Abstract
Physical cues from the extracellular environment that influence cell shape and directional migration are transduced into changes in cytoskeletal organization and biochemistry through integrin-based cell adhesions to extracellular matrix (ECM). Paxillin is a focal adhesion (FA) scaffold protein that mediates integrin anchorage to the cytoskeleton, and has been implicated in regulation of FA assembly and cell migration. To determine whether paxillin is involved in coupling mechanical distortion with directional movement, cell shape was physically constrained by culturing cells on square-shaped fibronectin-coated adhesive islands surrounded by non-adhesive barrier regions that were created with a microcontact printing technique. Square-shaped cells preferentially formed FAs and extended lamellipodia from their corner regions when stimulated with PDGF, and loss of paxillin resulted in loss of this polarized response. Selective expression of the N- and C-terminal domains of paxillin produced opposite, but complementary, effects on suppressing or promoting lamellipodia formation in different regions of square cells, which corresponded to directional motility defects in vitro. Paxillin loss or mutation was also shown to affect the formation of circular dorsal ruffles, and this corresponded to changes in cell invasive behavior in 3D. This commentary addresses the implications of these findings in terms of how a multifunctional FA scaffold protein can link physical cues to cell adhesion, protrusion and membrane trafficking so as to control directional migration in 2D and 3D. We also discuss how microengineered ECM islands and in vivo model systems can be used to further elucidate the functions of paxillin in directional migration.
Keywords: paxillin, focal adhesion, lamellipodia, dorsal ruffles, migration, motility, microcontact printing, membrane trafficking, mechanotransduction
Directional cell migration is critical for embryogenesis, tissue growth and homeostasis, wound healing and other normal physiological processes and is frequently deregulated in diseases such as cancer. While much is known about migration guided by chemical cues, the ways in which physical signals, such as extracellular matrix (ECM) orientation and physical forces (e.g., mechanical strain), direct cell motility are less well understood. In past studies, we showed that physical interactions between cells and the ECM control directional migration, in part by governing where cells form focal adhesions (FAs) and by spatially constraining lamellipodia formation to adjacent regions.1 These experiments were performed using microcontact-printed substrates patterned with ECM protein-coated islands surrounded by non-adhesive areas. We have shown that cells confined to square cell-sized islands align F-actin filaments along their diagonal axes and assemble FAs in corner regions (Fig. 1A). Furthermore, cells stimulated by a growth factor remodel FAs and extend motile processes preferentially from corner regions (Fig. 1B). The initial experiments by Parker et al.1 were performed using cells plated on gold substrates patterned with self-assembled monolayers of adhesive or polyethylene glycol (PEG)-terminated alkanethiols, fabricated using poly-dimethylsiloxane (PDMS) stamps cast from photolithographed silicon wafers.2 We recently replicated these findings3 using a simpler method of microcontact printing, in which ECM proteins are stamped directly onto activated PDMS-coated glass and unstamped areas are blocked with a non-adhesive detergent.4
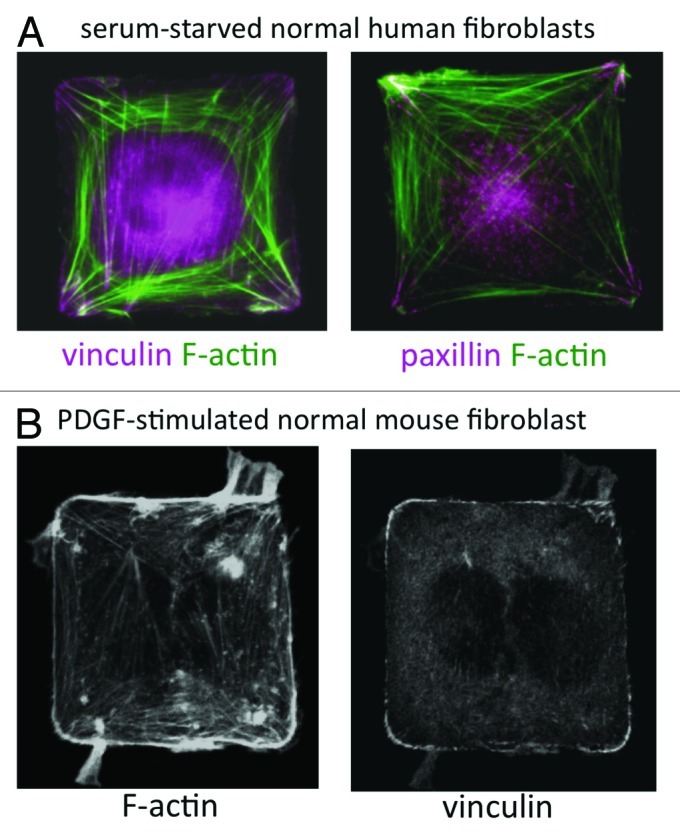
Figure 1. (A) Wild-type human lung IMR-90 fibroblasts plated on 50 μm2 fibronectin islands in serum-free medium. F-actin was labeled with Alexa-488 phalloidin (green) and FA proteins (vinculin and paxillin) were labeled by immunostaining. (B) Wild-type mouse embryonic fibroblast on a 50 μm2 fibronectin island, stimulated with PDGF (25 ng/ml) for 30 min. F-actin was labeled with Alexa-488 phalloidin and vinculin was labeled by immunostaining.
Although we rarely encounter perfectly square-shaped fibroblasts in vivo, they provide a useful model system in which to study how cell distortion influences complex cellular functions, such as migration. Constraining cell shape on polygonal adhesive islands induces a robust artificial polarization, which makes it possible to analyze biophysical relationships that would be difficult to dissect in a more physiological milieu. The advantages of using microcontact printed substrates to control cell shape are 3-fold. First, micropatterning generates a morphologically homogenous population, thereby reducing cell-to-cell and microenvironmental variation. Second, we can reproducibly quantify subtle or stochastic phenotypes induced by chemical or genetic perturbations in statistically significant numbers of cells. Third, and most importantly, in the square-cell model system, a priori knowledge of where FAs and lamellipodia should form (e.g., in the corners of square cells) allows us to detect uncoupling between shape, FA localization and motile process formation in drug-treated or genetically modified cells.
Cytoskeletal-ECM tension is mediated through FAs and focal contacts,5 and we previously demonstrated that traction forces are concentrated in the corners of square cells.1 Brock et al.6 also showed that spatially regulated activation of Rac and Rho signaling is required for coupling FA location and lamellipodia position. Finally, using arrays of micrometer-sized fibronectin islands, Xia et al.7 confirmed that migrating cells traveled in the direction of aligned FAs and that Rac activation spatially localized along newly formed FAs at the leading edge. However, the mechanism coupling FA position and lamellipodia localization remained unclear. We recently explored this mechanism and demonstrated that the multifunctional FA scaffold protein paxillin, which is known to recruit Rac and Rho regulators to FAs6 is involved in regulating directional membrane process formation in square-shaped cells.3 Based on these findings, we then tested predictions about paxillin’s role in 2D and 3D migration using scrape wound and matrigel invasion assays.
Paxillin Couples Cell Distortion to Directional Extension of Cell Protrusions
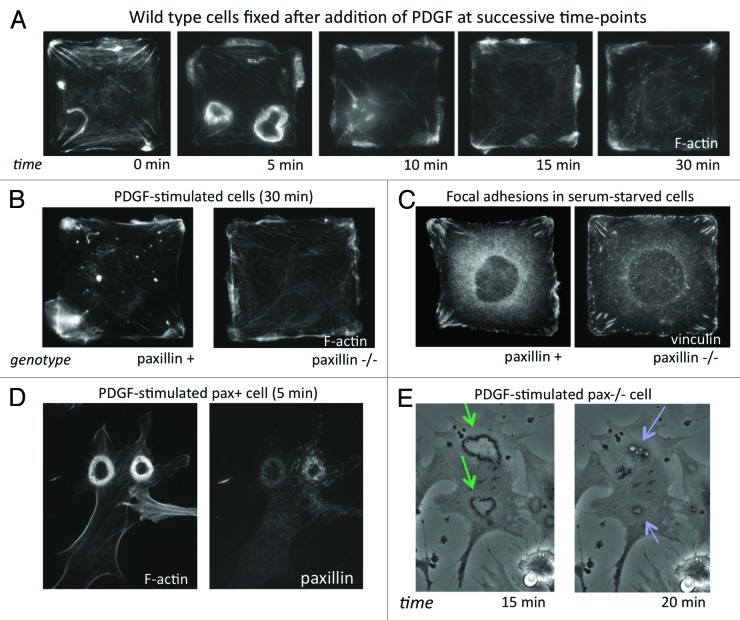
In our recent publication in PLoS One,3 we showed that physical control of directional migration requires the FA scaffold protein paxillin. Time-course analysis of fibroblasts cultured on single-cell sized, square ECM islands created by microcontact printing revealed that normal square cells activated Rac along their periphery and formed both dorsal and lateral membrane ruffles without spatial orientation from 5–10 min after stimulation with PDGF (Fig. 2A). In contrast, paxillin knockout (pax−/−) mouse embryonic fibroblasts (MEFs) and paxillin-knockdown human fibroblasts failed to restrict Rac activity to the corner regions, and instead formed small lamellipodia along their entire periphery (Fig. 2B). Consistent with previous reports,8 pax−/− cells also showed impaired spatial constraint of FA formation, with more small FAs in side regions and shorter FAs in corners (Fig. 2C). Thus, paxillin seems to be essential for establishing cell polarization based on substrate geometry, in which FA positioning is guided by differences in local cytoskeletal tension. However, paxillin likely plays other roles in actively promoting protrusions in response to chemical stimuli as well.
Figure 2. (A) Time-course of the motile response to PDGF (25 ng/ml) stimulation in wild type (pax+) mouse embryonic fibroblasts (MEFs). Cells were fixed without (0 min) and at 5, 10, 15 and 30 min after addition of PDGF and stained with Alexa-488 phalloidin to label F-actin. (B) Pax+ and pax−/− MEFs stimulated with PDGF for 30 min, fixed and stained with Alexa-488 phalloidin. (C) Vinculin labeling by immunofluorescence in serum-starved pax+ and pax−/− cells on square islands showing differences in FA distribution. (D) Pax+ MEF fixed 5 min after addition of PDGF and stained for F-actin and paxillin showing large circular dorsal ruffles (CDRs). (E) Phase-contrast time-lapse video of live pax−/− MEFs stimulated with PDGF (20X). Formation of CDRs (green arrows) persisted for more than 15 min and protrusions were internalized into macropinocytic vesicles (blue arrows) by about 20 min. In pax+ cells, CDR formation and internalization was complete by 10 min after addition of PDGF.
Expression of the paxillin N-terminus (paxN) or C-terminus (paxC) truncation mutants (Fig. 3) produced opposite, but complementary, effects on lamellipodia formation. PaxN primarily suppressed membrane extension along the sides of square cells, while paxC enhanced lamellipodia formation, particularly in corners. Unexpectedly, we found that pax−/− and paxN cells also formed more circular dorsal ruffles (CDRs) than control cells at early times and showed repeated rounds of CDR formation. CDRs are actin-driven membrane ruffles that extend from the dorsal surface of cells upon activation of receptor tyrosine kinases (e.g., PDGFR or EGFR), then contract and are internalized into macropinocytic vesicles.9 They contain many of the same proteins as lamellipodia, such as paxillin (Fig. 2D), but are distinct protrusive structures. PaxC cells formed fewer CDRs and extended larger lamellipodia even in the absence of PDGF. In two-dimensional (2D) scrape wound assays, pax−/− cells migrated at similar speeds to controls but lost directional persistence. Directional motility was rescued by re-expressing full-length paxillin or the N-terminus alone, but paxN cells exhibited reduced migration speed. In contrast, pax−/− and paxN cells exhibited increased migration in a three-dimensional (3D) matrigel invasion assay, with paxN cells invading the matrix even in the absence of PDGF. These studies indicate that paxillin is critical for integrating physical cues from the ECM with chemical motility signals by spatially constraining where cells form motile processes, and thereby regulates directional migration. This work also suggests that CDRs may correspond to invasive protrusions that drive cell migration through ECM in 3D microenvironments.
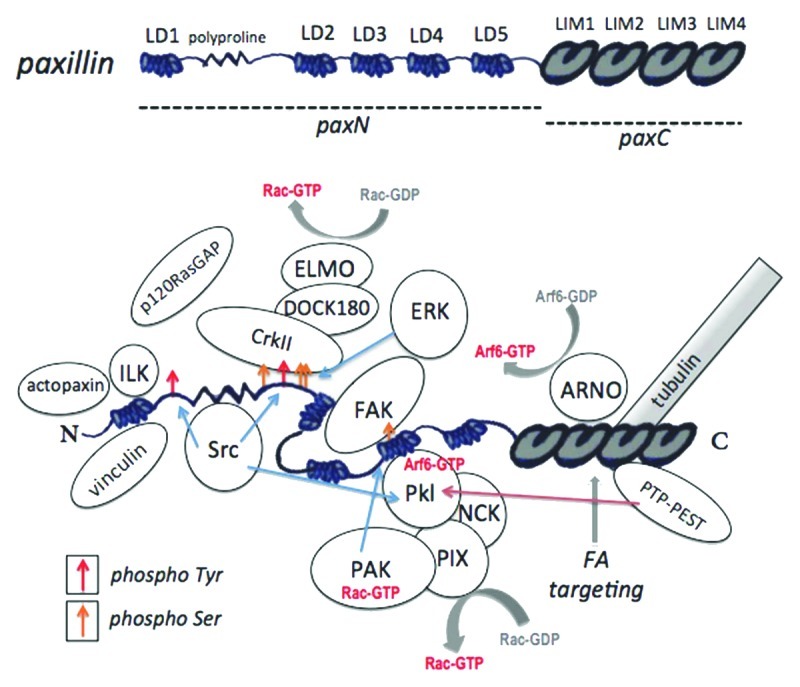
Figure 3. Paxillin domains and interacting proteins. Top: Paxillin protein domains. The regions comprising paxN and paxC mutant constructs are indicated. Bottom: Paxillin-protein interactions and phosphorylation sites. Tyr31 and Tyr118, targets of Src and FAK that provide binding sites for CrkII and p120RasGAP, are indicated by red arrows. Serine phosphorylation sites, including the PAK target site Ser273, are indicated by yellow arrows. Phosphorylation at this site alters affinity for FAK, Git1 and Pkl. For a review of paxillin-protein interactions, see Deakin and Turner (2008).8
Interestingly, both polarized lamellipodia formation and CDR suppression were rescued when the paxN and paxC truncation mutants were co-expressed in the same cell, which suggests that the two halves of paxillin can act independently of one another. In support of this hypothesis, Cortesio et al.10 recently reported that calpain cleaves paxillin between the LD1 and LD2 motifs, and that the resulting long fragment inhibits migration and FA turnover. Cleavage of paxillin following PDGF receptor activation may be required to limit the recruitment of lamellipodia-forming complexes to a polarized leading edge. However, the role of paxillin cleavage in controlling directional motility is as yet unclear. Our paxillin C-terminal construct did not contain the LD4 and LD5 motifs, which are present in the paxillin calpain cleavage product, and calpain-2 is known to cleave a number of other FA proteins.11 Still, the presence of at least one physiological cleavage product, which resembles the delta isoform, suggests that paxillin’s scaffold function may be regulated by dynamic structural and conformational changes.
As cells spread on an adhesive surface, they establish polarity by strengthening some adhesions through actin-generated tension while disassembling others; in square cells, this results in corner-localized FAs. Paxillin knockout cells have defects in adhesion remodeling,12 which may account for their mild defect in restricting FAs to corners in square islands. PaxN cells had a highly contractile phenotype, with extremely long, corner-localized FAs, and did not form fan-shaped lamellipodia. PaxC cells failed to restrict FA localization to corners and formed lamellipodia even in the absence of growth factor. A related protein, Hic-5, which was detected in the FAs of paxillin-deficient cells, has been shown to be upregulated in paxillin knockout cells. Like paxillin, Hic-5 binds FAK, Git-1 and PTP-PEST, but seems to play an antagonistic role in migration, suppressing lamellipodia extension.13,14 One model that would explain these data is that cytosolic paxillin (which may lack tyrosine phosphorylation) delivers Rac-mediated actin polymerization complexes, which antagonize Hic-5 and drive lamellipodia formation to peripheral FAs. The C-terminal fragment alone might compete with Hic-5 to promote lamellipodia formation, while the cytosolic N-terminus could sequester proteins away from FAs, favoring CDR formation at the dorsal membrane and non-Rac mediated protrusions (e.g., driven by Cdc42 or formins) near corner FAs (Fig. 4).
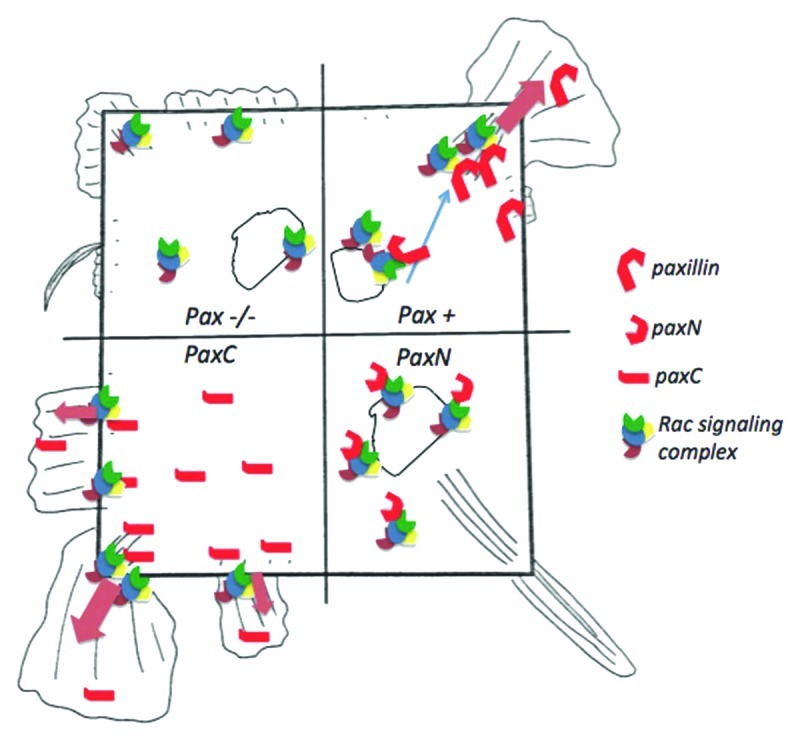
Figure 4. Model of the effects of paxillin and paxillin mutants on lamellipodia formation in square cells. The square, representing a shape-constrained cell, is divided into four quadrants, which correspond to paxillin−/−, paxillin+/+, paxN and paxC cell conditions. In control (pax+) cells (top right), paxillin may bind cytosolic or membrane-bound Rac-activating complexes and recruit them to focal adhesion (FA) sites. For example, upon PDGF receptor activation paxillin could bind Pkl-PIX-PAK complexes containing Arf6-GTP and Rac-GTP and deliver them to corner regions to promote localized lamellipodia formation. In paxillin−/− cells (top left), some active Rac complexes may drive limited, non-localized lamellipodia extension at the cell periphery, but delivery from endosomal compartments to the plasma membrane may be impaired. The C-terminal paxC fragment (bottom left) could compete with Hic-5, PTP-PEST or Rac inhibiting proteins, promoting enhanced lamellipodia formation even in the absence of growth factors. Conversely, the N-terminal paxN fragment (bottom right) could sequester Rac-activating proteins and lamellipodia machinery in the cytosol. The small, spiky protrusions in these cells could be driven by Cdc42 or RhoA instead of by Rac.
Does Paxillin Direct Membrane Trafficking in Motile Cells?
The increase in CDR formation and defects in macropinocytic vesicle trafficking we observed in paxillin knockout (Fig. 2E) and paxN cells implicate paxillin in another key process in cell migration: membrane trafficking. Likely candidates linking paxillin with the recycling of membrane components to the plasma membrane are the Arf6 GAPs, Pkl (which forms a complex with the Rac GEF PIX and the Rac effector PAK) and the related protein Git1.15 Pkl and Git1 have been shown to be required for directional motility in several cell types, and their precise functions in migrating cells are probably also context-dependent.15,16 Paxillin was recently shown to associate with the Arf6 GEF cytohesin-2/ARNO in mouse 3T3-L1 cells through its C-terminal LIM2 domain.17 Paxillin also associates with the exocyst complex in metastatic prostate cancer cells via Sec5.18 Furthermore, paxillin has been reported to play a role in autophagy, another membrane trafficking process, through association with Atg1.19 Thus, paxillin is poised to act as an important intermediary for integrating membrane trafficking with cytoskeletal dynamics and FA remodeling during migration. It will be informative to elucidate the role of paxillin in Arf6 signaling, particularly with respect to Src-mediated Pkl/Git phosphorylation and PTP-PEST-mediated dephosphorylation.20
A recent report describes a novel trafficking pathway in which β1 and β3 integrins and cortactin rapidly localize to CDRs in response to PDGF stimulation (within 3–5 min), and are then internalized into macropinosomes and recycled to the protrusive plasma membrane.21 Even more recently, the recruitment of integrins to CDRs was shown to depend on Src activation at FAs and on ILK, another paxillin-binding protein.22 We have also observed that GFP-paxillin and GFP-zyxin rapidly localize to dorsal ruffles upon stimulation with PDGF, which suggests that this pathway could be used for the recycling other FA proteins. The increased propensity to form CDRs and their increased lifetimes in mutant cells implicates paxillin in regulating the spatiotemporal dynamics of protein trafficking. A defect in targeting proteins to the leading edge could explain why paxillin-deficient cells form small, non-localized lateral lamellipodia and multiple leading edges. From these data we would predict that paxN cells are unable to redistribute Rac-driven actin-polymerization machinery to leading edges, and that they use a RhoG- or Cdc42-based mode of directional migration.23
Changes in the conformation of paxillin—via steric intramolecular interactions, phosphorylation states, mechanical forces, protein or lipid binding and/or proteolytic cleavage—would enable the regulated recruitment of different signaling and effector proteins to different regions of the polarized cell and thus drive efficient, directional migration. These studies raise intriguing questions about the conformational changes that regulate paxillin scaffold functions and about its dynamic roles in different subcellular compartments. Does cytosolic paxillin actively suppress Rac activation and lamellipodia formation, and does it activate other GTPase signaling pathways? Does paxillin recruited to CDRs or endosomes promote Arf6-mediated delivery of structural and signaling proteins to the leading edge of migrating cells? The paxillin LIM domains bind tubulin, so it could be involved in directing cargo, such as activated Arf6GTP-Pkl-PIX-PAK-RacGTP complexes and integrins, to FAs via microtubules. While it is tempting to speculate, further studies are needed to elucidate the complex relationship between FAs, membrane trafficking and directional migration.
Circular Dorsal Ruffles and 3D Matrix Invasion
It has been suggested that CDRs are related to invasive protrusive structures, such as invadopodia.24 We therefore tested the effects of paxillin deletion and mutation on 3D migration using a matrigel plug assay. Paxillin knockout MEFs were more invasive in response to PDGF than control cells; they migrated into matrigel plugs in greater numbers and to greater depths than paxillin-rescued cells. PaxN cells, which formed even more CDRs than knockouts, were highly invasive in matrigel even in the absence of PDGF stimulation. Accordingly, paxC cells formed fewer CDRs and were less invasive than knockouts. We also observed that when confluent monolayers overlaid with matrigel were stimulated with PDGF, cells overexpressing GFP-paxN formed extensive spiky “dorsal” protrusions that extended into 3D matrix.
These findings implicate paxillin in determining whether cells will migrate along a 2D planar substrate or invade a 3D matrix, which is critical during tissue formation and cancer metastasis. These data also support the hypothesis that CDRs in tissue culture represent 2D manifestations of physiologically relevant 3D protrusive structures. Paxillin and Hic-5 were shown to be important for switching between two modes of 3D migration in MDA-MB-231 breast tumor cells: paxillin knockdown induced an elongated, mesenchymal-like mode and Hic-5 knockdown induced a round, ameboid-like mode.14 Importantly, a recent study showed that expression of Hic-5, but not paxillin, promoted invadopodia formation in MCF10A cells.25 In our study, cells lacking paxillin or expressing only the N-terminus, which form small or no lamellipodia, may be primed to use RhoA-driven modes of migration, mediated by Hic-5, which would account for their increased invasion into matrigel.
Future Directions
Recent advances in automated image analysis, coupled with the greater availability of micropatterned substrates, make assays of the kind described here increasingly amenable for use in screening. For example, now that we have demonstrated that protrusive phenotypes observed in square cells are indicative of directional motility defects, it would be feasible to test a panel of mutants to identify the critical domains of paxillin involved in each step in this complex process. Our studies confirm that the paxillin LD4 motif, which binds FAK and the Arf6 GAPs (Fig. 3), is important for spatial restriction of lamellipodia in square cells (consistent with its reported role in cell migration26), but not for suppression of CDRs. However, there are many other domains in paxillin whose functions are not well understood. The use of microcontact printed substrates with genetic knockdown screens would uncover new genetic interactions and help to elucidate molecular mechanisms for a range of phenotypes. A RNAi screen in wild-type and paxillin-deficient cells also could be used to identify the key players involved in paxillin-mediated organization of FAs, and the promotion or suppression of lamellipodia and CDRs.
Paxillin deletion is embryonic lethal in mice,12 so it is clearly required for important developmental processes in vivo. An optimal system to study the role of paxillin in directional cell migration in vivo is the modified matrigel plug assay,27 where matrigel is cast in a PDMS mold and implanted subcutaneously into the back of a mouse (Fig. 5A). Host cells can then migrate from the skin into the gel. Local knockdown or overexpression of proteins can be accomplished with direct injection of siRNA targeting paxillin or a plasmid encoding paxillin complexed with a transfection reagent (e.g., jetPEI).27 Cell morphology (i.e., number of cells, aspect ratio, angle of orientation and branching) and depth of migration through the gel can be quantified in hematoxylin and eosin stained sections and cell types can be identified by immunohistochemistry (Fig. 5B). In addition, it would be interesting to adapt this system to include tumor cells or tumor releasate as a chemoattractant for host cells, because paxillin has been associated with metastasis in some cancers.28,29
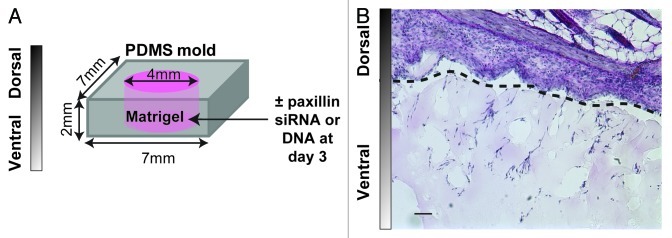
Figure 5. (A) Schematic of a modified matrigel plug assay. Matrigel is cast in a 7 × 7 × 2 mm PDMS mold inside a 4 mm well and implanted on a mouse back for 7–14 d. Paxillin siRNA or DNA (10 μg) is injected locally at day 3. (B) Micrograph of hematoxylin and eosin-stained implant/skin section after 7 d. Skin is shown above the dotted line and matrigel with host cells is shown below the dotted line. Scale bar = 50 µm.
To address the role of paxillin in directional migration in vascular network formation we can use the mouse neonatal retinal angiogenesis assay.27 Because retinal angiogenesis occurs after birth in mice, this system is advantageous for studying vascular growth and patterning in vivo. Paxillin siRNA or DNA in complex with a transfection reagent can be injected intravitreally in neonates. Vascular network formation in the retina is then assessed two days after injection using flat-mounted, fluorescein-conjugated isolectin staining and immunohistochemical analysis. Another simple system to study mesenchymal migration during organ formation is the embryonic tooth, in which proper development requires dental mesenchymal cells to migrate in response to both attractive and repulsive cues.30 Knockdown can be achieved by transplacental delivery of siRNA.31 Finally, given that our studies show a critical role for paxillin in fibroblast migration during wound healing in vitro, it likely plays a role in wound healing in vivo. A simple model system described by Eckes et al.32 can be modified by injecting paxillin siRNA or DNA either locally or systemically prior to wounding.
Use of extremely artificial or “highly stylized” model systems, such as micropatterned square cells, makes it possible to quantify complex, stochastic phenotypes with relative precision and reproducibility. This is a particularly useful strategy for studying proteins like paxillin, which have no intrinsic enzymatic function but serve as molecular scaffolds for dozens of different signaling pathways. From these experiments, we can make testable predictions about cellular processes in increasingly complex environments, from 2D culture assays to 3D in vitro assays to in vivo models. Understanding the role of paxillin in the multifarious cellular processes that are involved in directional cell motility will shed light on fundamental processes in disease, development, and basic cell biology.
Acknowledgments
This work was supported by NIH grant PO1-CA045548.
Glossary
Abbreviations:
- FA
focal adhesion
- ECM
extracellular matrix
- CDR
circular dorsal ruffle
- MEF
mouse embryonic fibroblast
- paxN
paxillin N-terminal truncation mutant
- paxC
paxillin C-terminal truncation mutant
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/21672
References
- 1.Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, et al. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 2002;16:1195–204. doi: 10.1096/fj.02-0038com. [DOI] [PubMed] [Google Scholar]
- 2.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng. 2001;3:335–73. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 3.Sero JE, Thodeti CK, Mammoto A, Bakal C, Thomas S, Ingber DE. Paxillin mediates sensing of physical cues and regulates directional cell motility by controlling lamellipodia positioning. PLoS One. 2011;6:e28303. doi: 10.1371/journal.pone.0028303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan JL, Liu W, Nelson CM, Raghavan S, Chen CS. Simple approach to micropattern cells on common culture substrates by tuning substrate wettability. Tissue Eng. 2004;10:865–72. doi: 10.1089/1076327041348365. [DOI] [PubMed] [Google Scholar]
- 5.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 6.Brock AL, Ingber DE. Control of the direction of lamellipodia extension through changes in the balance between Rac and Rho activities. Mol Cell Biomech. 2005;2:135–43. [PubMed] [Google Scholar]
- 7.Xia N, Thodeti CK, Hunt TP, Xu Q, Ho M, Whitesides GM, et al. Directional control of cell motility through focal adhesion positioning and spatial control of Rac activation. FASEB J. 2008;22:1649–59. doi: 10.1096/fj.07-090571. [DOI] [PubMed] [Google Scholar]
- 8.Deakin NO, Turner CE. Paxillin comes of age. J Cell Sci. 2008;121:2435–44. doi: 10.1242/jcs.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orth JD, Krueger EW, Weller SG, McNiven MA. A novel endocytic mechanism of epidermal growth factor receptor sequestration and internalization. Cancer Res. 2006;66:3603–10. doi: 10.1158/0008-5472.CAN-05-2916. [DOI] [PubMed] [Google Scholar]
- 10.Cortesio CL, Boateng LR, Piazza TM, Bennin DA, Huttenlocher A. Calpain-mediated proteolysis of paxillin negatively regulates focal adhesion dynamics and cell migration. J Biol Chem. 2011;286:9998–10006. doi: 10.1074/jbc.M110.187294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franco S, Perrin B, Huttenlocher A. Isoform specific function of calpain 2 in regulating membrane protrusion. Exp Cell Res. 2004;299:179–87. doi: 10.1016/j.yexcr.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, et al. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22:901–15. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibanuma M, Mori K, Nose K. HIC-5: A Mobile Molecular Scaffold Regulating the Anchorage Dependence of Cell Growth. Int J Cell Biol. 2012;2012:426138. doi: 10.1155/2012/426138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deakin NO, Turner CE. Distinct roles for paxillin and Hic-5 in regulating breast cancer cell morphology, invasion, and metastasis. Mol Biol Cell. 2011;22:327–41. doi: 10.1091/mbc.E10-09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoefen RJ, Berk BC. The multifunctional GIT family of proteins. J Cell Sci. 2006;119:1469–75. doi: 10.1242/jcs.02925. [DOI] [PubMed] [Google Scholar]
- 16.Yu JA, Deakin NO, Turner CE. Emerging role of paxillin-PKL in regulation of cell adhesion, polarity and migration. Cell Adh Migr. 2010;4:342–7. doi: 10.4161/cam.4.3.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torii T, Miyamoto Y, Sanbe A, Nishimura K, Yamauchi J, Tanoue A. Cytohesin-2/ARNO, through its interaction with focal adhesion adaptor protein paxillin, regulates preadipocyte migration via the downstream activation of Arf6. J Biol Chem. 2010;285:24270–81. doi: 10.1074/jbc.M110.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiczka KS, Yeaman C. Ral-regulated interaction between Sec5 and paxillin targets Exocyst to focal complexes during cell migration. J Cell Sci. 2008;121:2880–91. doi: 10.1242/jcs.031641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen GC, Lee JY, Tang HW, Debnath J, Thomas SM, Settleman J. Genetic interactions between Drosophila melanogaster Atg1 and paxillin reveal a role for paxillin in autophagosome formation. Autophagy. 2008;4:37–45. doi: 10.4161/auto.5141. [DOI] [PubMed] [Google Scholar]
- 20.Jamieson JS, Tumbarello DA, Hallé M, Brown MC, Tremblay ML, Turner CE. Paxillin is essential for PTP-PEST-dependent regulation of cell spreading and motility: a role for paxillin kinase linker. J Cell Sci. 2005;118:5835–47. doi: 10.1242/jcs.02693. [DOI] [PubMed] [Google Scholar]
- 21.Gu Z, Noss EH, Hsu VW, Brenner MB. Integrins traffic rapidly via circular dorsal ruffles and macropinocytosis during stimulated cell migration. J Cell Biol. 2011;193:61–70. doi: 10.1083/jcb.201007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azimifar SB, Böttcher RT, Zanivan S, Grashoff C, Krüger M, Legate KR, et al. Induction of membrane circular dorsal ruffles requires co-signalling of integrin-ILK-complex and EGF receptor. J Cell Sci. 2012;125:435–48. doi: 10.1242/jcs.091652. [DOI] [PubMed] [Google Scholar]
- 23.Monypenny J, Zicha D, Higashida C, Oceguera-Yanez F, Narumiya S, Watanabe N. Cdc42 and Rac family GTPases regulate mode and speed but not direction of primary fibroblast migration during platelet-derived growth factor-dependent chemotaxis. Mol Cell Biol. 2009;29:2730–47. doi: 10.1128/MCB.01285-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–57. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 25.Pignatelli J, Tumbarello DA, Schmidt RP, Turner CE. Hic-5 promotes invadopodia formation and invasion during TGF-β-induced epithelial-mesenchymal transition. J Cell Biol. 2012;197:421–37. doi: 10.1083/jcb.201108143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West KA, Zhang H, Brown MC, Nikolopoulos SN, Riedy MC, Horwitz AF, et al. The LD4 motif of paxillin regulates cell spreading and motility through an interaction with paxillin kinase linker (PKL) J Cell Biol. 2001;154:161–76. doi: 10.1083/jcb.200101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, et al. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–8. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salgia R, Li JL, Ewaniuk DS, Wang YB, Sattler M, Chen WC, et al. Expression of the focal adhesion protein paxillin in lung cancer and its relation to cell motility. Oncogene. 1999;18:67–77. doi: 10.1038/sj.onc.1202273. [DOI] [PubMed] [Google Scholar]
- 29.Shi J, Wang S, Zhao E, Shi L, Xu X, Fang M. Paxillin expression levels are correlated with clinical stage and metastasis in salivary adenoid cystic carcinoma. J Oral Pathol Med. 2010;39:548–51. doi: 10.1111/j.1600-0714.2009.00859.x. [DOI] [PubMed] [Google Scholar]
- 30.Mammoto T, Mammoto A, Torisawa YS, Tat T, Gibbs A, Derda R, et al. Mechanochemical control of mesenchymal condensation and embryonic tooth organ formation. Dev Cell. 2011;21:758–69. doi: 10.1016/j.devcel.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gratsch TE, De Boer LS, O’Shea KS. RNA inhibition of BMP-4 gene expression in postimplantation mouse embryos. Genesis. 2003;37:12–7. doi: 10.1002/gene.10221. [DOI] [PubMed] [Google Scholar]
- 32.Eckes B, Colucci-Guyon E, Smola H, Nodder S, Babinet C, Krieg T, et al. Impaired wound healing in embryonic and adult mice lacking vimentin. J Cell Sci. 2000;113:2455–62. doi: 10.1242/jcs.113.13.2455. [DOI] [PubMed] [Google Scholar]