Abstract
G protein-coupled receptor kinase 2 (GRK2) is emerging as a key integrative node in cell migration control. In addition to its canonical role in the desensitization of G protein-coupled receptors involved in chemotaxis, novel recently identified GRK2 substrates and interacting partners appear to mediate the GRK2-dependent modulation of diverse molecular processes involved in motility, such as gradient sensing, cell polarity or cytoskeletal reorganization. We have recently identified an interaction between GRK2 and histone deacetylase 6 (HDAC6), a major cytoplasmic α-tubulin deacetylase involved in cell motility and adhesion. GRK2 dynamically associates with and phosphorylates HDAC6 to stimulate its α-tubulin deacetylase activity at specific cellular localizations such as the leading edge of migrating cells, thus promoting local tubulin deacetylation and enhanced motility. This GRK2-HDAC6 functional interaction may have important implications in pathological contexts related to aberrant epithelial cell migration.
Keywords: GRK2, HDAC6, tubulin acetylation, cell migration, polarity, microtubules
Many cell types are able to undergo molecular and morphological polarization and to trigger motion in response to chemotactic gradients. Such oriented migration or chemotaxis is a fundamental process in embryogenesis, immunity and wound healing. However, it also contributes to pathological conditions such as cancer or inflammatory diseases.1,2 Directional sensing involves the detection of asymmetric extracellular cues by different membrane receptors, many of them chemokine receptors belonging to the G protein-coupled receptors superfamily (GPCR). In turn, stimulated chemotactic receptors generate localized activation of intracellular signaling effectors, leading to cell polarization (i.e., the establishment of distinct functionally and morphological specialized domains at the front and the rear of the cell body), membrane protrusion and the generation of forces required to move the cell toward the chemotactic stimuli.3,4
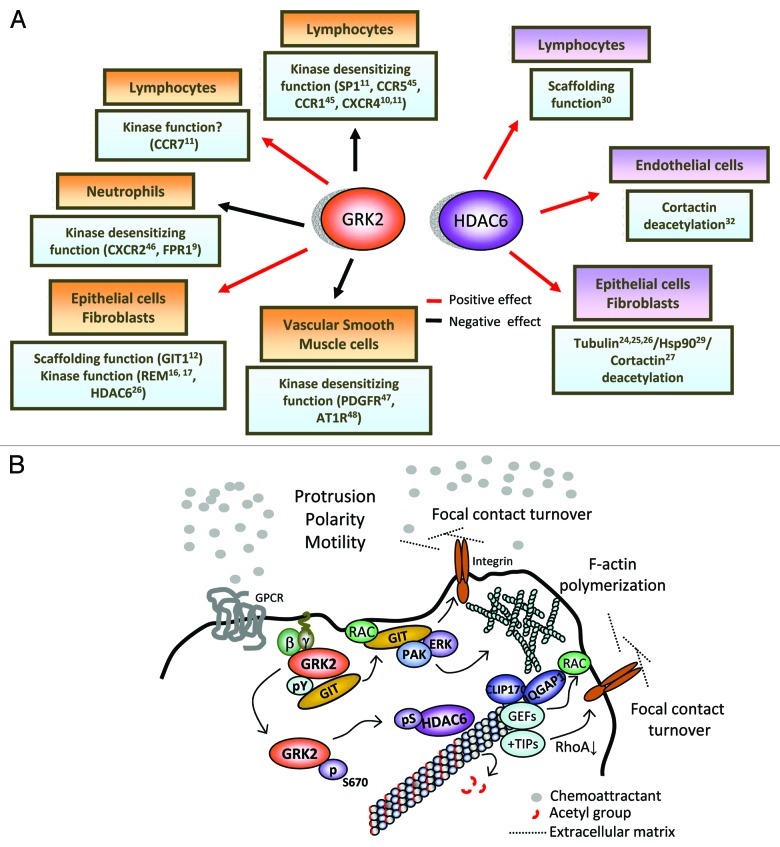
Receptor desensitization and internalization have been considered to play an important role in chemotaxis, since these processes modulate the intensity and duration of agonist stimulation.5,6 GPCR desensitization initiates with the phosphorylation of ligand-bound receptors by a group of seven serine/threonine kinases termed G protein-coupled receptor kinases (GRKs), of which GRK2 is the most ubiquitous member. This phosphorylation event enables the association of arrestins, which leads to receptor uncoupling from G proteins (i.e., desensitization). Arrestins also engage endocytic adaptors to trigger transient internalization of receptors, which then may be recycled back to the membrane (re-sensitization) or targeted for degradation.7,8 Consistent with such canonical negative role, enhanced expression of GRK2 has been shown to inhibit the chemotactic response of “professional” migratory cells of the immune system (at least to some chemokines, Fig. 1).
Figure 1. (A) Schematic representation of the relevant molecular partners/substrates of GRK2 and HDAC6 involved in the migration of different cell types. The overall effect of GRK2 and HDAC6 on cell migration (either positive or negative), as well as the relative contribution of their catalytic or scaffolding-dependent activities, will be dependent on the cell type and the signaling context. See main text for details. (B) Model depicting the intertwinement of GRK2-mediated regulation of GIT-1 scaffolding functions and of HDAC6’s tubulin-deacetylase activity in directed cell motility. In the lamellipodium, GRK2 would be recruited in a Gβγ-dependent manner to sites of the plasma membrane wherein chemotactic activation is taking place. At such specific locations, the dynamic association of GRK2 to the GIT1 scaffold (enhanced upon tyrosine phosphorylation of GRK2 and decreased upon phosphorylation by ERK at S670), would facilitate the localized activation of the Rac/PAK/MEK/ERK pathway, leading to increased focal contact turnover and cortical F-actin polymerization. Concomitantly, phosphorylation of GRK2 at S670 by MAPK would switch on the ability of GRK2 to phosphorylate co-localized HDAC6. Phosphorylated HDAC6 would display a higher deacetylase activity toward tubulin, contributing to keep down the acetylation of pioneer, highly dynamic MTs specifically at the lamellipodium. The presence of hypoacetylated MTs would stimulate cortical F-actin polymerization by helping to recruit at their plus-ends different Rac activators, such as IQGAP1 via the MT-interacting +TIP protein CLIP-170 or other small G proteins-GEF activities.49 In addition, targeting of focal contacts by dynamic cortical MTs at the lamellipodium prevents their maturation into focal adhesions. Theses contacting MTs release “relaxing” signals that trigger dissolution of focal contacts, probably as result of the local, +TIP protein-mediated downregulation of RhoA.50 The concerted action of hypoacetylated MTs and GIT-1 signalosomes at the leading edge of migrating cells could contribute to generate/reinforce cortical polarity and cellular protrusion.
However, how receptor desensitization participates in the different processes underpinning chemotactic movement has not been fully deciphered. Importantly, once oriented movement is initiated, locomotion needs to be maintained until cells reach destination. A recent work proposed that GRK2 levels are important for defining the stop signal of migration. Chemotaxis usually displays a biphasic dose-dependent behavior in response to chemoattractants, with inhibition appearing at higher doses of these compounds. The threshold for such inhibition could be dictated by GRK2 expression levels and the related extent of receptor desensitization and internalization. Therefore, in the absence of this kinase cessation of migration would not be achieved properly, what might result in sustained stimulated locomotion.9
Remarkably, emerging evidence indicate that the impact of GRK2 on cell migration is highly dependent on the stimuli and/or cell type considered, even leading to outcomes opposed to those aforementioned.10 For instance, while genetic deletion of GRK2 enhanced chemotactic responses of both T and B cells to sphingosine-1-phosphate (S1P), oriented migration of these cells toward CCL21 was decreased.11 On the other hand, we have reported a positive role for GRK2 in the migration of epithelial cells and fibroblasts.12 How can these discrepancies be explained on the basis of the conventional “GPCR-desensitizing role” of GRK2? It is now known that tumor or immune cells are not in permanent locomotion during the migratory process,13,14 but rather alternate periods of motility with stationary intervals or breaks that can vary in length and frequency in a cell type-specific or stimuli-dependent way. Such breaks may serve to build new pseudopodia and to re-evaluate the external chemotactic gradient, what would require the presence of fully active receptors at the cell surface. In this context, it is possible that enhanced GRK2 functionality could contribute to reduce the extent/frequency of such breaks (as this kinase also initiates receptor re-sensitization), thus facilitating “processivity” of motion in some stimuli-specific contexts. Alternatively, there is now compelling evidence that different chemokine receptors, as well as the same receptor in different cell types, can engage distinct signal transduction routes to promote motility, which could be positively influenced by GRKs in a desensitization-independent manner. In this regard, novel substrates and interacting partners that might underlie the positive contribution of GRK2 in cell migration have been identified.15 For instance, phosphorylation of ezrin16 and radixin17 by GRK2 results in membrane ruffling as well as membrane protrusion and motility of epithelial cells. Besides such phosphorylation-dependent events, we have described that GRK2 positively regulates migration of epithelial cells and fibroblasts in a kinase-independent manner. Such effect involves the GRK2-dependent stimulation of the scaffold function of GIT1 in the activation of the Rac/PAK/MEK/ERK1/2 pathway.12 Dynamic GRK2/GIT1 association in response to integrin and S1P receptor-mediated stimuli promotes cortical F-actin rearrangement and focal adhesion turnover, both critical events for efficient protrusion and locomotion.
A Novel GRK2/HDAC6 Interaction Regulates the Microtubule Cytoskeletal Network during Cell Migration
We have recently unveiled that another important process influenced by GRK2 is the establishment and maintenance of cell polarity by means of the regulation of microtubules (MTs). During cell migration, the microtubule cytoskeleton is also polarized, displaying different dynamics, posttranslational modifications and distinct sets of associated proteins between protruding and retracting regions of the cell.18,19 These asymmetries contribute to deliver cell-intrinsic cues from MTs necessary to reinforce and maintain cortical polarity, as well as to reduce adhesiveness by disassembly of focal adhesions and to remodel focal contacts at the rear and at the leading edge of the cell.20 Regarding post-translational modifications, tubulin subunits become acetylated in the stable subset of MTs arranged in the lamella region, while highly dynamic, “pioneer” MTs facing the lamellipodium are de-acetylated.21,26 The extent of tubulin acetylation in such different MT subsets has been suggested to be involved in the regulation of MTs dynamics and migration,22 although the underlying molecular mechanism has not been convincingly defined. Tubulin deacetylation is actively performed by HDAC6, a class IIa cytoplasmic histone deacetylase, which overexpression stimulates the migration of different cell types in response to a variety of signals.23-25
Our group has recently identified a novel pathway by which GRK2 regulates HDAC6 activity during chemotactic migration and cell spreading.26 This finding adds a new, GPCR-independent component to the relevant GRK2 interactome involved in epithelial cell migration, and also strengthens the functional link between tubulin acetylation and migration. We have found that GRK2 directly interacts with and phosphorylates HDAC6 at defined serine residues. This phosphorylation enhances both the extent and kinetics of HDAC6-mediated α-tubulin deacetylation, and is required for full HDAC6-tubulin deacetylase activity in situ. Moreover, expression of HDAC6 mutants with impaired phosphorylation by GRK2 fails to mimic the enhanced chemotactic motility promoted by wild-type HDAC6 in cells migrating toward fibronectin, similar to the effect of a tubulin-deacetylase inactive mutant. These data strongly suggest that HDAC6 phosphorylation by GRK2 plays a relevant role in the positive modulation of cell motility by these proteins.
In turn, the modulatory effect of GRK2 on HDAC6 is dynamically regulated by the phosphorylation status at serine 670 of GRK2 itself, which is rapidly upregulated in response to pro-migratory stimuli in parallel to tubulin de-acetylation. Particularly interesting is our observation that GRK2-S670A (a mutant defective in phosphorylation at this regulatory site) showed a reduced ability to phosphorylate HDAC6 compared with wt-GRK2, despite phosphorylation of other canonical substrates (GPCR or tubulin) and binding to HDAC6 were not significantly affected. Therefore, this modification of GRK2 acts as a key switch that specifically modulates the ability of GRK2 to catalyze the phosphorylation of HDAC6. Interestingly, both phospho-S670-GRK2 and HDAC6 are specifically co-recruited to chemoattractant-induced pseudopodia and both proteins co-localize in the leading edge of polarized, motile epithelial cells, a region that is devoid of acetylated MTs. Thus, we propose that the dynamic GRK2 phosphorylation at the leading edge triggered by different pro-migratory stimuli would translate into dynamic, local HDAC6-mediated de-acetylation of tubulin at the plus-ends of MTs, thus helping to maintain the cortical polarization underlying pseudopodia extension and directed migration.
In addition to tubulin, HDAC6 is also able to trigger de-acetylation of cortactin and Hsp90 in order to modulate cell migration.27,28 Unexpectedly, GRK2 does not stimulate the capacity of HDAC6 to de-acetylate cortactin, being the positive HDAC6-mediated effect of GRK2 in migration independent of the acetylation status of cortactin.26 These data suggest that tubulin is the relevant target of HDAC6 underlying the effects of GRK2 in migration. However, it remains an open question whether de-acetylation of other HDAC6 substrates such as Hsp90, a chaperone known to interact with GRK229 and that promotes actin remodeling during cell migration,28 or even catalytic-independent functions of HDAC6 involved in motility,30 could also be altered by GRK2 in a kinase- or scaffold-dependent manner.
A better understanding of how different substrate/partners of HDAC6 contribute to migration in a given cellular context, as well as of the impact of regulatory factors able to modulate precise HDAC6 activities, may have important implications in pathological contexts (see below). Interestingly, while in certain cell types such as fibroblasts or epithelial cells the control of cell motility by HDAC6 clearly relies on the regulation of tubulin acetylation and actin remodeling by inducing deacetylation of cortactin27 and Hsp9028,31 in endothelial cells tubulin deacetylation doesn’t seem to play a role32 (Fig. 1). Remarkably, we have observed that in the latter cell type enhanced GRK2 expression does not support migration in response to a variety of chemotactic stimuli (unpublished data). Therefore, our data suggest that the positive role of GRK2 in the migration of adherent cell types would depend on the relevant HDAC6 interactome involved in a given cell type or physiological situation.
On top of that, an important question to be addressed is how GRK2-mediated regulation of HDAC6 intertwines with that of GIT-1 in order to orchestrate cell polarity and adhesion dynamics in different cell types. Since the relative extent of actin- and microtubule-rich regions varies with cell type, it is reasonable to assume that their contribution to the migration machinery will also be different.19 For instance, microtubules have no role in the protrusion activity of keratinocytes, whereas they are prominently involved in the migration of astrocytes. In the context of such “locomotion” heterogeneity, the contribution of the regulatory actions of GRK2 mediated by GIT and HDAC6 on actin cytoskeleton and MT, respectively, might be differently balanced according to the migratory stimuli and protrusion forces involved (i.e., interplay between actin polymerization and microtubule dynamics). In this regard, we have recently observed that concurrent regulation of GIT-1 and HDAC6-dependent activities by GRK2 takes place in the migration of Hela cells toward fibronectin. By using specific inhibitors of HDAC6-mediated tubulin deacetylation and the overexpression of a GIT-1 mutant unable to mediate the stimulatory effects of GRK2 on chemotactic signaling, we have estimated that GIT-1 and HDAC6 components account in an additive manner for 30–40% and 35–45%, respectively, of the overall positive effect of GRK2 in the migration of this particular cell type.26 Consistently, migration is reduced ca. 60–70% when both GRK2-mediated migratory components (GIT-1 and HDAC6/tubulin) are simultaneously downplayed by silencing GRK2 expression.12 Based on these observations, we propose a model to integrate these different pro-migratory functions of GRK2 (Fig. 1). Upon chemotactic receptor activation, GRK2 would be recruited in a Gβγ-dependent manner to the lamellipodium plasma membrane. At such specific locations, chemokine receptor stimulation would promote the transient interaction of GRK2 with GIT-1 in a phosphorylation-regulated manner. An initial c-Src-mediated tyrosine phosphorylation of GRK2 enhances its binding to GIT-1, whereas subsequent phosphorylation at S670 by MAPK disrupts this interaction.12 In turn, MAPK phosphorylation switches on the ability of GRK2 to phosphorylate HDAC6 co-localized at the lamellipodium, what would result in a higher local HDAC6 de-acetylase activity toward tubulin.26 The presence of hypo-acetylated MTs at the lamellipodium together with functional GIT-1 signalosomes would stimulate cortical Rac and F-actin polymerization, as well as dynamic focal adhesion turnover in order to favor cell migration.
HDAC6 and GRK2 as New Potential Pharmacological Targets to Halt Cell Migration: Two are Better than One
In sum, both GRK2 and HDAC6 seem to modulate diverse molecular processes involved in motility (gradient sensing, adhesion, polarity and cytoskeletal reorganization) in a multifaceted way, by engaging in a variety of signaling routes and through the regulation of different partners, with the involvement of both their catalytic and scaffolding activities. Therefore, it is likely that altered activity/expression of these proteins might critically contribute to deregulate cell migration in relevant pathologies such as chronic inflammation or cancer. In line with this notion, overexpression of HDAC6 has been reported in ovarian carcinomas, breast tumors, oral squamous carcinomas and primary acute myeloid leukemia,33 while altered levels of GRK2 have been found in rheumatoid arthritis, multiple sclerosis and diverse neoplastic diseases as human granulosa cell tumors, thyroid and prostate cancer or some breast tumors.34 One of the more life-threatening aspects of cancer is the invasive migration and metastasis of malignant cells. Therefore, it is tempting to speculate that the reported interplay of GRK2 and HDAC6 might also be implicated in invasive migration. In this regard, GRK2 was found upregulated in different malignant mammary cell lines that display aberrant migration compared with normal cells,35 and GRK2 inhibition by expression of a peptide derived from the carboxyl-terminus of GRK2 (GRK2ct or βARK1ct), suppressed both tumor formation and growth36 as well as invasive migration of tumor breast cells.37
Overall, these data suggest that concurrent HDAC6 and GRK2 upregulation in human tumor malignancies may favor migration and invasion, and point to HDAC6 and GRK2 as new potential therapeutic targets for suppressing cancer growth and metastasis. In fact, several inhibitors with different profiles of selectivity toward distinct classes of HDACs are already in early phase clinical trials for a broad range of liquid and solid tumors.38 The hydroxamic acids tubacin and tubastatin-A25,39 and the naphthoquinone analog NQN-140 have been described to elicit a potent and selective HDAC6 inhibition. Interestingly, some of these compounds seem to act as “partial” HDAC6 inhibitors, blocking deacetylation of only some substrates. While NQN-1 induces hyper-acetylation of Hsp90 and tubulin,40 tubacin and tubastatin-A only prevent tubulin-deacetylation, without altering the extent of cortactin26 or Hsp90 acetylation.40,41 Such biased inhibition of HDAC6 must be taken into account when used as a tool to investigate the involvement of HDAC6 in a given cellular response. On the other hand, it should be possible to take therapeutic advantage of the specificity of these HDAC6’s inhibitors. For instance, it could be predicted that HDAC6 inhibitors similar to tubacin would be less effective than NQN-1-like compounds in inhibiting angiogenesis, since HDAC6 preferentially de-acetylates cortactin in migrating endothelial cells.32
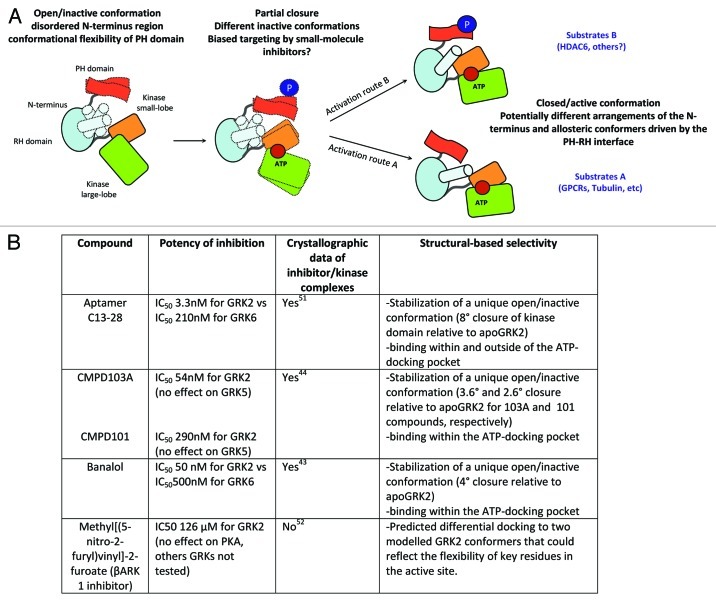
On the contrary, the identification of specific GRK2 pharmacological inhibitors with sufficient potency/selectivity has not been reported to date. Most of kinase inhibitors target the ATP-binding site, which is highly conserved among different GRKs subfamilies and very similar to other kinases of the AGC family. The catalytic domain of GRK2 is in contact with the two other domains of the kinase, the N-terminal RH domain and the C-terminal PH domain.42 Interestingly, structural analysis of different kinases in complex with balanol, a general AGC kinase inhibitor, reveals that banalol recognized-conformations could differ in different GRK isoforms and these divergences could be exploited to develop more selective inhibitors.43 A recent study showing that the heterocyclic compounds CMPD103A and CMPD101 display higher selectivity to inhibit GRK2/3 vs. GRK1 and GRK4 subfamilies by binding to a unique inactive conformation of GRK2 further support this concept.44 Such unique kinase domain conformation might be influenced by differential intra-molecular interactions of the N-terminal RH domain with the small and large lobes of the kinase domain. When GRK2 is activated by the binding of substrates, lipids or Gβγ subunits, the RH domain has been proposed to act as an allosteric transducer domain by altering the relative orientation of the kinase small and large lobes.42 Allosteric communication has also been suggested to take place between the C-tail PH domain and the RH domain of GRK2. Therefore, it is theoretically possible that structural alterations caused by covalent modifications at the C-terminus could be transmitted to the catalytic domain, resulting in a different inactive conformation and/or conformational closure/activation of the kinase domain. Our data show that phosphorylation at S670 causes a switch in the repertoire of GRK2 substrates.26 This phosphorylation could promote the acquisition of a distinctive competent conformation at the active site (and not in docking sites) that would allow phosphorylation of HDAC6. It has been speculated that different substrates could drive a different kinase domain closure and stabilization of the GRK2’s active site through unique pathways, with the involvement of different allosteric changes.43 In this scenario, GRK2pS670 could channel a particular route of allosteric activation driven by a specific subset of substrates. Whether such phosphorylation is also mandatory for other substrates of GRK2 remains to be established, but this new finding supports the provocative concept that the design of “biased inhibitors” of GRK2 might be possible, leading to the preferential switch-off of phosphorylation of particular substrates, without altering the overall cellular kinase activity (Fig. 2).
Figure 2. (A) Proposed model for the potential occurrence of different active/inactive conformers at the GRK2 kinase domain. The full kinase domain closure induced by the presence of ATP and the substrate is needed to render GRK2 catalytically competent. Inactive GRK2 adopts an open conformation with a disordered N-terminus (indicated by dotted shapes) and poor arrangement of the AST (active site tether) region that passes over the small and large kinase lobes. Both regions become increasingly ordered as GRK2 approaches its active/closed conformation. Moreover, kinase closure is allosterically induced/ influenced by the binding of a variety of substrates (GPCRs, cytosolic proteins) that would establish specific contacts with GRK2. In addition, the C-terminal PH domain might participate in the allosteric activation of GRK2 due to its interaction with the RH domain, and this interface can be altered by the docking of phospholipids, Gβγ subunits or posttranslational modifications. We hypothesize that the extent of GRK2 phosphorylation at Ser670 may alter the allosteric communication relayed by the PH-RH axis, what might route the activation process through slightly different conformations of the active site, resulting in distinct substrate selectivity and potential differential inhibitor sensitivity (see text for details). (B) Crystallographic analysis of several GRK2-inhibitor complexes compared with the GRK2-ATP complex (apoGRK2) indicate that selective inhibitors could “freeze” the kinase at different unique inactive conformations that might arise during closure of the kinase domain. It must be note that for crystallization purposes, a mutant Ser670→Ala670 variant of GRK2 has been routinely used in these studies.
In sum, since the functional interaction of GRK2 and HDAC6 triggers more potent chemotactic responses in epithelial cells, it is tempting to suggest that treatment of certain types of cancer (and of other diseases related to aberrant cell migration) would benefit from the combined inhibition of these proteins. Moreover, the possibility of developing “substrate-conditioned” inhibitors for both HDAC6 and GRK2 would allow to select ´ la carte the more suitable and effective combinations of these inhibitors to tackle aberrant migration, taking into account the specific partners of HDAC6 or GRK2 implicated in each one of the pathological contexts.
Acknowledgments
Our laboratory is funded by grants from Ministerio de Educación y Ciencia (SAF2011-23800), Fundación Ramón Areces, The Cardiovascular Network (RECAVA) of Ministerio Sanidad y Consumo-Instituto Carlos III (RD06-0014/0037) and Comunidad de Madrid Indisnet Network (S2011/BMD-2332) to F.M. and Fundación Eugenio Rodriguez Pascual, Fundación Areces and Instituto de Salud Carlos III (PI11/00859) to P.P.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/21585
References
- 1.Raman D, Sobolik-Delmaire T, Richmond A. Chemokines in health and disease. Exp Cell Res. 2011;317:575–89. doi: 10.1016/j.yexcr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Kay RR, Langridge P, Traynor D, Hoeller O. Changing directions in the study of chemotaxis. Nat Rev Mol Cell Biol. 2008;9:455–63. doi: 10.1038/nrm2419. [DOI] [PubMed] [Google Scholar]
- 4.Berzat A, Hall A. Cellular responses to extracellular guidance cues. EMBO J. 2010;29:2734–45. doi: 10.1038/emboj.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol. 2009;10:819–30. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotton M, Claing A. G protein-coupled receptors stimulation and the control of cell migration. Cell Signal. 2009;21:1045–53. doi: 10.1016/j.cellsig.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–82. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 8.Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther. 2012;133:40–69. doi: 10.1016/j.pharmthera.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Ma B, Malik AB, Tang H, Yang T, Sun B, et al. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat Immunol. 2012;13:457–64. doi: 10.1038/ni.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penela P, Ribas C, Aymerich I, Mayor F., Jr New roles of G protein-coupled receptor kinase 2 (GRK2) in cell migration. Cell Adh Migr. 2009;3:19–23. doi: 10.4161/cam.3.1.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnon TI, Xu Y, Lo C, Pham T, An J, Coughlin S, et al. GRK2-dependent S1PR1 desensitization is required for lymphocytes to overcome their attraction to blood. Science. 2011;333:1898–903. doi: 10.1126/science.1208248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penela P, Ribas C, Aymerich I, Eijkelkamp N, Barreiro O, Heijnen CJ, et al. G protein-coupled receptor kinase 2 positively regulates epithelial cell migration. EMBO J. 2008;27:1206–18. doi: 10.1038/emboj.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Entschladen F, Gunzer M, Scheuffele CM, Niggemann B, Zänker KS. T lymphocytes and neutrophil granulocytes differ in regulatory signaling and migratory dynamics with regard to spontaneous locomotion and chemotaxis. Cell Immunol. 2000;199:104–14. doi: 10.1006/cimm.1999.1605. [DOI] [PubMed] [Google Scholar]
- 14.Niggemann B, Drell TL, 4th, Joseph J, Weidt C, Lang K, Zaenker KS, et al. Tumor cell locomotion: differential dynamics of spontaneous and induced migration in a 3D collagen matrix. Exp Cell Res. 2004;298:178–87. doi: 10.1016/j.yexcr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Penela P, Murga C, Ribas C, Lafarga V, Mayor F., Jr The complex G protein-coupled receptor kinase 2 (GRK2) interactome unveils new physiopathological targets. Br J Pharmacol. 2010;160:821–32. doi: 10.1111/j.1476-5381.2010.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cant SH, Pitcher JA. G protein-coupled receptor kinase 2-mediated phosphorylation of ezrin is required for G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Mol Biol Cell. 2005;16:3088–99. doi: 10.1091/mbc.E04-10-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahsai AW, Zhu S, Fenteany G. G protein-coupled receptor kinase 2 activates radixin, regulating membrane protrusion and motility in epithelial cells. Biochim Biophys Acta. 2010;1803:300–10. doi: 10.1016/j.bbamcr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegrist SE, Doe CQ. Microtubule-induced cortical cell polarity. Genes Dev. 2007;21:483–96. doi: 10.1101/gad.1511207. [DOI] [PubMed] [Google Scholar]
- 19.Etienne-Manneville S. From signaling pathways to microtubule dynamics: the key players. Curr Opin Cell Biol. 2010;22:104–11. doi: 10.1016/j.ceb.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T, Noritake J, Kaibuchi K. Regulation of microtubules in cell migration. Trends Cell Biol. 2005;15:76–83. doi: 10.1016/j.tcb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Wadsworth P. Regional regulation of microtubule dynamics in polarized, motile cells. Cell Motil Cytoskeleton. 1999;42:48–59. doi: 10.1002/(SICI)1097-0169(1999)42:1<48::AID-CM5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Zilberman Y, Ballestrem C, Carramusa L, Mazitschek R, Khochbin S, Bershadsky A. Regulation of microtubule dynamics by inhibition of the tubulin deacetylase HDAC6. J Cell Sci. 2009;122:3531–41. doi: 10.1242/jcs.046813. [DOI] [PubMed] [Google Scholar]
- 23.Valenzuela-Ferńndez A, Cabrero JR, Serrador JM, Sánchez-Madrid F. HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 2008;18:291–7. doi: 10.1016/j.tcb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–8. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 25.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci U S A. 2003;100:4389–94. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lafarga V, Aymerich I, Tapia O, Mayor F, Jr., Penela P. A novel GRK2/HDAC6 interaction modulates cell spreading and motility. EMBO J. 2012;31:856–69. doi: 10.1038/emboj.2011.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao YS, Hubbert CC, Lu J, Lee YS, Lee JY, Yao TP. Histone deacetylase 6 regulates growth factor-induced actin remodeling and endocytosis. Mol Cell Biol. 2007;27:8637–47. doi: 10.1128/MCB.00393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo J, Benovic JL. G protein-coupled receptor kinase interaction with Hsp90 mediates kinase maturation. J Biol Chem. 2003;278:50908–14. doi: 10.1074/jbc.M307637200. [DOI] [PubMed] [Google Scholar]
- 30.Cabrero JR, Serrador JM, Barreiro O, Mittelbrunn M, Naranjo-Súrez S, Martín-Cófreces N, et al. Lymphocyte chemotaxis is regulated by histone deacetylase 6, independently of its deacetylase activity. Mol Biol Cell. 2006;17:3435–45. doi: 10.1091/mbc.E06-01-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyault C, Zhang Y, Fritah S, Caron C, Gilquin B, Kwon SH, et al. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev. 2007;21:2172–81. doi: 10.1101/gad.436407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaluza D, Kroll J, Gesierich S, Yao TP, Boon RA, Hergenreider E, et al. Class IIb HDAC6 regulates endothelial cell migration and angiogenesis by deacetylation of cortactin. EMBO J. 2011;30:4142–56. doi: 10.1038/emboj.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aldana-Masangkay GI, Sakamoto KM. The role of HDAC6 in cancer. J Biomed Biotech. 2011;2011:875824. doi: 10.1155/2011/875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Métayé T, Gibelin H, Perdrisot R, Kraimps JL. Pathophysiological roles of G-protein-coupled receptor kinases. Cell Signal. 2005;17:917–28. doi: 10.1016/j.cellsig.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Salcedo A, Mayor F, Jr., Penela P. Mdm2 is involved in the ubiquitination and degradation of G-protein-coupled receptor kinase 2. EMBO J. 2006;25:4752–62. doi: 10.1038/sj.emboj.7601351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bookout AL, Finney AE, Guo R, Peppel K, Koch WJ, Daaka Y. Targeting Gbetagamma signaling to inhibit prostate tumor formation and growth. J Biol Chem. 2003;278:37569–73. doi: 10.1074/jbc.M306276200. [DOI] [PubMed] [Google Scholar]
- 37.Kirui JK, Xie Y, Wolff DW, Jiang H, Abel PW, Tu Y. Gbetagamma signaling promotes breast cancer cell migration and invasion. J Pharmacol Exp Ther. 2010;333:393–403. doi: 10.1124/jpet.109.164814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickinson M, Johnstone RW, Prince HM. Histone deacetylase inhibitors: potential targets responsible for their anti-cancer effect. Invest New Drugs. 2010;28(Suppl 1):S3–20. doi: 10.1007/s10637-010-9596-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J Am Chem Soc. 2010;132:10842–6. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inks ES, Josey BJ, Jesinkey SR, Chou CJ. A novel class of small molecule inhibitors of HDAC6. ACS Chem Biol. 2012;7:331–9. doi: 10.1021/cb200134p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280:26729–34. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 42.Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gbetagamma. Science. 2003;300:1256–62. doi: 10.1126/science.1082348. [DOI] [PubMed] [Google Scholar]
- 43.Tesmer JJ, Tesmer VM, Lodowski DT, Steinhagen H, Huber J. Structure of human G protein-coupled receptor kinase 2 in complex with the kinase inhibitor balanol. J Med Chem. 2010;53:1867–70. doi: 10.1021/jm9017515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thal DM, Yeow RY, Schoenau C, Huber J, Tesmer JJ. Molecular mechanism of selectivity among G protein-coupled receptor kinase 2 inhibitors. Mol Pharmacol. 2011;80:294–303. doi: 10.1124/mol.111.071522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vroon A, Heijnen CJ, Kavelaars A. GRKs and arrestins: regulators of migration and inflammation. J Leukoc Biol. 2006;80:1214–21. doi: 10.1189/jlb.0606373. [DOI] [PubMed] [Google Scholar]
- 46.Fan J, Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med. 2003;9:315–21. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 47.Peppel K, Zhang L, Huynh TT, Huang X, Jacobson A, Brian L, et al. Overexpression of G protein-coupled receptor kinase-2 in smooth muscle cells reduces neointimal hyperplasia. J Mol Cell Cardiol. 2002;34:1399–409. doi: 10.1006/jmcc.2002.2092. [DOI] [PubMed] [Google Scholar]
- 48.Guo J, Chen H, Ho J, Mancini J, Sontag T, Laporte SA, et al. TGFbeta-induced GRK2 expression attenuates AngII-regulated vascular smooth muscle cell proliferation and migration. Cell Signal. 2009;21:899–905. doi: 10.1016/j.cellsig.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 49.Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, et al. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–85. doi: 10.1016/S0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- 50.Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 1999;146:1033–44. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tesmer VM, Lennarz S, Mayer G, Tesmer JJG. Molecular mechanisms for inhibition of G protein-coupled receptor kinase 2 by a selective RNA aptamer. Structure. 2012;20:1300–9. doi: 10.1016/j.str.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iino M, Furugori T, Mori T, Moriyama S, Fukuzawa A, Shibano T. Rational design and evaluation of new lead compound structures for selective betaARK1 inhibitors. J Med Chem. 2002;45:2150–9. doi: 10.1021/jm010093a. [DOI] [PubMed] [Google Scholar]