Abstract
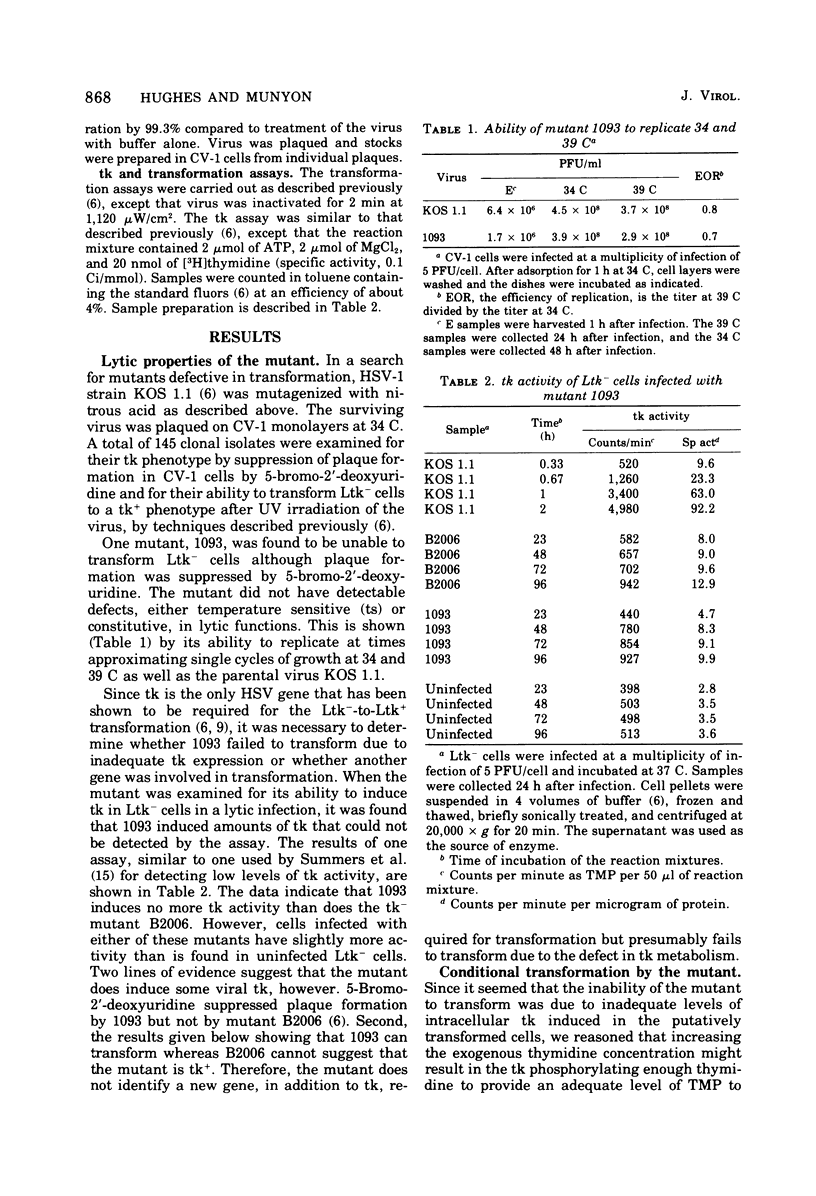
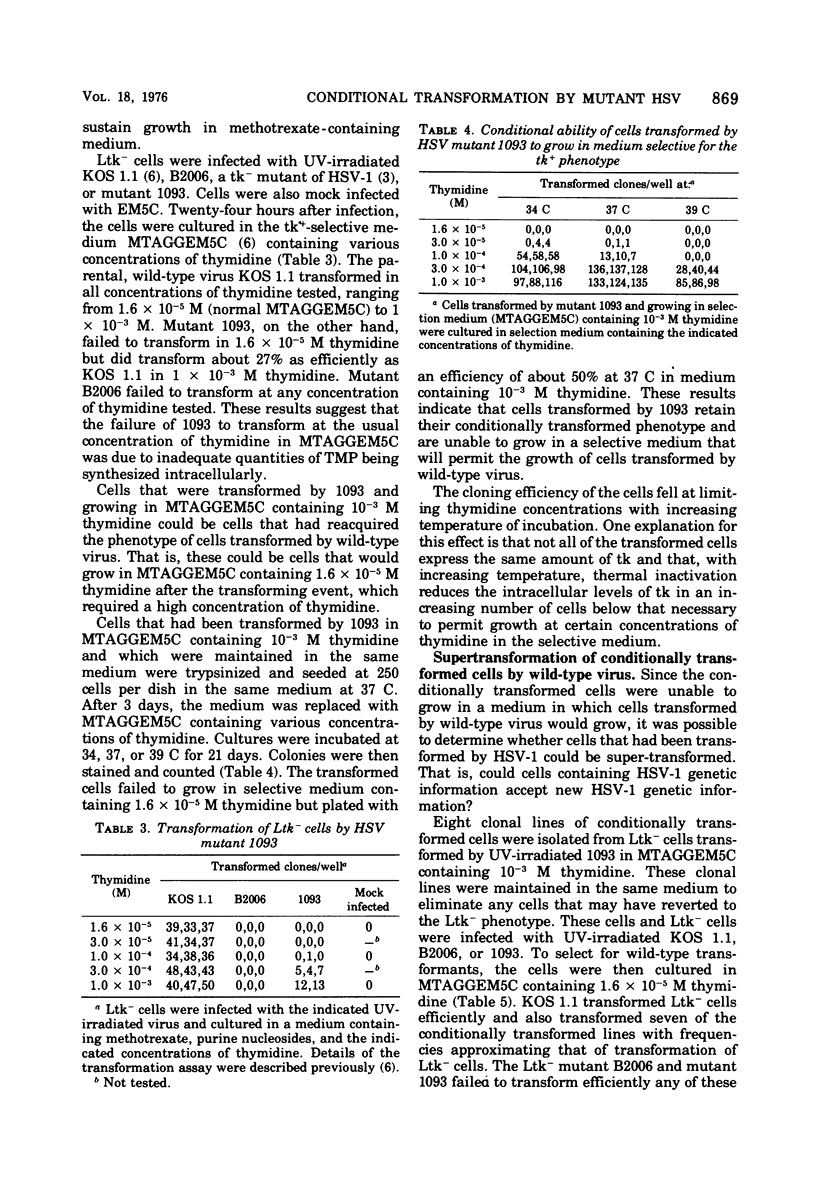
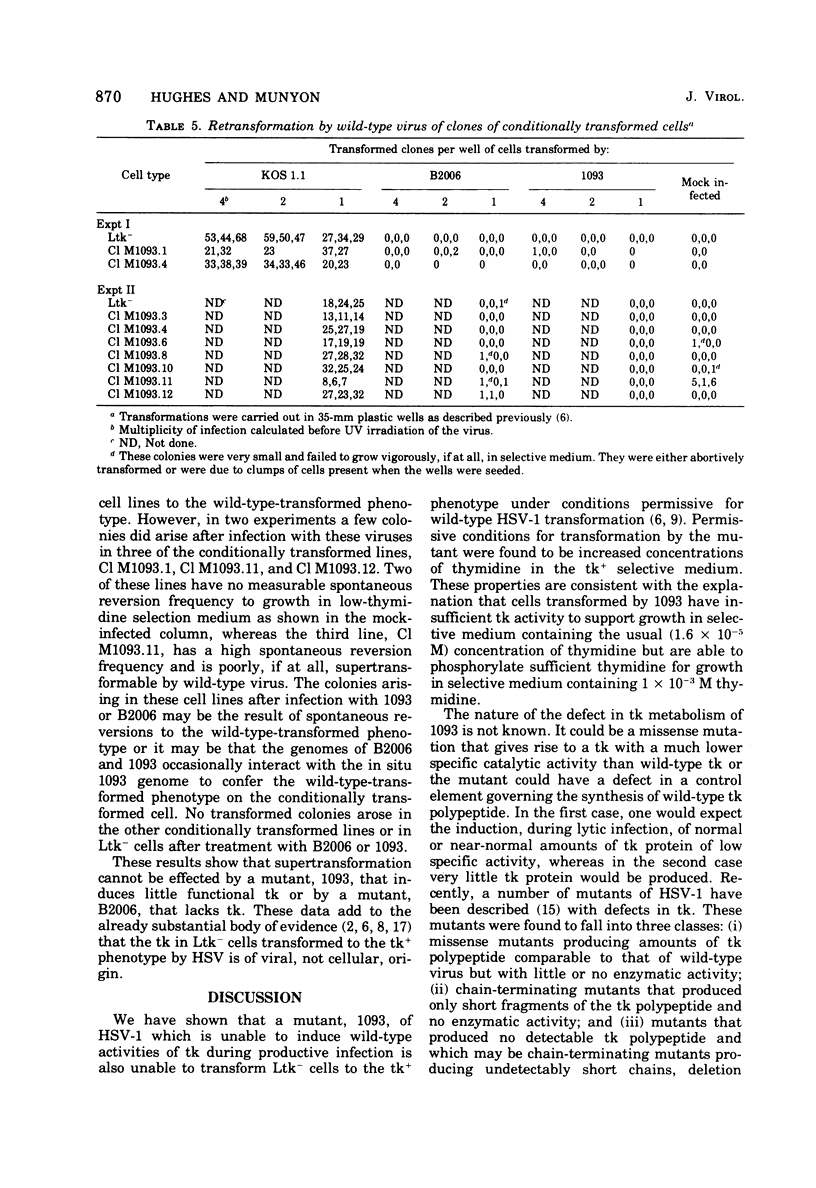
After nitrous acid mutagenesis of herpes simplex virus type 1 (HSV-1), a mutant, 1093, was isolated which, during productive infection, induced very low levels of thymidine kinase (tk). The mutant virus was found, after UV irradiation, to be unable to transform L cells lacking tk (Ltk-) to a tk+ phenotype as chararcterized by growth of the cells in a modified HAT-selective medium containing 1.6 X 10(-5) M thymidine. Cells transformed by wild-type virus grew vigorously under the same conditions. The mutant was able to transform Ltk- cells if the medium contained 10(-3) M thymidine. These transformed cells maintained their conditional character and would not grow in low concentrations of thymidine in selective medium. Therefore, this mutant is conditional on the thymidine concentration in the selection medium in its ability to transform Ltk- cells to a tk+ phenotype. The conditionally transformed cells could be supertransformed with wild-type UV-irradiated HSV-1 to a phenotype which would grow in low-thymidine selective medium. The frequency of supertransformation closely approximated the frequency of transformation of Ltk- cells by wild-type virus. Supertransformation at high frequency could not be effected by mutant 1093 or the tk- mutant B2006. These results indicate that the presence of HSV-1 genetic information in HSV-1-transformed cells does not preclude the acquisition by these cells of at least one additional HSV-1 gene, that for tk.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUBBS D. R., KIT S. MUTANT STRAINS OF HERPES SIMPLEX DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Apr;22:493–502. doi: 10.1016/0042-6822(64)90070-4. [DOI] [PubMed] [Google Scholar]
- Davis D. B., Munyon W., Buchsbaum R., Chawda R. Virus type-specific thymidine kinase in cells biochemically transformed by herpes simplex virus types 1 and 2. J Virol. 1974 Jan;13(1):140–145. doi: 10.1128/jvi.13.1.140-145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbs D. R., Kit S. Isolation of double lysogens from 3T3 cells transformed by plaque morphology mutants of SV40. Proc Natl Acad Sci U S A. 1970 Mar;65(3):536–543. doi: 10.1073/pnas.65.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. G., Jr, Munyon W. H. Temperature-sensitive mutants of herpes simplex virus type 1 defective in lysis but not in transformation. J Virol. 1975 Aug;16(2):275–283. doi: 10.1128/jvi.16.2.275-283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Buchsbaum R., Paoletti E., Mann J., Kraiselburd E., Davis D. Electrophoresis of thymidine kinase activity synthesized by cells transformed by herpes simplex virus. Virology. 1972 Sep;49(3):683–689. doi: 10.1016/0042-6822(72)90525-9. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Zeigel R., Buchsbaum R., Paoletti E. Biochemical transformation of L-cells with ultraviolet-irradiated herpes simplex virus. Fed Proc. 1972 Nov-Dec;31(6):1669–1673. [PubMed] [Google Scholar]
- Prasad I., Zouzias D., Basilico C. Simian virus 40 integration sites in the genome of virus-transformed mouse cells. J Virol. 1975 Oct;16(4):897–904. doi: 10.1128/jvi.16.4.897-904.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger H. C., Basilico C. Mutation causing temperature-sensitive expression of cell transformation by a tumor virus (SV40-3T3 mouse cells-growth control). Proc Natl Acad Sci U S A. 1972 Jan;69(1):109–114. doi: 10.1073/pnas.69.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Takemoto K. K., Todaro G. J. The resistance of SV40-transformed 3T3 to supertransformation. Proc Soc Exp Biol Med. 1971 Mar;136(3):742–746. doi: 10.3181/00379727-136-35355. [DOI] [PubMed] [Google Scholar]
- Subak-Sharpe J. H., Brown S. M., Ritchie D. A., Timbury M. C., Macnab J. C., Marsden H. S., Hay J. Genetic and biochemical studies with herpesvirus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):717–730. doi: 10.1101/sqb.1974.039.01.085. [DOI] [PubMed] [Google Scholar]
- Summers W. P., Wagner M., Summers W. C. Possible peptide chain termination mutants in thymide kinase gene of a mammalian virus, herpes simplex virus. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4081–4084. doi: 10.1073/pnas.72.10.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Yamanishi K. Transformation of hamster embryo and human embryo cells by temperature sensitive mutants of herpes simplex virus type 2. Virology. 1974 Sep;61(1):306–311. doi: 10.1016/0042-6822(74)90267-0. [DOI] [PubMed] [Google Scholar]
- Thouless M. E., Chadha K. C., Munyon W. H. Serological specificity of thymidine kinase activity in herpes simplex virus-transformed L cells. Virology. 1976 Jan;69(1):350–351. doi: 10.1016/0042-6822(76)90225-7. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Ogino T., Takahashi M. Induction of cellular DNA synthesis by a temperature-sensitive mutant of herpes simplex virus type 2. Virology. 1975 Oct;67(2):450–462. doi: 10.1016/0042-6822(75)90446-8. [DOI] [PubMed] [Google Scholar]


