Abstract
Modifications of the hypothalamo-pituitary-adrenal axis and associated changes in circulating levels of glucocorticoids form a key component of the response of an organism to stressful challenges. Increased levels of glucocorticoids promote gluconeogenesis, mobilization of amino acids, and stimulation of fat breakdown to maintain circulating levels of glucose necessary to mount a stress response. In addition to profound changes in the physiology and function of multiple tissues, stress and elevated glucocorticoids can also inhibit reproduction, a logical effect for the survival of self. Precise levels of glucocorticoids are required for proper gonadal function; where the balance is disrupted, so is fertility. Glucocorticoids affect gonadal function at multiple levels in hypothalamo-pituitary-gonadal axis: 1) the hypothalamus (to decrease the synthesis and release of GnRH); 2) the pituitary gland (to inhibit the synthesis and release of LH and FSH); 3) the testis/ovary (to modulate steroidogenesis and/or gametogenesis directly). Furthermore, maternal exposure to prenatal stress or exogenous glucocorticoids can lead to permanent modification of hypothalamo-pituitary-adrenal function and stress-related behaviors in offspring. Glucocorticoids are vital to many aspects of normal brain development, but fetal exposure to superabundant glucocorticoids can result in life-long effects on neuroendocrine function. This review focuses on the molecular mechanisms believed to mediate glucocorticoid inhibition of reproductive functions and the anatomical sites at which these effects take place.
Keywords: Glucocorticoid, Hypothalamus, Pituitary, Testis, Ovary, Uterus
Introduction
The secretion of glucocorticoids is a classic endocrine response to stress. Glucocorticoids synthesized in the adrenal cortex in response to adrenocorticotrophic hormone (ACTH) stimulate gluconeogenesis to provide energy for the “flight or fight” response. The response to stress combines suppressive and stimulating actions of glucocorticoids for the ultimate goal of preservation of self. Consequently, these steroids exert a diverse range of functions throughout the body, many of which have important implications for fertility. Reallocation of resources during the stress response suppresses the reproductive axis, which gives higher priority to an individual’s survival rather than the maintenance of species. Numerous patient studies regarding couples conceiving children suggests that chronic stress can lead to reproductive dysfunction. Various stressors, including infection, malnutrition, anxiety and depression, trigger a rise in glucocorticoids that suppress reproductive functions along the hypothalamo-pituitary-gonadal (HPG) axis. [1, 2]. At the level of the hypothalamus, glucocorticoids inhibit the release of gonadotropin-releasing hormone (GnRH) release [3, 4]. The effect of glucocorticoids in the pituitary is secondary to its effect on GnRH secretion but results in a decline in circulating luteinizing hormone (LH) levels [5]. At the cellular level, glucocorticoids exert their effects through binding the glucocorticoid receptor (GR), a member of the nuclear steroid receptor superfamily that functions as a ligand-dependent transcription factor to regulate the expression of glucocorticoid-responsive genes (reviewed by Nicolaides [6]). GR regulates expression of genes either positively and negatively depending on the glucocorticoid response element sequence, promoter contex, or alternatively, binding DNA indirectly through other transcription factors. The localization of the GR to specific cell types within the testis and ovary suggest a direct influence on reproductive function at the gonadal level [7, 8]. Glucocorticoids act directly at the level of the testis or ovaries through inhibition of steroid hormone production or glucocorticoid-induced apoptosis [9, 10]. This review focuses on the physiological role of stress-induced glucocorticoid action at each level along the HPG axis.
Hypothalamo-Pituitary-Gonadal Axis
The hypothalamus is a critical processing center, receiving numerous inputs from endocrine feedback loops that provide for integrated function among organs, complex coordinated homeostatic mechanisms, and behavior important for development and reproduction [11]. The hypothalamus directs many of its actions though secretion of GnRH, which travels down the anterior portion of the pituitary via the hypophyseal portal system and binds to receptors on the secretory cells of the adenohypophysis. GnRH from the hypothalamus acts on the pituitary gland to stimulate the synthesis and release LH and FSH (Figure 1). In the testis, LH binds its receptor on Leydig cells to stimulate testosterone production. Within the seminiferous tubule, FSH binds its receptor in the Sertoli Cell to stimulate spermatogenesis and influence the number of LH receptors through paracrine regulation. In the ovary, FSH controls follicular granulosa cell growth and estradiol production, while LH controls oocyte maturation, ovulation, and follicular luteinization (reviewed in [12]).
Figure I.
The Hypothalamo-Pituitary-Gonadal Axis. The Hypothalamo-Pituitary-Gonadal axis includes the effects of the hypothalamus, pituitary, and gonads as a feed forward/back entity. The hypothalamus produces gonadotropin-releasing hormone, which signals through the anterior portion of the pituitary gland to produce luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In females, FSH and LH act primarily to activate the ovaries to produce estrogen and inhibin. In males, LH stimulates the Leydig cells of the testes to produce testosterone and FSH signals through the Sertoli cells to support spermatogenesis. The sex steroids hormones, testosterone and estrogen, and FSH-stimulated inhibin feed back to inhibit the release of GnRH and pituitary secretion of LH and FSH.
The gonads play a key role in HPG axis by directing feedback mechanisms to the hypothalamus and pituitary, as well as regulating hormones of the reproductive system in an autocrine or paracrine manner. The sex hormones, estrogen and testosterone, direct proper gonadal function at physiological levels, but when in excess, can also feedback to suppress their own production. High levels of testosterone drive down GnRH release from the hypothalamus to negatively regulate the production of LH. Similarly, high levels of estrogens suppress the release of GnRH, completing an endocrine feedback loop through its return to the hypothalamus. High levels of progesterone, produced in response to LH, employ the same negative feedback loop used by estrogen and testosterone. The gonads also secrete other hormones that influence pituitary control of reproduction, such as inhibin, activin, and follistatin. Inhibin and activin, members of the transforming growth factor s (TGFs) family, were identified as components of gonadal fluids that exhibit the ability to suppress or stimulate, respectively, follicle-stimulating hormone (FSH) secretion from pituitary gonadotropes [13]. Although structurally unrelated to inhibin and activin, follistatin is recognized as activin-binding protein that can bio-neutralize and thereby modulate all actions of activins [14]. The collective feed forward actions of GnRH, LH, and FSH and feedback mechanisms of testosterone, estrogen, and inhibin complete the loop of the HPG axis, which is required for maintenance of reproductive status.
Glucocorticoids Regulate the HPG Axis
The onset of stress initiates the inhibition of reproductive behavior and physiology for the preservation of self. The stress-induced rise in glucocorticoids represses GnRH secretion, which can result in hypogonadotropic hypogonadism. In Cushing’s disease, a clinical disorder of hypercortisolemia, hypogonadism is common. The response to GnRH is impaired and testosterone levels are low in men. The response to glucocorticoids in the brain is directed by the specific regions and cell types that contain GR. The hippocampus, a site of abundant GR expression in the brain, mediates a variety of behavioral responses to glucocorticoids and provides indirect inhibitory feedback to the hypothalamus [15, 16]. GR, also present in hypothalamic neurons, directly contribute to glucocorticoid mediated down-regulation of the HPG axis. This conclusion is supported by studies that show GR present in GnRH-containing hypothalamic cell lines functioning as ligand-activated transcriptional regulators [17]. Glucocorticoid treatment in a hypothalamic cell lines repress endogenous GnRH mRNA and the transcriptional activity of transfected GnRH promoter-reporter gene vectors. The element required for glucocorticoid repression of mouse GnRH gene transcription, the distal negative glucocorticoid response element (nGRE), is not bound directly by GR but is recognized by Oct-1 [18]. Therefore, one mechanism of glucocorticoid repression of GnRH does not involve direct DNA binding by the GR but rather novel protein-protein interactions between the GR and the DNA-bound Oct-1 transcription factor. In addition to suppressing GnRH synthesis, glucocorticoids decrease the activity of the GnRH pulse-generating center [3]. Together this suggests that the primary site for the inhibitory actions of glucocorticoids on gonadotropin secretion resides at a suprapituitary level, probably through an interruption of hypothalamic GnRH release.
A novel negative regulator of the HPG axis known as gonadotropin-inhibitory hormone (GnIH) was recently discovered in quail, and orthologous neuropeptides known as RFamide-related peptides (RFRPs) have also been identified in rodents and primates [19, 20]. Acute and chronic stress stimulate expression of RFRP in the adult male rat hypothalamus, where expression of RFRP and GR overlap [21]. In birds and mammals, RFRP plays a functional role in suppression of HPG function in vivo. Systemic administration of RFRP and stress suppress LH release and sexual behavior. Adrenalectomy prevents stress-induced increase in hypothalamic RFRP expression and subsequent suppression of LH.
The inhibitory effects on LH secretion by chronic or acute treatment with glucocorticoids have been well documented in a variety of species. However, it is also reported that endogenous glucocorticoids released in response to acute stress may protect gonadotropin secretion [22]. This is attributed to a specific mechanism that involves regulation of prostaglandins (PGs) in the brain, which mediate the suppressive effect on LH pulsatility common to infectious, hypoglycemic, and restraint stress. In response toinfectious stress, namely treatment with tumor necrosis factor-α (TNF-α), adrenalectomy enhances the inhibitory effect of TNF-α on pulsatile LH secretion [23]. Pretreatment with glucocorticoids lessens TNF-α-induced suppression of LH secretion in intact rats, indicating that threshold glucocorticoid levels are necessary for the maintenance of LH secretion and reproductive function during inflammatory stress [24]. Pretreatment with iodomethacin, a PGs synthesis inhibitor, blocks most of the inhibitory effect of TNF-α on the LH surge. Synthesis of PGs can be inhibited by glucocorticoids through suppression of the induction of the cyclooxygenase inhibitor 2 (COX-2). COX-2 expression in blood vessels in the brain is enhanced by hypoglycemic and restraint stress, supporting a role for PGs as mediators of acute stresses in the brain [25]. Increased release of glucocorticoids in response to stress may counter act the effects of stress-induced PGs synthesis to maintain, rather than suppress, reproductive function under acute stress conditions.
In the pituitary, mechanisms exist for glucocorticoid regulation of transcription of the GnRH receptor (GnRHR) gene [26]. Reporter and chromatin immunoprecipitation assays show that dexamethasone treatment, a synthetic glucocorticoid used clinically, can increase transcription of GnRHR by nuclear translocation and interaction of the GR with the activating protein-1 (AP-1) region of the mGnRHR gene. Furthermore, GnRH and dexamethasone act synergistically at the GnRHR promoter through steroid receptor coactivator-1 recruitment to the AP-1 region. This represents another mechanism by which glucocorticoids can regulated GnRH activity. Pituitary cells are also the direct target of glucocorticoid action, although responses here are divergent for LH and FSH. Primary pituitary cultures from female rats treated with glucocorticoids demonstrate differential responses; basal secretion of LH is inhibited and basal secretion of FSH is enhanced [27]. Furthermore, sex differences exist for glucocorticoid regulation of LH secretion from the pituitary. Gonadectomized rams are more sensitive than gonadectomized ewes to the inhibitory effects of glucocorticoids on LH secretion [28]. The sex differences are specific to effects of glucocorticoids on LH pulses that may indicate sex differences in the mechanisms of action of glucocorticoids to suppress LH in sheep. In females, pituitary release of FSH is critical for follicular development [29]. Production of the s-subunit of FSH is the rate-limiting step in FSH synthesis, and while glucocorticoids are notable regulators of FSHs by themselves, co-treatment of activin with glucocorticoids promotes a synergistic effect [30, 31]. Glucocorticoids can further increase FSH secretion by decreasing plasma concentrations of inhibin and follistatin [32, 33].
Stress-associated reproductive disorders are associated with reduced pulsatile secretion of LH and FSH from the pituitary gland, concomitant with decreased expression and release of GnRH from the hypothalamus. Thus, supraphysiologic glucocorticoid exposure can directly affect the HPG axis by central actions on the hypothalamus and pituitary. Studies suggest a different mechanism that under acute stress glucocorticoids protect rather than inhibit LH secretion. This may be specific to PGs-mediated suppression of the HPG axis, but additional studies are needed to elucidate the role specific levels of glucocorticoids have on HPG axis in the brain.
Glucocorticoid Action in the Testis
Stress provokes an elevation in glucocorticoid concentration which precedes a decline in testosterone concentration in the male. Suppression of testosterone production by glucocorticoids occurs not only at the hypothalamic and pituitary level, but also directly in the testes. In the testis, the GR is localized to multiple interstial cell types, including Leydig cells, macrophages, fibroblasts, smooth muscle cells, and endothelial cells of blood vessels [7]. Furthermore, GR is present in zygotene and early pachytene primary spermatocytes during stages XIII–XIV and I–III of the spermatogenic cycle. While GR is not expressed by other spermatogenic cells or the spermatogenic nurturing Sertoli cell, GR is present in other male reproductive accessory tissues, such as the epididymis, vas deferens, and prostate. Some of the earliest studies on the male gonad and sexual dysfunction associated with elevated circulating cortisol levels were made on men with Cushing’s syndrome [34]. Patients develop Cushing’s syndrome in response to prolonged exposure to cortisol, either through excess production of ACTH or long-term exogenous administration of glucocorticoids. Patients with Cushing’s syndrome present with low plasma testosterone concentrations, with little alteration in pituitary luteinizing hormone (LH) levels. Levels of testosterone are further suppressed with the diagnostic administration of dexamethasone [9]. Stress in the form of anesthesia, surgery, anticipation of battle, or physical training also show the same reciprocal relationship of glucocorticoid rise and testosterone decrease [35].
Patients with Cushing’s syndrome and the localization of GR to Leydig cells provide clues to the mechanisms that stress and glucocorticoids employ to inhibit male fertility. These insights are consistent with the findings others have described relating to glucocorticoid-regulated LH receptor expression and influence on local testosterone production. Interestingly, studies in males training for military combat under constant threat of attack, sleep deprivation, and physical exertion, which are associated with elevated levels of serum cortisol, demonstrate maximal testosterone suppression but levels of LH similar to baseline [36]. This data suggests that stress-induced variations in serum glucocorticoid content may not have a significant effect on circulating levels of LH and on its binding properties to its receptor, indicating that glucocorticoid repressive effects on Leydig cell steroidogenic capacity can not simply be explained as decreased stimulation of the Leydig cell by LH [37]. However, it is known that glucocorticoids can directly inhibit testicular LH receptor content in both intact and hypophysectomized rats and testicular steroidogenesis [9, 38]. While the precise molecular mechanism by which glucocorticoids decrease testosterone production is not entirely understood, numerous studies have shown that glucocorticoids directly inhibit the transcription of genes encoding testosterone biosynthetic enzymes, such as cytochrome P450-dependent cholesterol side chain cleavage enzyme (P450 SCC), the cholesterol transporting steroidogenic acute regulatory protein (StAR), and cytochrome P450-dependent 17α-hydroxylase/C17-C20lyase (CYP17) [39–41]. Interestingly, the promoters of some of these steroidogenic genes repressed by glucocorticoids do not contain classical glucocorticoid response elements, which may suggest an indirect mechanism. Glucocorticoids antagonize cAMP-induced StAR transcription in Leydig cells through dexamethasone-induced binding of active GR to the nuclear receptor NR4A1, forming a transcriptionally inactive complex [42]. It is important to note that the harmful effects of stress and high levels of circulating glucocorticoids on testosterone levels can also be attributed to rapid non-genomic mechanisms. Glucocorticoid treatment in mouse Leydig cells can inhibit cAMP formation within 15 minutes, and a rapid decline in testosterone production is seen by 30 minutes [43]. Since the genomic mechanism of glucocorticoids would be expected to require a longer period of time to register at the protein level, these rapid actions of glucocorticoids may occur via the “putative” membrane corticosteroid receptor, as has been demonstrated for the estrogen receptor, or through release of a receptor interacting protein following ligand binding [44].
Elevated levels of glucocorticoids are also associated with decreased testosterone biosynthesis by Leydig cells. The degree to which glucocorticoids inhibit Leydig cell function is determined by the amount of GR in the cell, the intracellular concentration of glucocorticoids, and the oxidative activity of 11β-hydroxysteroid dehydrogenase (11β-HSD), an enzyme that catalyzes both oxidative and reductive reactions of glucocorticoids. Two 11β-HSD isoforms exist by which Leydig cells regulate their intracellular concentration of glucocorticoid levels, 11β-HSD1 and -2 [45, 46]. The catalytic direction of 11s-HSD1 is determined by the cell type and intracellular milieu [47]. It has been clearly shown that 11β-HSD1 in Leydig cells behaves predominantly as a dehydrogenase, while 11β-HSD1 in liver cells behaves predominantly as a reductase [48].11β-HSD2, having no detectable reductase activity, is known to be an exclusively oxidative enzyme. 11s-HSD has therefore been described as a gatekeeper of testicular steroidogenesis in times of stress. Under stressful conditions, the oxidative capacity of 11β-HSD in Leydig cells may be exceeded by high levels of glucocorticoids leading to suppression of testosterone biosynthesis. There is also evidence for a dynamic coupling between 11s-HSD and the enzymes responsible for testosterone biosynthesis. This action would also account for the rapid effects of glucocorticoids on the suppression of testosterone production.
In addition to the rapid effects of glucocorticoids on testosterone production, glucocorticoids have also been reported to induce Leydig cell apoptosis, reducing the number of Leydig cells per testis; they also promote apoptosis of spermatogonia within the seminiferous tubules [49, 50]. It is well known that glucocorticoids induce apoptosis in thymocytes in vitro and in vivo through reductions in the mitochondrial membrane potential and the generation of reactive oxygen species (ROS) [51, 52]. Glucocorticoid-induced apoptosis in Leydig cells involves a reduction in the mitochondrial membrane potential, generation of ROS, as well as, the activation of the Fas system and cleavage of procaspase-3 [53]. In humans, severe psychological stress is associated with decreased sperm concentration, which could be attributed to glucocorticoid induced-apoptosis of Leydig cells or apoptosis of testicular spermatogonia [54]. Expression of GR in spermatocytes of discrete stages of spermatogenesis suggests that glucocorticoids can directly influence the spermatogenic cycle in a stage-specific manner. Transient increase in serum corticosterone and reduction of serum testosterone caused by immobilization stress can enhance testicular germ cell apoptosis in rats, although the exact mechanism of apoptosis within these cells is not currently known [55]. One study points to dexamethasone increased expression of BAX, a proapoptotic gene, in testicular germ cells [56]. Stages VII–VIII are the most susceptible to dexamethasone-induced apoptosis, which correspond to a GR-expressing spermatogenic stage. Viability at these stages is also considered to be androgen dependent, thus, the increase in BAX expression in testicular germ cells is probably related to the inhibitory effect of glucocorticoids on androgen biosynthesis in Leydig cells. In addition, higher doses of dexamethasone result in apoptosis of androgen-independent stages of the spermatogenic cycle, as well as, a limited portion of Sertoli cells. Bax expression is not increased in the apoptotic Sertoli cells suggesting that other factors are involved in glucocorticoid-induced apoptosis in these testicular cells.
The identification of GR in the nuclei of macrophages, resident cells of the testis, is of no surprise due to the well characterized immune modulating actions of glucocorticoids. Glucocorticoids are able to modulate macrophage function depending on concentration and can thereby control the host's immune responses to pathogens. Macrophage function is enhanced at low corticosterone levels while high concentrations are immunosuppressive [57]. Part of their immunosuppressive actions include suppressing the expression of macrophage migration inhibitory factor (MIF), an important pro-inflammatory cytokine found to be expressed at sites of inflammation, in human T lymphoblasts (CEM C7A) and human lung epithelial cells (A549) [58]. However, at low concentrations of glucocorticoids, MIF production is induced rather than repressed by macrophages [59]. MIF is also expressed in the testis by the Leydig cell and this cell type responds to glucocorticoid treatment by stimulating MIF expression. MIF secretion by the Leydig cell modulates Sertoli cell inhibin production, therefore, the MIF response to glucocorticoids by the Leydig cells may play a role in another feedback mechanism along the HPG axis [60].
Apart from elevated glucocorticoids, adrenal insufficiency in men as a result of Addison’s disease, chronic adrenal insufficiency, or adrenalectomy results in testicular deficiency [61, 62]. Surprisingly, studies on the effect of adrenalectomy on testicular function showed a reduction in Leydig cell testosterone production and sperm density, similar to over-exposure to glucocorticoids. Corticosterone deficiency decreases Leydig cell steroidogenic enzymes and peptide receptors gene expression and thus testosterone production [63]. While levels of the steroidogenic enzyme StAR remained the same, mRNA levels of P450 SCC and 17β-HSD were significantly decreased in Leydig cells of corticosterone-deficient animals. Both P450 SCC and 17β-HSD are involved in key steps of steroid hormone biosynthesis in the Leydig cell and their decreased expression and activity may be responsible for decreased testosterone production in cases of corticosterone deficiency.
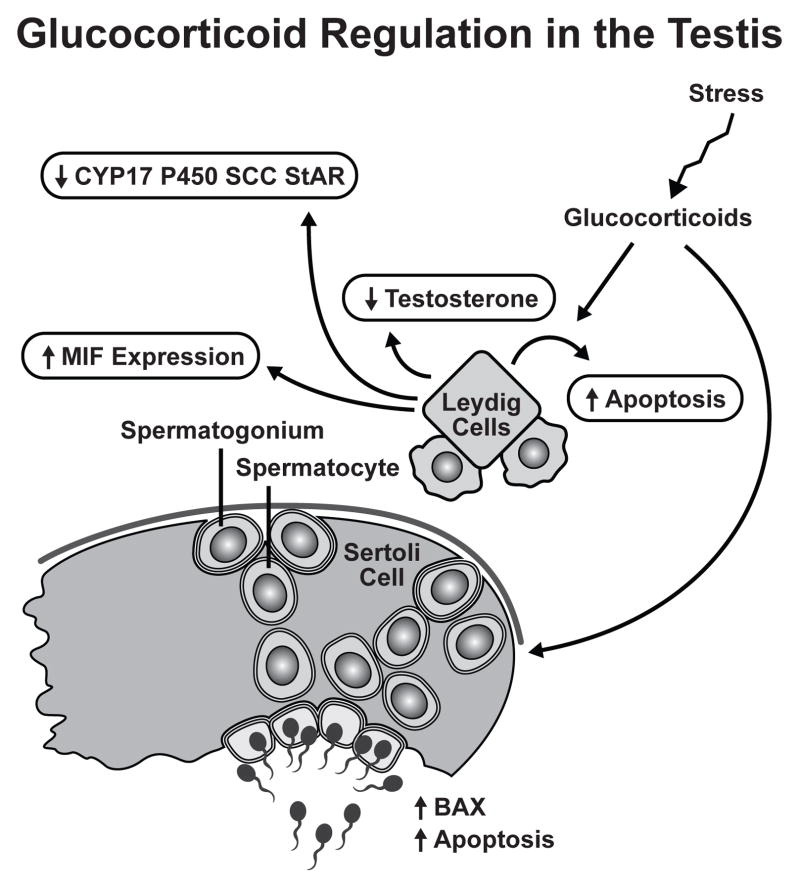
Clinical cases of Cushing’s and Addison’s disease point to the requirement for a precise amount of glucocorticoids for proper testicular function and fertility. Leydig cells represent the primary GR expressing cell type in the testis and are the main targets of glucocorticoid action. Glucocorticoids mediate their effects in the Leydig cell through the GR in classical genomic mechanisms, as it the case for MIF and Bax expression, and by rapid non-genomic mechanisms. Throughout the entire testis apoptosis plays a role in normal testicular physiology, however, glucocorticoids can modulate fertility through stress-induced reduction of cell number. It follows that in times of emotional or physical stress resources would be diverted from the reproductive process to those needed for self preservation. These mechanisms involved in glucocorticoid regulation of male fertility are summarized in Figure 3.
Figure III.
Glucocorticoid Regulation in the Testis. The Leydig cell is the primary target of glucocorticoid regulation in the testis. The direct inhibitory effect of glucocorticoids involves multiple mechanisms: (1) inhibition of testosterone biosynthesis, (2) reduction in steroidogenic enzymes CYP17, P450 SCC, and StAR, (3) induction of Leydig cell apoptosis, and (4) stimulation of MIF expression. Glucocorticoids can regulate other cell types within the testis. Germ cells are susceptible to glucocorticoid-induced apoptosis possibly through BAX regulation.
Glucocorticoids Target the Ovary
Throughout reproductive maturity, the ovary experiences regulation by glucocorticoids that exert both agonistic and antagonistic effects on ovarian function. Glucocorticoids are able to directly modulate ovarian function in three unique ways. As reviewed above, the primary way glucocorticoids influence ovarian function is indirect through altering levels of circulating gonadotropins by acting on the hypothalamus and pituitary. The second mechanism of glucocorticoid regulation is also indirect and involves affecting levels of metabolic hormones and growth factors, such as insulin-like growth factor-1. Finally, glucocorticoids can directly modulate ovarian function through the presence of its receptor in ovarian cell types.
The ovary does not appear to produce glucocorticoids locally; therefore, regulation by one of the two 11s-HSDs is one mechanism of local regulation of glucocorticoid action in the ovary. The presence of 11s-HSD has been demonstrated in many ovarian cell types, including the oocyte, cumulus cells, granulosa cells, theca cells, granulosa-lutein cells, corpus luteum and ovarian surface epithelium [64–66]. Within the ovary, 11s-HSD1 and 11s-HSD2 are developmentally regulated. Specifically, granulosa cells that have not experienced an ovulatory LH surge express 11s-HSD2, whereas luteinizing granulosa cells express 11s-HSD1 with no detectable expression of11s-HSD2 [8, 67]. This data indicates that levels of active glucocorticoids are suppressed during follicular development and maturation, while levels are increased during the ovulatory process triggered by the LH surge. As ovulation may be analogous to an inflammatory event, increased generation of anti-inflammatory glucocorticoids by the reductase 11s-HSD1 may be a physiological mechanism to limit the ovarian inflammatory process. Interleukin-1β (IL-1β), a cytokine crucial to the ovulatory process, also upregulates basal and LH-stimulated expression of 11s-HSD1 in cultured rat granulosa cells, which may be part of the inflammatory cascade of ovulation [8].
Local levels and steroidal regulation of cofactor enzymes also influence the activity of 11s-HSD during the periovulatory switch in enzyme isoforms. 11s-HSD1 can act as either a reductase or dehydrogenase depending on the relative abundance of nicotinamide adenine dinucleotide phosphate (NADP+) and reduced NADP (NADPH), while the dehydrogenase activity of 11s-HSD2 requires NAD+. During follicular maturation, P450 aromatase oxidizes NADPH to NADP+ to synthesize estradiol, supporting the oxidative activities of 11s-HSD1 [68]. Human follicular fluid contain hydrophilic stimuli and hydrophobic inhibitors of NADP+-dependent 11s-HSD1 dehydrogenase activity [69]. High activity of the hydrophilic enzyme stimulator is associated with low relative levels of active to inactive cortisol, indicating a role for these follicular factors in determining the direction of 11s-HSD activity
Glucocorticoid action in the ovary also depends on binding to its receptor; therefore, the abundance of functional GR available in target cells is another important mechanism of regulation. The GR is present in follicles, corpus luteum, and ovarian surface epithelium cells of rat and human ovaries [70]. The expression of the receptor in the follicle and corpus luteum is consistent during follicular maturation, ovulation, and pregnancy in the rat, indicating that the principle regulatory mechanisms in these cell types is the level of active glucocorticoids [8]. However, in cultured human ovarian surface epithelial cells, GR gene expression is regulated by the inflammatory cytokine IL-1α [71]. The human ovarian surface epithelium, a simple, squamous-to-cuboidal cell layer covering the entire ovary, is breached and regenerated every time a follicle ovulates. This single-layered epithelium contains a local system for generating anti-inflammatory glucocorticoids through increased conversion of cortisone to cortisol by 11β-HSD1, as part of the process to minimize injury of the ovarian surface during ovulation [72]. The means by which glucocorticoids protect the ovary include: increased expression of 11β-HSD1, increased GR suppression of the COX-2 gene, and suppression of IL-1α matrix metalloproteinase gene expression [73]. The interesting dynamic of IL-1α to both stimulate expression of the anti-inflammatory signaling protein GR and 11β-HSD1 and serve as the antagonistic substrate for cell remodeling suggests a novel mechanism for localizing and limiting proteolytic damage to the ovarian surface during ovulation.
As in the steroidogenic cells of the testis, glucocorticoids act directly on ovarian cells to inhibit LH action and steroid biosynthesis [74]. In cultured human granulosalutein cells, cortisol inhibits LH-stimulated steroidogenesis in a concentration dependent manner and this interaction is modulated by 11β-HSD. In cultured rat granulosa cells, glucocorticoids suppress FSH-induced P450 aromatase activity and LH receptor numbers [10, 75]. In contrast, glucocorticoids enhance FSH-stimulated progesterone synthesis. The synergistic effect of glucocorticoids on FSH-mediated progesterone synthesis can be accounted for by enhanced production and impaired metabolism of progesterone. Glucocorticoids stimulate 3β-HSD activity, which converts pregnenolone to progesterone, and inhibits 20α-HSD activity, which reduces progesterone to an inactive form. In addition to steroidogenesis, glucocorticoids can regulate oogenesis along multiple stages of ovulation.
Mammalian oocytes are maintained in a stage of meiotic arrest until shortly before ovulation and only after the preovulatory surge of LH does the oocyte resume meiotic maturation and become fertilizable. The direct effect of glucocorticoids on oocyte maturation has been investigated in mammals but differences were noted between animal models. Glucocorticoids inhibit meiotic development in pig oocytes but have little to no effect in sheep oocytes, so some effects may be species specific [76, 77]. To evaluate the effect of glucocorticoids on human oocytes, levels of intrafollicular glucocorticoids were correlated to oocyte maturation and fertilizability in women undergoing in vitro fertilization. Follicular fluid from follicles whose oocytes were not fertilized had levels of cortisol significantly higher than the levels in follicular fluid from follicles containing successfully fertilized oocytes [78]. These results suggest that glucocorticoids may antagonize oocyte maturation.
Interestingly, within the ovary, ovulation and the resulting folliculoluteal transition has been equated to an inflammatory response, as a result of the acute hemodynamic, cellular, and biochemical changes that occur at the site of follicle rupture. Due to the well documented anti-inflammatory effects of glucocorticoids and the expression of the GR in the corpus luteum, glucocorticoids may affect maintenance of the corpus luteum or the immune cell-mediated processes during luteolysis. Glucocorticoids inhibit rather than stimulate the remodeling associated with luteolysis and increase the survival of luteinized granulosa cells and may have role in maintenance of the corpora lutea during maternal recognition of pregnancy [79]. In rat and human granulosa cells which have undergone luteinization following exposure to an ovulatory dose of LH in vivo, there is no detectable expression of 11β-HSD2 but predominant expression of 11β-HSD1 [67]. 11β-HSD1 expression increases progressively as the cells undergo functional luteinization, which corresponds to increased levels of available glucocorticoids and a switch in expression from mineralocorticoid receptors in the follicle to GR in luteinized cells [8, 80]. In fact, a rise in free cortisol that is 50 times higher in follicular fluid after the LH surge is indicative that expression levels of the 11sHSD enzymes in the ovary are an accurate measure for the direction of glucocorticoid metabolism [81].
Beyond control at the hypothalamic and pituitary level, the ovary is also equipped with local regulatory mechanisms of glucocorticoid action. The primary regulatory mechanism consists of changes in the expression of the two isoforms of 11β-HSD that catalyze the conversion between active and inactive glucocorticoids. During follicular maturation, the dehydrogenase activity of 11β-HSD restricts levels of active glucocorticoids. Meanwhile at ovulation, the 11β-HSD increases levels of active glucocorticoids, which mediates the inflammatory response associated with oocyte rupture of the ovarian surface epithelium. Further actions of glucocorticoids in the ovary include local regulation of steroidogenesis, oocyte maturation, maintenance of the corpora lutea, and luteal regression. Accordingly, glucocorticoid action in the ovary is integral part of its physiology, which requires precise levels of available glucocorticoids. Stress-related increase in levels of glucocorticoids negatively effect fertility in women, where not only ovarian function is compromised but also uterine.
Other Targets of Glucocorticoid Action and Potential Roles in Fertility
Coordinated actions of steroid hormones in the female regulate uterine cell proliferation and differentiation to establish implantation. Embryo implantation requires a series of well-coordinated events, including: apposition of the uterine luminal epithelium to the blastocyst, trophectoderm adhesion and invasion of the luminal epithelium, and invasion of the stroma by the trophectoderm. Progesterone and estrogen have long been established as essential regulators of this process; however, glucocorticoids are emerging as important regulators of hormone action in the uterus. Glucocorticoids can block estrogen-induce uterine growth and differentiation, as well as embryo implantation in the rat [82–84]. Estrogen administration to immature rats induces edema of the stroma and myometrium and changes comparable to an acute inflammatory response [85]. When co-administered with estrogen, dexamethasone completely blocks the proinflammatory-like effects of estrogen, along with the vasodilation and edema. Although dexamethasone is able to block many of the biological effects of estrogen in the uterus, when uteri are analyzed by microarray analysis, very few genes display antagonistic regulation. Of those antagonistically regulated genes, none stand out as candidates for mediating the proinflammatory effects induced by estrogen but repressed by dexamethasone. However, glucocorticoids have since been shown to regulate the complement system, a key mediator of innate immunity [86]. Estrogen treatment induces vasodilation and vascular permeability in the uterus, along with potent bactericidal activity of the uterine laminal fluid. Co-administration with dexamethasone is able to completely block estrogen-induced amount and latent activity of complement proteins in the rat uterus. Studies in breast cancer cell lines also provide insight into the dynamic antagonism of estrogen and glucocorticoids [87]. Activation of the GR by dexamethasone induces expression and activity of the estrogen sulfotransferase enzyme (SULT1E1) important for the metabolic deactivation of estrogens. GR-mediated SULT1E1 gene activation facilitated estrogen deactivation and inhibits estrogen-dependent breast cancer growth in cell culture and in vivo. Whether a similar mechanism of estrogen deprivation by glucocorticoids occurs in the uterus and is involved in glucocorticoid regulation of the innate immune response has yet to be determined.
Maternal Stress and Fetal Outcomes
The immune response in the female is unique in that the reproductive tract is exposed to allogenic sperm and a fetus that are immunologically distinct. To meet this specific challenge, the immune system within the uterus is controlled precisely by steroid hormones to optimize both maternal and fetal survival. In early pregnancy, the actions of glucocorticoids are balanced between the positive effects that would promote pregnancy, suppress uterine natural killer cells, and promote trophoblast growth/invasion, and effects that would compromise pregnancy, inhibition of cytokine-PGs signaling, induction of apoptosis, and inhibition of embryonic and placental growth. It is obvious that glucocorticoid signaling is an intricate part of all stages of pregnancy and that overexposure puts both the mother and fetus at risk. There are multiple mechanisms by which glucocorticoid concentrations can increase: administration of synthetic glucocorticoids to the mother, stress-induced elevation of maternal cortisol levels, and impaired cortisol metabolism within the fetus. It is well established that changes in the prenatal environment related to stress are able to modify hypothalamo-pituitary-adrenal (HPA) function of the mother [88]. However, continued analysis of human epidemiological data suggests that the fetal environment can also influence susceptibility in offspring to later conditions, such as glucose intolerance, hypertension, and metabolic syndrome (reviewed in [89]). Etiology of such symptoms suggests that programming of the HPA axis in utero is linked to development of insulin resistance, diabetes, and cardiovascular disease in adulthood. It is interesting to ponder the utility of such fetal programming by the maternal environment. One possibility is that a pregnant mammal exposed to a hostile environment requires increased vigilance for survival. The stress signals transmitted to the fetus would therefore program offspring for stress-related behaviors and altered development of the HPA axis in order to enhance survival after birth. It is a sophisticated mechanism in which our offspring are able to adapt to the environment into which they are born into. However, as humans with vast available resources during pregnancy, a compromised or modified pregnancy may or may not confer any additional benefit to offspring but will more likely convey a detrimental behavioral, endocrine, cardiovascular, or metabolic regulation.
A large number of animal studies have investigated the effects of changes in the fetal environment on HPA function and related conditions. Within given species, effects in the offspring are highly dependent on the nature of the exposure to glucocorticoids (i.e. maternal stress, exposure to exogenous glucocorticoids, malnutrition), as well as the intensity and duration of exposure during pregnancy. In guinea pigs, an acute period of maternal nutrient restriction corresponding to the period of maximal fetal brain growth results in offspring with modified HPA function that is highly sex-specific [90]. Adult male guinea pigs born to nutrient-restricted mothers exhibit reduced basal and stress-induced HPA activity, whereas female offspring from the same litters exhibited elevated basal and activated HPA activity. With regard to timing of exposure, brief exposure to maternal stress at 70% gestation in guinea pigs results in adult male offspring with elevated basal cortisol levels but normal adrenocortical responses to stress. However, the same stress administered at 90% gestation results in male offspring with normal basal levels of cortisol but increased HPA responsiveness to challenge [91]. Outcomes in offspring are also dependent on the stage in the reproductive cycle when the analysis of the given outcome is measured. Normal cycling adult female guinea pigs exposed to prenatal stress exhibit reduced salivary cortisol response but only during the estrous phase of the cycle [92]. This may explain the under-representation of females in the literature. There also appears to be a strong influence of postnatal environment on prenatal conditions where foster parents can reduce or reverse the effects of the prenatal stress. Differences in the observed influence of prenatal stress on adult behaviors among species may also arise from the unique profiles of fetal body and brain development that exist between species. In humans, the rapid phase of fetal brain development occurs from weeks 27 to 30 of pregnancy and extends into the postnatal period, whereas in the rat or mouse maximal brain growth is not initiated until postnatal life. Therefore a period of maternal stress in a rodent would correspond to a very different phase in the fetal brain of a human.
High prenatal maternal cortisol levels also predict sensitivity of cortisol responses to stress in the offspring. A sample of 10-year-old children from the Avon Longitudinal Study of Parents and Children demonstrated a link between prenatal anxiety during late pregnancy and differences in basal salivary cortisol levels [94]. A retrospective study of young adults whose mothers experienced severe stress during pregnancy in the form of death or severe illness of a relative, exhibited a higher cortisol response to a psychosocial stress test than those in the control group [95]. Additionally, studies have shown that offspring of women pregnant at the time of a disaster tend to be smaller, born prematurely, and have a wide range of subsequent behavioral and physiological abnormalities [96]. Offspring born from early gestation coinciding with the Dutch famine, a 5-month period of severe food shortage during World War II, had an associated increase in coronary artery disease, atherogenic lipid profile, and altered clotting, with an enhanced blood pressure response to stress. Pregnant women exposed to the World Trade Center collapse in September 2001 who developed posttraumatic stress disorder (PTSD) had offspring with lower awakening and evening cortisol concentrations than those who did not develop (PTSD) [97].
The question remains as to how these effects of maternal stress could be transmitted to the subsequent generation. Fetal programming likely occurs through several mechanisms including: altered maternal endocrine adaptation to pregnancy and transmission of epigenetic modifications to the offspring. Maternal stress leads to numerous endocrine changes in the mother, including increases in plasma ACTH and glucocorticoids. Some of the effects on the fetus may be indirect through modification of placental function. The placenta forms a structural and biochemical barrier to maternal endogenous glucocorticoids but inhibition of placental 11β-HSD2, the primary barrier protecting the fetus from high maternal glucocorticoid concentrations, leads to endocrine and behavioral outcomes of offspring that are similar to those of excess fetal glucocorticoid exposure [98]. 11β-HSD2 mRNA is highly expressed in the labyrinthine zone, the major site of maternal/fetal exchange, in a spatiotemporal manner [99]. Interestingly, the silencing of 11β-HSD2 at day E16.5 in the mouse coincides with the onset of 11β-HSD1 gene expression and the peak of the corticosterone surge during late gestation. The onset of 11β-HSD1 expression in the placenta coincides with the corticosterone surge during late gestation, which is believed to be involved in the final maturation of vital organs such as the lungs and initiation of parturition.
Uterine quiescence during most of pregnancy is maintained by anti-inflammatory actions of fetal hormones, while fetal parturition is associated with an inflammatory response within the maternal uterus and cervix, including stimulation of macrophage migration to the uterus, release of cytokines, and activation of inflammatory transcription factors. In sheep, the HPA axis matures during the last 2–3 weeks of gestation and the increased production of cortisol by the fetal adrenal gland is suggested to serve as a signal for the initiation of labor [100]. Elevated fetal cortisol production enhances cyclooxygenase-2 expression, which increases production of PGs. These stimulate the expression of CYP17 to enhance the production of C19-steroids, which are metabolized to estrogens. Increased levels of estrogens enhance uterine contractility by antagonizing the progesterone receptor [101]. Promoting fetal cortisol production establishes a positive-feedback loop, where steroids return via umbilical circulation to the placenta where cortisol promotes further corticotrophin-releasing hormone secretion. The surge in fetal cortisol late in gestation also increases the maturation of the fetal lungs and their capacity to synthesize surfactant, which may also serve as a signal for the initiation of labor [102]. However, in cases where the fetus is distressed or compromised, activation of the fetal HPA axis can accelerate fetal organ maturation and promote the onset of parturition, providing a means of escape for fetal survival.
Preterm birth accounts for almost 13% of all live births in the United States. These women are routinely treated with synthetic glucocorticoids between 24 and 34 weeks gestation to reduce respiratory distress syndrome in newborns. Glucocorticoids are also given preconceptually to women with recurrent miscarriages However, the fact that glucocorticoids play a central role in modulating HPG function, leads to questions regarding the impact of synthetic glucocorticoid treatment during pregnancy. Unlike the endogenous glucocorticoids, synthetic glucocorticoids are not metabolized by placental 11β-HSD2, and therefore, enter fetal circulation [103]. Maternal treatment with glucocorticoids in vivo can induce placenta apoptosis and glucocorticoid-induced growth inhibition in rats [104]. Placental apoptosis was also induced by the 11β-HSD2 inhibitor Carbenoxolone, which indicates that placental apoptosis may also be stimulated by increased placental exposure to endogenous glucocorticoids.
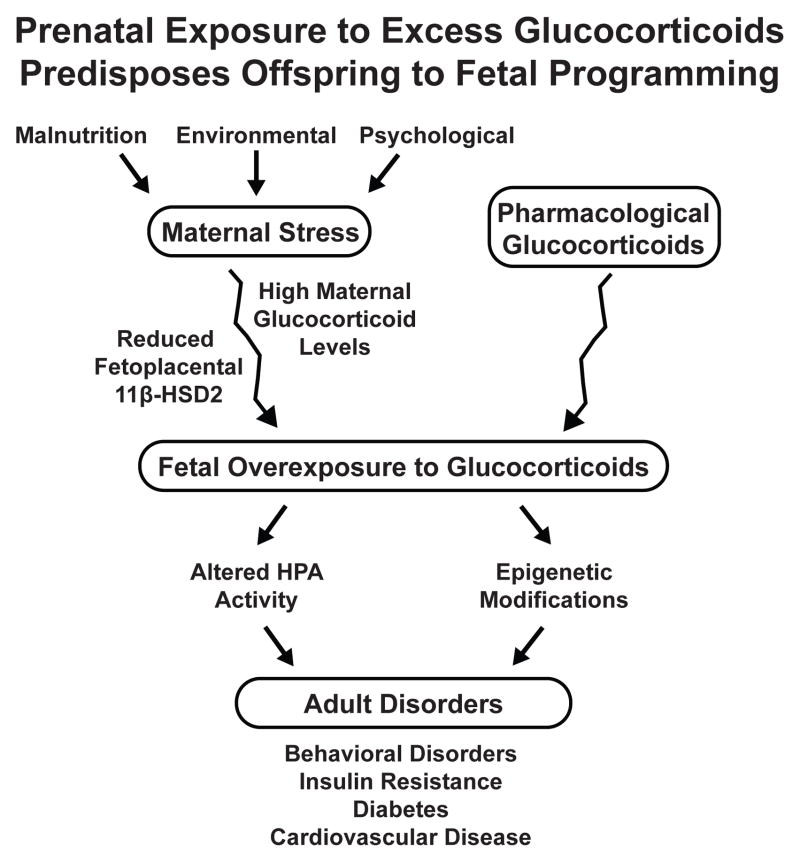
Glucocorticoids exert potent effects on cellular function in essentially all organ systems, particularly in terms of differentiation and homeostasis. These actions are of particular importance in mammalian pregnancy, considering glucocorticoids are known to influence metabolic adaptation of the mother, maturation of the fetus, and timing of parturition. Because glucocorticoids provide key signals in cellular differentiation, prenatal exposure to high levels of glucocorticoids has been directly linked to the subsequent development of disease in adult life (Figure 4). The mechanisms of endocrine programming are complex involving multiple pathways and are reviewed by Kapoor [89]. Despite gaps in our knowledge, understanding these mechanisms may explain laboratory findings and human observations that environmental influences and emotional stress in one generation can influence disease risk in the next.
Figure IV.
Prenatal Exposure to Excess Glucocorticoids Predispose Offspring to Fetal Programming. Excessive fetal glucocorticoid exposure may result from a stressful maternal environment, reduced fetoplacental 11β-HSD2 activity, or exogenously administered glucocorticoids. A modified fetal environment can directly affect development of the brain and neuroendocrine structures, likely involving epigenetic modifications. These changes can predispose offspring to various metabolic, cardiovascular, and neurobiological pathophysiologies.
Concluding Remarks
Glucocorticoids are physiological hormones that are essential for life [105]. While originally named for their ability to stimulate glucose formation in the liver, glucocorticoids exert a variety of effects on target cells, mediating many aspects of homeostasis, regulating the immune system, and playing a critical role in the systemic stress response. In response to psychological and physiological stressors, serum concentrations of glucocorticoids are elevated. As seen in patients with Cushing’s syndrome, couples undergoing prolonged time to conception, and offspring stressed in utero, stress levels of glucocorticoids can disrupt and suppress endocrine signaling in the HPG axis. These effects are seen at multiple points along the axis, including suppression of the pituitary gonadotropes through receptors in the hypothalamus and direct influence in the testes and ovaries. Furthermore, maternal exposure to an adverse environment during pregnancy causes glucocorticoid-induced modifications of HPA activity and behavioral changes that may enhance early survival of offspring but at the greater cost of susceptibility to subsequent vascular or metabolic disease in adulthood.
As reviewed above, the role of glucocorticoids in reproduction has been extensively studied through in vitro cell culture, animal models, and patient data. What is missing from this analysis is direct examination of glucocorticoid signaling in a tissue and cell type specific manner. Due to the early lethality of the GR knockout mouse, the role of the GR in glucocorticoid physiology and during reproduction can not be studied [105]. Until the development of a site-specific, inducible GR mutant in reproductive tissues, studies will be restricted to global depletion of glucocorticoids in developmentally complete adrenalectomized animals or descriptive patient data. Fully understanding the mechanism of glucocorticoid action at each level within the HPG axis will allow for the development of intervention strategies that mitigate the adverse effects of stress on reproductive function.
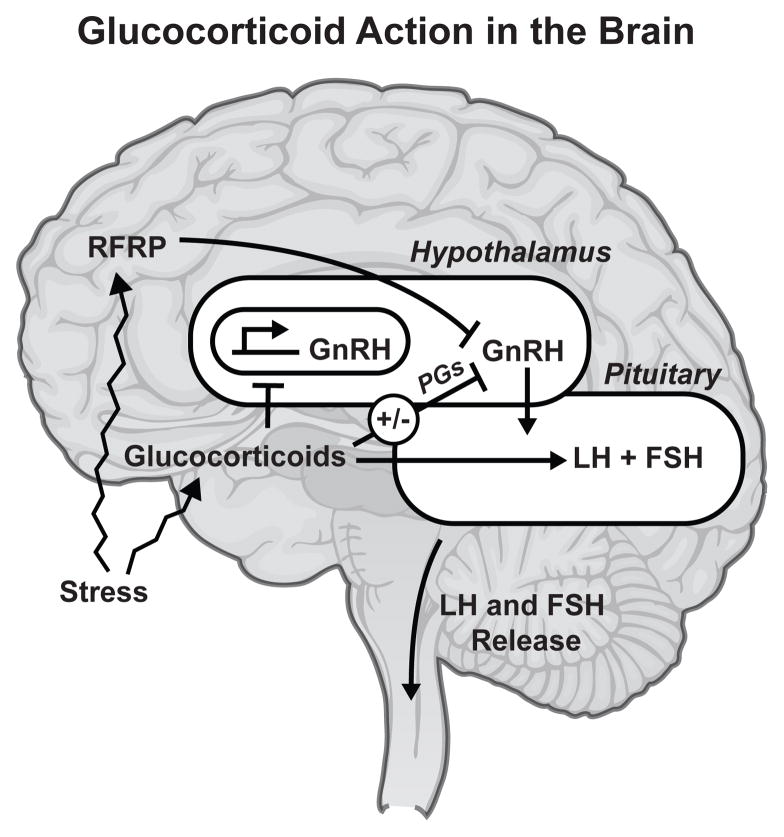
Figure II.
Glucocorticoid Action in the Brain. In the reproductive system, the brain's hypothalamus produces GnRH, which stimulates the pituitary gland to produce LH and FSH, which in turn stimulate production of testosterone, estradiol and sexual behavior. Stress induces elevated circulating levels of glucocorticoids, which act directly on the hypothalamus to suppress GnRH production. Glucocorticoids also stimulate expression of RFRP, a gonadotropin-inhibitory hormone, which also acts to reduce GnRH production as well as to directly lower pituitary secretion of LH and FSH. Glucocorticoids may also play a protective role in maintaining the HPG axis during acute stress through suppression of PGs.
Acknowledgments
We gratefully acknowledge Dr. Robert Oakley for discussion and assistance with manuscript preparation. This research was supported by the Intramural Research Program of the NIH National Institute of Environmental Health Sciences.
References
- 1.Rabin D, et al. Stress and reproduction: physiologic and pathophysiologic interactions between the stress and reproductive axes. Adv Exp Med Biol. 1988;245:377–87. doi: 10.1007/978-1-4899-2064-5_29. [DOI] [PubMed] [Google Scholar]
- 2.Collu R, Gibb W, Ducharme JR. Effects of stress on the gonadal function. J Endocrinol Invest. 1984;7(5):529–37. doi: 10.1007/BF03348463. [DOI] [PubMed] [Google Scholar]
- 3.Dubey AK, Plant TM. A suppression of gonadotropin secretion by cortisol in castrated male rhesus monkeys (Macaca mulatta) mediated by the interruption of hypothalamic gonadotropin-releasing hormone release. Biol Reprod. 1985;33(2):423–31. doi: 10.1095/biolreprod33.2.423. [DOI] [PubMed] [Google Scholar]
- 4.Kamel F, Kubajak CL. Modulation of gonadotropin secretion by corticosterone: interaction with gonadal steroids and mechanism of action. Endocrinology. 1987;121(2):561–8. doi: 10.1210/endo-121-2-561. [DOI] [PubMed] [Google Scholar]
- 5.Briski KP, Sylvester PW. Acute inhibition of pituitary LH release in the male rat by the glucocorticoid agonist decadron phosphate. Neuroendocrinology. 1991;54(4):313–20. doi: 10.1159/000125908. [DOI] [PubMed] [Google Scholar]
- 6.Nicolaides NC, et al. The human glucocorticoid receptor: molecular basis of biologic function. Steroids. 75(1):1–12. doi: 10.1016/j.steroids.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz R, et al. Localization of the glucocorticoid receptor in testis and accessory sexual organs of male rat. Mol Cell Endocrinol. 1993;95(1–2):115–20. doi: 10.1016/0303-7207(93)90036-j. [DOI] [PubMed] [Google Scholar]
- 8.Tetsuka M, et al. Expression of 11beta-hydroxysteroid dehydrogenase, glucocorticoid receptor, and mineralocorticoid receptor genes in rat ovary. Biol Reprod. 1999;60(2):330–5. doi: 10.1095/biolreprod60.2.330. [DOI] [PubMed] [Google Scholar]
- 9.Bambino TH, Hsueh AJ. Direct inhibitory effect of glucocorticoids upon testicular luteinizing hormone receptor and steroidogenesis in vivo and in vitro. Endocrinology. 1981;108(6):2142–8. doi: 10.1210/endo-108-6-2142. [DOI] [PubMed] [Google Scholar]
- 10.Hsueh AJ, Erickson GF. Glucocorticoid inhibition of FSH-induced estrogen production in cultured rat granulosa cells. Steroids. 1978;32(5):639–48. doi: 10.1016/0039-128x(78)90074-0. [DOI] [PubMed] [Google Scholar]
- 11.Gharib SD, et al. Molecular biology of the pituitary gonadotropins. Endocr Rev. 1990;11(1):177–99. doi: 10.1210/edrv-11-1-177. [DOI] [PubMed] [Google Scholar]
- 12.Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 120(4):963–72. doi: 10.1172/JCI41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCullagh DR. Dual Endocrine Activity of the Testes. Science. 1932;76(1957):19–20. doi: 10.1126/science.76.1957.19. [DOI] [PubMed] [Google Scholar]
- 14.Michel U, Farnworth P, Findlay JK. Follistatins: more than follicle-stimulating hormone suppressing proteins. Mol Cell Endocrinol. 1993;91(1–2):1–11. doi: 10.1016/0303-7207(93)90248-i. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld P, et al. Ontogeny of the type 2 glucocorticoid receptor in discrete rat brain regions: an immunocytochemical study. Brain Res. 1988;470(1):119–27. doi: 10.1016/0165-3806(88)90207-6. [DOI] [PubMed] [Google Scholar]
- 16.McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986;66(4):1121–88. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- 17.Chandran UR, et al. Glucocorticoid receptor-mediated repression of gonadotropin-releasing hormone promoter activity in GT1 hypothalamic cell lines. Endocrinology. 1994;134(3):1467–74. doi: 10.1210/endo.134.3.8119188. [DOI] [PubMed] [Google Scholar]
- 18.Chandran UR, et al. The glucocorticoid receptor is tethered to DNA-bound Oct-1 at the mouse gonadotropin-releasing hormone distal negative glucocorticoid response element. J Biol Chem. 1999;274(4):2372–8. doi: 10.1074/jbc.274.4.2372. [DOI] [PubMed] [Google Scholar]
- 19.Tsutsui K, et al. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275(2):661–7. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- 20.Kriegsfeld LJ, et al. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A. 2006;103(7):2410–5. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirby ED, et al. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci U S A. 2009;106(27):11324–9. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuwaki T, et al. Maintenance of gonadotropin secretion by glucocorticoids under stress conditions through the inhibition of prostaglandin synthesis in the brain. Endocrinology. 2006;147(3):1087–93. doi: 10.1210/en.2005-1056. [DOI] [PubMed] [Google Scholar]
- 23.Matsuwaki T, et al. Glucocorticoid maintains pulsatile aecretion of luteinizing hormone under infectious stress condition. Endocrinology. 2003;144(8):3477–82. doi: 10.1210/en.2002-221111. [DOI] [PubMed] [Google Scholar]
- 24.Matsuwaki T, et al. Glucocorticoid counteracts the suppressive effect of tumor necrosis factor-alpha on the surge of luteinizing hormone secretion in rats. J Endocrinol. 2004;181(3):509–13. doi: 10.1677/joe.0.1810509. [DOI] [PubMed] [Google Scholar]
- 25.Konsman JP, et al. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J Comp Neurol. 2004;472(1):113–29. doi: 10.1002/cne.20052. [DOI] [PubMed] [Google Scholar]
- 26.Kotitschke A, et al. Genomic and nongenomic cross talk between the gonadotropin-releasing hormone receptor and glucocorticoid receptor signaling pathways. Mol Endocrinol. 2009;23(11):1726–45. doi: 10.1210/me.2008-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suter DE, Schwartz NB. Effects of glucocorticoids on secretion of luteinizing hormone and follicle-stimulating hormone by female rat pituitary cells in vitro. Endocrinology. 1985;117(3):849–54. doi: 10.1210/endo-117-3-849. [DOI] [PubMed] [Google Scholar]
- 28.Stackpole CA, et al. Sex difference in the suppressive effect of cortisol on pulsatile secretion of luteinizing hormone in sheep. Endocrinology. 2006;147(12):5921–31. doi: 10.1210/en.2006-0667. [DOI] [PubMed] [Google Scholar]
- 29.Kumar TR, et al. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201–4. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 30.McGillivray SM, et al. Activin and glucocorticoids synergistically activate follicle-stimulating hormone beta-subunit gene expression in the immortalized LbetaT2 gonadotrope cell line. Endocrinology. 2007;148(2):762–73. doi: 10.1210/en.2006-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ringstrom SJ, et al. Cortisol in vivo increases FSH beta mRNA selectively in pituitaries of male rats. Endocrinology. 1991;129(5):2793–5. doi: 10.1210/endo-129-5-2793. [DOI] [PubMed] [Google Scholar]
- 32.Tohei A, Kogo H. Dexamethasone increases follicle-stimulating hormone secretion via suppression of inhibin in rats. Eur J Pharmacol. 1999;386(1):69–74. doi: 10.1016/s0014-2999(99)00722-0. [DOI] [PubMed] [Google Scholar]
- 33.Kawakami S, Fujii Y, Winters SJ. Follistatin production by skin fibroblasts and its regulation by dexamethasone. Mol Cell Endocrinol. 2001;172(1–2):157–67. doi: 10.1016/s0303-7207(00)00371-3. [DOI] [PubMed] [Google Scholar]
- 34.Smals AG, Kloppenborg PW, Benraad TJ. Plasma testosterone profiles in Cushing's syndrome. J Clin Endocrinol Metab. 1977;45(2):240–5. doi: 10.1210/jcem-45-2-240. [DOI] [PubMed] [Google Scholar]
- 35.Aono T, et al. Influence of surgical stress under general anesthesia on serum gonadotropin levels in male and female patients. J Clin Endocrinol Metab. 1976;42(1):144–8. doi: 10.1210/jcem-42-1-144. [DOI] [PubMed] [Google Scholar]
- 36.Bernton E, et al. Adaptation to chronic stress in military trainees. Adrenal androgens, testosterone, glucocorticoids, IGF-1, and immune function. Ann N Y Acad Sci. 1995;774:217–31. [PubMed] [Google Scholar]
- 37.Orr TE, Mann DR. Effects of restraint stress on plasma LH and testosterone concentrations, Leydig cell LH/hCG receptors, and in vitro testicular steroidogenesis in adult rats. Horm Behav. 1990;24(3):324–41. doi: 10.1016/0018-506x(90)90013-n. [DOI] [PubMed] [Google Scholar]
- 38.Saez JM, et al. Effects of in vivo administration of dexamethasone, corticotropin and human chorionic gonadotropin on steroidogenesis and protein and DNA synthesis of testicular interstitial cells in prepuberal rats. Endocrinology. 1977;101(4):1256–63. doi: 10.1210/endo-101-4-1256. [DOI] [PubMed] [Google Scholar]
- 39.Hales DB, Payne AH. Glucocorticoid-mediated repression of P450scc mRNA and de novo synthesis in cultured Leydig cells. Endocrinology. 1989;124(5):2099–104. doi: 10.1210/endo-124-5-2099. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, et al. The role of arachidonic acid in steroidogenesis and steroidogenic acute regulatory (StAR) gene and protein expression. J Biol Chem. 2000;275(26):20204–9. doi: 10.1074/jbc.M003113200. [DOI] [PubMed] [Google Scholar]
- 41.Payne AH, Sha LL. Multiple mechanisms for regulation of 3 beta-hydroxysteroid dehydrogenase/delta 5----delta 4-isomerase, 17 alpha-hydroxylase/C17-20 lyase cytochrome P450, and cholesterol side-chain cleavage cytochrome P450 messenger ribonucleic acid levels in primary cultures of mouse Leydig cells. Endocrinology. 1991;129(3):1429–35. doi: 10.1210/endo-129-3-1429. [DOI] [PubMed] [Google Scholar]
- 42.Martin LJ, Tremblay JJ. Glucocorticoids antagonize cAMP-induced Star transcription in Leydig cells through the orphan nuclear receptor NR4A1. J Mol Endocrinol. 2008;41(3):165–75. doi: 10.1677/JME-07-0145. [DOI] [PubMed] [Google Scholar]
- 43.Dong Q, et al. Rapid glucocorticoid mediation of suppressed testosterone biosynthesis in male mice subjected to immobilization stress. J Androl. 2004;25(6):973–81. doi: 10.1002/j.1939-4640.2004.tb03170.x. [DOI] [PubMed] [Google Scholar]
- 44.Norfleet AM, et al. Estrogen receptor-alpha detected on the plasma membrane of aldehyde-fixed GH3/B6/F10 rat pituitary tumor cells by enzyme-linked immunocytochemistry. Endocrinology. 1999;140(8):3805–14. doi: 10.1210/endo.140.8.6936. [DOI] [PubMed] [Google Scholar]
- 45.Ge RS, et al. Developmental changes in glucocorticoid receptor and 11beta-hydroxysteroid dehydrogenase oxidative and reductive activities in rat Leydig cells. Endocrinology. 1997;138(12):5089–95. doi: 10.1210/endo.138.12.5614. [DOI] [PubMed] [Google Scholar]
- 46.Ge RS, et al. 11{beta}-Hydroxysteroid dehydrogenase 2 in rat leydig cells: its role in blunting glucocorticoid action at physiological levels of substrate. Endocrinology. 2005;146(6):2657–64. doi: 10.1210/en.2005-0046. [DOI] [PubMed] [Google Scholar]
- 47.Hu GX, et al. Rapid mechanisms of glucocorticoid signaling in the Leydig cell. Steroids. 2008;73(9–10):1018–24. doi: 10.1016/j.steroids.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ge RS, et al. Identification of a kinetically distinct activity of 11beta-hydroxysteroid dehydrogenase in rat Leydig cells. Endocrinology. 1997;138(6):2435–42. doi: 10.1210/endo.138.6.5165. [DOI] [PubMed] [Google Scholar]
- 49.Gao HB, et al. Glucocorticoid induces apoptosis in rat leydig cells. Endocrinology. 2002;143(1):130–8. doi: 10.1210/endo.143.1.8604. [DOI] [PubMed] [Google Scholar]
- 50.Yazawa H, Sasagawa I, Nakada T. Apoptosis of testicular germ cells induced by exogenous glucocorticoid in rats. Hum Reprod. 2000;15(9):1917–20. doi: 10.1093/humrep/15.9.1917. [DOI] [PubMed] [Google Scholar]
- 51.Compton MM, Cidlowski JA. Identification of a glucocorticoid-induced nuclease in thymocytes. A potential “lysis gene” product. J Biol Chem. 1987;262(17):8288–92. [PubMed] [Google Scholar]
- 52.Castedo M, et al. Mitochondrial perturbations define lymphocytes undergoing apoptotic depletion in vivo. Eur J Immunol. 1995;25(12):3277–84. doi: 10.1002/eji.1830251212. [DOI] [PubMed] [Google Scholar]
- 53.Gao HB, et al. Mechanisms of glucocorticoid-induced Leydig cell apoptosis. Mol Cell Endocrinol. 2003;199(1–2):153–63. doi: 10.1016/s0303-7207(02)00290-3. [DOI] [PubMed] [Google Scholar]
- 54.Zorn B, et al. Psychological factors in male partners of infertile couples: relationship with semen quality and early miscarriage. Int J Androl. 2008;31(6):557–64. doi: 10.1111/j.1365-2605.2007.00806.x. [DOI] [PubMed] [Google Scholar]
- 55.Yazawa H, et al. Effect of immobilization stress on testicular germ cell apoptosis in rats. Hum Reprod. 1999;14(7):1806–10. doi: 10.1093/humrep/14.7.1806. [DOI] [PubMed] [Google Scholar]
- 56.Mahmoud H, et al. Dexamethasone effects on Bax expression in the mouse testicular germ cells. Folia Histochem Cytobiol. 2009;47(2):237–41. doi: 10.2478/v10042-009-0041-z. [DOI] [PubMed] [Google Scholar]
- 57.Lim HY, et al. Glucocorticoids exert opposing effects on macrophage function dependent on their concentration. Immunology. 2007;122(1):47–53. doi: 10.1111/j.1365-2567.2007.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alourfi Z, et al. Glucocorticoids suppress macrophage migration inhibitory factor (MIF) expression in a cell-type-specific manner. J Mol Endocrinol. 2005;34(2):583–95. doi: 10.1677/jme.1.01647. [DOI] [PubMed] [Google Scholar]
- 59.Calandra T, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377(6544):68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 60.Meinhardt A, et al. Macrophage migration inhibitory factor production by Leydig cells: evidence for a role in the regulation of testicular function. Endocrinology. 1996;137(11):5090–5. doi: 10.1210/endo.137.11.8895383. [DOI] [PubMed] [Google Scholar]
- 61.Lescoat G, Lescoat D, Garnier DH. Influence of adrenalectomy on maturation of gonadotrophin function in the male rat. J Endocrinol. 1982;95(1):1–6. doi: 10.1677/joe.0.0950001. [DOI] [PubMed] [Google Scholar]
- 62.Sugino Y, et al. Genotyping of congenital adrenal hyperplasia due to 21-hydroxylase deficiency presenting as male infertility: case report and literature review. J Assist Reprod Genet. 2006;23(9–10):377–80. doi: 10.1007/s10815-006-9062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parthasarathy C, Balasubramanian K. Effects of corticosterone deficiency and its replacement on Leydig cell steroidogenesis. J Cell Biochem. 2008;104(5):1671–83. doi: 10.1002/jcb.21733. [DOI] [PubMed] [Google Scholar]
- 64.Albiston AL, et al. Cloning and tissue distribution of the human 11 beta-hydroxysteroid dehydrogenase type 2 enzyme. Mol Cell Endocrinol. 1994;105(2):R11–7. doi: 10.1016/0303-7207(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 65.Benediktsson R, et al. 11 beta-Hydroxysteroid dehydrogenase in the rat ovary: high expression in the oocyte. J Endocrinol. 1992;135(1):53–8. doi: 10.1677/joe.0.1350053. [DOI] [PubMed] [Google Scholar]
- 66.Condon J, et al. Ontogeny and sexual dimorphic expression of mouse type 2 11beta-hydroxysteroid dehydrogenase. Mol Cell Endocrinol. 1997;127(2):121–8. doi: 10.1016/s0303-7207(97)04000-8. [DOI] [PubMed] [Google Scholar]
- 67.Michael AE, et al. Isoforms of 11beta-hydroxysteroid dehydrogenase in human granulosalutein cells. Mol Cell Endocrinol. 1997;132(1–2):43–52. doi: 10.1016/s0303-7207(97)00118-4. [DOI] [PubMed] [Google Scholar]
- 68.Michael AE, Thurston LM, Rae MT. Glucocorticoid metabolism and reproduction: a tale of two enzymes. Reproduction. 2003;126(4):425–41. doi: 10.1530/rep.0.1260425. [DOI] [PubMed] [Google Scholar]
- 69.Thurston LM, et al. Ovarian modulators of 11beta-hydroxysteroid dehydrogenase (11betaHSD) activity in follicular fluid from gonadotrophin-stimulated assisted conception cycles. Reproduction. 2002;124(6):801–12. doi: 10.1530/rep.0.1240801. [DOI] [PubMed] [Google Scholar]
- 70.Schreiber JR, Nakamura K, Erickson GF. Rat ovary glucocorticoid receptor: identification and characterization. Steroids. 1982;39(5):569–84. doi: 10.1016/0039-128x(82)90057-5. [DOI] [PubMed] [Google Scholar]
- 71.Rae MT, et al. Antiinflammatory steroid action in human ovarian surface epithelial cells. J Clin Endocrinol Metab. 2004;89(9):4538–44. doi: 10.1210/jc.2003-032225. [DOI] [PubMed] [Google Scholar]
- 72.Yong PY, et al. Regulation of 11beta-hydroxysteroid dehydrogenase type 1 gene expression in human ovarian surface epithelial cells by interleukin-1. Hum Reprod. 2002;17(9):2300–6. doi: 10.1093/humrep/17.9.2300. [DOI] [PubMed] [Google Scholar]
- 73.Rae MT, et al. Glucocorticoid receptor-mediated regulation of MMP9 gene expression in human ovarian surface epithelial cells. Fertil Steril. 2009;92(2):703–8. doi: 10.1016/j.fertnstert.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 74.Michael AE, et al. Direct inhibition of ovarian steroidogenesis by cortisol and the modulatory role of 11 beta-hydroxysteroid dehydrogenase. Clin Endocrinol (Oxf) 1993;38(6):641–4. doi: 10.1111/j.1365-2265.1993.tb02147.x. [DOI] [PubMed] [Google Scholar]
- 75.Schoonmaker JN, Erickson GF. Glucocorticoid modulation of follicle-stimulating hormone-mediated granulosa cell differentiation. Endocrinology. 1983;113(4):1356–63. doi: 10.1210/endo-113-4-1356. [DOI] [PubMed] [Google Scholar]
- 76.Yang JG, Chen WY, Li PS. Effects of glucocorticoids on maturation of pig oocytes and their subsequent fertilizing capacity in vitro. Biol Reprod. 1999;60(4):929–36. doi: 10.1095/biolreprod60.4.929. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez R, et al. The effect of glucocorticoids on ERK-1/2 phosphorylation during maturation of lamb oocytes and their subsequent fertilization and cleavage ability in vitro. Reprod Toxicol. 29(2):198–205. doi: 10.1016/j.reprotox.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 78.Jimena P, et al. Adrenal hormones in human follicular fluid. Acta Endocrinol (Copenh) 1992;127(5):403–6. doi: 10.1530/acta.0.1270403. [DOI] [PubMed] [Google Scholar]
- 79.Myers M, et al. Role of luteal glucocorticoid metabolism during maternal recognition of pregnancy in women. Endocrinology. 2007;148(12):5769–79. doi: 10.1210/en.2007-0742. [DOI] [PubMed] [Google Scholar]
- 80.Thurston LM, et al. Expression of 11beta-hydroxysteroid dehydrogenase (11betaHSD) proteins in luteinizing human granulosalutein cells. J Endocrinol. 2003;178(1):127–35. doi: 10.1677/joe.0.1780127. [DOI] [PubMed] [Google Scholar]
- 81.Harlow CR, Jenkins JM, Winston RM. Increased follicular fluid total and free cortisol levels during the luteinizing hormone surge. Fertil Steril. 1997;68(1):48–53. doi: 10.1016/s0015-0282(97)81474-4. [DOI] [PubMed] [Google Scholar]
- 82.Bever AT, Hisaw FL, Velardo JT. Inhibitory action of desoxycorticosterone acetate, cortisone acetate, and testosterone on uterine growth induced by estradiol-17beta. Endocrinology. 1956;59(2):165–9. doi: 10.1210/endo-59-2-165. [DOI] [PubMed] [Google Scholar]
- 83.Bitman J, Cecil HC. Differential inhibition by cortisol of estrogen-stimulated uterine responses. Endocrinology. 1967;80(3):423–9. doi: 10.1210/endo-80-3-423. [DOI] [PubMed] [Google Scholar]
- 84.Johnson DC, Dey SK. Role of histamine in implantation: dexamethasone inhibits estradiol-induced implantation in the rat. Biol Reprod. 1980;22(5):1136–41. doi: 10.1093/biolreprod/22.5.1136. [DOI] [PubMed] [Google Scholar]
- 85.Rhen T, et al. Dexamethasone blocks the rapid biological effects of 17beta-estradiol in the rat uterus without antagonizing its global genomic actions. Faseb J. 2003;17(13):1849–70. doi: 10.1096/fj.02-1099com. [DOI] [PubMed] [Google Scholar]
- 86.Rhen T, Cidlowski JA. Estrogens and glucocorticoids have opposing effects on the amount and latent activity of complement proteins in the rat uterus. Biol Reprod. 2006;74(2):265–74. doi: 10.1095/biolreprod.105.045336. [DOI] [PubMed] [Google Scholar]
- 87.Gong H, et al. Glucocorticoids antagonize estrogens by glucocorticoid receptor-mediated activation of estrogen sulfotransferase. Cancer Res. 2008;68(18):7386–93. doi: 10.1158/0008-5472.CAN-08-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levine S, Alpert M, Lewis GW. Infantile experience and the maturation of the pituitary adrenal axis. Science. 1957;126(3287):1347. doi: 10.1126/science.126.3287.1347. [DOI] [PubMed] [Google Scholar]
- 89.Kapoor A, et al. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572(Pt 1):31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lingas RI, Matthews SG. A short period of maternal nutrient restriction in late gestation modifies pituitary-adrenal function in adult guinea pig offspring. Neuroendocrinology. 2001;73(5):302–11. doi: 10.1159/000054647. [DOI] [PubMed] [Google Scholar]
- 91.Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo-pituitary-adrenal axis activity in male guinea pig offspring. J Physiol. 2005;566(Pt 3):967–77. doi: 10.1113/jphysiol.2005.090191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kapoor A, Matthews SG. Prenatal stress modifies behavior and hypothalamic-pituitary-adrenal function in female guinea pig offspring: effects of timing of prenatal stress and stage of reproductive cycle. Endocrinology. 2008;149(12):6406–15. doi: 10.1210/en.2008-0347. [DOI] [PubMed] [Google Scholar]
- 93.Austin MP, Leader LR, Reilly N. Prenatal stress, the hypothalamic-pituitary-adrenal axis, and fetal and infant neurobehaviour. Early Hum Dev. 2005;81(11):917–26. doi: 10.1016/j.earlhumdev.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 94.O'Connor TG, et al. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58(3):211–7. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 95.Entringer S, et al. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm Behav. 2009;55(2):292–8. doi: 10.1016/j.yhbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 96.Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol. 2005;20(3):345–52. doi: 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 97.Yehuda R, et al. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J Clin Endocrinol Metab. 2005;90(7):4115–8. doi: 10.1210/jc.2005-0550. [DOI] [PubMed] [Google Scholar]
- 98.Burton PJ, Waddell BJ. Dual function of 11beta-hydroxysteroid dehydrogenase in placenta: modulating placental glucocorticoid passage and local steroid action. Biol Reprod. 1999;60(2):234–40. doi: 10.1095/biolreprod60.2.234. [DOI] [PubMed] [Google Scholar]
- 99.Thompson A, V, Han K, Yang K. Spatial and temporal patterns of expression of 11beta-hydroxysteroid dehydrogenase types 1 and 2 messenger RNA and glucocorticoid receptor protein in the murine placenta and uterus during late pregnancy. Biol Reprod. 2002;67(6):1708–18. doi: 10.1095/biolreprod.102.005488. [DOI] [PubMed] [Google Scholar]
- 100.Liggins GC, et al. The mechanism of initiation of parturition in the ewe. Recent Prog Horm Res. 1973;29:111–59. doi: 10.1016/b978-0-12-571129-6.50007-5. [DOI] [PubMed] [Google Scholar]
- 101.Challis JRG, et al. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21(5):514–50. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 102.Liggins GC. Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol. 1969;45(4):515–23. doi: 10.1677/joe.0.0450515. [DOI] [PubMed] [Google Scholar]
- 103.Moss TJ, et al. Pharmacokinetics of betamethasone after maternal or fetal intramuscular administration. Am J Obstet Gynecol. 2003;189(6):1751–7. doi: 10.1016/s0002-9378(03)00825-1. [DOI] [PubMed] [Google Scholar]
- 104.Waddell BJ, et al. Apoptosis in rat placenta is zone-dependent and stimulated by glucocorticoids. Biol Reprod. 2000;63(6):1913–7. doi: 10.1095/biolreprod63.6.1913. [DOI] [PubMed] [Google Scholar]
- 105.Cole TJ, et al. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9(13):1608–21. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]