Abstract
Cell migration is a critical step of normal developmental processes and disease progression. Often, migrating cells interact and maintain contact with neighboring cells. However, the precise roles of cell-cell adhesion in cell migration have thus far been poorly defined. Often in aggressive cancers, N-cadherin is prominently upregulated, yet, these highly motile cells have limited cell-cell adhesion when plated on a stiff 2D substrate. But, the same cells in a 3D matrix migrate as a multicellular cluster. This new observation suggests that N-cadherin-mediated cell-cell adhesion supports cell interactions between migrating cells in a more physiologically relevant 3D matrix, but not on a 2D substrate. While N-cadherin is an integral part of neural synapses, the ectopic expression of N-cadherin in transformed epithelial cells plays an equally important part in initiating pro-migratory signaling, and providing strong yet flexible cell cohesion essential for persistent cell migration in a 3D matrix. The 3D cell migration analysis for studying cell-to-cell interactions exposes the roles of N-cadherin in multicellular migration, and reveals novel insights into cell migration-dependent normal and pathological processes.
Keywords: 3D matrix, Epithelial-to-mesenchymal transition, N-cadherin, cell migration, cell-cell adhesion
Introduction
All multicellular organisms depend on cell movement as a driving force for embryogenesis, tissue remodel and repair. Migrating cells maintain contact with neighboring cells, which is thought to provide a spatial cue for collective cell migration. Even the earliest study in cell migration of precardiac mesoderm cell clusters of a chick embryo suggested the importance of cell-cell junctions for cohesive cell movement.1 The underlying mechanism of cell-cell adhesion is supported by a large collection of cell-cell adhesion proteins,2 which in turn provides the selective cell-to-cell interactions necessary for cell rearrangement in tissues.3 While the detailed mechanisms of single cell migration have been explored, little is known about the mechanisms and regulation of cell-cell adhesion during collective cell migration.
Traditional strategies for studying cell migration have focused on examining migrating cells on a two-dimensional (2D) surface. Although flattened cells on a 2D surface provide better visualization for fine cytoskeletal structures, these stiff substrates are not an ideal surface for studying cell-to-cell interactions of migrating cells. On a 2D substrate, highly migratory cells frequently detach and scatter from their neighbors (Fig. 1),4 whereas in a 3D matrix, the same cells maintain cell-cell contacts and migrate collectively as a cell cluster (Fig. 1),5 This unique collective cell morphology is likely due to the soft 3D matrix (relative to a stiff coverslip) that dissipates or absorbs the traction force exerted on the matrix. With effective cellular traction force reduced in the soft matrix (e.g., collagen or Matrigel™ matrix) migrating cells are thus prevented from moving away from each other. By examining cell migration in a more physiological 3D matrix, the roles of cell-cell adhesion in this collective cell movement are exposed.
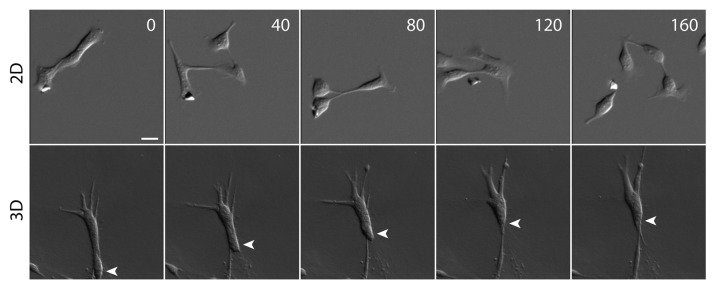
Figure 1. Cell migration phenotype on 2D surface and in 3D matrix. While migrating cells detach frequently from neighboring cells on a collagen-coated 2D surface, migrating cells maintain cell-cell contacts in a 3D collagen matrix. The cells are hepatocyte growth factor (HGF)-treated MDCK epithelial cells that have undergone a complete EMT. White arrowhead tracks the position of a migrating cell. Scale bar, 20 μm. Time in minutes.
The question remains as to which molecules or types of cell-cell junctions facilitate cell-to-cell interactions between migratory epithelial cells. During developmental processes and cancer metastasis, epithelial cells alter their gene expression profile and lose their typical epithelial morphology to adopt a more invasive, mesenchymal phenotype, a process known as epithelial-to-mesenchymal transition (EMT). This morphological transformation is accompanied by the loss of typical epithelial cell-cell junctions and the downregulation of E-cadherin, but in its place, the upregulation of N-cadherin. This cadherin switching is not limited to E-to-N cadherin and epithelial cells.6 For example, neural crest cells lose their typical N-cadherin expression, and instead express cadherin-6B and cadherin-7 when entering a more migratory state.7 Therefore, depending on whether it is natively or ectopically expressed, the same cadherins may play different roles in embryonic development, cell differentiation and cancer cell invasion. In cancer, the switch from E-cadherin to N-cadherin expression is a hallmark of cancer progression and is often observed in metastatic tumors.6,8 Therefore, this newly upregulated N-cadherin emerged as a potential regulator of collective cancer cell migration.
N-Cadherin-Mediated Pro-Migratory Signaling
The role of N-cadherin in collective cell migration is not limited to adhesive roles. For example, N-cadherin-deficient cells do not form cell clusters, and the resultant single cells in the 3D matrix have a round shape and do not migrate.5 This is consistent with previous studies which showed that transient expression of N-cadherin in breast carcinoma cells increases cell motility and invasiveness in a Boyden chamber,9,10 and the increased motility observed in various breast carcinoma cell lines is independent of E-cadherin levels.10 Interestingly, this immobile phenotype of N-cadherin-deficient cells is not due to the lack of cell-cell adhesion since calcium removal disrupts cell-cell adhesion between N-cadherin expressing invasive cells in 3D matrix, yet the individual cells continue to migrate independently of their neighboring cells.5 This suggests that disruption of cell-cell adhesion does not completely halt cell migration and that the roles of N-cadherin in cell migration is not limited to supporting cell-to-cell interactions.
Which domain of N-cadherin is important for pro-migratory signaling? The exogenous expression of the cytoplasmic or extracellular domain of N-cadherin induces cell migration in N-cadherin deficient cells (Fig. 2A),5 suggesting that both the cytoplasmic and extracellular domain promote cell migration, but presumably through independent mechanisms. Previous studies have shown that the N-cadherin extracellular domain directly interacts with the fibroblast growth factor (FGF) receptor to regulate the mitogen-activated protein kinase-extracellular signal regulated kinase (MAPK-ERK) pathway (Fig. 2B),11 a signaling pathway that correlates with MMP production and cell invasion.12 In addition, N-cadherin-expression suppresses Akt3 expression and phosphorylation, which promotes cell migration,13 although the detailed pathway is still unclear.
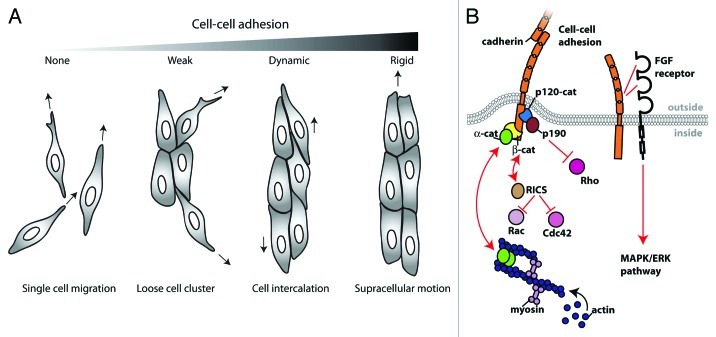
Figure 2. N-cadherin-mediated cell-cell adhesion regulates collective cell migration. (A) Cell clusters adopt different migration phenotypes depending on the strength of N-cadherin mediated cell-cell adhesion. In the absence of N-cadherin-mediated cell-cell adhesion (e.g., the extracellular domain deleted N-cadherin mutant expressing cells), the cells are migratory but remain single cells. With weak cell-cell adhesion (e.g., the cytoplasmic deleted N-cadherin mutant cells), migratory cells often form cell clusters but cell-cell interactions are transient. In wildtype cells, cell-cell adhesion is strong enough to minimize cell scattering, but dynamic enough to support cell intercalation within cell clusters. The expression of an N-cadherin-α-catenin chimera induces strong cell-cell adhesion that prevents cell intercalation, but promotes supracellular movement of the entire cluster. (B) Model depicting different pathways through which N-cadherin can regulate cell migration. N-cadherin 4th extracellular domain has been shown to interact with fibroblast growth factor receptor (FGF receptor), which is involved in the pro-migratory MAPK/ERK pathway. N-cadherin cytoplasmic domain interacts with binding partners such as β-catenin (β-cat), which interacts with α-catenin (α-cat), a molecule that can bind and organize the actin network. Furthermore, N-cadherin cytoplasmic domain may be regulating cell migration through the regulation of GTPase activity. N-cadherin binding partner, β-catenin also binds RICS, a GTPase activating protein for cdc42 and Rac1. Or, N-cadherin binding partner, p120-catenin (p120-cat) binds p190RhoGAP (p190), which is a GTPase activating protein for RhoA.
The cytoplasmic domain of cadherins regulates Rho GTPases, and this regulation is cadherin type-specific. Unlike the formation of E-cadherin junctions that activates Rac1 and cdc42,14,15 the formation of N-cadherin junctions activates RhoA, but not Rac1 and cdc42 in myoblasts.16 Furthermore, previous studies have shown that p120-catenin in the N-cadherin complex of fibroblasts associates with a RhoA-specific GTPase activating protein, p190RhoGAP,17 while β-catenin in the postsynaptic density of neurons interacts with RICS (RhoGAP involved in the β-catenin-N-cadherin and NMDA receptor signaling), a GTPase-activating protein specific to cdc42 and Rac1 GTPases (Fig. 2B).18 Since these previous studies often focused on specialized cells that natively express N-cadherin, and only analyzed cells on a 2D substrate, additional tests are necessary to verify whether highly invasive post EMT cells in a 3D environment use similar Rho GTPase regulations.
One key function of N-cadherin is establishing cell polarity by limiting protrusive activity at cell-cell contacts, which is critical for persistent, multicellular movement. In a 3D matrix, disruption of N-cadherin-mediated cell-cell adhesion results in the dissociation of cell clusters, and the resultant individual cells extend protrusions in multiple directions causing them to no longer migrate together in a single direction.5 Similarly, during Xenopus embryogenesis, neural cell cluster dissociation by calcium removal or N-cadherin inhibition results in cells extending protrusions on top of each other, the loss of Rac1 activity at the cell’s free edge and significantly reduced efficiency of chemotactic migration.19 Consistent with this, hippocampal neurons lacking αN-catenin, an actin regulator in the cadherin complex, results in hyperactive filopodia protrusions from the spine heads in contact with axons.20 These data indicate that cadherin mediated cell-cell adhesion may be required for the suppression of membrane protrusion, thus promoting collective cell polarization, and migration of the cluster in the same direction.
The Regulation of N-Cadherin-Mediated Cell-Cell Adhesion
The physical adhesion between migratory cells must be strong to withstand external forces exerted by neighboring migratory cells. N-cadherin knockdown cells expressing the extracellular domain of N-cadherin are able to form small clusters, but the cells frequently dissociate from cell clusters compared with wild-type cells in a 3D matrix (Fig. 2A).5 This suggests that although the extracellular domain of N-cadherin may be sufficient for initial cell adhesion between cells, but the N-cadherin cytoplasmic domain is important for strengthening the adhesion, presumably by regulating the actin cytoskeleton in the vicinity of N-cadherin junctions (Fig. 2B).
The cadherin cytoplasmic domain is the key regulator of strength and structural integrity of cell-cell junctions in multicellular structures through its regulation of the actin cytoskeleton. For example, when the N-cadherin-deficient cells are rescued with an N-cadherin and α-catenin fusion protein to artificially promote N-cadherin and actin interaction, these rescued cells do not move or intercalate within the cell cluster compared with cells expressing wild-type N-cadherin (Fig. 2A).5 If this N-cadherin chimera is providing a stable cadherin-actin linkage, then the dynamics of the cadherin-actin interactions play a crucial role in cell intercalation. Previous in vitro biochemical analysis has shown that α-catenin does not bind the E-cadherin complex and the actin cytoskeleton simultaneously,21 but rather binds and regulates actin filament organization independently of its interaction with the cadherin complex (Fig. 2B).22 Therefore, a simple static model of the cadherin-actin linkage via α-catenin is unrealistic and the cadherin-actin interaction is most likely highly dynamic in live cells.
It is possible that this dynamic cadherin-actin linkage may be temporally strengthened to withstand intermittent forces exerted by neighboring cells. For example, E-cadherin-mediated cell-cell junctions stiffen in response to applied shear stress and this mechano-regulation of cadherin junctions depends on vinculin.23 This mechano-sensitive property of cadherin junctions may be in part regulated via a conformational change in α-catenin. Under a force-free condition (e.g., in the presence of myosin II inhibitor), α-catenin is in a closed conformation that masks the vinculin binding site, whereas under a force-bearing condition, α-catenin is in an open conformation with an accessible vinculin binding domain.24 Accordingly, vinculin localization to cell-cell junctions depends on the external forces.24,25
Another potential force-responsive regulating protein at cadherin junctions is zyxin.26 In a 3D matrix, zyxin localizes to the sites of force-bearing N-cadherin junctions between transformed epithelial cells (Fig. 3). Zyxin is recruited to actin stress fibers upon uniaxial stretching of cells on a compliant substrate27 or through the application of external force on the cell dorsal surface.28,29 Furthermore, zyxin responds to not only external forces as previously shown but also internal actin-myosin contractile forces.30 In addition, stretch-induced actin polymerization at focal adhesion sites is dependent on zyxin and its interaction with the actin-binding Ena/VASP proteins.31 Therefore, in response to forces exerted by migratory cells, the mechano-sensitive cadherin complex may be regulating its interaction with the actin cytoskeleton through various adaptor proteins, which in turn generates a unique actin network in collectively migrating cells.
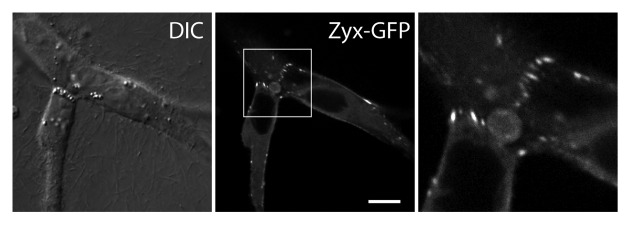
Figure 3. Zyxin accumulates at sites of force-bearing N-cadherin mediated cell-cell junctions in transformed epithelial cells migrating in a 3D matrix. Zyxin-GFP expressing MDCK cells are embedded in a collagen gel, and imaged using a confocal microscope. Zyxin accumulates as focal adhesion-like puncta at cell-cell contacts in a 3D matrix. Scale bar, 10 μm.
In transformed epithelial cells, clustered cells organize actin into bundles of stress fibers that are aligned parallel to the direction of cell elongation and traction force, and appear to terminate at cell-cell junctions.5 N-cadherin-mediated cell-cell junctions may be important for connecting these actin bundles and coordinating the actin organization continuously across multiple cells within a cluster. Consistently, a supracellular organization of actin has also been described in other systems including radial actin fibers, which form a network across epithelial sheets,32 and cortical actin that extends across multiple melanoma cells within a cluster.33 In addition, for the collective motion of cell clusters on a 2D substrate, myosin II activity at the sites of cell-cell adhesion is downregulated.34 This unique actin-myosin II organization across multiple cells is likely important for the coordination of contractile forces and cell migration as a collective group in a 3D matrix.
Future Perspectives
Cell migration studies have traditionally used single cells on 2D surfaces, leaving the mechanisms of multicellular migration in 3D matrix less well understood. However, research trends are progressing toward the use of 3D matrices, which expose types of cell movement that are not readily accessible on 2D substrates. Current 3D cell migration studies involve the use of matrices with a relatively simple and homogeneous composition. Future investigations in the development of heterogeneous extracellular matrices will better mimic the physiological environment and more realistic cell movement.
From recent studies, it is becoming evident that cell-cell adhesion proteins such as N-cadherin contribute more than simply an adhesive role between neighboring cells. The extensive roles of N-cadherin include signaling migration, regulating strong yet dynamic cell-cell junctions and organizing actin throughout cells in a cluster. However, the precise mechanistic pathways behind how N-cadherin contributes to these diverse roles remain to be examined. For example, how cells generate the supracellular actin organization and use such actin network for coordinated movement of the cell cluster. Also, the specific molecules involved in the N-cadherin pro-migration signaling pathway need to be uncovered.
Elucidating the mechanisms of collective cell migration is important because metastasizing cancer cells such as mammary carcinomas migrate as elongated multicellular chains.35-37 This collective tumor cell invasion may be an effective strategy for transporting a sizable number of cells to a new location for colonization, thus facilitating metastasis. Consistent with this, carcinoma chain migration is often associated with high metastatic capacity and a poor prognosis.38 Similarly, in melanoma explants cultured in a 3D matrix, collective chain migration occurred most frequently in samples derived from melanoma tumors, which had penetrated deeper in the tissue, and posed a high risk to the patient.33 Although cell-cell adhesion molecules are thought to be important for mediating junctions within the cell clusters, the precise roles of cell-cell adhesion in multicellular migration are only beginning to emerge. Studying multicellular migration will, not only improve our understanding of normal developmental processes, but also provide insights into how to better target and minimize pathological cell migration, thereby improving current strategies for suppressing tumor cell invasion and metastasis.
Acknowledgments
The Yamada laboratory is supported by a Beckman Young Investigator Award, a NIH EUREKA GM094798, and the fund from the University of California Cancer Research Coordinating Committee.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/21766
References
- 1.Dehaan RL. Migration patterns of the precardiac mesoderm in the early chick embrvo. Exp Cell Res. 1963;29:544–60. doi: 10.1016/S0014-4827(63)80016-6. [DOI] [PubMed] [Google Scholar]
- 2.Oda H, Takeichi M. Evolution: structural and functional diversity of cadherin at the adherens junction. J Cell Biol. 2011;193:1137–46. doi: 10.1083/jcb.201008173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeichi M. Self-organization of animal tissues: cadherin-mediated processes. Dev Cell. 2011;21:24–6. doi: 10.1016/j.devcel.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 4.de Rooij J, Kerstens A, Danuser G, Schwartz MA, Waterman-Storer CM. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J Cell Biol. 2005;171:153–64. doi: 10.1083/jcb.200506152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shih W, Yamada S. N-cadherin-mediated cell-cell adhesion promotes cell migration in a three-dimensional matrix. J Cell Sci. 2012;125:3661–70. doi: 10.1242/jcs.103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727–35. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa S, Takeichi M. Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development. 1995;121:1321–32. doi: 10.1242/dev.121.5.1321. [DOI] [PubMed] [Google Scholar]
- 8.Derycke LD, Bracke ME. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int J Dev Biol. 2004;48:463–76. doi: 10.1387/ijdb.041793ld. [DOI] [PubMed] [Google Scholar]
- 9.Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000;148:779–90. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631–44. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez-Heras E, Howell FV, Williams G, Doherty P. The fibroblast growth factor receptor acid box is essential for interactions with N-cadherin and all of the major isoforms of neural cell adhesion molecule. J Biol Chem. 2006;281:35208–16. doi: 10.1074/jbc.M608655200. [DOI] [PubMed] [Google Scholar]
- 12.Hulit J, Suyama K, Chung S, Keren R, Agiostratidou G, Shan W, et al. N-cadherin signaling potentiates mammary tumor metastasis via enhanced extracellular signal-regulated kinase activation. Cancer Res. 2007;67:3106–16. doi: 10.1158/0008-5472.CAN-06-3401. [DOI] [PubMed] [Google Scholar]
- 13.Chung S, Yao J, Suyama K, Bajaj S, Qian X, Loudig OD, et al. N-cadherin regulates mammary tumor cell migration through Akt3 suppression. Oncogene. 2012 doi: 10.1038/onc.2012.65. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe T, Sato K, Kaibuchi K. Cadherin-mediated intercellular adhesion and signaling cascades involving small GTPases. Cold Spring Harb Perspect Biol. 2009;1:a003020. doi: 10.1101/cshperspect.a003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braga VM, Yap AS. The challenges of abundance: epithelial junctions and small GTPase signalling. Curr Opin Cell Biol. 2005;17:466–74. doi: 10.1016/j.ceb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouvière C. N-cadherin-dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J Cell Biol. 2002;158:953–65. doi: 10.1083/jcb.200202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, et al. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–39. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 18.Okabe T, Nakamura T, Nishimura YN, Kohu K, Ohwada S, Morishita Y, et al. RICS, a novel GTPase-activating protein for Cdc42 and Rac1, is involved in the beta-catenin-N-cadherin and N-methyl-D-aspartate receptor signaling. J Biol Chem. 2003;278:9920–7. doi: 10.1074/jbc.M208872200. [DOI] [PubMed] [Google Scholar]
- 19.Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, et al. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell. 2010;19:39–53. doi: 10.1016/j.devcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/S0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- 21.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–15. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, et al. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107–15. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–42. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 25.Sumida GM, Tomita TM, Shih W, Yamada S. Myosin II activity dependent and independent vinculin recruitment to the sites of E-cadherin-mediated cell-cell adhesion. BMC Cell Biol. 2011;12:48. doi: 10.1186/1471-2121-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirata H, Tatsumi H, Sokabe M. Zyxin emerges as a key player in the mechanotransduction at cell adhesive structures. Commun Integr Biol. 2008;1:192–5. doi: 10.4161/cib.1.2.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol. 2005;171:209–15. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colombelli J, Besser A, Kress H, Reynaud EG, Girard P, Caussinus E, et al. Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization. J Cell Sci. 2009;122:1665–79. doi: 10.1242/jcs.042986. [DOI] [PubMed] [Google Scholar]
- 29.Smith MA, Blankman E, Gardel ML, Luettjohann L, Waterman CM, Beckerle MC. A zyxin-mediated mechanism for actin stress fiber maintenance and repair. Dev Cell. 2010;19:365–76. doi: 10.1016/j.devcel.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uemura A, Nguyen TN, Steele AN, Yamada S. The LIM domain of zyxin is sufficient for force-induced accumulation of zyxin during cell migration. Biophys J. 2011;101:1069–75. doi: 10.1016/j.bpj.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirata H, Tatsumi H, Sokabe M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J Cell Sci. 2008;121:2795–804. doi: 10.1242/jcs.030320. [DOI] [PubMed] [Google Scholar]
- 32.Vaezi A, Bauer C, Vasioukhin V, Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev Cell. 2002;3:367–81. doi: 10.1016/S1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 33.Hegerfeldt Y, Tusch M, Bröcker EB, Friedl P. Collective cell movement in primary melanoma explants: plasticity of cell-cell interaction, beta1-integrin function, and migration strategies. Cancer Res. 2002;62:2125–30. [PubMed] [Google Scholar]
- 34.Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, et al. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol. 2011;13:49–58. doi: 10.1038/ncb2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberg RA. The biology of cancer (Garland Science, New York; 2007). [Google Scholar]
- 36.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–57. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 37.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto E, Kohama G, Sunakawa H, Iwai M, Hiratsuka H. Mode of invasion, bleomycin sensitivity, and clinical course in squamous cell carcinoma of the oral cavity. Cancer. 1983;51:2175–80. doi: 10.1002/1097-0142(19830615)51:12<2175::AID-CNCR2820511205>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]