Abstract
Synapse formation, maintenance and plasticity are critical for the correct function of the nervous system and its target organs. During development, these processes enable the establishment of appropriate neural circuits. During adulthood, they allow adaptation to both physiological and environmental changes. In this review, we discuss emerging roles for two families of classical axon and vascular guidance cues in synaptogenesis and synaptic plasticity, the semaphorins and the vascular endothelial growth factors (VEGFs). Their contribution to synapse formation and function add a new facet to the spectrum of overlapping and complementary roles for these molecules in development, adulthood and disease.
Keywords: semaphorin, VEGF, synapse, plasticity, synaptogenesis, blood vessel
Introduction
The contact sites through which neurons pass electrical or chemical signals to other cells are termed synapses. Presynaptic sites are usually located on axons, while post-synaptic sites are found on dendrites, neuronal somata or non-neuronal targets that require innervation, including muscle and blood vessels. Because synapses consist of a presynaptic and postsynaptic site, they permit the assembly of neuronal circuits with directionality and hierarchy. The ordered formation of appropriate types and numbers of synapses in the correct locations is therefore essential for normal nervous system function.
Most synapses form during embryonic and postnatal development, but new synapses can also be added in adulthood, for example during memory formation and learning (Fig. 1A). Newly formed synapses are either maintained or eliminated in processes that involve interactions between pre- and post-synaptic cells and are integrated with regulatory mechanisms that monitor synaptic activity. In the process of synaptic pruning, several synapses may be removed collectively alongside the axon branches or dendrites that carry them (Fig. 1B). Finally, increased or decreased levels of neurotransmitter release at synapses, or an altered response to those neurotransmitters, collectively strengthen or weaken synapses in a mechanism termed synaptic plasticity. Scientific research over the past three decades suggests that a large collection of different patterning cues synergize to create appropriate synaptic complexity and specificity for neural networks to perform optimally. The semaphorins and vascular endothelial growth factors (VEGFs) are two families of neuronal and vascular patterning cues that regulate synapse formation, maintenance, elimination and plasticity.
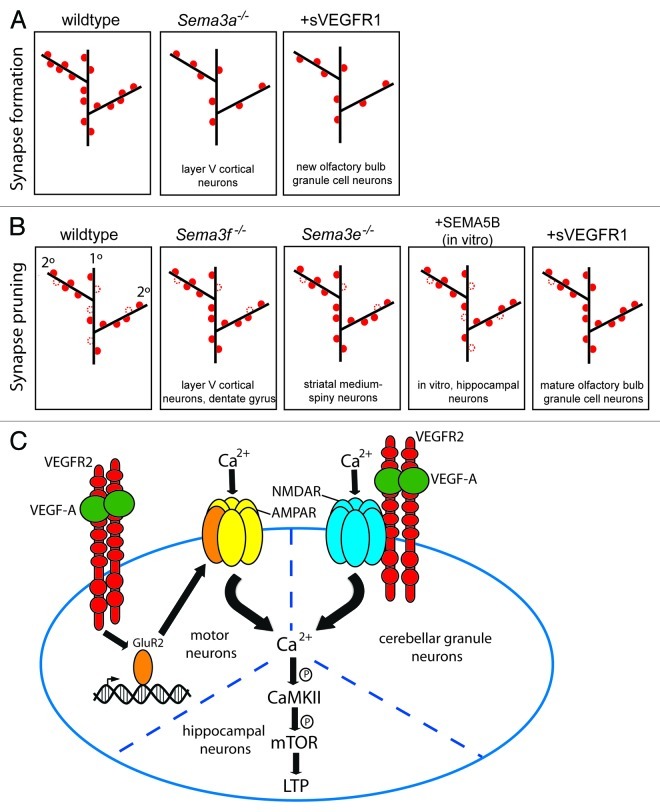
Figure 1. Roles of semaphorins and VEGFs in synapse formation, elimination and plasticity. (A) During late vertebrate embryonic and early postnatal stages in vertebrates, synapses (red dot) form between axons and neuronal dendrites (black lines). On dendrites, the postsynaptic site is located on membranous protrusions called spines. Sema3a−/− mice were reported to have reduced spine density in layer V cortical neurons. Inactivation of VEGF-A in the mouse brain with a soluble VEGFR1 receptor reduces spine density in newly born olfactory bulb granule cell neurons. (B) After synapse formation, some spines are pruned to eliminate synapses (red dotted circle). Sema3f−/− mice have more spines on primary apical dendrites (labeled 1°) of layer V cortical and dentate gyrus neurons, indicating reduced pruning, but normal spines on secondary dendrites (2°). Sema3e−/− mice also have increased spine density on primary dendrites, but in direct-pathway medium-spiny neurons. In vitro, overexpression of Sema5B also reduces spine number in rat hippocampal neurons. Inactivation of VEGF-A in the mouse brain with sVEGFR1 receptor increases spine number in mature olfactory bulb granule cell neurons, suggesting reduced pruning. (C) Three different VEGF-A effects relevant to synaptic plasticity have been identified; here, these VEGF-A functions are represented in one virtual postsynaptic cell. In developing mouse cerebellar granule cell neurons, VEGF-A acts through VEGFR2 to modulate Ca2+ influx through NMDA receptors. In cultured rat motor neurons, VEGF-A modulates Ca2+ influx through AMPA receptors by stimulating the transcription of the receptor subunit GluR2; VEGFR2 may mediate this effect. In cultured rat hippocampal neurons, VEGF-A-induced Ca2+ influx stimulates phosphorylation (indicated with P) of CaMKII and mTOR, leading to LTP. It is not known if these different means to alter Ca2+ influx may be integrated in one type of neuron, or if they represent independent pathways that are employed by distinct neuronal subtypes.
The semaphorins belong to a large family of glycoproteins that includes both secreted and membrane-bound forms and are characterized by the presence of a so-called SEMA domain; they are categorized into eight classes, with invertebrates using classes 1, 2 and 5 semaphorins and vertebrates using classes 3–7.1 To convey their signals, semaphorins bind transmembrane receptors of the plexin (PLXN) family or composite receptors consisting of a plexin and a ligand-binding co-receptor of the neuropilin (NRP) family, NRP1 or NRP2. Although initially discovered due to their function as chemorepellents in axon guidance,2 semaphorins have also been implicated in vascular growth and function.3
The VEGF family includes several cysteine knot glycoproteins that are termed VEGF-A, VEGF-B, VEGF-C and VEGF-D and are structurally unrelated to the semaphorins.4 The VEGFs are best known as growth and patterning factors for blood vessels and lymphatic vessels.4 In addition, they were more recently shown to pattern subsets of neurons.5 For example, VEGF-A, the most widely studied VEGF family member, is essential for blood vascular development, but also regulates neuronal generation, survival, migration and axon guidance.5 The VEGFs bind to transmembrane tyrosine kinase receptors termed VEGFR1 (FLT1), VEGFR2 (KDR, FLK1) and VEGFR3, but share with class 3 semaphorins the ability to bind NRP1 and NRP2.6 Extending the concept of receptor sharing, VEGFR2 can also be recruited into plexin/neuropilin receptor complexes to modulate the signaling response induced by semaphorins.7 This review discusses recent evidence for roles for semaphorins and VEGFs in synaptic development and plasticity to update and complement previous excellent reviews.8-10
Control of Synapse Formation by Semaphorins
Early evidence that semaphorins control synaptic development in vivo came from work on the Drosophila giant fiber, which controls the fight-or-flight response induced by visual stimuli. Using SEMA1A loss-of-function mutants, researchers showed that giant fiber axons were either mistargeted or reached their target interneurons without forming synapses.11 This defect was rescued by expressing SEMA1A in the Sema1a−/− postsynaptic target interneuron.11 Interestingly, the SEMA1A cytoplasmic signaling domain is necessary for successful synapse formation, and expressing SEMA1A presynaptically also rescues synapses in Sema1a−/− mutants; it has therefore been proposed that that SEMA1A may act as both a ligand and a receptor during giant fiber synapse formation, although the precise mechanism is still unclear.11
Treatment of primary mouse cortical neurons with SEMA3A increases the density of dendritic spines as well as clustering and co-localization of the presynaptic marker synapsin-1 with the postsynaptic marker PSD-95.12 In agreement with these in vitro findings, one study reported that the spine density of layer V cortical neurons was reduced in Sema3a−/− adult mouse brains12 (Fig. 1A). However, another study determined the distribution of synapses along specific dendritic sections and did not find any changes in spine density in cortical layer V or other parts of the Sema3a-null brain.13
Several class 4 semaphorins co-localize and interact with PSD-95 in vitro.14-16 While SEMA4B knockdown in rat hippocampal neuron cultures reduced the density of both excitatory (glutaminergic) and inhibitory (GABAergic) synapses, knockdown of SEMA4D reduced the density of GABAergic synapses only.17 Different class 4 semaphorin family members may therefore induce the formation and maturation of select subsets of synapses, perhaps due to differential expression of their receptors and downstream signaling pathways in their target neurons.
Control of Synapse Formation by VEGF
A role for VEGF-A in synapse formation was initially suggested by work on cultured primary cortical neurons, which demonstrated increased neurite extension and upregulation of dendrite-enriched microtubule associated protein MAP2 in vitro.18 In addition to guiding subsets of CNS axons to their targets in vivo,19,20 VEGF-A may therefore also promote synapse formation or maturation. Thus, sympathetic nerve density on resistance arteries is normal in Vegfad/d mice with low levels of VEGF-A, but synapses between the sympathetic nerve endings and smooth muscle cells in these arteries are abnormal, with a wider synaptic cleft.21 Consistent with defective vasoregulation of resistance arteries by sympathetic nerves, these mice cannot maintain their core body temperature under cold stress or upregulate cerebral blood flow after short-term oxygen shortage.21 More direct evidence for a role of VEGF-A in controlling synapse formation was provided by experiments in which the forced expression of a soluble VEGFR1 protein in the adult mouse brain sequestered VEGF-A and concomitantly decreased dendritic spine number and density on newly-born granule cell neurons in the olfactory bulb, independently of effects on neurogenesis or blood vessels.22
Roles for Semaphorins in Synaptic Maintenance and Elimination
Semaphorins are well known inducers of axon pruning, and additionally have been implicated in synapse elimination, also known as synaptic pruning.10 For example, SEMA3A treatment of primary mouse hippocampal neurons reduces the accumulation of the synaptic markers synaptophysin and PSD-95 in puncta, indicating synapse elimination.23 These observations suggest that SEMA3A can promote synapse elimination in hippocampal neurons, even though it has been reported, controversially, to induce synapse formation in cortical neurons (see above).
SEMA3F, its receptor NRP2 and the signaling co-receptor PLXNA3 are all required for synaptic elimination through spine pruning in vivo. Thus, adult Sema3f−/−, Nrp2−/− and Plxna3−/− mouse brains have an increased number and abnormal distribution of spines on primary dendrites of cortical layer V and dentate gyrus neurons13 (Fig. 1B). Moreover, SEMA3F treatment reduces the total number of spines and PSD-95-positive puncta in established primary cultures of dentate gyrus neurons, indicating a role in synapse elimination.13 Strikingly, the colocalization of the excitatory synapse markers PSD-95 and vGlut1 was decreased, whereas two markers for inhibitory synapses, vGAT and gephrin, were unchanged.13 These observations show that SEMA3F functions to specifically eliminate excitatory synapses in cultured dentate gyrus neurons.
A higher density of glutamatergic synapses is observed on the primary dendrites of direct-pathway medium spiny neurons in the striatum of mice lacking either SEMA3E or its receptor PLXND124 (Fig. 1B). Thus, SEMA3E signaling through PLXND1 appears to inhibit the formation or promote the elimination of thalamostriatal synapses,24 in analogy to the role of SEMA3F signaling in dentate gyrus neurons.13
SEMA5B may also promote synapse elimination, as its knockdown significantly increases and its overexpression decreases synapse number in cultured rat hippocampal neurons25 (Fig. 1B). Time-lapse imaging of hippocampal neurons treated with SEMA5B showed that this decrease in synapse number was due to the rapid elimination of synapses and not a secondary consequence of a failure to form new synapses.25 Yet, the role of SEMA5B in vivo remains to be confirmed.
Possible Roles for VEGFs in Synaptic Maintenance and Elimination
VEGFs may also contribute to synaptic elimination (Fig. 1B). Thus, sequestering VEGF-A by transgenic expression of soluble VEGFR1 in adult mice increases spine density in already-established olfactory bulb granule cells (Fig. 1B), even though this treatment impairs spine formation in newly born olfactory bulb granule cells22 (Fig. 1A). VEGF-A may therefore contribute both to synapse formation and, potentially, elimination at different developmental stages of the same type of neuron.
VEGF-A may also be essential for synaptic maintenance, at least for motor neurons. Thus Vegfad/d mice with reduced levels of VEGF-A suffer from motor neuron degeneration,26 and this correlates with the downregulation of genes involved in synapse formation, assembly and plasticity in motor neurons at the onset of motor dysfunction.27 In agreement with a role for VEGF-A in synaptic maintenance, the intraventricular infusion of exogenous VEGF-A preserves neuromuscular junctions in G93A-SOD1 mice, a transgenic model of motor neuron degeneration.28
Together, current knowledge suggests opposing roles for VEGF-A in synapse pruning and maintenance for different neuronal subtypes. It has not yet been investigated if these differential effects correlate with the expression of specific sets of VEGF-A receptors in particular neurons.
Semaphorins in Synaptic Plasticity
Several lines of evidence suggest that semaphorins regulate synaptic plasticity. First, the expression of class 3 semaphorins in the cortex (SEMA3A) and hippocampus (SEMA3C and SEMA3F) is reduced in rat models of epilepsy,29,30 while rats housed in enriched environments increase the expression of NRP1 in the hippocampus.31 Second, several ex vivo studies using hippocampal slices suggest a role for class 3 semaphorins in synaptic plasticity. Thus, treatment of adult mouse hippocampal slices with SEMA3A dose dependently decreases the number of field excitatory postsynaptic currents (EPSCs).23 Interestingly, SEMA3F has the opposite effect, as treatment of adult mouse hippocampal slices with SEMA3F increases the amplitude and frequency of mini EPSCs.32 Third, genetic evidence suggests a role for class 3 semaphorins in vivo. Thus, dentate gyrus and cortical layer V slices from Nrp2−/− mice show increased frequency of mini ESPCs, and Sema3f−/− mice are prone to seizures.13 In agreement, long-term potentiation (LTP) in cultured hippocampal neurons induces miR-188, a microRNA that downregulates NRP2 protein levels, and when transfected into these neurons rescues the NRP2-mediated decrease in dendritic spine density and synaptic transmission.33
Providing yet another line of evidence for the role of class 3 semaphorins in regulating synaptic plasticity in vivo, mice lacking either SEMA3E or PLXND1 show an increased frequency of mini EPSCs and, additionally, of evoked ESPCs in direct pathway medium spiny neurons.24 Together, these studies suggest that class 3 semaphorin family members cooperate to alter synaptic plasticity in the adult brain by differentially affecting the same type of neurons or acting on distinct types of neurons.
Importantly, two independent studies using paired pulse experiments showed that the increased synaptic transmission in mice lacking SEMA3F or SEMA3E is not due to the modulation of synaptic strength.13,24 Instead, the effect of these semaphorins on synaptic transmission was attributed to an increased density and altered distribution of primary dendritic spines.13,24 Evidence that semaphorins control synaptic transmission directly and independently of synapse formation is therefore still lacking. Interestingly, however, the cyclin-dependent kinase CDK5, which has been linked to LTP, is a downstream target of semaphorins,34 and direct roles for semaphorins in modulating synaptic activity and plasticity may therefore emerge in the future.
VEGF in Synaptic Plasticity
The knockdown of VEGF-D in the mouse hippocampus decreases the frequency and amplitude of mini EPSCs and impairs memory formation; moreover, its knockdown in primary hippocampal neuron cultures reduces dendritic length, but not spine density.35 These observations are consistent with a model in which VEGF-D controls synaptic plasticity by changing dendrite structure. Adding detail to the molecular mechanism, VEGF-D-induced dendritic branching downstream of neuronal activity involves nuclear calcium signaling and the calcium/calmodulin-dependent protein kinase IV.35
VEGF-A may also modulate synaptic plasticity. For example, treatment of adult rat hippocampal neuron cultures with exogenous VEGF-A enhances LTP,36 while sequestering VEGF-A with a soluble VEGFR1 receptor in the adult mouse hippocampus reduces LTP, independently of neurogenesis and vascular changes.37 Moreover, viral or transgenic overexpression of VEGF-A in adult rats or mice enhances hippocampal spatial memory formation,31,37 and the intrathecal administration of VEGF-A to adult rats induces a long-lasting increase in nerve burst amplitude in respiratory phrenic nerves.38
Similar to the semaphorins, VEGF-A is also upregulated after neuronal activity in mice, for example in the hippocampus and in motor neurons after seizures39,40 and in cultured neurons and brain slices following membrane depolarization.36 Additionally, exogenous VEGF-A reduces the amplitude and frequency of field excitatory and inhibitory postsynaptic potentials (EPSPs and IPSPs) in rat hippocampal and motor neurons in brain slices and enhances mini EPSC frequency in neonatal rat hippocampal neuron cultures.39,41,42 Whether these effects are caused by changes in spine density or dendrite number, as observed for semaphorins, remains to be addressed.
Some evidence supports the possibility that VEGF-A modulates synaptic transmission directly by altering calcium influx (Fig. 1C). Thus, VEGF-A treatment increases the transcription of the glutamate AMPA receptor subunit GluR2, which decreases AMPA receptor permeability to Ca2+ in cultured rat motor neurons.43 Additionally, VEGF-A and its receptor VEGFR2 associate with the glutamate NMDA receptor in developing mouse cerebellar granule cells before synapse formation to modulate NMDA receptor-mediated currents and Ca2+ influx in these cells.44 VEGF-A treatment also increases Ca2+ flux into cultured rat hippocampal neurons and activates CamKII and mTOR, two molecules known to operate in signaling pathways that promote LTP.36 However, it is not yet known if these mechanisms also occur in the same neuron and are functionally coordinated, or if they are specific to different neuronal subtypes. Finally, it is not yet known whether VEGF-A can modulate glutamate receptor-based synaptic activity in functional adult synapses in vivo.
Discussion and Future Directions
While we now know that VEGF-A and VEGF-D contribute to synaptic development, maintenance and plasticity, the role of other VEGF family members remains to be clarified. For example, VEGF-B and VEGF-C have been implicated in neurogenesis,45,46 but possible roles in the synapse have not yet been investigated. Similarly, only a small subset of proteins in the large semaphorin family has been studied for possible roles in the synapse, and many more roles may therefore be uncovered. The sources of semaphorins and VEGFs during synapse formation, maintenance and pruning also remain unknown. Finally, it will be important to examine if there is cooperation, competition or redundancy between both classes of secreted glycoproteins, in particular as they share some subunits of their receptor complexes, such as NRP1, NRP2, PLXND1 and VEGFR2.
The analysis of genetic mutants of these VEGF-A receptors for defects in synapse formation, elimination or plasticity will also help to determine if VEGF-A is generally important because of its direct effects on synapses, or if it affects synapses more often indirectly, i.e., by acting through blood vessels or other VEGF-responsive cell types that release synaptic modulators. Several studies implicating VEGF-A in dendritogenesis, LTP modulation and neuroprotection after seizures have excluded vascular effects as the primary cause of these phenotypes and are therefore consistent with direct effects of VEGF-A on synapses.22,37,40 However, synaptogenesis has been shown to occur alongside angiogenesis after cerebral ischemia, a condition that strongly upregulates VEGF-A,47 and the hypoxia-induced upregulation of the transcription factor HIF1A, which is upstream of VEGF-A, enhances spontaneous firing in hippocampal cultures.41 Thus, under specific physiological circumstances, VEGF-A may promote synaptogenesis also indirectly by inducing new blood vessel growth, similar to its proposed role in neurogenesis.48 Adding further levels of complexity, hypoxia can also inhibit synapse formation in the newborn rat brain, which is associated with changes in the expression of genes involved in synaptic function and maturation.49 Semaphorins can also modulate blood vessel growth and function,50-52 but it has not yet been examined if they contribute to synaptic development and plasticity indirectly through their vascular roles.
While studies of VEGF and semaphorins in normal synaptic development and maintenance are a focus of ongoing research, future research will undoubtedly aim to understand their contribution to diseases in which synaptic function is affected. The dysregulation or dysfunction of semaphorins and VEGFs has been observed in several neural disorders. For example, upregulation of SEMA3A has been linked to schizophrenia and Alzheimer disease,53,54 downregulation of Sema5a has been observed in autism55 and Sema3f binding by the fragile X mental retardation protein (FMRP) has been implicated in fragile X mental retardation pathology.56 Furthermore, genetic variation of VEGFA has been associated with motor neuron disease57 and Alzheimer disease.58 Whether the link of semaphorins and VEGFs to these disorders involves synaptic effects remains to be investigated.
As new roles of VEGFs and semaphorins in synaptic health and dysfunction are being uncovered, these molecules may become new targets for therapeutic intervention in neurological disease.
Acknowledgments
M.T. is supported by a PhD studentship from the Wellcome Trust (092839/Z/10/Z), C.R. by a Junior Investigator Award from The Wellcome Trust (095623/Z/11/Z) and F.M. by a project grant from the BBSRC to C.R. (BB/I008373/1).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/22408
References
- 1.Zhou Y, Gunput R-AF, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008;33:161–70. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Pasterkamp RJ, Kolodkin AL. Semaphorin junction: making tracks toward neural connectivity. Curr Opin Neurobiol. 2003;13:79–89. doi: 10.1016/S0959-4388(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 3.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–65. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Mackenzie F, Ruhrberg C. Diverse roles for VEGF-A in the nervous system. Development. 2012;139:1371–80. doi: 10.1242/dev.072348. [DOI] [PubMed] [Google Scholar]
- 6.Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437:169–83. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- 7.Bellon A, Luchino J, Haigh K, Rougon G, Haigh J, Chauvet S, et al. VEGFR2 (KDR/Flk1) signaling mediates axon growth in response to semaphorin 3E in the developing brain. Neuron. 2010;66:205–19. doi: 10.1016/j.neuron.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Mann F, Chauvet S, Rougon G. Semaphorins in development and adult brain: Implication for neurological diseases. Prog Neurobiol. 2007;82:57–79. doi: 10.1016/j.pneurobio.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Shen K, Cowan CW. Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol. 2010;2:a001842. doi: 10.1101/cshperspect.a001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanderhaeghen P, Cheng H-J. Guidance molecules in axon pruning and cell death. Cold Spring Harb Perspect Biol. 2010;2:a001859. doi: 10.1101/cshperspect.a001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godenschwege TA, Hu H, Shan-Crofts X, Goodman CS, Murphey RK. Bi-directional signaling by Semaphorin 1a during central synapse formation in Drosophila. Nat Neurosci. 2002;5:1294–301. doi: 10.1038/nn976. [DOI] [PubMed] [Google Scholar]
- 12.Morita A, Yamashita N, Sasaki Y, Uchida Y, Nakajima O, Nakamura F, et al. Regulation of dendritic branching and spine maturation by semaphorin3A-Fyn signaling. J Neurosci. 2006;26:2971–80. doi: 10.1523/JNEUROSCI.5453-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, et al. Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature. 2009;462:1065–9. doi: 10.1038/nature08628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burkhardt C, Müller M, Badde A, Garner CC, Gundelfinger ED, Püschel AW. Semaphorin 4B interacts with the post-synaptic density protein PSD-95/SAP90 and is recruited to synapses through a C-terminal PDZ-binding motif. FEBS Lett. 2005;579:3821–8. doi: 10.1016/j.febslet.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 15.Inagaki S, Ohoka Y, Sugimoto H, Fujioka S, Amazaki M, Kurinami H, et al. Sema4c, a transmembrane semaphorin, interacts with a post-synaptic density protein, PSD-95. J Biol Chem. 2001;276:9174–81. doi: 10.1074/jbc.M009051200. [DOI] [PubMed] [Google Scholar]
- 16.Schultze W, Eulenburg V, Lessmann V, Herrmann L, Dittmar T, Gundelfinger ED, et al. Semaphorin4F interacts with the synapse-associated protein SAP90/PSD-95. J Neurochem. 2001;78:482–9. doi: 10.1046/j.1471-4159.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- 17.Paradis S, Harrar DB, Lin Y, Koon AC, Hauser JL, Griffith EC, et al. An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron. 2007;53:217–32. doi: 10.1016/j.neuron.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenstein JM, Mani N, Khaibullina A, Krum JM. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci. 2003;23:11036–44. doi: 10.1523/JNEUROSCI.23-35-11036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erskine L, Reijntjes S, Pratt T, Denti L, Schwarz Q, Vieira JM, et al. VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron. 2011;70:951–65. doi: 10.1016/j.neuron.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz de Almodovar C, Fabre PJ, Knevels E, Coulon C, Segura I, Haddick PC, et al. VEGF mediates commissural axon chemoattraction through its receptor Flk1. Neuron. 2011;70:966–78. doi: 10.1016/j.neuron.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storkebaum E, Ruiz de Almodovar C, Meens M, Zacchigna S, Mazzone M, Vanhoutte G, et al. Impaired autonomic regulation of resistance arteries in mice with low vascular endothelial growth factor or upon vascular endothelial growth factor trap delivery. Circulation. 2010;122:273–81. doi: 10.1161/CIRCULATIONAHA.109.929364. [DOI] [PubMed] [Google Scholar]
- 22.Licht T, Eavri R, Goshen I, Shlomai Y, Mizrahi A, Keshet E. VEGF is required for dendritogenesis of newly born olfactory bulb interneurons. Development. 2010;137:261–71. doi: 10.1242/dev.039636. [DOI] [PubMed] [Google Scholar]
- 23.Bouzioukh F, Daoudal G, Falk J, Debanne D, Rougon G, Castellani V. Semaphorin3A regulates synaptic function of differentiated hippocampal neurons. Eur J Neurosci. 2006;23:2247–54. doi: 10.1111/j.1460-9568.2006.04783.x. [DOI] [PubMed] [Google Scholar]
- 24.Ding JB, Oh WJ, Sabatini BL, Gu C. Semaphorin 3E-Plexin-D1 signaling controls pathway-specific synapse formation in the striatum. Nat Neurosci. 2012;15:215–23. doi: 10.1038/nn.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connor TP, Cockburn K, Wang W, Tapia L, Currie E, Bamji SX. Semaphorin 5B mediates synapse elimination in hippocampal neurons. Neural Dev. 2009;4:18. doi: 10.1186/1749-8104-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–8. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 27.Brockington A, Heath PR, Holden H, Kasher P, Bender FL, Claes F, et al. Downregulation of genes with a function in axon outgrowth and synapse formation in motor neurones of the VEGFdelta/delta mouse model of amyotrophic lateral sclerosis. BMC Genomics. 2010;11:203. doi: 10.1186/1471-2164-11-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng C, Sköld MK, Li J, Nennesmo I, Fadeel B, Henter JI. VEGF reduces astrogliosis and preserves neuromuscular junctions in ALS transgenic mice. Biochem Biophys Res Commun. 2007;363:989–93. doi: 10.1016/j.bbrc.2007.09.088. [DOI] [PubMed] [Google Scholar]
- 29.Barnes G, Puranam RS, Luo Y, McNamara JO. Temporal specific patterns of semaphorin gene expression in rat brain after kainic acid-induced status epilepticus. Hippocampus. 2003;13:1–20. doi: 10.1002/hipo.10041. [DOI] [PubMed] [Google Scholar]
- 30.Holtmaat AJ, Gorter JA, De Wit J, Tolner EA, Spijker S, Giger RJ, et al. Transient downregulation of Sema3A mRNA in a rat model for temporal lobe epilepsy. A novel molecular event potentially contributing to mossy fiber sprouting. Exp Neurol. 2003;182:142–50. doi: 10.1016/S0014-4886(03)00035-9. [DOI] [PubMed] [Google Scholar]
- 31.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–35. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 32.Sahay A, Kim CH, Sepkuty JP, Cho E, Huganir RL, Ginty DD, et al. Secreted semaphorins modulate synaptic transmission in the adult hippocampus. J Neurosci. 2005;25:3613–20. doi: 10.1523/JNEUROSCI.5255-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee K, Kim JH, Kwon OB, An K, Ryu J, Cho K, et al. An activity-regulated microRNA, miR-188, controls dendritic plasticity and synaptic transmission by downregulating neuropilin-2. J Neurosci. 2012;32:5678–87. doi: 10.1523/JNEUROSCI.6471-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, et al. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–6. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauceri D, Freitag HE, Oliveira AM, Bengtson CP, Bading H. Nuclear calcium-VEGFD signaling controls maintenance of dendrite arborization necessary for memory formation. Neuron. 2011;71:117–30. doi: 10.1016/j.neuron.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 36.Kim BW, Choi M, Kim YS, Park H, Lee HR, Yun CO, et al. Vascular endothelial growth factor (VEGF) signaling regulates hippocampal neurons by elevation of intracellular calcium and activation of calcium/calmodulin protein kinase II and mammalian target of rapamycin. Cell Signal. 2008;20:714–25. doi: 10.1016/j.cellsig.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, et al. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci U S A. 2011;108:5081–6. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dale-Nagle EA, Satriotomo I, Mitchell GS. Spinal vascular endothelial growth factor induces phrenic motor facilitation via extracellular signal-regulated kinase and Akt signaling. J Neurosci. 2011;31:7682–90. doi: 10.1523/JNEUROSCI.0239-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCloskey DP, Hintz TM, Scharfman HE. Modulation of vascular endothelial growth factor (VEGF) expression in motor neurons and its electrophysiological effects. Brain Res Bull. 2008;76:36–44. doi: 10.1016/j.brainresbull.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicoletti JN, Shah SK, McCloskey DP, Goodman JH, Elkady A, Atassi H, et al. Vascular endothelial growth factor is up-regulated after status epilepticus and protects against seizure-induced neuronal loss in hippocampus. Neuroscience. 2008;151:232–41. doi: 10.1016/j.neuroscience.2007.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang YF, Yang CH, Huang CC, Tai MH, Hsu KS. Pharmacological and genetic accumulation of hypoxia-inducible factor-1alpha enhances excitatory synaptic transmission in hippocampal neurons through the production of vascular endothelial growth factor. J Neurosci. 2010;30:6080–93. doi: 10.1523/JNEUROSCI.5493-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCloskey DP, Croll SD, Scharfman HE. Depression of synaptic transmission by vascular endothelial growth factor in adult rat hippocampus and evidence for increased efficacy after chronic seizures. J Neurosci. 2005;25:8889–97. doi: 10.1523/JNEUROSCI.2577-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogaert E, Van Damme P, Poesen K, Dhondt J, Hersmus N, Kiraly D, et al. VEGF protects motor neurons against excitotoxicity by upregulation of GluR2. Neurobiol Aging. 2010;31:2185–91. doi: 10.1016/j.neurobiolaging.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Meissirel C, Ruiz de Almodovar C, Knevels E, Coulon C, Chounlamountri N, Segura I, et al. VEGF modulates NMDA receptors activity in cerebellar granule cells through Src-family kinases before synapse formation. Proc Natl Acad Sci U S A. 2011;108:13782–7. doi: 10.1073/pnas.1100341108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Bras B, Barallobre MJ, Homman-Ludiye J, Ny A, Wyns S, Tammela T, et al. VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat Neurosci. 2006;9:340–8. doi: 10.1038/nn1646. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: evidence from knockout mice and growth factor administration. Dev Biol. 2006;289:329–35. doi: 10.1016/j.ydbio.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–51. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 48.Louissaint A, Jr., Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–60. doi: 10.1016/S0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 49.Curristin SM, Cao A, Stewart WB, Zhang H, Madri JA, Morrow JS, et al. Disrupted synaptic development in the hypoxic newborn brain. Proc Natl Acad Sci U S A. 2002;99:15729–34. doi: 10.1073/pnas.232568799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7:107–16. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, et al. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–8. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 52.Reidy KJ, Villegas G, Teichman J, Veron D, Shen W, Jimenez J, et al. Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development. Development. 2009;136:3979–89. doi: 10.1242/dev.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eastwood SL, Law AJ, Everall IP, Harrison PJ. The axonal chemorepellant semaphorin 3A is increased in the cerebellum in schizophrenia and may contribute to its synaptic pathology. Mol Psychiatry. 2003;8:148–55. doi: 10.1038/sj.mp.4001233. [DOI] [PubMed] [Google Scholar]
- 54.Good PF, Alapat D, Hsu A, Chu C, Perl D, Wen X, et al. A role for semaphorin 3A signaling in the degeneration of hippocampal neurons during Alzheimer’s disease. J Neurochem. 2004;91:716–36. doi: 10.1111/j.1471-4159.2004.02766.x. [DOI] [PubMed] [Google Scholar]
- 55.Melin M, Carlsson B, Anckarsater H, Rastam M, Betancur C, Isaksson A, et al. Constitutional downregulation of SEMA5A expression in autism. Neuropsychobiology. 2006;54:64–9. doi: 10.1159/000096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–99. doi: 10.1016/S0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- 57.Ferńndez-Santiago R, Sharma M, Mueller JC, Gohlke H, Illig T, Anneser J, et al. Possible gender-dependent association of vascular endothelial growth factor (VEGF) gene and ALS. Neurology. 2006;66:1929–31. doi: 10.1212/01.wnl.0000219756.71928.25. [DOI] [PubMed] [Google Scholar]
- 58.Del Bo R, Ghezzi S, Scarpini E, Bresolin N, Comi GP. VEGF genetic variability is associated with increased risk of developing Alzheimer’s disease. J Neurol Sci. 2009;283:66–8. doi: 10.1016/j.jns.2009.02.318. [DOI] [PubMed] [Google Scholar]