Abstract
Small Rho-GTPases are enzymes that are bound to GDP or GTP, which determines their inactive or active state, respectively. The exchange of GDP for GTP is catalyzed by so-called Rho-guanine nucleotide exchange factors (GEFs). Rho-GEFs are characterized by a Dbl-homology (DH) and adjacent Pleckstrin-homology (PH) domain that serves as enzymatic unit for the GDP/GTP exchange. Rho-GEFs show different GTPase specificities, meaning that a particular GEF can activate either multiple GTPases or only one specific GTPase. We recently reported that the Rho-GEF Trio, known to be able to exchange GTP on Rac1, RhoG and RhoA, regulates lamellipodia formation to mediate cell spreading and migration in a Rac1-dependent manner. In this commentary, we review the current knowledge of Trio in several aspects of cell biology.
Keywords: Trio, Rac1, RhoG, lamellipodia, Rho-GEF
Introduction
The Rho-GEF Trio was originally identified in 1996 as a binding partner of the transmembrane tyrosine phosphatase LAR.1 Trio is a large protein of 350 kD that harbors three domains with putative enzymatic activity, hence the name Trio. Trio encodes two Dbl-homology-Pleckstrin-homology (DH-PH) Rho-GEF units with different specificities. The N-terminal DH-PH unit (TrioD1) mediates GDP to GTP exchange on Rac1 and RhoG, whereas the C-terminal DH-PH unit (TrioD2) activates RhoA.1-3 In addition to the two GEF units, Trio also includes an N-terminal putative lipid-transfer SEC14 domain, several spectrin-repeats, two SH3-domains, an Ig-like domain and a C-terminal serine/threonine kinase domain (Fig. 1).4-8 Using DomPred Protein Domain Prediction Server (freely available at http://bioinf.cs.ucl.ac.uk/dompred) and based on the protein sequence, we predict that Trio has nine spectrin-repeats at the N-terminus. Shortly after the discovery of Trio, a closely related protein was identified that was named Kalirin.9-11 Trio and Kalirin share 68% nucleotide and 65% amino acid sequence identity, but whereas Trio is ubiquitously expressed, Kalirin expression is mainly confined to the central nervous system.1,10 Surprisingly, the N-terminal GEF unit is almost identical between the two proteins, showing 92% homology at the protein level, whereas the C-terminal GEF unit shows 67% homology.
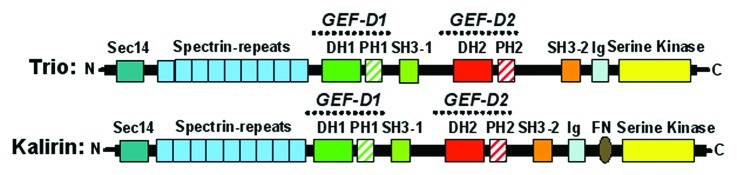
Figure 1. Schematic representation of the structure of Rho-GEFs Trio and Kalirin. Trio and Kalirin both express two DH-PH units (green/red) and a serine-kinase domain (yellow). Both DH-PH units are flanked by a SH3 domain (lime/rose). Trio and Kalirin harbor a SEC14 domain (royal blue) and spectrin-repeats (sky blue) at the N-terminus.
Isoforms of Trio
Several isoforms of both Kalirin and Trio have been identified. For both proteins, a single gene is responsible for the expression of Trio and Kalirin. However, due to alternative splicing and the use of different promoters, several isoforms are formed.12,13 Kalirin-7 (also known as Duo), -9 and -12 are expressed in the brain and differ in length at their C-terminus.13 Several Trio isoforms, Trio A, B and D, are strongly expressed in the brain and during development, whereas Trio C, also known as Solo/Trio8, is exclusively expressed in the cerebellum.12,14 All these splice variants include the N-terminal SEC14 and spectrin-repeats as well as the first DH-PH GEF domain. A fifth isoform, Trio E, has been found in neuroblastoma cells and comprises the C-terminal GEF unit including the kinase domain.12 Interestingly, a sixth isoform named Tgat expresses the C-terminal GEF unit only and is found in patients with adult T-cell leukemia.15
Trio and Regulatory Mechanisms
As mentioned above, Trio is a large protein that harbors next to the two GEF and kinase domains several other domains that may be involved in protein or lipid interaction. Up to now, the mechanisms by which the individual domains of Trio are activated and the functional consequences of this for Trio as a single protein are unclear. In this section we will discuss the potential contribution of phosphorylation, inter- and intra-molecular interactions and presence of two GEF domains with different specificities.
Trio and phosphorylation
Our recent work suggests that during cell spreading Trio is activated upon the engagement of integrins, in particular integrin β1, since in our studies the cells were plated on fibronectin-coated surfaces in serum-free conditions.16 Research by the group of Der showed that for the exchange factor Vav1, tyrosine phosphorylation by Lck is crucial for its GEF function in vitro.17 However, although Trio harbors several tyrosine residues, it is not known if tyrosine phosphorylation is required for Trio-mediated GTP exchange.
Medley and colleagues showed that the kinase domain of Trio, known to interact with LAR, is constitutively phosphorylated on tyrosine residues.6 The levels of phosphorylation were further increased when FAK was co-expressed. Trio interacted with FAK through two distinct regions: the SH3-Ig-like region and the serine/threonine kinase domain (Fig. 1). The authors furthermore showed that upon phosphorylation, Trio shifted to a more detergent-insoluble fraction.6 This indicates that Trio tyrosine phosphorylation may trigger its interaction with the actin cytoskeleton. However, it remains unclear if FAK affects the GEF activity of Trio.
Trio interaction partners
Although the molecular mechanism of the activation of the TrioD1 GEF domain is thus far unclear, its ability to activate Rac1 was shown to be regulated by interactions with several proteins. Rac1 activation by Trio is negatively regulated by interactions with the F-actin capping protein CARMIL,18 the motor protein Myosin II19 and the F-actin binding protein Tara.20 In contrast, association of the integral membrane protein Kidins220/ARMS (kinase-D-interacting substrate of 220 kDa/ankyrin repeat-rich membrane spanning) with the spectrin-repeats of Trio was demonstrated to promote Rac1 activation.21 In addition, the F-actin cross-linker protein Filamin interacted with the PH-domain of the TrioD1 GEF unit and was required for TrioD1-induced membrane dynamics.22 In another study by Bellanger and colleagues, they showed that the PH domain of TrioD1 is involved both in regulating the catalytic activity of TrioD1 and in determining the sub-cellular localization of its associated DH domain.23 Thus, the N-terminal PH domain of Trio may serve as a cytoskeletal targeting signal. Since Rac1 has also been shown to bind to the actin-binding protein Filamin,24-26 Filamin may function as a scaffold for Trio-mediated Rac1 activation in a similar manner as has been reported for the interaction of filamin with the GEF Vav2.26
Trio and the SEC14 domain
Trio, together with the Rho-GEFs Kalirin and Dbs, are the only Rho-GEFs in the family of > 80 Rho-GEF members that comprise a SEC14 homology domain (Fig. 1).27 Proteins encompassing a SEC14 domain are widely expressed in plants, yeast, invertebrates and mammals, suggesting that this domain is highly conserved.28 SEC14 domains, also known as CRAL-TRIO domains, were shown to mediate the interaction between proteins and specific phospholipids,8,29,30 such as PtdIns, PtdCho, PtdSer and a number of different phosphorylated forms of PtdIns. Work by the group of Whitehead showed that removal of the SEC14 domain of Dbs induced Dbs distribution to the periphery of the cell, whereas full length Dbs was found in peri-nuclear regions and co-localized with Golgi markers.31 Thus, the SEC14 domain in Rho-GEFs may promote membrane targeting and thereby determine local Rho-GEF activity. The authors furthermore showed that the SEC14 homology domain forms intracellular contacts with the PH-domain of Dbs. Next, they showed that these contacts must be released to achieve full transformation activity by Dbs.31 Whether the SEC14 domain of Trio plays a similar regulatory role in the activity of the N-terminal DH-PH domain of Trio is unknown.
Trio and the spectrin-repeats
Spectrin-repeats are three-helix bundle structures that occur in many different proteins, either as single copies, for Dbs, or in tandem repeats, for Trio and Kalirin.32 These repeats can act in a structural way, by coordinating cytoskeletal interactions with high spatial precision, as well as an intermediary for interactions with several regulatory proteins.32 For Kalirin, it is known that the spectrin-repeats bind to the cytosolic part of peptidylglycine α-amidating mono-oxygenase (PAM).10 Later, PAM was also found to interact with the spectrin-repeats of Trio.33 PAM is a secretory granule membrane protein and overexpression results in reduced filopodia and cortical actin in AtT-20 cells.34,35 Recently, a second protein Disrupted-in-Schizophrenia 1 (DISC1) is found to bind to the first part, i.e., the amino-half, of the spectrin-repeats of Trio.36 Interestingly, by binding of DISC1 to Trio, the N-terminal GEF domain is relieved from intramolecular inhibition and able to activate Rac1.
Neubrand and colleagues showed that Kidins220/ARMS binds to the spectrin-repeats of Trio.21 The authors additionally showed that Kidins220/ARMS can also bind to Kalirin. They hypothesized that Kidins220/ARMS mediates the cellular distribution of Trio in neuronal cells.
As with the SEC14 domain, the relevance of the Trio spectrin-repeats is still largely unclear, although the study of Chen and coworkers suggest that the repeats may bind to the first GEF domain and thereby preventing GTPase activation.36 To fully understand its working mechanism, future studies are required.
Trio and its two GEF domains
Trio and its close relative Kalirin are unique in that they can activate both Rac1 and RhoA with two separate GEF units within the same molecule. Since overexpression of a full-length Trio construct induces primarily Rac1- and RhoG-dependent phenotypical changes,16,37 it is likely that the activation of RhoA by the TrioD2 GEF domain is tightly regulated within the Trio molecule. Indeed, the PH-domain that is adjacent to the TrioD2 DH-domain was shown to negatively regulate RhoA activation.23 This auto-inhibition was shown to be relieved by binding of the Gαq-subunit of heterotrimeric G-proteins to a C-terminal extension of the PH-domain resulting in RhoA activation.38,39
However, it remains unclear why Trio expresses two catalytic GEF domains that target small GTPases with apparent antagonistic downstream effects, i.e., RhoA and Rac1. At this point one can only speculate about their function. It is broadly accepted that Rho-GEFs determine local activity of downstream GTPases. Therefore, it may be that the activity of Rac1 and RhoA are required at the same location but not at the same time. Recently, it became clear that Rac1 and RhoA are both activated at the leading edge of a migrating cell.40,41 Using biosensor techniques for Rac1 and RhoA revealed that first RhoA and then Rac1 is activated.42 In such a situation, it may be efficient to have only one GEF present with two distinct GEF domains that can activate both GTPases in a spatially and temporally coordinated manner. However, future experiments are needed to prove such hypothesis.
For Kalirin, it has been reported that Kalirin7 localizes to postsynaptic densities (PSD),43 where it is tyrosine phosphorylated by EphB2 tyrosine kinase receptor.44 Although the phosphorylation does not affect the GEF activity, it does change the distribution of Kalirin and thereby changes its mode of action. The Rho-GEF Trio may be regulated in a similar way. Debant and colleagues indicated that Trio may be phosphorylated on serine and threonine residues.1 However, it is unclear if changes in phosphorylation status of Trio affect its distribution or activity. Another interesting feature of Kalirin is its ability to bind specifically to iNOS, preventing dimerization of iNOS, resulting in inhibition of iNOS activity.45 This is an example of Kalirin acting as a scaffold protein. The group of Debant showed that Trio targets Filamin in order to regulate the actin cytoskeleton.22 Thus, Rho-GEFs such as Trio and Kalirin cannot only act as proteins with enzymatic activity but may also be used by other proteins for correct cellular targeting.
Trio and Neuronal Development
Upon the finding that UNC-73, an important regulator of axon guidance during nervous system development in C. elegans, is an ortholog of mammalian Trio,46 several studies followed demonstrating a role for Trio in axon guidance and neuronal development in Drosophila and mammals.37,47-51 Trio was shown to mediate Rac1 and/or RhoG activation during neuronal growth cone migration and axon guidance downstream of several guidance receptors, including the Netrin receptor,52-54 Notch,55,56 SAX-3/ROBO18,57 and the NGF receptor.21,37 In addition, Trio was demonstrated to be an essential regulator of skeletal muscle development.51,58,59 Mice deficient for Trio died between embryonic day E15.5 and birth and showed, besides aberrant organization of the hippocampus and olfactory bulb, defects in secondary myogenesis.51 Trio was later demonstrated to interact with M-cadherin and to regulate myoblast fusion by mediating Rac1 activation downstream of M-cadherin engagement.58 These latter findings indicate that Trio may also be involved in regulating cell-cell contacts.
Trio and Cancer
Seipel and coworkers have shown that the N-terminal GEF domain of Trio induces migration in 3T3 fibroblast cells and promotes anchorage-independent growth.4 Our recent data underscore these findings.16 Together with the knowledge that Rac1 is involved in transformation and tumor progression,60 these data suggest that the ability of Trio to activate Rac1 and induce cell migration is linked to tumor progression.
Trio was found to be highly expressed in glioblastoma,61 breast tumors,62,63 soft tissue sarcomas64 and urinary bladder tumors.65 In addition, Trio levels are also significantly increased in breast cancer patients with poor predictive outcome.64 Moreover, Salhia and coworkers showed that reduction of Trio using siRNA perturbed the migration capacity of glioblastoma cells in vitro.61 These studies show that the expression of endogenous Trio is increased in several types of cancer, but do not clarify if Trio activity is also hampered.
Tgat, an alternative splice variant encoding only the DH domain of the TrioD2 GEF unit of Trio, was identified in patients with adult T-cell leukemia and was demonstrated to induce cell transformation and tumor formation,15,66,67 indicating that also the C-terminal GEF domain of Trio may potentially regulate cancer progression.
Although Trio may potentially be an interesting target in anti-tumor therapy, it remains to be proven if increased protein levels or mutations indeed lead to changes in Trio activity.
Trio and Leukocytes
Since Trio controls spreading and migration of HeLa cells, its role in these processes may potentially also be extrapolated to other types of migratory cells, such as leukocytes. Upon analysis of Trio expression in different types of leukocytes, we were unable to detect endogenous, full-length Trio protein in freshly isolated neutrophils, monocytes and naïve lymphocytes (Fig. 2). Interestingly, we did detect Trio in several leukemic cell lines of both myeloid and lymphoid origin, correlating Trio expression also with leukemic cancers (Fig. 2). Trio was also detected in immature dendritic cells that were differentiated from primary monocytes (Fig. 2). The expression of Trio in cell lines does not prove that Trio is involved in leukemia. However, it is an intriguing hypothesis that Trio expression is increased in these cell types and may promote the exchange rate on Rac1, RhoG and/or RhoA. Future studies will be required to show if Trio is a potential regulator of leukemic cell migration.
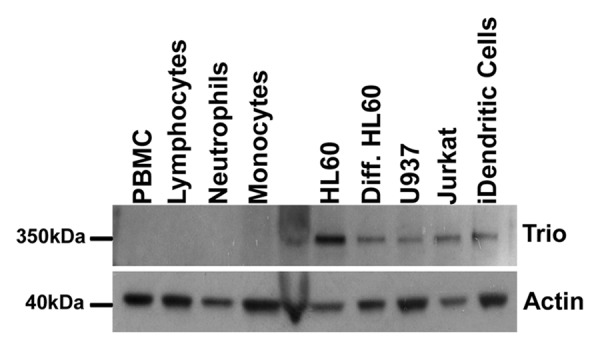
Figure 2. Trio expression in leukocytes and leukemic cell lines. Trio (350 kDa) protein expression in primary peripheral blood mononuclear cells (PBMC; lane 1), naïve lymphocytes (lane 2), neutrophils (lane 3) and monocytes (lane 4), followed by HL60, HL60 differentiated to neutrophil-like with 1.3% (v/v) DMSO, U937 cells, Jurkat and immature dendritic cells. Approximately 100,000 cells were loaded per lane. Actin (40 kDa) was used as a control for equal sample loading.
Acknowledgments
We thank Dr. P.L. Hordijk for critically reading the manuscript. J.D. van B. is supported by the Dutch Heart Foundation (grant no. 2005T039), LSBR fellowship (fellowship no. 1028) and NWO Veni grant 916.76.053. J. van R. is supported by AMC Research B.V.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/21418
References
- 1.Debant A, Serra-Pagès C, Seipel K, O’Brien S, Tang M, Park SH, et al. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci U S A. 1996;93:5466–71. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellanger JM, Lazaro JB, Diriong S, Fernandez A, Lamb N, Debant A. The two guanine nucleotide exchange factor domains of Trio link the Rac1 and the RhoA pathways in vivo. Oncogene. 1998;16:147–52. doi: 10.1038/sj.onc.1201532. [DOI] [PubMed] [Google Scholar]
- 3.Blangy A, Vignal E, Schmidt S, Debant A, Gauthier-Rouvière C, Fort P. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J Cell Sci. 2000;113:729–39. doi: 10.1242/jcs.113.4.729. [DOI] [PubMed] [Google Scholar]
- 4.Seipel K, Medley QG, Kedersha NL, Zhang XA, O’Brien SP, Serra-Pages C, et al. Trio amino-terminal guanine nucleotide exchange factor domain expression promotes actin cytoskeleton reorganization, cell migration and anchorage-independent cell growth. J Cell Sci. 1999;112:1825–34. doi: 10.1242/jcs.112.12.1825. [DOI] [PubMed] [Google Scholar]
- 5.Medley QG, Serra-Pagès C, Iannotti E, Seipel K, Tang M, O’Brien SP, et al. The trio guanine nucleotide exchange factor is a RhoA target. Binding of RhoA to the trio immunoglobulin-like domain. J Biol Chem. 2000;275:36116–23. doi: 10.1074/jbc.M003775200. [DOI] [PubMed] [Google Scholar]
- 6.Medley QG, Buchbinder EG, Tachibana K, Ngo H, Serra-Pagès C, Streuli M. Signaling between focal adhesion kinase and trio. J Biol Chem. 2003;278:13265–70. doi: 10.1074/jbc.M300277200. [DOI] [PubMed] [Google Scholar]
- 7.McPherson CE, Eipper BA, Mains RE. Multiple novel isoforms of Trio are expressed in the developing rat brain. Gene. 2005;347:125–35. doi: 10.1016/j.gene.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 8.Saito K, Tautz L, Mustelin T. The lipid-binding SEC14 domain. Biochim Biophys Acta. 2007;1771:719–26. doi: 10.1016/j.bbalip.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Alam MR, Caldwell BD, Johnson RC, Darlington DN, Mains RE, Eipper BA. Novel proteins that interact with the COOH-terminal cytosolic routing determinants of an integral membrane peptide-processing enzyme. J Biol Chem. 1996;271:28636–40. doi: 10.1074/jbc.271.45.28636. [DOI] [PubMed] [Google Scholar]
- 10.Alam MR, Johnson RC, Darlington DN, Hand TA, Mains RE, Eipper BA. Kalirin, a cytosolic protein with spectrin-like and GDP/GTP exchange factor-like domains that interacts with peptidylglycine alpha-amidating monooxygenase, an integral membrane peptide-processing enzyme. J Biol Chem. 1997;272:12667–75. doi: 10.1074/jbc.272.19.12667. [DOI] [PubMed] [Google Scholar]
- 11.Colomer V, Engelender S, Sharp AH, Duan K, Cooper JK, Lanahan A, et al. Huntingtin-associated protein 1 (HAP1) binds to a Trio-like polypeptide, with a rac1 guanine nucleotide exchange factor domain. Hum Mol Genet. 1997;6:1519–25. doi: 10.1093/hmg/6.9.1519. [DOI] [PubMed] [Google Scholar]
- 12.Portales-Casamar E, Briançon-Marjollet A, Fromont S, Triboulet R, Debant A. Identification of novel neuronal isoforms of the Rho-GEF Trio. Biol Cell. 2006;98:183–93. doi: 10.1042/BC20050009. [DOI] [PubMed] [Google Scholar]
- 13.McPherson CE, Eipper BA, Mains RE. Genomic organization and differential expression of Kalirin isoforms. Gene. 2002;284:41–51. doi: 10.1016/S0378-1119(02)00386-4. [DOI] [PubMed] [Google Scholar]
- 14.Sun YJ, Nishikawa K, Yuda H, Wang YL, Osaka H, Fukazawa N, et al. Solo/Trio8, a membrane-associated short isoform of Trio, modulates endosome dynamics and neurite elongation. Mol Cell Biol. 2006;26:6923–35. doi: 10.1128/MCB.02474-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshizuka N, Moriuchi R, Mori T, Yamada K, Hasegawa S, Maeda T, et al. An alternative transcript derived from the trio locus encodes a guanosine nucleotide exchange factor with mouse cell-transforming potential. J Biol Chem. 2004;279:43998–4004. doi: 10.1074/jbc.M406082200. [DOI] [PubMed] [Google Scholar]
- 16.van Rijssel J, Hoogenboezem M, Wester L, Hordijk PL, Van Buul JD. The N-terminal DH-PH domain of Trio induces cell spreading and migration by regulating lamellipodia dynamics in a Rac1-dependent fashion. PLoS One. 2012;7:e29912. doi: 10.1371/journal.pone.0029912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abe K, Whitehead IP, O’Bryan JP, Der CJ. Involvement of NH(2)-terminal sequences in the negative regulation of Vav signaling and transforming activity. J Biol Chem. 1999;274:30410–8. doi: 10.1074/jbc.274.43.30410. [DOI] [PubMed] [Google Scholar]
- 18.Vanderzalm PJ, Pandey A, Hurwitz ME, Bloom L, Horvitz HR, Garriga G. C. elegans CARMIL negatively regulates UNC-73/Trio function during neuronal development. Development. 2009;136:1201–10. doi: 10.1242/dev.026666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CS, Choi CK, Shin EY, Schwartz MA, Kim EG. Myosin II directly binds and inhibits Dbl family guanine nucleotide exchange factors: a possible link to Rho family GTPases. J Cell Biol. 2010;190:663–74. doi: 10.1083/jcb.201003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yano T, Yamazaki Y, Adachi M, Okawa K, Fort P, Uji M, et al. Tara up-regulates E-cadherin transcription by binding to the Trio RhoGEF and inhibiting Rac signaling. J Cell Biol. 2011;193:319–32. doi: 10.1083/jcb.201009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neubrand VE, Thomas C, Schmidt S, Debant A, Schiavo G. Kidins220/ARMS regulates Rac1-dependent neurite outgrowth by direct interaction with the RhoGEF Trio. J Cell Sci. 2010;123:2111–23. doi: 10.1242/jcs.064055. [DOI] [PubMed] [Google Scholar]
- 22.Bellanger JM, Astier C, Sardet C, Ohta Y, Stossel TP, Debant A. The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin. Nat Cell Biol. 2000;2:888–92. doi: 10.1038/35046533. [DOI] [PubMed] [Google Scholar]
- 23.Bellanger JM, Estrach S, Schmidt S, Briançon-Marjollet A, Zugasti O, Fromont S, et al. Different regulation of the Trio Dbl-Homology domains by their associated PH domains. Biol Cell. 2003;95:625–34. doi: 10.1016/j.biolcel.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Ohta Y, Suzuki N, Nakamura S, Hartwig JH, Stossel TP. The small GTPase RalA targets filamin to induce filopodia. Proc Natl Acad Sci U S A. 1999;96:2122–8. doi: 10.1073/pnas.96.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon YJ, Choi JS, Lee JY, Yu KR, Ka SH, Cho Y, et al. Filamin B serves as a molecular scaffold for type I interferon-induced c-Jun NH2-terminal kinase signaling pathway. Mol Biol Cell. 2008;19:5116–30. doi: 10.1091/mbc.E08-06-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Del Valle-Pérez B, Martínez VG, Lacasa-Salavert C, Figueras A, Shapiro SS, Takafuta T, et al. Filamin B plays a key role in vascular endothelial growth factor-induced endothelial cell motility through its interaction with Rac-1 and Vav-2. J Biol Chem. 2010;285:10748–60. doi: 10.1074/jbc.M109.062984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 28.Aravind L, Neuwald AF, Ponting CP. Sec14p-like domains in NF1 and Dbl-like proteins indicate lipid regulation of Ras and Rho signaling. Curr Biol. 1999;9:R195–7. doi: 10.1016/S0960-9822(99)80127-4. [DOI] [PubMed] [Google Scholar]
- 29.Bankaitis VA, Mousley CJ, Schaaf G. The Sec14 superfamily and mechanisms for crosstalk between lipid metabolism and lipid signaling. Trends Biochem Sci. 2010;35:150–60. doi: 10.1016/j.tibs.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mousley CJ, Tyeryar KR, Vincent-Pope P, Bankaitis VA. The Sec14-superfamily and the regulatory interface between phospholipid metabolism and membrane trafficking. Biochim Biophys Acta. 2007;1771:727–36. doi: 10.1016/j.bbalip.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kostenko EV, Mahon GM, Cheng L, Whitehead IP. The Sec14 homology domain regulates the cellular distribution and transforming activity of the Rho-specific guanine nucleotide exchange factor Dbs. J Biol Chem. 2005;280:2807–17. doi: 10.1074/jbc.M411139200. [DOI] [PubMed] [Google Scholar]
- 32.Djinovic-Carugo K, Gautel M, Ylänne J, Young P. The spectrin repeat: a structural platform for cytoskeletal protein assemblies. FEBS Lett. 2002;513:119–23. doi: 10.1016/S0014-5793(01)03304-X. [DOI] [PubMed] [Google Scholar]
- 33.Xin X, Ferraro F, Bäck N, Eipper BA, Mains RE. Cdk5 and Trio modulate endocrine cell exocytosis. J Cell Sci. 2004;117:4739–48. doi: 10.1242/jcs.01333. [DOI] [PubMed] [Google Scholar]
- 34.Ciccotosto GD, Schiller MR, Eipper BA, Mains RE. Induction of integral membrane PAM expression in AtT-20 cells alters the storage and trafficking of POMC and PC1. J Cell Biol. 1999;144:459–71. doi: 10.1083/jcb.144.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alam MR, Steveson TC, Johnson RC, Bäck N, Abraham B, Mains RE, et al. Signaling mediated by the cytosolic domain of peptidylglycine alpha-amidating monooxygenase. Mol Biol Cell. 2001;12:629–44. doi: 10.1091/mbc.12.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen SY, Huang PH, Cheng HJ. Disrupted-in-Schizophrenia 1-mediated axon guidance involves TRIO-RAC-PAK small GTPase pathway signaling. Proc Natl Acad Sci U S A. 2011;108:5861–6. doi: 10.1073/pnas.1018128108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estrach S, Schmidt S, Diriong S, Penna A, Blangy A, Fort P, et al. The Human Rho-GEF trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Curr Biol. 2002;12:307–12. doi: 10.1016/S0960-9822(02)00658-9. [DOI] [PubMed] [Google Scholar]
- 38.Rojas RJ, Yohe ME, Gershburg S, Kawano T, Kozasa T, Sondek J. Galphaq directly activates p63RhoGEF and Trio via a conserved extension of the Dbl homology-associated pleckstrin homology domain. J Biol Chem. 2007;282:29201–10. doi: 10.1074/jbc.M703458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams SL, Lutz S, Charlie NK, Vettel C, Ailion M, Coco C, et al. Trio’s Rho-specific GEF domain is the missing Galpha q effector in C. elegans. Genes Dev. 2007;21:2731–46. doi: 10.1101/gad.1592007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraynov VS, Chamberlain C, Bokoch GM, Schwartz MA, Slabaugh S, Hahn KM. Localized Rac activation dynamics visualized in living cells. Science. 2000;290:333–7. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- 41.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–72. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 42.Welch CM, Elliott H, Danuser G, Hahn KM. Imaging the coordination of multiple signalling activities in living cells. Nat Rev Mol Cell Biol. 2011;12:749–56. doi: 10.1038/nrm3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabiner CA, Mains RE, Eipper BA. Kalirin: a dual Rho guanine nucleotide exchange factor that is so much more than the sum of its many parts. Neuroscientist. 2005;11:148–60. doi: 10.1177/1073858404271250. [DOI] [PubMed] [Google Scholar]
- 44.Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–74. doi: 10.1016/S0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- 45.Ratovitski EA, Alam MR, Quick RA, McMillan A, Bao C, Kozlovsky C, et al. Kalirin inhibition of inducible nitric-oxide synthase. J Biol Chem. 1999;274:993–9. doi: 10.1074/jbc.274.2.993. [DOI] [PubMed] [Google Scholar]
- 46.Steven R, Kubiseski TJ, Zheng H, Kulkarni S, Mancillas J, Ruiz Morales A, et al. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell. 1998;92:785–95. doi: 10.1016/S0092-8674(00)81406-3. [DOI] [PubMed] [Google Scholar]
- 47.Awasaki T, Saito M, Sone M, Suzuki E, Sakai R, Ito K, et al. The Drosophila trio plays an essential role in patterning of axons by regulating their directional extension. Neuron. 2000;26:119–31. doi: 10.1016/S0896-6273(00)81143-5. [DOI] [PubMed] [Google Scholar]
- 48.Bateman J, Shu H, Van Vactor D. The guanine nucleotide exchange factor trio mediates axonal development in the Drosophila embryo. Neuron. 2000;26:93–106. doi: 10.1016/S0896-6273(00)81141-1. [DOI] [PubMed] [Google Scholar]
- 49.Liebl EC, Forsthoefel DJ, Franco LS, Sample SH, Hess JE, Cowger JA, et al. Dosage-sensitive, reciprocal genetic interactions between the Abl tyrosine kinase and the putative GEF trio reveal trio’s role in axon pathfinding. Neuron. 2000;26:107–18. doi: 10.1016/S0896-6273(00)81142-3. [DOI] [PubMed] [Google Scholar]
- 50.Newsome TP, Schmidt S, Dietzl G, Keleman K, Asling B, Debant A, et al. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell. 2000;101:283–94. doi: 10.1016/S0092-8674(00)80838-7. [DOI] [PubMed] [Google Scholar]
- 51.O’Brien SP, Seipel K, Medley QG, Bronson R, Segal R, Streuli M. Skeletal muscle deformity and neuronal disorder in Trio exchange factor-deficient mouse embryos. Proc Natl Acad Sci U S A. 2000;97:12074–8. doi: 10.1073/pnas.97.22.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Forsthoefel DJ, Liebl EC, Kolodziej PA, Seeger MA. The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Development. 2005;132:1983–94. doi: 10.1242/dev.01736. [DOI] [PubMed] [Google Scholar]
- 53.Briançon-Marjollet A, Ghogha A, Nawabi H, Triki I, Auziol C, Fromont S, et al. Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance. Mol Cell Biol. 2008;28:2314–23. doi: 10.1128/MCB.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng YJ, He WQ, Tang J, Tao T, Chen C, Gao YQ, et al. Trio is a key guanine nucleotide exchange factor coordinating regulation of the migration and morphogenesis of granule cells in the developing cerebellum. J Biol Chem. 2010;285:24834–44. doi: 10.1074/jbc.M109.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Le Gall M, De Mattei C, Giniger E. Molecular separation of two signaling pathways for the receptor, Notch. Dev Biol. 2008;313:556–67. doi: 10.1016/j.ydbio.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song JK, Giniger E. Noncanonical Notch function in motor axon guidance is mediated by Rac GTPase and the GEF1 domain of Trio. Dev Dyn. 2011;240:324–32. doi: 10.1002/dvdy.22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watari-Goshima N, Ogura K, Wolf FW, Goshima Y, Garriga G. C. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat Neurosci. 2007;10:169–76. doi: 10.1038/nn1834. [DOI] [PubMed] [Google Scholar]
- 58.Charrasse S, Comunale F, Fortier M, Portales-Casamar E, Debant A, Gauthier-Rouvière C. M-cadherin activates Rac1 GTPase through the Rho-GEF trio during myoblast fusion. Mol Biol Cell. 2007;18:1734–43. doi: 10.1091/mbc.E06-08-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouquier N, Vignal E, Charrasse S, Weill M, Schmidt S, Léonetti JP, et al. A cell active chemical GEF inhibitor selectively targets the Trio/RhoG/Rac1 signaling pathway. Chem Biol. 2009;16:657–66. doi: 10.1016/j.chembiol.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 60.Jaffe AB, Hall A. Rho GTPases in transformation and metastasis. Adv Cancer Res. 2002;84:57–80. doi: 10.1016/S0065-230X(02)84003-9. [DOI] [PubMed] [Google Scholar]
- 61.Salhia B, Tran NL, Chan A, Wolf A, Nakada M, Rutka F, et al. The guanine nucleotide exchange factors trio, Ect2, and Vav3 mediate the invasive behavior of glioblastoma. Am J Pathol. 2008;173:1828–38. doi: 10.2353/ajpath.2008.080043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lane J, Martin TA, Mansel RE, Jiang WG. The expression and prognostic value of the guanine nucleotide exchange factors (GEFs) Trio, Vav1 and TIAM-1 in human breast cancer. Int Semin Surg Oncol. 2008;5:23. doi: 10.1186/1477-7800-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sosa MS, Lopez-Haber C, Yang C, Wang H, Lemmon MA, Busillo JM, et al. Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer. Mol Cell. 2010;40:877–92. doi: 10.1016/j.molcel.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adamowicz M, Radlwimmer B, Rieker RJ, Mertens D, Schwarzbach M, Schraml P, et al. Frequent amplifications and abundant expression of TRIO, NKD2, and IRX2 in soft tissue sarcomas. Genes Chromosomes Cancer. 2006;45:829–38. doi: 10.1002/gcc.20343. [DOI] [PubMed] [Google Scholar]
- 65.Zheng M, Simon R, Mirlacher M, Maurer R, Gasser T, Forster T, et al. TRIO amplification and abundant mRNA expression is associated with invasive tumor growth and rapid tumor cell proliferation in urinary bladder cancer. Am J Pathol. 2004;165:63–9. doi: 10.1016/S0002-9440(10)63275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamada K, Moriuchi R, Mori T, Okazaki E, Kohno T, Nagayasu T, et al. Tgat, a Rho-specific guanine nucleotide exchange factor, activates NF-kappaB via physical association with IkappaB kinase complexes. Biochem Biophys Res Commun. 2007;355:269–74. doi: 10.1016/j.bbrc.2007.01.147. [DOI] [PubMed] [Google Scholar]
- 67.Bouquier N, Fromont S, Zeeh JC, Auziol C, Larrousse P, Robert B, et al. Aptamer-derived peptides as potent inhibitors of the oncogenic RhoGEF Tgat. Chem Biol. 2009;16:391–400. doi: 10.1016/j.chembiol.2009.02.006. [DOI] [PubMed] [Google Scholar]


