followed by an intraperitoneal injection of irinotecan (I) at two different dose levels (75 mg/kg or 37.5 mg/kg) or PBS 1 h later. The treatment was repeated weekly until day 36. A) Tumor volumes. n = 5. irinotecan (37.5 mg/kg) vs. JO-1+irinotexan (37.5 mg/kg): P<0.01 on day 38; P<0.001 day 38 for 75 mg/kg. B–D) At the end of the monitoring period animals were sacrificed and blood cell counts as well as tissue histology were analyzed. I(37.5) – irinotecan (37.5 mg/kg), I(75) – irinotecan (75 mg/kg). B) Platelet and white blood cell counts in treated mice at the day of sacrifice (day 38). n=5, irinotecan (37.5 mg/kg) vs. JO-1+irinotexan (37.5 mg/kg): **P<0.01. irinotecan (75 mg/kg) vs. JO-1+irinotexan (75 mg/kg): ***P<0.001. C) Representative spleen sections, H&E stained. Note depletion of cells in germinal centers in I(75) treated mice. Magnification: 20x. D) Representative intestine sections.
E–G) nab-paclitaxel (ABX): Immunodeficient CB17-SCID/beige mice were subcutaneously injected with 3×106 MDA-MB-231 cells. Mice were treated from day 23 on, when the tumors reached a volume of ~ 90 mm3. They were injected every other day with JO-1 (2 mg/kg) or PBS intravenously, followed 1h later by nab-paclitaxel (ABX) at different dose levels (10 and 5 mg/kg). E) Tumor volumes. n = 5. ABX (5 mg/kg) vs JO-1 + ABX (5 mg/kg): P<0.001 from day 28 on; ABX (10 mg/kg) vs. JO-1 + ABX (10 mg/kg): P<0.01 from Day 30 on. F) White blood cell counts in treated mice at the day of sacrifice (day 36). n=5, ABX(10) vs JO-1+ABX(10): *p<0.05. G) Representative sternum sections. H&E stained. Note depletion of bone marrow cells in ABX(10) treated mice.
H-K) liposomal doxorubicin (Doxil). A total of 4×106 A549 cells were injected subcutaneously into CB17-SCID/beige mice. Once the tumor reached a volume of 65 mm3, the mice were injected intravenously with 2 mg/kg JO-1 or PBS, followed by an intravenous injection of liposomal doxorubicin (1 or 3 mg/kg) or PBS one hour later. The treatment was repeated on day 17, 20 and 22. H) Tumor volumes. n = 5. Liposomal doxorubicin (1 mg/kg) intravenously vs. JO-1 + Liposomal doxorubicin (1 mg/kg): P < 0.05 from day 23 on; Liposomal doxorubicin (3 mg/kg) vs. JO-1 + Liposomal doxorubicin (3 mg/kg): P < 0.05 from day 24 on. I–K) Toxicology studies Doxil (1) – liposomal doxorubicin (1 mg/kg), Doxil (3) – liposomal doxorubicin (3 mg/kg). I) serum enzymes: aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP). n=3. Liposomal doxorubicin (1 mg/kg) vs JO-1+Liposomal doxorubicin (1 mg/kg): **P<0.01; Liposomal doxorubicin (3 mg/kg) vs JO-1+Liposomal doxorubicin (3 mg/kg): **P<0.01. The elevated transaminase levels in PBS- treated control animals could be explained by the presence of relatively large xenograft tumors in these mice which potentially can produce factors that act on hepatocytes. J) Representative sternum sections. K) Representative intestine sections.
Next, we evaluated nab-paclitaxel in the MDA-MB-231 breast cancer model. Nab-paclitaxel was administered at two different dose levels (5 and 10 mg/kg) alone or in combination with JO-1 (Figs. 2E–F). JO-1 co-therapy significantly increased the anti-tumor effects of nab-paclitaxel (Fig. 2E). The combination of JO-1 plus nab-paclitaxel (5 mg/kg) was significantly more effective than nab-paclitaxel alone given at a dose of 10 mg/kg. JO-1 plus nab-paclitaxel (10 mg/kg) halted tumor growth. Compared to paciltaxel, nab-paclitaxel demonstrated a better safety profile based on blood cell analysis (data not shown). However, the highest dose of nab-paclitaxel (10 mg/kg) still decreased the white blood cell count, which was prevented by JO-1 pre-treatment (Fig. 2F). The myeloprotective effect was also shown in sternum sections. In contrast to the depletion of bone marrow cells seen in mice treated with ABX alone, animals that received JO-1/ABX co-therapy displayed a normal histology (Fig. 2G). Notably, no toxic side effects of nab-paclitaxal were found in the other tissues analyzed, i.e. intestine, kidney and spleen.
The third model involved liposomal doxorubicin in the A549 lung cancer model (Figs. 2H-K). Similar to the results above, JO-1 pre-treatment significantly improved liposomal doxorubicin therapy at both dose levels of the chemotherapy drug (1 mg/kg and 3 mg/kg) (Fig. 2H). JO-1 mitigated adverse side effects induced by liposomal doxorubicin. For example, markers for liver damage, including AST, ALT, and ASP were significanly decreased in animals treated with JO-1 and liposomal doxorubicin compared to mice treated with liposomal doxorubicin alone (Fig. 2I). Severe tissue damage, caused by liposomal doxorubicin, found in the bone marrow and intestinal epithelium was greatly reduced in mice that received JO-1 injections (Figs. 2J and K).
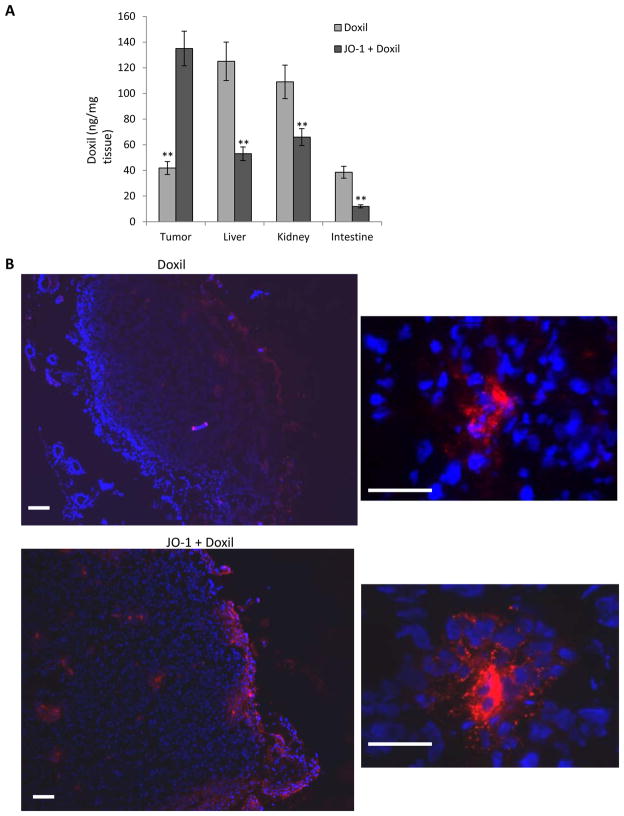
Importantly, in all three studies shown in Fig. 2, we found that JO-1 co-therapy greatly reduced the toxic side effects associated with chemotherapy. We speculate that the ability of JO-1 to open up intercellular junctions in tumors and increase the uptake of chemotherapeutics reduces the drug exposure to normal tissue thus providing a larger therapeutic window. In support of this speculation, using an ELISA to measure PEGylated compounds in tissues (13), we found significantly more liposomal doxorubicin in tumors and less in other tissues in mice that received JO-1 prior to intravenous liposomal doxorubicin injection (Fig. 3A). Immunofluorescence analysis of tissue sections revealed more Doxil signals in tumors of JO-1+Doxil treated mice (Fig. 3B). In these animals, Doxil was found more dispersed over a greater distance from blood vessels, suggesting better intratumoral penetration and absorption by tumor tissue (Fig. 3B).
Figure 3. Liposomal doxorubicin/Doxil biodistribution.
Mice carrying HCC1954 tumors were intravenously injected with PBS, Doxil (1 mg/kg) or, JO-1 (2 mg/kg) followed by Doxil one hour later. Two hours after PBS or Doxil injection, mice were sacrificed, blood was flushed from the system, and organs were collected to analyze the presence of Doxil in tissues using antibodies against PEG. A) Tissue lysates were subjected to ELISA for JO-1. n=3. The differences between the two groups are significant (**P<0.01). B) Immunfluorescence analysis of tumor sections with anti-PEG antibodies. The scale bar is 20 μm. Notably, free PEG is poorly detected by ELISA or immunohistochemistry (13). Effective binding is greatly enhanced in PEG conjugates such as Doxil.
JO-1 biodistribution, tumor therapy, and safety studies in hDSG2 transgenic mice
A more adequate model for safety and efficacy studies with JO-1 are human DSG2 (hDSG2) transgenic mice with MMC-hDSG2 tumors. JO-1 binds to MMC-DSG2 cells and triggers reorganization of junctions in a similar way as seen in human epithelial cells, indicating that JO-1 signaling through hDSG2 can override junction regulation in mouse cells (12). Transplantation of MMC-hDSG2 cells into the mammary fat pad of hDSG2 transgenic mice resulted in tumors that resembled key features of breast cancer in humans, e.g. nests of hDSG2bright epithelial cells that were surrounded by tumor stroma (Fig. 4A). Intravenous injection of JO-1 resulted in efficient accumulation of JO-1 in MMC-hDSG2 tumor nests as seen on tumor sections analyzed 6 hours after injection (Fig. 4A). Immunoreactive JO-1 was also found in epithelial cells of the intestine in hDSG2 transgenic mice (Fig. 4B), which is consistent with our recent biodistribution study with Ad3-GFP virus (12). We measured JO-1 concentrations in tissue and tumor lysates by ELISA at 6 hours after injection in the following 4 groups of animals: i) hDSG2-transgenic mice with MMC-hDSG2 tumors (Fig. 4C “hDSG2-pos”), ii) hDSG2-transgenic mice without tumors (Fig. 4D “hDSG2-pos”), iii) non-transgenic littermates with MMC tumors (Fig. 4C “hDSG2-neg”), and iv) non-transgenic littermates without tumors (Fig. 4D “hDSG2-neg”). We found highly selective, hDSG2-dependent accumulation of JO-1 in MMC-hDSG2 tumors established in hDSG2 transgenic mice. About 10-fold less JO-1 was found in the intestine and colon of hDSG2 transgenic mice. JO-1 was also detected in the liver and spleen of both hDSG2-positive and –negative mice, suggesting an hDSG2 independent uptake. Immunohistochemistry analyses, showed that JO-1 in the liver is mostly present in sinusoidal spaces and F4/80-positive Kupffer cells (Suppl. Fig. 4) and not taken up by parenchymal liver cells. Similarly, in the spleen, JO-1 signals appear in the peripheral zone of germinal centers (Suppl. Fig. 4) and are mostly likely the result of uptake by splenic macrophages. The amount of JO-1 in the intestine and colon was more than 2-fold higher in hDSG2 transgenic mice without MMC-hDSG2 tumors, supporting our hypothesis that the tumor acts as a “sink” for JO-1. From the JO-1 biodistribution studies in MMC-hDSG2 tumor-bearing hDSG2 mice, the question arose why JO-1 predominantly accumulates in the tumor. Immunofluorescence studies on tumor and intestine sections of hDSG2 transgenic mice, showed that epithelial tumor cells lack strict polarization, in contrast to normal epithelial cells (Figs. 4E and F). As a consequence, in tumors, hDSG2 appears to be readily accessible, while in intestinal epithelial cells most of the hDSG2 is trapped in junctions, reflected by overlapping signals for hDSG2 and the junction marker claudin 7 (Fig. 4F).
Figure 4. JO-1 biodistribution studies in hDSG2 transgenic mice.
A–C) A total of 4×106 MMC-hDSG2 cells were injected into the mammary fat pad of hDSG2 transgenic mice. When tumors reached a volume of 100 mm3, JO-1 (2 mg/kg) was injected intravenously. Mice were sacrificed 6 hours later, blood was removed from the circulation, and organs were harvested. A and B) Sections of MMC-hDSG2 tumors and intestine stained for JO-1 (red) and hDSG2 (green). C and D). JO-1 biodistribution: JO-1 (2 mg/kg) was injected intravenously. Six hours later, blood was flushed from the circulation and tissue lysates were analyzed for JO-1 by ELISA. n=3. ***P<0.001, n.s. non-signficant. E and F) Tumor (E) and intestine (F) sections from MMC-hDSG2 tumor bearing hDSG2 transgenic mice were stained for the adherens junction marker claudin 7 (red) and hDSG2 (green). Representative sections are shown. The scale bars are 20 μm.
We also measured clearance of JO-1 from blood after intravenous injection (Suppl. Fig. 5). These studies show that the half-life of JO-1 in hDSG2 transgenic mice is approximately 6 hours. JO-1 clearance from blood is slower in hDSG2 transgenic mice than in non-transgenic littermates, which could be due to the fact that hDSG2 is expressed on platelets and subfractions of leukocytes (12).
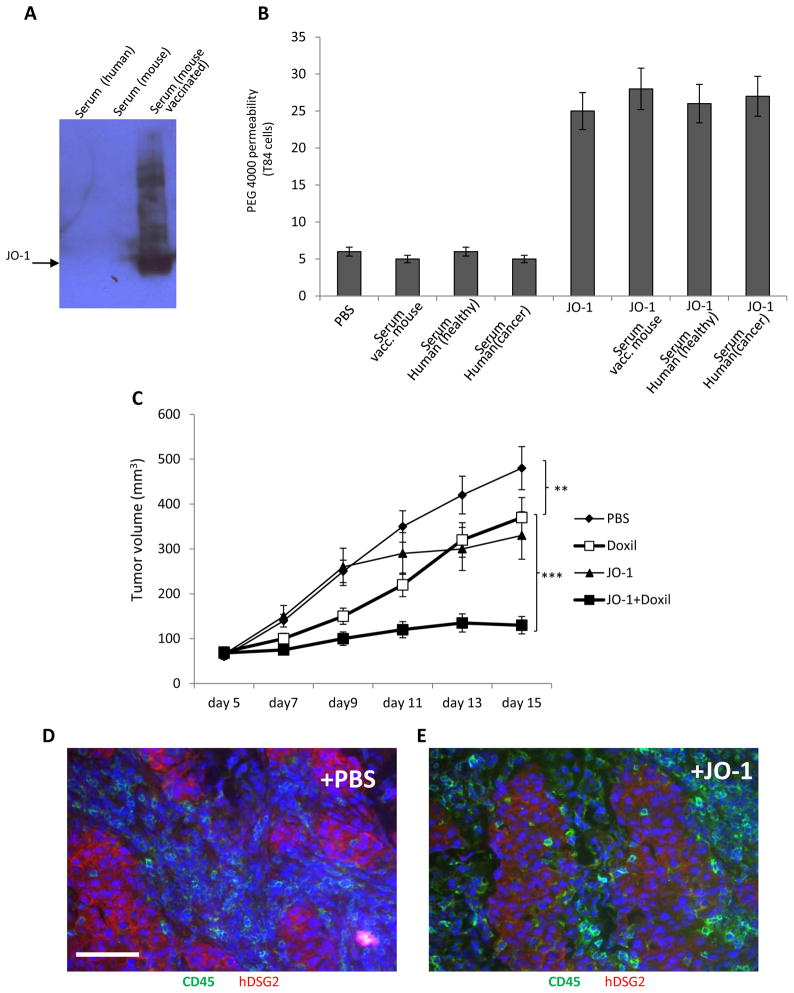
As JO-1 is a viral protein, adaptive immune responses might develop in humans, particularly after repeated injection. Despite the fact that approximately one third of humans have neutralizing antibodies against Ad3 (8), our studies showed that these antibodies did not interact with JO-1 (Fig. 5A). Furthermore, polyclonal anti-JO-1 antibodies generated by vaccination of mice did not affect the enhancing effect of JO-1 transepithelial transport of PEG 4000 in polarized colon cancer cultures (Fig. 5B). Our hDSG2-transgenic mouse/MMC-hDSG2 model allowed us to test the effect of anti-JO-1 antibodies on JO-1 activity in vivo (Fig. 5C). When JO-1 antibodies were detectable in serum of JO-1 vaccinated mice, MMC-hDSG2 tumor cells were injected into the mammary fat pad. As seen in the xenograft tumor models, JO-1 increased the efficacy of Doxil therapy in vaccinated, hDSG2-transgenic mice with pre-established MMC-hDSG2 tumors (Fig. 5C). Interestingly, JO-1 injection alone had a significant therapeutic effect in this immunocompetent tumor model. Because this effect was not seen in the immunodeficient tumor models (Fig. 1), we speculated that JO-1 facilitates anti-tumor immune responses. In support of this speculation, we found significantly more tumor-infiltrating leukocytes in MMC-hDSG2 tumor nests in JO-1 injected mice (Figs. 5E and F). Clearly, detailed T-cell studies are required to support this speculation. (Notably, MMC-hDSG2 cells express rat Neu (18), which could serve as an antigen.)
Fig. 5. Effect of anti-JO-1 antibodies on JO-1 activity.
A) Analysis of serum for reactivity with JO-1 by Western blot. JO-1 was run on SDS-PAGE and filters were incubated with pooled human serum containing neutralizing antibodies against Ad3 virus (lane 1), serum from naïve mice (lane 2), or serum from JO-1 vaccinated mice (see Materials and Methods) (lane 3). B) JO-1 enhances transepithelial transport of PEG (4,000 Da) in vitro in the presence of anti-JO-1 antibodies. Pooled serum from 10 healthy donors (“healthy”) and 10 breast cancer patients (“cancer”) or serum from a JO-1 vaccinated mouse (“vacc. mouse”) was used in this study. Human colon epithelial T84 cells were incubated in transwell plates until transepithelial resistance was constant. JO-1 (5 μg/ml) or PBS mixed with heat-inactivated serum (at a final concentration of 20%) was added to the inner chamber, followed by 14C-PEG-4,000 1 hour later. Radioactive counts were measured 1 hour later in the outer chamber. n=3 C) Effect of JO-1 in vaccinated hDSG2 transgenic mice with MMC-hDSG2 tumors. Mice were vaccinated with JO-1 as described in A). When JO-1 antibodies were detectable in serum, MMC-hDSG2 tumor cells were injected into the mammary fat pad. Treatment with JO-1 (2 mg/kg), Doxil (1.5 mg/kg), or JO-1 plus Doxil was started 5 days after tumor cell injection. n=5. **P<0.01, ***P<0.001. D and E) MMC-hDSG2 tumor sections 2 days after PBS (E) or JO-1 (F) injection stained for hDSG2 (red) and the pan-leukocyte marker CD45 (green). The scale bar is 20μm. The number of CD45-positive cells in tumor nests was 7.8(+/−3.4) cells per mm2 in PBS injected mice and 35+/−12 cells per mm2 in JO-1 injected mice (n=3).
As seen before in hDSG2-transgenic mice that received intravenous Ad3-GFP injection, JO-1 injection also caused a mild diarrhea that started within 3 hours after injection and subsided by day 2. There were no significant changes in blood parameters at 24 hours (Suppl. Figs. 6A and B) and 3 days (not shown) after injection of JO-1 at a dose of 2mg/kg, i.e. a dose that conferred an enhancing effect on Doxil therapy. After injection of JO-1 at a dose of 10mg/kg, blood analysis detected leucopenia, decrease in serum glucose levels, and increase in serum GPT levels. Liver damage is also reflected by the presence of steatosis in the liver (Suppl. Fig. 6C). Notably, the potential danger of provoking a serious immune response as a consequence of the administration of multiple doses of JO-1 to patients has to be taken into consideration.
Overall, our studies in hDSG2 transgenic mice show that JO-1 (2mg/kg) co-therapy is safe and effective in the presence of anti-JO-1 antibodies.
Discussion
We showed that JO-1 co-therapy allows doses of chemotherapeutics to be decreased without compromising anti-tumor effects and that it provides protective effects to normal tissues resulting in an improved safety profile for chemotherapy. We also demonstrated that JO-1 can overcome resistance of xenograft tumors to a chemotherapy drug. Our data indicate that JO-1 predominantly accumulates in tumors. A number of factors could account for this, including i) overexpression of DSG2 on tumor cells, ii) better accessibility of DSG2 on tumor cells because of a lack of strict cell polarization, and iii) a high degree of vascularization and vascular permeabilty in tumors. Because of its preferential action on epithelial junctions of tumors, JO-1 appears to create as “sink” for chemotherapy drugs, thereby reducing the exposure of normal tissue to these drugs.
Before JO-1 co-therapy is used in cancer treatment, certain issues will need to be addressed.
JO-1 immunogenicity
This might not be a critical issue if JO-1 is used in combination with chemotherapy, which suppresses immune responses to foreign proteins (even at doses that are markedly lower than drug doses used for cancer treatment). This expectation is supported by studies with oncolytic adenovirus vectors in which immunosuppression allowed for repeated vector application (19). Furthermore, we have demonstrated that JO-1 remains active in vitro and in vivo even in the presence of anti-JO-1 antibodies generated by vaccination of mice. This might be due to the fact that the JO-1 interaction with DSG2 is of very high avidity and cannot be disrupted by polyclonal anti-JO-1 antibodies. (Notably JO-1 is a dimer of a trimeric fiber knob (10).) It can however not be excluded that repeated JO-1 injection into immunocompetent patients results in the development of competing, high-affinity antibodies. This problem can potentially be addressed by generating next generation JO-1 proteins that have immunodominant epitopes removed or higher affinity to DSG2. Furthermore, we have recently identified the DSG2 interacting residues within JO-1 and based on this knowledge, it might be possible to transplant the DSG2-interacting Ad3 fiber loops into less-immunogenic scaffolds.
Role of DSG2 in cancers
In agreement with other studies (6, 7), we have found a higher DSG2 expression in malignant tissues than in the surrounding normal epithelial tissue (Suppl. Fig. 1). Furthermore, a recent study on squamous cell cancer demonstrated that, in contrast to other desmosomal proteins, DSG2 was up-regulated in invasive cancer (20). An NCBI website reports DSG2 expression in esophageal, colorectal, head and neck, kidney, uterine, bladder, prostate, GI tract, breast, cervical, lung, ovarian, pancreatic, and skin tumors, while DSG2 was absent in adrenal tumors, sarcoma, glioma, leukemia, lymphoma, retinoblastoma, and soft tissue/muscle tissue tumors. (http://www.ncbi.nlm.nih.gov/UniGene/ESTProfileViewer.cgi?uglist=Hs.412597). There are, however, also studies reporting a reduction in the amounts of DSG2 in invasive pancreatic or gastric cancer (21, 22), and it has been argued that epithelial features, including tight junctions, are lost in metastatic cancer. These arguments do not consider that in advanced carcinomas mesenchymal cells can regain characteristics of epithelial cells via mesenchymal-to-epithelial transition (23). The epithelial phenotype of cancer cells and their ability to form physical barriers represent a protective mechanism for cancer cells. We have analyzed more than 60 primary and metastatic breast cancer samples and found over-expression of DSG2 in all of these samples. A fraction of these biopsies are shown in Suppl. Fig. 1.
Risk of metastasis
JO-1 binding to DSG2 on tumor cells triggers pathways involved in epithelial-to-mesenchymal transition (EMT), a process which has been associated with tumor metastasis. However, to date none of our in vivo studies has shown any evidence of increased tumor growth or metastasis after treatment with JO-1. Furthermore, at day 3 after JO-1 injection into mice bearing Her2/neu-positive HCC1954 tumors, there was no significant increase in the percentage of circulating Her2/neu- positive cells in the blood (Suppl. Fig. 7). Tumor metastasis requires more than transient activation of EMT pathways. Detachment from epithelial cancers and migration of tumor cells is only possible after long-term crosstalk between malignant cells and the tumor microenvironment, resulting in changes in the tumor stroma and phenotypic reprogramming of epithelial cells into mesenchymal cells (24).
Toxic side effects
Except for a mild, transient diarrhea, intravenous injection of JO-1 (at a dose of 2mg/kg) had no critical side effects on other tissues or hematological parameters in hDSG2 transgenic mice. We speculate that DSG2 in tissues other than the tumor and certain epithelial cells in the GI tract is not accessible to intravenously injected JO-1. Clearly, the demonstration of safety of JO-1 in combination with chemotherapy drugs in non-human primates is required before this approach can be considered in humans. In this context we have recently reported the DSG2 in macaques is expressed in the same patterns as in humans and that it is recognized by JO-1 (12).
Overall, we demonstrated that JO-1 improves the efficacy and safety of a number of cancer chemotherapy drugs, implying that JO-1 co-therapy has the potential to improve the life quality of patients that receive cancer treatment. Moreover, it may allow patients to continue chemotherapy, which has been halted or delayed due to toxicity. Finally, cancer treatment with newer drugs, such as nab-paclitaxel and and liposomal doxorubicin is expensive and presents a substantial burden to both patients and the costs of healthcare in general. JO-1 has potential to decrease costs of cancer therapy by reducing the amount of chemotherapy required as well as costs associated with treatment related toxicity. These savings are likely to exceed the additional cost of JO-1 treatment.
Supplementary Material
Translational relevance.
Most cancers originate from epithelial tissues and retain features of differentiated epithelial cells such as tight junctions, i.e. zipper-like structures that tightly link the individual malignant cells. This barricading feature can be used by tumors to exclude therapeutics from penetrating the tumor. We designed the recombinant protein JO-1 (“junction opener-1”). JO-1 binds to the junction protein desmoglein 2 (DSG2) in epithelial junctions, triggers the cleavage of adhesive DSG2 dimers, and activates intracellular signaling pathways resulting in a decreased expression of junction proteins. JO-1 co-administration increased the efficacy of several chemotherapy drugs in tumor models for breast, lung, and prostate cancer. Furthermore, JO-1 co-therapy allowed chemotherapy doses to be decreased without compromising the anti-tumor effects, and provided protective effects to normal tissues. JO-1 co-therapy has the potential to improve both the efficacy of treatment and the life quality of cancer patients that receive chemotherapy.
Acknowledgments
We thank Melanie Wurm for helpful advice.
The study was supported by NIH grant R01 CA080192 (AL), R01 HLA078836 (AL), the Pacific Ovarian Cancer Research Consortium/Specialized Program of Research Excellence in Ovarian Cancer Grant P50 CA83636, by a grant from the Fred Hutchinson Cancer Research Center, and a grant from the Marsha Rivkin Center for Ovarian Cancer Research. I.B. is a recipient of a postdoctoral fellowship award from ”Deutsche Krebshilfe” (108988).
References
- 1.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 2.Lavin SR, McWhorter TJ, Karasov WH. Mechanistic bases for differences in passive absorption. J Exp Biol. 2007;210:2754–64. doi: 10.1242/jeb.006114. [DOI] [PubMed] [Google Scholar]
- 3.Green SK, Karlsson MC, Ravetch JV, Kerbel RS. Disruption of cell-cell adhesion enhances antibody-dependent cellular cytotoxicity: implications for antibody-based therapeutics of cancer. Cancer Res. 2002;62:6891–900. [PubMed] [Google Scholar]
- 4.Fessler SP, Wotkowicz MT, Mahanta SK, Bamdad C. MUC1* is a determinant of trastuzumab (Herceptin) resistance in breast cancer cells. Breast Cancer Res Treat. 2009;118:113–24. doi: 10.1007/s10549-009-0412-3. [DOI] [PubMed] [Google Scholar]
- 5.Oliveras-Ferraros C, Vazquez-Martin A, Cufi S, Queralt B, Baez L, Guardeno R, et al. Stem cell property epithelial-to-mesenchymal transition is a core transcriptional network for predicting cetuximab (Erbitux) efficacy in KRAS wild-type tumor cells. J Cell Biochem. 2011;112:10–29. doi: 10.1002/jcb.22952. [DOI] [PubMed] [Google Scholar]
- 6.Biedermann K, Vogelsang H, Becker I, Plaschke S, Siewert JR, Hofler H, et al. Desmoglein 2 is expressed abnormally rather than mutated in familial and sporadic gastric cancer. J Pathol. 2005;207:199–206. doi: 10.1002/path.1821. [DOI] [PubMed] [Google Scholar]
- 7.Harada H, Iwatsuki K, Ohtsuka M, Han GW, Kaneko F. Abnormal desmoglein expression by squamous cell carcinoma cells. Acta Derm Venereol. 1996;76:417–20. doi: 10.2340/0001555576417420. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Li ZY, Liu Y, Persson J, Beyer I, Moller T, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med. 2011;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trinh HV, Lesage G, Chennamparampil V, Vollenweider B, Burckhardt CJ, Schauer S, et al. Avidity binding of human adenovirus serotypes 3 and 7 to the membrane cofactor CD46 triggers infection. J Virol. 2011 doi: 10.1128/JVI.06181-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Li Z, Yumul R, Lara S, Hemminki A, Fender P, et al. Multimerization of adenovirus serotype 3 fiber knob domains is required for efficient binding of virus to desmoglein 2 and subsequent opening of epithelial junctions. J Virol. 2011;85:6390–402. doi: 10.1128/JVI.00514-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyer I, van Rensburg R, Strauss R, Li Z, Wang H, Persson J, et al. Epithelial junction opener JO-1 improves monoclonal antibody therapy of cancer. Cancer Res. 2011;71:7080–90. doi: 10.1158/0008-5472.CAN-11-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Beyer I, Persson J, Song H, Li Z, van Rensburg R, et al. A new human DSG2-transgenic mouse model for studying the tropism and pathology of DSG2-interacting adenoviruses. Journal of Virology. 2012 doi: 10.1128/JVI.00205-12. published online ahead of print on 28 March 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su YC, Chen BM, Chuang KH, Cheng TL, Roffler SR. Sensitive quantification of PEGylated compounds by second-generation anti-poly(ethylene glycol) monoclonal antibodies. Bioconjug Chem. 2010;21:1264–70. doi: 10.1021/bc100067t. [DOI] [PubMed] [Google Scholar]
- 14.Beyer I, van Rensburg R, Strauss R, Li Z, Wang H, Persson J, et al. Epithelial junction opener JO-1 improves monoclonal antibody therapy of cancer. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-11-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, et al. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328:1031–5. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strauss R, Li ZY, Liu Y, Beyer I, Persson J, Sova P, et al. Analysis of epithelial and mesenchymal markers in ovarian cancer reveals phenotypic heterogeneity and plasticity. PLoS One. 2011;6:e16186. doi: 10.1371/journal.pone.0016186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emmenegger U, Francia G, Chow A, Shaked Y, Kouri A, Man S, et al. Tumors that acquire resistance to low-dose metronomic cyclophosphamide retain sensitivity to maximum tolerated dose cyclophosphamide. Neoplasia. 2011;13:40–8. doi: 10.1593/neo.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knutson KL, Almand B, Dang Y, Disis ML. Neu antigen-negative variants can be generated aftaer neu-specific antibody therapy in neu transgenic mice. Cancer Res. 2004;64:1146–51. doi: 10.1158/0008-5472.can-03-0173. [DOI] [PubMed] [Google Scholar]
- 19.Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips NJ, Wold WS. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol Ther. 2008;16:1665–73. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurzen H, Munzing I, Hartschuh W. Expression of desmosomal proteins in squamous cell carcinomas of the skin. J Cutan Pathol. 2003;30:621–30. doi: 10.1034/j.1600-0560.2003.00122.x. [DOI] [PubMed] [Google Scholar]
- 21.Ramani VC, Hennings L, Haun RS. Desmoglein 2 is a substrate of kallikrein 7 in pancreatic cancer. BMC Cancer. 2008;8:373. doi: 10.1186/1471-2407-8-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yashiro M, Nishioka N, Hirakawa K. Decreased expression of the adhesion molecule desmoglein-2 is associated with diffuse-type gastric carcinoma. Eur J Cancer. 2006;42:2397–403. doi: 10.1016/j.ejca.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–26. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 24.Guarino M. Epithelial-mesenchymal transition and tumour invasion. Int J Biochem Cell Biol. 2007;39:2153–60. doi: 10.1016/j.biocel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, Yin S, Banerjee S, Sarkar F, Reddy KB. Enhanced anticancer effect of the combination of cisplatin and TRAIL in triple-negative breast tumor cells. Mol Cancer Ther. 2011;10:550–7. doi: 10.1158/1535-7163.MCT-10-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.