Abstract
Integrin-dependent and -independent MMP-9 and uPAR signaling plays a key role in glioma cell migration and invasion. In this article, we comment on all the possible pathways and molecules associated with MMP-9- and uPAR-mediated glioma cell migration with a special emphasis on integrins, a family of cell adhesion molecules. Our recent research investigations highlighted the substantial benefit of silencing both MMP-9 and uPAR together compared with their individual treatments in glioma. Simultaneous knockdown of both MMP-9 and uPAR regulated a majority of the molecules associated with glioma cell migration and significantly reduced the migration potential of glioma cells. Our results point out that the bicistronic construct, which can simultaneously silence both MMP-9 and uPAR offers a great therapeutic potential and is worth developing as a new drug for treating GBM patients.
Keywords: MMP-9, uPAR, glioma, integrin, migration, invasion, knockdown, shRNA
Cancer cell migration and invasion are initial steps in metastasis, which is a primary cause of cancer-related death. Strategies to treat infiltrating gliomas, such as chemotherapy and gene therapy, have remained largely unsuccessful and the property that makes glioma resistant to treatment is the tendency of the tumor cells to invade normal brain tissue.1 Approximately 60% of all primary brain tumors in adults are malignant gliomas (anaplastic astrocytoma, anaplastic oligodendroglioma and glioblastoma multiforme). Glioblastoma multiforme (GBM) is the most common and highly aggressive malignant neoplasm of the central nervous system. GBM cells secrete matrix metalloproteinases (MMPs). A significant correlation between MMP-9 levels and the histological grade of malignancy has already been reported.2-5 Our recent studies clearly demonstrated the role of MMP-9 and the associated molecular mechanisms in cancer cell migration.6-9
In the context of cell motility, the extracellular matrix (ECM) is both a requirement and a physical barrier for cell movement. The ECM provides physical support and organization to tissues. It is a complex assembly of proteins and polysaccharides that are secreted, assembled and modeled by cells. A well-defined brain ECM exists in the form of a true basement membrane, cerebral vasculature and the glial limitans externa. The cerebral vascular basement membrane, which surrounds the blood vessels of the brain, contains type-IV and type-V collagens, laminin, fibronectin and heparan-sulfate proteoglycans.10 Type IV collagen and laminin, which are mainly present in the capillaries and large blood vessels, are the main constituents of most basement membranes. Laminin describes a large group of adhesion glycoproteins that are found in all basement membranes and in hyperplastic blood vessels in gliomas, gliosarcomas and meningiomas, as an integral part of the glial limitans externa. Fibronectin is found at the gliomesenchymal junction of tumors and in tumor-associated blood vessels. Advanced stages of glioblastoma have been shown to express vitronectin, a component of the ECM that is usually absent from normal brain and early-stage gliomas. Tenascin-C, another ECM proteoglycan, is synthesized by glial and neural-crest cells, as well as by satellite cells of the peripheral nervous system. Cells express plasma membrane receptors such as integrins, a family of cell adhesion molecules that bind to ECM components. Cell migration therefore often involves the coordination of ECM proteolysis, adhesion and signaling. Integrins are involved in interactions between the cell and the surrounding ECM and play a central role in cell migration. Integrins expressed in tumor cells contribute to tumor progression and metastasis by increasing tumor cell migration, invasion, proliferation and survival.11 Interactions between integrins expressed by glioma cells and the ECM and the activity of MMPs form the basis for glioma cell migration and invasion.12
Similar to MMP-9, the expression of urokinase-type plasminogen activator receptor (uPAR) is much more robust in high-grade than in low-grade human gliomas.13 Localization of uPAR mRNA in astrocytoma cells and the endothelial cells within brain tumor tissue has been reported.13 uPAR regulates proteolysis by binding the extracellular protease uPA and also activates many intracellular signaling pathways.14 Coordination of uPAR with ECM proteolysis and cell signaling underlies its important function in cell migration, proliferation and survival. The most important transmembrane receptors associated with uPAR signaling are the integrin family of ECM receptors. Integrins are essential uPAR signaling co-receptors and the interactions between uPAR-β1 and uPAR-β3 have an important role in signaling for cell migration and invasion.14 uPAR localizes to integrin-containing adhesion complexes and co-immunoprecipitates with integrins and integrin-associated signaling molecules such as FAK and Src family kinases.15-21 uPAR-β1 integrin interactions are associated with the activation of FAK and ERK, whereas uPAR-β3 integrin interactions are associated with the activation of Rac.14 uPAR-β1 integrin signaling to ERK and Src increases the expression of uPA and MMPs through AP1 transcription factors.22-24 Although the activation of ERK by uPAR has been considered primarily to promote cell proliferation or protease expression, myosin light chain kinase (MLCK), the cytoplasmic ERK target can regulate cell motility. uPAR signaling activates MLCK and contributes to induction of cell motility in human tumor cells.25 uPAR-β3 integrin interaction has an important role in signaling for cell migration through activation of the Rho family small GTPase Rac. Blockade of the uPAR function in tumor cell lines inhibits Rac activation and cell motility, whereas ectopic expression of uPAR drives Rac activation.14 uPAR and integrin-driven activation of Rac allows assembly of filamentous actin (F-actin)-containing membrane protrusions that extend the leading edge of the cell forward, while pericellular proteolysis removes ECM barriers that would impede the extension of these protrusions.
Upregulation of α2β1, α3β1, α5β1, αVβ3 and α6β1 integrins on GBM have already been reported.26,27 Collagens, fibronectin, laminin, vitronectin, invasin, osteopontin, prothrombin and thrombospondin serve as extracellular ligands for these integrins.28 It was recently found that α9β1 integrin played a significant role in the progression of glioblastoma.29 Integrin α9β1 is classified within a two member sub-family of integrins highlighted in part by its specialized role in cell migration.30 Tenascin is a ligand for α9β1 integrin. α9β1 has distinguished its functionality from other integrins by facilitating accelerated cell migration.31 Unlike other integrin heterodimers, α9β1 was able to both increase cell migration and inhibit cell spreading.32,33 Our recent studies clearly demonstrated the role played by α9β1 integrin in glioma cell migration.6,8 α9β1 ligation can activate signaling through Src and FAK-mediated tyrosine phosphorylation of multiple proteins including p130Cas and paxillin.33,34 Unlike other integrins, α9β1 has been proposed to utilize inducible nitric oxide synthase (iNOS)-nitric oxide (NO) and spermidine/spermine acetyl transferase (SSAT)-inward rectifier potassium channel (Kir) pathways along with common integrin signaling proteins such as Src and FAK to transduce cell migration.30
Recently, we have reported the physical interactions that exist among uPAR, MMP-9, β1 integrin, α9 integrin and SSAT in the context of glioma cell migration.8,35 Cooperation between MMP-9 and integrins is known to activate αVβ3, which strongly enhanced tumor migration.36 Further, uPAR knockdown in glioma cells reduced the expression of αVβ3 and associated glioma cell migration.37 Although the interaction of uPAR with integrins is well reported by several investigators, the physical association of MMP-9 with these molecules remains unclear. In addition to mediating glioma cell migration via integrins, MMP-9 acts as a processing enzyme for CD44 cleavage.9 CD44 is a single chain, transmembrane glycoprotein that is widely expressed in physiological and pathological conditions. CD44 is implicated in cell-cell and cell-matrix adhesion, migration and signaling. CD44 expression is prominent in GBM tissue samples.9 In addition, we noticed a strong physical interaction between MMP-9 and CD44. Direct interaction of MMP-9 with CD44 promotes cleavage of the later into extracellular and intracellular domains that are involved in glioma cell migration and adhesion, respectively. It was suggested that the cleaved extracellular domain of CD44 induces cell crawling at the leading edge on a hyaluronic acid matrix, along with lamellipod extension which induces mechanical stretching of cells, triggering extracellular calcium ion flux through stretch-activated calcium channels.38 MMP-9 knockdown in these glioma cells inhibited MMP-9-mediated proteolytic cleavage of CD44. Therefore, the reduced glioma cell migration after MMP-9 knockdown could also attribute to the inhibition of CD44 cleavage. In this scenario, it appears that the simultaneous knockdown of both MMP-9 and uPAR offers a substantial reduction in cancer cell migration compared with their individual knockdowns.
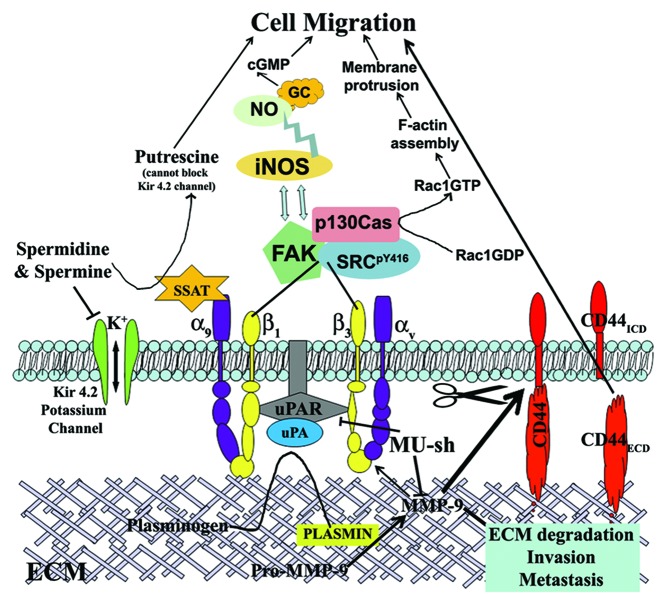
In the recent past, we reported that the transcriptional inactivation of both MMP-9 and uPAR in combination by shRNA-mediated gene silencing offered a prominent and significant reduction in glioma cell migration and invasion.6 The reduced glioma cell migration could be attributed to the regulation of several pathways and molecules associated with cell migration, which are downstream to both MMP-9 and uPAR (Fig. 1). MMP-9 and uPAR knockdown in glioma cells reduced FAK, Src and F-actin expressions.6 In addition, the combined inhibition of MMP-9 and uPAR reduced SSAT expression in glioma cells that results in the elevated intracellular levels of spermidine and spermine with subsequent blockade of Kir 4.2 potassium channel.8 Although the study revealed the involvement of SSAT-potassium channel pathway in glioma cell migration mediated by MMP-9 and uPAR, we failed to show that it is directly mediated via α9β1 integrin. However, in our earlier study, we could show that the blockade of α9β1 integrin significantly inhibited the increased migration potential of glioma cells in MMP-9/uPAR overexpressed cells. Although part of the inhibition in migration potential after α9β1 integrin blockade is attributed to SSAT-potassium channel pathway involvement, we cannot rule out the possibility of α9β1-iNOS pathway involvement in MMP-9-/uPAR-mediated glioma cell migration. Our future studies will elucidate the significance of α9β1-iNOS pathway involvement in MMP-9-/uPAR-mediated cancer cell migration. In addition to the regulation of cancer cell migration and invasion, combined inhibition of MMP-9 and uPAR by gene silencing technology reduced glioma cell proliferation, tumor growth and angiogenesis and induced apoptosis.6,8,39-42 Taken together, simultaneous inhibition of MMP-9 and uPAR by plasmid shRNA construct (pMU or MU-sh) significantly inhibited cancer cell migration by controlling all the possible mechanisms, and this construct appears to have a great therapeutic potential to develop as a new drug for treating GBM patients.
Figure 1. Schematic presentation of the possible role and the mechanisms by which MMP-9/uPAR plasmid shRNA (MU-sh) regulate glioma cell migration. ECM, extracellular matrix; ECD, extracellular domain; ICD, intracellular domain; SSAT, spermidine/spermine-N1-acetyl transferase; GC, guanylyl cyclase; NO, nitric oxide; NOS, nitric oxide synthase.
Acknowledgments
We thank Debbie McCollum for manuscript preparation and Diana Meister and Sushma Jasti for manuscript review. This research was supported by a grant from National Institute of Neurological Disorders and Stroke (NINDS), NS047699 (to J.S.R.). The contents are solely the responsibility of the authors and do not necessarily represent the official views of National Institutes of Health. The funders had no role in preparation of the manuscript or decision to publish.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/21673
References
- 1.Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery. 1996;39:235–50, discussion 250-2. doi: 10.1097/00006123-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Kachra Z, Beaulieu E, Delbecchi L, Mousseau N, Berthelet F, Moumdjian R, et al. Expression of matrix metalloproteinases and their inhibitors in human brain tumors. Clin Exp Metastasis. 1999;17:555–66. doi: 10.1023/A:1006760632766. [DOI] [PubMed] [Google Scholar]
- 3.Raithatha SA, Muzik H, Muzik H, Rewcastle NB, Johnston RN, Edwards DR, et al. Localization of gelatinase-A and gelatinase-B mRNA and protein in human gliomas. Neuro Oncol. 2000;2:145–50. doi: 10.1093/neuonc/2.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rooprai HK, Van Meter T, Rucklidge GJ, Hudson L, Everall IP, Pilkington GJ. Comparative analysis of matrix metalloproteinases by immunocytochemistry, immunohistochemistry and zymography in human primary brain tumours. Int J Oncol. 1998;13:1153–7. doi: 10.3892/ijo.13.6.1153. [DOI] [PubMed] [Google Scholar]
- 5.Rao JS, Steck PA, Mohanam S, Stetler-Stevenson WG, Liotta LA, Sawaya R. Elevated levels of M(r) 92,000 type IV collagenase in human brain tumors. Cancer Res. 1993;53(Suppl):2208–11. [PubMed] [Google Scholar]
- 6.Veeravalli KK, Chetty C, Ponnala S, Gondi CS, Lakka SS, Fassett D, et al. MMP-9, uPAR and cathepsin B silencing downregulate integrins in human glioma xenograft cells in vitro and in vivo in nude mice. PLoS One. 2010;5:e11583. doi: 10.1371/journal.pone.0011583. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Nalla AK, Gorantla B, Gondi CS, Lakka SS, Rao JS. Targeting MMP-9, uPAR, and cathepsin B inhibits invasion, migration and activates apoptosis in prostate cancer cells. Cancer Gene Ther. 2010;17:599–613. doi: 10.1038/cgt.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veeravalli KK, Ponnala S, Chetty C, Tsung AJ, Gujrati M, Rao JS. Integrin α9β1-mediated cell migration in glioblastoma via SSAT and Kir4.2 potassium channel pathway. Cell Signal. 2012;24:272–81. doi: 10.1016/j.cellsig.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chetty C, Vanamala SK, Gondi CS, Dinh DH, Gujrati M, Rao JS. MMP-9 induces CD44 cleavage and CD44 mediated cell migration in glioblastoma xenograft cells. Cell Signal. 2012;24:549–59. doi: 10.1016/j.cellsig.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- 11.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldbrunner RH, Bernstein JJ, Tonn JC. ECM-mediated glioma cell invasion. Microsc Res Tech. 1998;43:250–7. doi: 10.1002/(SICI)1097-0029(19981101)43:3<250::AID-JEMT7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M, Sawaya R, Mohanam S, Rao VH, Bruner JM, Nicolson GL, et al. Expression and localization of urokinase-type plasminogen activator receptor in human gliomas. Cancer Res. 1994;54:5016–20. [PubMed] [Google Scholar]
- 14.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11:23–36. doi: 10.1038/nrm2821. [DOI] [PubMed] [Google Scholar]
- 15.Aguirre Ghiso JA. Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene. 2002;21:2513–24. doi: 10.1038/sj.onc.1205342. [DOI] [PubMed] [Google Scholar]
- 16.Degryse B, Resnati M, Czekay RP, Loskutoff DJ, Blasi F. Domain 2 of the urokinase receptor contains an integrin-interacting epitope with intrinsic signaling activity: generation of a new integrin inhibitor. J Biol Chem. 2005;280:24792–803. doi: 10.1074/jbc.M413954200. [DOI] [PubMed] [Google Scholar]
- 17.Myöhänen HT, Stephens RW, Hedman K, Tapiovaara H, Rønne E, Høyer-Hansen G, et al. Distribution and lateral mobility of the urokinase-receptor complex at the cell surface. J Histochem Cytochem. 1993;41:1291–301. doi: 10.1177/41.9.8394852. [DOI] [PubMed] [Google Scholar]
- 18.Wei Y, Eble JA, Wang Z, Kreidberg JA, Chapman HA. Urokinase receptors promote beta1 integrin function through interactions with integrin alpha3beta1. Mol Biol Cell. 2001;12:2975–86. doi: 10.1091/mbc.12.10.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohuslav J, Horejsí V, Hansmann C, Stöckl J, Weidle UH, Majdic O, et al. Urokinase plasminogen activator receptor, beta 2-integrins, and Src-kinases within a single receptor complex of human monocytes. J Exp Med. 1995;181:1381–90. doi: 10.1084/jem.181.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 1999;147:89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carriero MV, Del Vecchio S, Capozzoli M, Franco P, Fontana L, Zannetti A, et al. Urokinase receptor interacts with alpha(v)beta5 vitronectin receptor, promoting urokinase-dependent cell migration in breast cancer. Cancer Res. 1999;59:5307–14. [PubMed] [Google Scholar]
- 22.Tang CH, Hill ML, Brumwell AN, Chapman HA, Wei Y. Signaling through urokinase and urokinase receptor in lung cancer cells requires interactions with beta1 integrins. J Cell Sci. 2008;121:3747–56. doi: 10.1242/jcs.029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh S, Johnson JJ, Sen R, Mukhopadhyay S, Liu Y, Zhang F, et al. Functional relevance of urinary-type plasminogen activator receptor-alpha3beta1 integrin association in proteinase regulatory pathways. J Biol Chem. 2006;281:13021–9. doi: 10.1074/jbc.M508526200. [DOI] [PubMed] [Google Scholar]
- 24.Wei Y, Tang CH, Kim Y, Robillard L, Zhang F, Kugler MC, et al. Urokinase receptors are required for alpha 5 beta 1 integrin-mediated signaling in tumor cells. J Biol Chem. 2007;282:3929–39. doi: 10.1074/jbc.M607989200. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen DH, Catling AD, Webb DJ, Sankovic M, Walker LA, Somlyo AV, et al. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. J Cell Biol. 1999;146:149–64. doi: 10.1083/jcb.146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gingras MC, Roussel E, Bruner JM, Branch CD, Moser RP. Comparison of cell adhesion molecule expression between glioblastoma multiforme and autologous normal brain tissue. J Neuroimmunol. 1995;57:143–53. doi: 10.1016/0165-5728(94)00178-Q. [DOI] [PubMed] [Google Scholar]
- 27.Mahesparan R, Read TA, Lund-Johansen M, Skaftnesmo KO, Bjerkvig R, Engebraaten O. Expression of extracellular matrix components in a highly infiltrative in vivo glioma model. Acta Neuropathol. 2003;105:49–57. doi: 10.1007/s00401-002-0610-0. [DOI] [PubMed] [Google Scholar]
- 28.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–8. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 29.Brown MC, Staniszewska I, Lazarovici P, Tuszynski GP, Del Valle L, Marcinkiewicz C. Regulatory effect of nerve growth factor in alpha9beta1 integrin-dependent progression of glioblastoma. Neuro Oncol. 2008;10:968–80. doi: 10.1215/15228517-2008-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta SK, Vlahakis NE. Integrin alpha9beta1: Unique signaling pathways reveal diverse biological roles. Cell Adh Migr. 2010;4:194–8. doi: 10.4161/cam.4.2.10900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, et al. Integrin alpha9beta1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis. J Biol Chem. 2007;282:15187–96. doi: 10.1074/jbc.M609323200. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Slepak M, Ginsberg MH. Binding of Paxillin to the alpha 9 Integrin Cytoplasmic Domain Inhibits Cell Spreading. J Biol Chem. 2001;276:37086–92. doi: 10.1074/jbc.M105114200. [DOI] [PubMed] [Google Scholar]
- 33.Young BA, Taooka Y, Liu S, Askins KJ, Yokosaki Y, Thomas SM, et al. The cytoplasmic domain of the integrin alpha9 subunit requires the adaptor protein paxillin to inhibit cell spreading but promotes cell migration in a paxillin-independent manner. Mol Biol Cell. 2001;12:3214–25. doi: 10.1091/mbc.12.10.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta SK, Vlahakis NE. Integrin alpha9beta1 mediates enhanced cell migration through nitric oxide synthase activity regulated by Src tyrosine kinase. J Cell Sci. 2009;122:2043–54. doi: 10.1242/jcs.041632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponnala S, Veeravalli KK, Chetty C, Dinh DH, Rao JS. Regulation of DNA repair mechanism in human glioma xenograft cells both in vitro and in vivo in nude mice. PLoS One. 2011;6:e26191. doi: 10.1371/journal.pone.0026191. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B. Activated integrin alphavbeta3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:9482–7. doi: 10.1073/pnas.1633689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adachi Y, Lakka SS, Chandrasekar N, Yanamandra N, Gondi CS, Mohanam S, et al. Down-regulation of integrin alpha(v)beta(3) expression and integrin-mediated signaling in glioma cells by adenovirus-mediated transfer of antisense urokinase-type plasminogen activator receptor (uPAR) and sense p16 genes. J Biol Chem. 2001;276:47171–7. doi: 10.1074/jbc.M104334200. [DOI] [PubMed] [Google Scholar]
- 38.Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004;95:930–5. doi: 10.1111/j.1349-7006.2004.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lakka SS, Gondi CS, Yanamandra N, Dinh DH, Olivero WC, Gujrati M, et al. Synergistic down-regulation of urokinase plasminogen activator receptor and matrix metalloproteinase-9 in SNB19 glioblastoma cells efficiently inhibits glioma cell invasion, angiogenesis, and tumor growth. Cancer Res. 2003;63:2454–61. [PubMed] [Google Scholar]
- 40.Lakka SS, Gondi CS, Dinh DH, Olivero WC, Gujrati M, Rao VH, et al. Specific interference of urokinase-type plasminogen activator receptor and matrix metalloproteinase-9 gene expression induced by double-stranded RNA results in decreased invasion, tumor growth, and angiogenesis in gliomas. J Biol Chem. 2005;280:21882–92. doi: 10.1074/jbc.M408520200. [DOI] [PubMed] [Google Scholar]
- 41.Gondi CS, Dinh DH, Gujrati M, Rao JS. Simultaneous downregulation of uPAR and MMP-9 induces overexpression of the FADD-associated protein RIP and activates caspase 9-mediated apoptosis in gliomas. Int J Oncol. 2008;33:783–90. [PMC free article] [PubMed] [Google Scholar]
- 42.Chetty C, Lakka SS, Bhoopathi P, Gondi CS, Veeravalli KK, Fassett D, et al. Urokinase plasminogen activator receptor and/or matrix metalloproteinase-9 inhibition induces apoptosis signaling through lipid rafts in glioblastoma xenograft cells. Mol Cancer Ther. 2010;9:2605–17. doi: 10.1158/1535-7163.MCT-10-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]