Abstract
Here, we present emerging ideas surrounding the interplay between the actin cytoskeleton and receptor transport and activation. The bulk of actin dynamics in cells is thought to contribute to architecture and mobility. Actin also contributes to trafficking, acting as a molecular scaffold, providing force to deform membranes, facilitating vesicle abscission or propelling a vesicle through the cytoplasm1,2 and recent studies highlight important connections between the directed trafficking of receptors and the impact on cell migration and actin dynamics. Additionally, a number of newly described actin nucleation promoting factors, such as the vesicle associated protein WASH, reveal unexpected roles of actin in membrane traffic and suggest that the cell dedicates a significant proportion of its regulation of actin dynamics to controlling trafficking.
Keywords: trafficking, endosomes, receptor trafficking, exocytosis, WASP family proteins, Arp2/3 complex
Multiple Endocytic Compartments Display Arp2/3-Mediated Actin Assembly Dynamics
Cells often communicate with their environment via transmembrane receptors that receive signals from the extracellular milieu and transmit them internally. Regulation occurs not only at the level of receptors binding to ligands, but can critically depend on the numbers of receptors displayed on the cell surface and the endocytic trafficking pathways shuttling receptors and receptor-ligand complexes away from and back to the plasma membrane (for recent reviews see refs. 3–5).
As a general rule, new actin assembly in cells occurs near a membrane interface. One of the main drivers of new actin filament formation adjacent to membranes is the Arp2/3 complex (actin-related protein 2/3 complex), which is activated by the WASP-family (Wiskott-Aldrich syndrome family) proteins (for recent reviews see refs. 1, 6 and 7). During cell migration, new filament assembly occurs mostly underneath the plasma membrane, but also on internal membrane systems. Actin on intracellular vesicles is often in very small patches (≤ 1 μM diameter) and is usually transient and dynamic, requiring very sensitive high-speed imaging. Only recently has technology developed to a point where we are able to begin to capture some of the intricacies of intracellular actin/membrane interactions.
Mammalian genomes encode at least five types of WASP family proteins and surprisingly, at least three (N-WASP, WASH and WHAMM), likely have important and evolutionarily conserved roles in membrane trafficking.3 WASP proteins generate branched actin networks by bringing the Arp2/3 complex and actin monomers together to nucleate a new branch from a mother filament.1 In addition to WASP proteins, formins (such as FMNL1/2 and Diaphanous), which nucleate and processively promote elongation of unbranched filaments also can polymerize actin on vesicular structures.8-12 Likewise, Spire1, which contains multiple WASP-homology domains and can nucleate actin independently of Arp2/3 complex, may cooperate with Arp2/3 complex, as well as formin-2 (Cappuccino) to somehow control progression of early to late endosomes.9
Here we highlight recent research surrounding the vesicle associated WASP-family protein WASH and we propose that actin assembly has an important role in trafficking of various receptor cargoes through multiple endocytic compartments. WASH was originally identified as a gene upregulated in breast cancer cells that is located in the subtelomeric regions of multiple human chromosomes.13,14 In humans, there are several WASH copies in the genome, with most of them currently curated as pseudogenes. WASH is found in a complex of five proteins, containing SWIP, FAM21, Strumpellin and CCDC53 (Fig. 1) and termed the WASH regulatory complex (SHRC).15-17
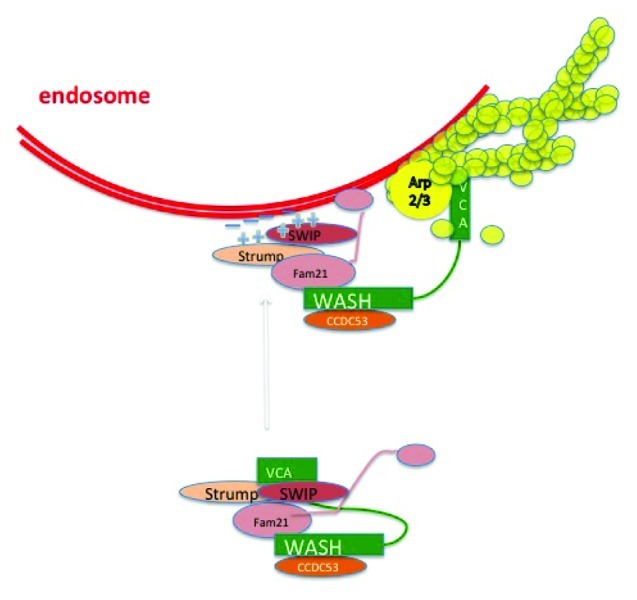
Figure 1. The WASH containing regulatory complex (SHRC) activates actin polymerization on endosomes. Schematic of the pentameric WASH containing SHRC complex in an inactivated state with sequestered VCA domain (depicted in analogy to the WAVE/Scar complex; see ref. 17 for description) and when polymerizing actin on an endosome. The complex is likely tethered to the membrane by multiple lipid-protein interactions.
Actin Has an Important Role in Multiple Endocytic Events/Stages and WASH Facilitates Receptor Retrieval Back to the Plasma Membrane
The most well understood aspect of actin in trafficking is probably the role of actin assembly in clathrin-mediated endocytosis. This has been extensively reviewed elsewhere,18-20 so we will only briefly summarize the main ideas. Clathrin coated pits associate close to the plasma membrane with both dendritic actin networks and actin comet tails.21 Notably, the comet tails observed in a recent correlative EM study were not always visible in the matching immunofluorescence pictures suggesting that small/subtle membrane associated actin structures still need high magnification microscopy to distinguish them from the high “background” of other cellular actin structures.21 N-WASP contributes to the generation of branched actin networks on clathrin-coated vesicles. Actin is thought to form a cage around the newly forming neck of a nascent vesicle together with BAR domain proteins and eventually dynamin. Actin polymerization helps to physically stabilize the tubular neck of a nascent vesicle as the clathrin coat assembles and the force from actin assembly pushes against the inherent membrane tension.22,23 Once dynamin assembles onto the neck, scission can begin via dynamin’s GTPase powered squeezing mechanism, although the actual mechanical forces involved here are still debated.24 Finally, when the new vesicle breaks free, the clathrin coat is disassembled and actin may then polymerize at one pole to push the new vesicle away from the plasma membrane as it journeys to become an early endosome.
Once the vesicle becomes an early endosome, it is likely to be associated with WASH rather than N-WASP. It isn’t clear how N-WASP dissociates and WASH associates or even when, but EEA1 positive early endosomal vesicles contain WASH puncta that co-localize with actin and Arp2/3 complex.15,16,25,26 WASH mediated actin polymerization is maintained on endosomes until the multivesicular body (MVB) stage, or presumably until late endosomes (LE) lose the ability to recycle receptors and merge/mature to lysosomes.27
Receptor transport back to the plasma membrane from an endocytic vesicle is much less defined than the endocytic internalization process. The common view is that tubulin is the cytoskeletal element contributing to outward vesicular traffic via kinesins, and WASH contains a putative tubulin binding motif, which may link actin and microtubule based transport (reviewed in ref.7). But secretion, in a variety of cell types, shows little change upon tubulin depolymerization.28,29 Rocketing of vesicles with endo/lysosomal content might be facilitated by N-WASP and actin comet tail formation30,31 and a recent publication on vesicular transport in oocytes showed evidence for a novel mechanism of outward vesicle movement that is facilitated by actin cables assembled by Formin2 and Spire 1/2.32 Thus, there are still many questions about the relative importance of actin and microtubules in delivery of vesicles back to the plasma membrane from internal endocytic compartments and likely different cell types and compartments have different mechanisms.
WASH localizes on many different endosomal compartments, including early endosomes, late endosomes and, in Dictyostelium, post lysosomes.15,16,25,26,33 It co-localizes with the retromer complex,16 in support of the idea that WASH-mediated actin networks work together with retromer in salvaging cargo such as receptors for retrograde transport. Furthermore, depletion of WASH led to a redistribution of CI-MPR (cation-independent mannose 6-phosphate receptor), a cargo of retromer that is retrieved from endosomes back to the trans Golgi.16 WASH strongly localizes to the Rab7 positive late endosome/multivesicular body (LE/MVB),26 a compartment that is also marked by the retromer complex, which is thought to interact with Rab7.34 Seaman and colleagues identified the SHRC subunit strumpellin, as a retromer interacting protein and showed that the other subunits of the WASH regulatory complex also co-interact with retromer complex.35,36 How WASH functions together with retromer is not fully understood, but a recent study links the FAM21 subunit of SHRC to retromer directly37 and the SHRC is likely to be important in the formation and/or organization of structures known as retromer tubules.38 Retromer tubules form when receptors are to be recycled back to the plasma membrane from endosomes. Temkin and colleagues found WASH in association with the retromer tubule associated sorting nexin SNX27 as an important component for recycling of the β-2 adrenergic receptor.38 However, this is a Rab4-dependent process,38 so may be distinct from the Rab7-dependent sorting of other receptors. The mechanism for WASH uncoupling from retrograde vesicles when they reach their destination is not known and it is unclear whether retromer and WASH always co-cycle together.
Actin Has Emerging Roles in Membrane Tubulation, Sorting of Receptor/Cargo Complexes and Determining the Fate of Particular Membrane Proteins
Increased imaging capability has allowed detection of multiple actin networks on various vesicle compartments, including tubules that emerge from endosomes, which often are associated with retrieval of cargo back to the plasma membrane or in the case of the CI-MPR, to the Golgi. The nature and function of actin on these emerging tubules and the small vesicles that bud from them is just beginning to be understood.
Deforming membranes to bud vesicles requires energy to overcome membrane tension and to induce curvature, movement and scission, which could come from actin polymerization.24 The Arp2/3 activator WHAMM (WASP homolog associated with actin, membranes and microtubules) localizes to the endoplasmic reticulum and Golgi complex39 and is proposed to form a link between microtubules and nascent membrane tubules forming in ER to Golgi transport. WHAMM is proposed to nucleate branched actin networks on nascent tubules and drive their active formation. In direct contrast, WASH is proposed to oppose membrane tubulation, since knockdown of WASH can result in increased appearance of tubules in cells.7,15,16 WASH was proposed to establish an actin network on a nascent tubule, allowing dynamin to be recruited and thus promote vesicle scission from the tubule. This idea fits with previous studies of BAR domain proteins showing that when actin assembly was prevented, the tubules failed to be cleaved.40 The arrival of branched actin allowed dynamin to be recruited and efficiently cleave nascent tubules. It is unclear if WHAMM and WASH actually work by such contrasting mechanisms, with WHAMM promoting tubulation and WASH promoting scission, but clearly further studies are needed.
Whether WASH and WHAMM promote tubulation or scission of vesicles, it is clear that at least WASH is important for efficient receptor recycling. Several endosomal receptor cargos have now been analyzed for their dependence on WASH function, actin polymerization and other members of the SHRC. All studies agreed that WASH depletion led to a redistribution of recycling receptors from the plasma membrane, Golgi or lysosomes to endosomal membranes indicating a retention of receptors at that level.15,16,26,41 WASH depleted cells display slower recycling kinetics of multiple cargoes.15,26 This was less pronounced for the constitutive recycling transferrin receptor, which was only modestly affected15,26 or even unaffected25 by WASH depletion, than for receptors that are regulated by signal transduction and degradation like the integrin α5β1.26,42-44 Perhaps surprisingly, it is increasingly becoming apparent that α5β1 integrins primarily recycle from a late endosomal compartment when cells are migrating in a 3D environment19,26,45 in agreement with WASH’s partial co-localization with Rab7 positive endosomes.15 Recycling from a mature MVB/LE compartment had been long suggested for trafficking of the MHCII in immune cells, which relies on the late endosomal environment for loading with cognate peptides.46
The mechanism by which WASH regulates sorting of receptors such as α5β1 integrin and targeting for recycling rather than degradation is not yet clear, but two lines of evidence point toward a possible role in actin-based sorting of receptors. First, Dictyostelium WASH is important for the recycling of the vacuolar ATPase that allows lysosomes to be neutralized and then exocytosed from cells.33 Insall and colleagues demonstrated that V-ATPase is an actin binding protein and hypothesized that actin networks created by WASH and Arp2/3 complex capture V-ATPase in patches on the surface of lysosomes and thus sort the V-ATPase for removal and recycling.33 Second, in mammalian cells, a recent study by Puthenveedu and colleagues identified determinants of receptor recycling and found that β-2-adrenergic receptors need to be sorted into actin rich domains on endosomes to allow efficient exit from the endosome.47 Actin binding, either directly by the receptor or via receptor-associated proteins, as in the case of the β-2-adrenergic receptor, was the only prerequisite for recycling of a subset of signaling receptors.
A systematic survey of endosomal transport discriminated between at least two separate types of receptor cargo.48 The first group, represented by the transferrin receptor, had characteristics of passive cargo where receptor accumulation was positively correlated with the size of the endosome the recycling of receptors presumably commenced at a constant rate. The second group, represented in the study by the EGFR (epidermal growth factor receptor), showed signs of guidance and adaptable regulation in the endosomal system. The mean EGFR cargo content remained constant independent of endosome size indicating tight regulation of EGFR localization.48 After activation, regulated receptors such as EGF receptor are ubiquitinated and subsequently sorted toward degradation.49 Ubiquitinated EGFR is sorted into intraluminal vesicles (ILVs) by the ESCRT (endosomal sorting complex required for transport) family of complexes and degraded after endosomal fusion with lysosomes.50 Thus degradation works by active sorting of receptors away from the limiting membrane of the endosome, suggesting that receptors are otherwise sorted for recycling to the plasma membrane or any other cellular destination.
The ESCRT complex and WASH can localize to the same endosome and our preliminary data do not indicate direct co-localization (Fig. 2). Actin binding of some receptors is necessary for efficient recycling from a distal endosomal compartment33,47 and we found that WASH overexpression correlated with increased cell surface expression of α5β1 integrin.26 Recycling endosomes, a term used here for the aforementioned endosomal population still capable of retrograde receptor traffic, have been shown to consist of distinct domains and protrusions enriched in specialized sets of molecules required for transport away from endosomes.51
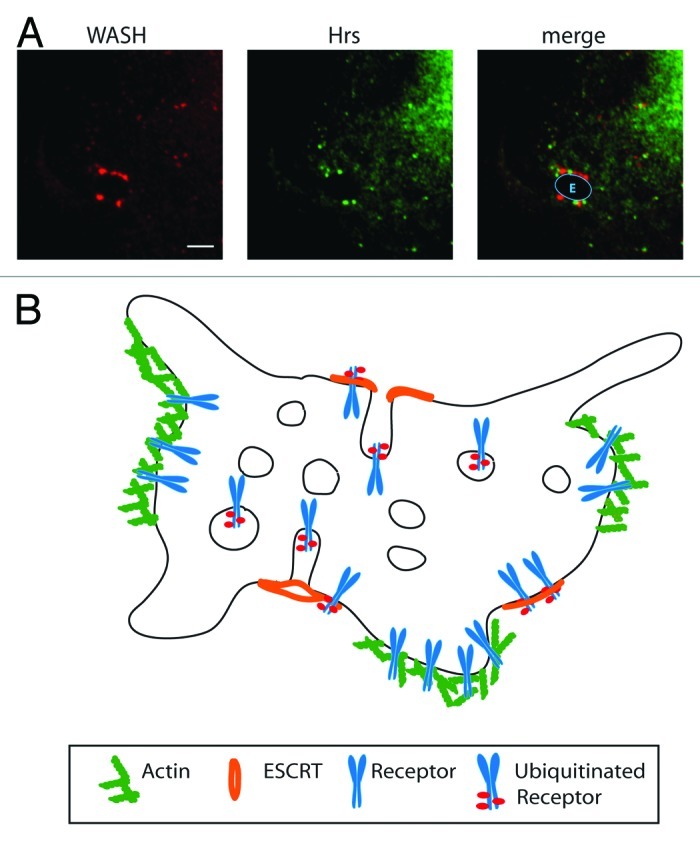
Figure 2. Actin mediated receptor sorting. (A) Immunofluorescence staining of the ESCRT-0 component Hrs and WASH on an endosomal membrane of a senescent IMR90 fibroblast. Scale bar 5 μM. Blue line indicates endosomal outline. E, endosome. (B) Model of multivesicular body with WASH and actin rich domains leading to recycling of receptors and ESCRT rich regions that lead to the sorting of ubiquitinated receptor into intraluminal vesicles.
We propose that actin and the WASH complex might act as a positive recycling guide opposing ESCRT mediated sorting into ILVs of MVBs. Reversible actin binding—extrinsic or intrinsic—would form an ideal way to sequester receptors away from each other. Whenever receptors are destined for degradation the actin binding would be inhibited and sorting into ESCRT rich region could proceed. The continuous occurrence of actin-rich domains from the plasma membrane to late endosomes would be an ideal method to hand over receptors that are destined for reuse.
In summary, it is emerging that mammalian cells devote a considerable component of their actin nucleation promoting proteins to vesicle trafficking functions. Actin is likely to be important in more than its currently established role in pushing against membranes to drive protrusion and movement and these likely include receptor sorting and directed trafficking. The actin nucleation promoting protein WASH has emerged as an important regulator of receptor trafficking. The roles of proteins such as WASH and the actin networks that they generate are still only beginning to be understood and likely are complex.
A recent study demonstrated the presence of short cortical actin filaments on the plasma membrane and predicted that they would spontaneously form aster-shaped clusters on the membrane surface, due to myosins or to attractive forces between the filaments.52 If receptors with engineered actin binding motifs are added to the system, nano-clusters spontaneously formed.52 This showed that actin networks on membrane surfaces are in general able to organize receptors into clusters and suggests a novel mechanism by which they could alter sorting and/or signaling.
Acknowledgments
L.M.M., S.D.J.C. and T.Z. are funded by a core grant from Cancer Research UK to L.M.M. and a pilot grant from Breast Cancer Campaign to L.M.M. and T.Z. Thanks to Peter van den Berghe and Jim Norman (Beatson Institute) for providing the IMR90 fibroblasts.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/21373
References
- 1.Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell. 2009;17:310–22. doi: 10.1016/j.devcel.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Anitei M, Hoflack B. Bridging membrane and cytoskeleton dynamics in the secretory and endocytic pathways. Nat Cell Biol. 2012;14:11–9. doi: 10.1038/ncb2409. [DOI] [PubMed] [Google Scholar]
- 3.Caswell P, Norman J. Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 2008;18:257–63. doi: 10.1016/j.tcb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Jones MC, Caswell PT, Norman JC. Endocytic recycling pathways: emerging regulators of cell migration. Curr Opin Cell Biol. 2006;18:549–57. doi: 10.1016/j.ceb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–22. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–51. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rottner K, Hänisch J, Campellone KG. WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond. Trends Cell Biol. 2010;20:650–61. doi: 10.1016/j.tcb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Kitzing TM, Wang Y, Pertz O, Copeland JW, Grosse R. Formin-like 2 drives amoeboid invasive cell motility downstream of RhoC. Oncogene. 2010;29:2441–8. doi: 10.1038/onc.2009.515. [DOI] [PubMed] [Google Scholar]
- 9.Morel E, Parton RG, Gruenberg J. Annexin A2-dependent polymerization of actin mediates endosome biogenesis. Dev Cell. 2009;16:445–57. doi: 10.1016/j.devcel.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Gardberg M, Talvinen K, Kaipio K, Iljin K, Kampf C, Uhlen M, et al. Characterization of Diaphanous-related formin FMNL2 in human tissues. BMC Cell Biol. 2010;11:55. doi: 10.1186/1471-2121-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colón-Franco JM, Gomez TS, Billadeau DD. Dynamic remodeling of the actin cytoskeleton by FMNL1γ is required for structural maintenance of the Golgi complex. J Cell Sci. 2011;124:3118–26. doi: 10.1242/jcs.083725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Borja M, Janssen L, Verwoerd D, Hordijk P, Neefjes J. RhoB regulates endosome transport by promoting actin assembly on endosomal membranes through Dia1. J Cell Sci. 2005;118:2661–70. doi: 10.1242/jcs.02384. [DOI] [PubMed] [Google Scholar]
- 13.Linardopoulou EV, Parghi SS, Friedman C, Osborn GE, Parkhurst SM, Trask BJ. Human subtelomeric WASH genes encode a new subclass of the WASP family. PLoS Genet. 2007;3:e237. doi: 10.1371/journal.pgen.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leirdal M, Shadidy M, Røsok O, Sioud M. Identification of genes differentially expressed in breast cancer cell line SKBR3: potential identification of new prognostic biomarkers. Int J Mol Med. 2004;14:217–22. [PubMed] [Google Scholar]
- 15.Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell. 2009;17:712–23. doi: 10.1016/j.devcel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Gomez TS, Billadeau DDA. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell. 2009;17:699–711. doi: 10.1016/j.devcel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia D, Gomez TS, Metlagel Z, Umetani J, Otwinowski Z, Rosen MK, et al. WASH and WAVE actin regulators of the Wiskott-Aldrich syndrome protein (WASP) family are controlled by analogous structurally related complexes. Proc Natl Acad Sci U S A. 2010;107:10442–7. doi: 10.1073/pnas.0913293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–33. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 19.Tu C, Ortega-Cava CF, Winograd P, Stanton MJ, Reddi AL, Dodge I, et al. Endosomal-sorting complexes required for transport (ESCRT) pathway-dependent endosomal traffic regulates the localization of active Src at focal adhesions. Proc Natl Acad Sci U S A. 2010;107:16107–12. doi: 10.1073/pnas.1009471107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yarar D, Waterman-Storer CM, Schmid SL. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol Biol Cell. 2005;16:964–75. doi: 10.1091/mbc.E04-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins A, Warrington A, Taylor KA, Svitkina T. Structural organization of the actin cytoskeleton at sites of clathrin-mediated endocytosis. Curr Biol. 2011;21:1167–75. doi: 10.1016/j.cub.2011.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai J, Sheetz MP. Regulation of endocytosis, exocytosis, and shape by membrane tension. Cold Spring Harb Symp Quant Biol. 1995;60:567–71. doi: 10.1101/SQB.1995.060.01.060. [DOI] [PubMed] [Google Scholar]
- 23.Upadhyaya A, Sheetz MP. Tension in tubulovesicular networks of Golgi and endoplasmic reticulum membranes. Biophys J. 2004;86:2923–8. doi: 10.1016/S0006-3495(04)74343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Sun Y, Oster GF, Drubin DG. Mechanochemical crosstalk during endocytic vesicle formation. Curr Opin Cell Biol. 2010;22:36–43. doi: 10.1016/j.ceb.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duleh SN, Welch MD. WASH and the Arp2/3 complex regulate endosome shape and trafficking. Cytoskeleton (Hoboken) 2010;67:193–206. doi: 10.1002/cm.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zech T, Calaminus SD, Caswell P, Spence HJ, Carnell M, Insall RH, et al. The Arp2/3 activator WASH regulates α5β1-integrin-mediated invasive migration. J Cell Sci. 2011;124:3753–9. doi: 10.1242/jcs.080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–59. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- 29.Bloom GS, Goldstein LS. Cruising along microtubule highways: how membranes move through the secretory pathway. J Cell Biol. 1998;140:1277–80. doi: 10.1083/jcb.140.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taunton J, Rowning BA, Coughlin ML, Wu M, Moon RT, Mitchison TJ, et al. Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J Cell Biol. 2000;148:519–30. doi: 10.1083/jcb.148.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozelle AL, Machesky LM, Yamamoto M, Driessens MH, Insall RH, Roth MG, et al. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr Biol. 2000;10:311–20. doi: 10.1016/S0960-9822(00)00384-5. [DOI] [PubMed] [Google Scholar]
- 32.Schuh M. An actin-dependent mechanism for long-range vesicle transport. Nat Cell Biol. 2011;13:1431–6. doi: 10.1038/ncb2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carnell M, Zech T, Calaminus SD, Ura S, Hagedorn M, Johnston SA, et al. Actin polymerization driven by WASH causes V-ATPase retrieval and vesicle neutralization before exocytosis. J Cell Biol. 2011;193:831–9. doi: 10.1083/jcb.201009119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seaman MN, Harbour ME, Tattersall D, Read E, Bright N. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J Cell Sci. 2009;122:2371–82. doi: 10.1242/jcs.048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harbour ME, Breusegem SY, Antrobus R, Freeman C, Reid E, Seaman MN. The cargo-selective retromer complex is a recruiting hub for protein complexes that regulate endosomal tubule dynamics. J Cell Sci. 2010;123:3703–17. doi: 10.1242/jcs.071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harbour ME, Breusegem SY, Seaman MN. Recruitment of the endosomal WASH complex is mediated by the extended ‘tail’ of Fam21 binding to the retromer protein Vps35. Biochem J. 2012;442:209–20. doi: 10.1042/BJ20111761. [DOI] [PubMed] [Google Scholar]
- 37.Jia D, Gomez TS, Billadeau DD, Rosen MK. Multiple repeat elements within the FAM21 tail link the WASH actin regulatory complex to the retromer. Mol Biol Cell. 2012;23:2352–61. doi: 10.1091/mbc.E11-12-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Temkin P, Lauffer B, Jäger S, Cimermancic P, Krogan NJ, von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol. 2011;13:715–21. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campellone KG, Webb NJ, Znameroski EA, Welch MD. WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport. Cell. 2008;134:148–61. doi: 10.1016/j.cell.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fricke R, Gohl C, Dharmalingam E, Grevelhörster A, Zahedi B, Harden N, et al. Drosophila Cip4/Toca-1 integrates membrane trafficking and actin dynamics through WASP and SCAR/WAVE. Curr Biol. 2009;19:1429–37. doi: 10.1016/j.cub.2009.07.058. [DOI] [PubMed] [Google Scholar]
- 41.Duleh SN, Welch MD. WASH and the Arp2/3 complex regulate endosome shape and trafficking. Cytoskeleton (Hoboken) 2010;67:193–206. doi: 10.1002/cm.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, et al. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–53. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 44.Lobert VH, Brech A, Pedersen NM, Wesche J, Oppelt A, Malerød L, et al. Ubiquitination of alpha 5 beta 1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev Cell. 2010;19:148–59. doi: 10.1016/j.devcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Dozynkiewicz MA, Jamieson NB, Macpherson I, Grindlay J, van den Berghe PV, von Thun A, et al. Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev Cell. 2012;22:131–45. doi: 10.1016/j.devcel.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murk JL, Stoorvogel W, Kleijmeer MJ, Geuze HJ. The plasticity of multivesicular bodies and the regulation of antigen presentation. Semin Cell Dev Biol. 2002;13:303–11. doi: 10.1016/S1084952102000605. [DOI] [PubMed] [Google Scholar]
- 47.Puthenveedu MA, Lauffer B, Temkin P, Vistein R, Carlton P, Thorn K, et al. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell. 2010;143:761–73. doi: 10.1016/j.cell.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collinet C, Stöter M, Bradshaw CR, Samusik N, Rink JC, Kenski D, et al. Systems survey of endocytosis by multiparametric image analysis. Nature. 2010;464:243–9. doi: 10.1038/nature08779. [DOI] [PubMed] [Google Scholar]
- 49.Haglund K, Dikic I. The role of ubiquitylation in receptor endocytosis and endosomal sorting. J Cell Sci. 2012;125:265–75. doi: 10.1242/jcs.091280. [DOI] [PubMed] [Google Scholar]
- 50.Rodahl LM, Stuffers S, Lobert VH, Stenmark H. The role of ESCRT proteins in attenuation of cell signalling. Biochem Soc Trans. 2009;37:137–42. doi: 10.1042/BST0370137. [DOI] [PubMed] [Google Scholar]
- 51.Jovic M, Sharma M, Rahajeng J, Caplan S. The early endosome: a busy sorting station for proteins at the crossroads. Histol Histopathol. 2010;25:99–112. doi: 10.14670/hh-25.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gowrishankar K, Ghosh S, Saha S, C R, Mayor S, Rao M. Active remodeling of cortical actin regulates spatiotemporal organization of cell surface molecules. Cell. 2012;149:1353–67. doi: 10.1016/j.cell.2012.05.008. [DOI] [PubMed] [Google Scholar]


