Abstract
Vascular endothelial growth factor A (VEGF-A) is well known for its key roles in blood vessel growth. Although most studies on VEGF and VEGF receptors have been focused on their functions in angiogenesis and in endothelial cells, the role of VEGF in cancer biology appears as an emerging area of importance. In this context, the presence of VEGF receptors in tumor cells strongly suggests that VEGF-A also promotes a wide range of functions, both in vitro and in vivo, all autocrine functions on tumor cells, including adhesion, survival, migration and invasion. Ultimately, refining our knowledge of VEGF signaling pathways in tumor cells should help us to understand why the current used treatments targeting the VEGF pathway in cancer are not universally effective in inhibiting metastasis tumors, and it should also provide new avenues for future therapies.
Keywords: VEGF, VEGF receptors, VEGF-A, breast tumor, tumor cells
Introduction
Vascular endothelial growth factor (VEGF-A) is a key mediator of angiogenesis, the effects of which on cancer growth and development have been well characterized.1,2 Alternative splicing of VEGF-A leads to several different isoforms, which are differentially expressed in various tumor types and have distinct functions in tumor blood vessel formation. Angiogenic isoforms are known as VEGF120, VEGF144, VEGF164 and VEGF188 in mice, and VEGF121, VEGF145, VEGF165 and VEGF189 in humans.3 Depending on the presence of genomic exons 6 and 7, these isoforms are either secreted as soluble forms (VEGF121 and VEGF165) or remain cell- or matrix-associated (VEGF189, VEGF206 and partially VEGF165).4,5 VEGF165 and VEGF121 are the most abundant isoforms and have been extensively studied. VEGF189 shows the strongest association to the cell membrane of all human VEGF isoforms. VEGF189 can also be cleaved in vitro into shorter bioactive forms by proteases, such as plasmin, urokinase plasminogen activator and MMPs,6-8 suggesting that native or cleaved proteins may have distinct roles.
Cellular responses to VEGF165 are mediated by two high-affinity type III tyrosine kinase receptors, VEGF-R2 (KDR/Flk1) and VEGF-R1 (Flt-1), and two receptors of the semaphorin receptor family, neuropilin-1 and neuropilin-2.9,10 Initially, VEGF-A (VEGF165) was believed to act exclusively on endothelial cells through VEGF-R1 and VEGF-R2. VEGF-R2 is the main signaling VEGF receptor in endothelial cells, but it has been shown that its activity is balanced by VEGF-R1, which sequesters excess VEGF through its extracellular domain.11,12 VEGF165 and VEGF189 isoforms have different binding affinities for the VEGF receptors.13 Native VEGF189 binds preferentially to VEGF-R1 and NRP1,13 raising the possibility that specific functions may be driven by different isoforms in physiology or in pathology.13-16
VEGF receptors are not restricted to vascular endothelial cells, and are also present in neurons, retinal epithelium, smooth muscle cells and tumor cells. The identification of VEGF receptor expression in a variety of solid tumors and in tumor cell lines has generated interest and autocrine functions of VEGF may be hypothesized in cancer.17-21 MCF-7 cells express VEGF-R1 and VEGF-R2. However, VEGFR1/2 expression is not always detectable in breast tumor cells. Moderate levels of VEGF-R1/Flt-1 and low levels of VEGF-R2/Flk-1/KDR mRNAs are present in a variety of breast cancer cell lines. In addition, neuropilins (NRP1 or NRP2) are expressed at high level in breast tumor cells (MDA-MB-231 and MDA-MB-435, respectively).13,17,20,22
In the present review, we report that VEGF-A expressed by different types of cancer cells acts through an autocrine signaling pathway mediated by VEGF receptors and/or NRP1 to promote tumorigenesis. VEGF can promote proliferation, survival, adhesion, migration and chemotaxis of breast cancer cells, independently from angiogenesis.
Autocrine Functions of VEGF in Breast Tumor Cells
Proliferation
Several studies demonstrate that VEGF-A induces tumor cell proliferation in mice models of breast cancer; increased tumor proliferation is observed in transgenic mice with VEGF165 targeted to mammary epithelial cells under the control of mouse mammary tumor virus (MMTV) promoter23 or in xenograft mice model generated by the injection of MDA-MB-231 tumor cells transfected with a VEGF165 or VEGF189 plasmid under a bicistronic eukaryotic vector.13 In vitro, increased proliferation is observed in VEGF165- or VEGF189-overexpressing MDA-MB-231 cells, as compared with control cells.13 VEGF also induces cell proliferation in vitro in T47D cells.23 MCF-7 breast cancer cells are estrogen (E2)-sensitive cells and E2-dependent for their proliferation. Estrogen signaling increases VEGF expression in MCF-7 cells, or MBA-MB231 transfected with ERα, through an estrogen-responsive element within the VEGF promoter.24 This effect is inhibited by 4-OH-tamoxifen (4-OH-T). Interestingly, higher VEGF secretion and increased VEGF-R2 signaling are observed in Tamoxifen-resistant cells compared with sensitive MCF-7 one.25 Pharmacological inhibition of p38 kinase in combination with 4-OHT gave an additive effect on inhibition of proliferation in tamoxifen-resistant cells, demonstrating an increased autocrine VEGF/VEGFR2 signaling through p38 mitogen-activated kinase in tamoxifen-resistant cells.25
Survival
VEGF is an autocrine survival factor for breast cancer cells.20,26-30 Several mechanisms have been proposed to support VEGF-induced survival in breast cancer cells. In normoxia and hypoxia, VEGF blockade using VEGF neutralizing antibodies or siRNA VEGF resulted in direct tumor cell apoptosis, implicating the PI3K pathway, downregulation of Bcl-2 expression and increase of the pro-apoptotic protein Bad.20,26,27 In some studies, VEGF signaling has been shown to induce the survival of tumor cells through VEGF-R1 or VEGF-R2.23,29 A specifically targeted knockdown of VEGF-R1 expression by siRNA (siVEGF-R1) in MCF-7 or MDA-MB-231 cells significantly decreases the survival of breast cancer cells through downregulation of protein kinase B (AKT) phosphorylation, while a targeted knockdown of VEGF-R2 or NRP1 expression has no effect on the survival of these cancer cells.29 Specifically, VEGFR1 was predominantly expressed internally, mainly in the nuclear envelope of breast cancer cell lines,29 suggesting that VEGF mediate intracrine survival in breast carcinoma cells through internally expressed VEGF-R1/FLT1. The role of neuropilin 1 in the survival of breast cancer cells (MDA-MB-231) was also described by several groups,20,30 and VEGF was shown to promote breast carcinoma survival by stimulating the PI3-kinase pathway.20 The role of NRP1 will be discussed in the next chapter due to the multifaceted role of neuropilins (see below). In our study,30 we also compared the role of different VEGF isoforms on the survival of MDA-MB-231. Contrasting roles of VEGF165 and VEGF189 isoforms were found to affect tumor survival. Unexpectedly, we found that VEGF189 reduced survival in stress conditions as compared with VEGF165,30 and the mechanisms remain to be established. Recently, the role of integrins was analyzed in several studies.31,32 Chung and Mercurio31 have reported that hypoxia-induced protection from apoptosis via VEGF was dependent on α6β1 integrins in MDA-MB-435 breast cancer cells. In three dimensional cultures, a β1 integrin inhibitory antibody induces apoptosis of breast cancer cells and inhibits tumor growth.32 Thus, the mechanisms of tumor survival, which is a fundamental process in metastasis, are probably heterogeneous depending on the nature of the tumor cell and/or VEGF receptors present in these cells.
Cell adhesion
The existence of multiple VEGF isoforms exhibiting different binding affinities toward heparin sulfate raises the possibility that individual isoforms may differently affect cell adhesion. Hutchings and Plouët33 showed that endothelial cells could adhere and spread on VEGF189 and VEGF165, but not on VEGF121. Adhesion was mediated by the α3β1 and αvβ3 integrins and other αv integrins but not by the cognate VEGF receptors.33 Using stable human breast carcinoma cells (MDA-MB-231) which overexpress VEGF165 or VEGF189 isoforms, we reported an increase of the adhesion of VEGF165-overexpressing MDA-MB-231, as compared with control cells; however, the adhesion of VEGF189-overexpressing MDA-MB-231 cells on fibronectin and vitronectin was increased as compared with VEGF165-overexpressing MDA-MB-231.13 Neutralizing antibodies against α5β1, but not αvβ3 and αvβ5, significantly inhibited the adhesion of V189 clones to fibronectin.13 Furthermore, the adhesion of V189 to vitronectin was significantly reduced by neutralizing antibodies against anti- αvβ5, but not by anti-α5β1 antibodies. The role of NRP1 in the VEGF-induced adhesion of MDA-MB-231 cells was suggested in our studies.13 Recently, a new VEGF-NRP2 signaling pathway was described in MDA-MB-435 cells;34 VEGF165-NRP2 activates the α6β1 integrin, making it able to form focal adhesions on laminin.34
Migration and invasion
An autocrine loop exists for VEGF to induce breast cancer cell migration/invasion. MCF-7 cells that are not a tumorigenic breast cancer cell line without estrogen supplementation and do not induce metastasis in mice have a low capacity of migration in vitro. MCF-7 cells express lower levels of VEGF than MDA-MB-231 cells which have high invasive and migration capacities. The first experiments demonstrating the autocrine effect of VEGF165 in the migration or invasion were described for MDA-MB-231 and T47D breast cancer cell lines.18,35 The expression of VEGF and its receptor NRP1 in MDA-MB-231 was correlated with the aggressiveness of the cells.35 This invasive property involves NRP1 but not VEGF-R2, which is poorly expressed in these cells.35 A possible mechanism is a competition between VEGF and Semaphorin 3A (SEMA 3A) for NRP1 in this cell line, as shown in experiments in which the protein levels of SEMA3A and VEGF in breast cancer cell lines were correlated with the chemotactic activity of the cells.35 Cells with the highest SEMA3A/VEGF protein ratio exhibited the lowest migration rate. In contrast, those that had the lowest ratio of SEMA3A/VEGF protein exhibited the highest migration rates. Other experiments have shown that the expression of VEGF induced the expression of CXCR4 which is responsible for the invasiveness.36
In our laboratory we isolated two subpopulations from the MDA-MB-231 parental cells through matrigel in vitro. In this way, we could isolate one population with poor invasive capacity (REF cells) from another with high capacity (INV cells). The characteristics of the invasive cells were a loss of extracellular matrix and endothelial attachment, an increase in survival and in metastasis colonization in nude mice model.37 The analysis of the genes differently regulated by these two cell lines revealed that the more invasive cells (INV) expressed VEGF more than 2-fold, as compared with REF cells. Also we could demonstrate by western blot analysis that the expression of the NRP1 receptors in the invasive cells was increased. However, we could not demonstrate an activation of CXCR4 in these cells. Also, we could not see differences in AKT activation between INV and REF cells.37 Cell migration and invasion result from a balance between cell adhesion and detachment, both of which are required for motility. If the adhesion force is too strong, the cells attach tightly to the substrate and are unable to disperse.
Indirectly, VEGF-induced migration has also been reported to be mediated by Snail, a transcription repressor of E-cadherin.38 VEGF and NRP1 increase snail expression by suppressing the activity of glycogen synthase kinase 3 (GSK3), a negative regulator of Snail. The subsequent attenuation of E-cadherin expression favors the emergence of a more invasive EMT phenotype.38 The autocrine pathway of VEGF in migration was also described for others cancer cell types. For example VEGF via VEGF-R2 followed by the activation of ras/ERK1 pathway increased the migration of PC3 prostate cell line.39 VEGF has been shown to increase the migration of hepatocarcinoma cells,40 and skin cancer cells.41 In conclusion, it seems clear that an autocrine loop exists for VEGF to induce cancer cell migration/invasion, but further experiments are required to understand the pathway(s) involved in the autocrine stimulation of migration/ invasion by VEGF.
Mechanisms of Autocrine Function in Cancer Cells: Role of Neuropilins
Neuropilin (NRP)-1 is a transmembrane protein expressed by endothelial cells and several other cell types, such as dendritic cells and malignant tumor cells. It has been shown that NRP1 regulates both endothelial cells and tumor cell functions. NRP1 is an essential receptor for angiogenesis. In cultured endothelial cells, NRP1 enhances VEGF-R2 signaling by binding to VEGF165 and promoting a complex formation between the three molecules.42,43 In vivo blockade of neuropilin-1 function induced an effect additive to that of anti-VEGF to inhibit tumor growth.44 NRP2 shares many properties with NRP-1. The binding of NRP2 with VEGFR-2 and VEGFR-3 has also been reported to promote endothelial cell survival and migration.45 Blockade of NRP2 function inhibits tumor cell metastasis.46
NRP1 and NRP2, which are multifunctional proteins frequently expressed by cancer cells, have been linked to tumor progression47,48 or metastasis.46 Evidence indicates that NRPs may contribute to the function of cancer cells by several specific mechanisms. NRP1 or NRP2 are strongly expressed in aggressive breast cancer cells, MDA-MB-231 or MDA-MB-435 respectively. The increase of NRP in tumor cells was found to correlate with survival, migration and chemoresistance.49 However, the complexity of NRP-VEGF interactions is underlined by the fact that NRP1 binds members of two different ligand families: the VEGF family and the class 3 semaphorin family (Sema 3A and Sema3B) of axonal guidance regulators.50 The multifaceted role of neuropilins in cancer has been reviewed recently by Ellis47 and Tamagnone.48
Neuropilins as VEGF receptors
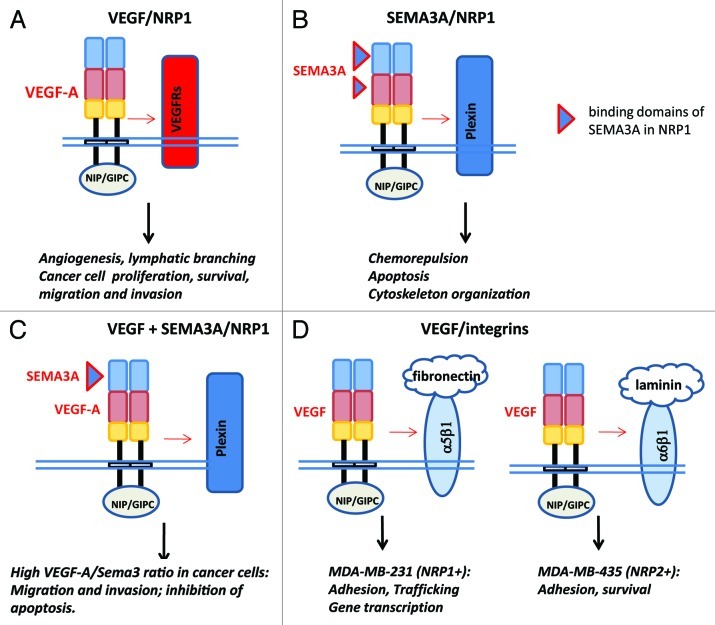
Using the BIACore system, we recently demonstrated that both VEGF165 and 189 binds directly to neuropilin-1.13 VEGF189 binds more strongly to NRP1 than VEGF165.13 In contrast, VEGF121 does not bind to NRP1. Thus, NRP1 appears as a receptor that recognized the heparin-binding VEGF splice forms. The binding of VEGF189 to NRP2 has not been reported until now. NRP1 was initially reported to bind to the exon 7-encoded region of VEGF-A (164/165). However, analysis of the crystal structure of the exon 7/8-encoded VEGF-A heparin binding domain in complex revealed that exon 8-encoded residues within the NRP1-b1 domain are implicated.51 The two neuropilins have a similar domain structure, containing an A domain (containing a1/a2), a B domain (containing b1/b2), a C domain that is thought to be possibly important for the interaction of NRP to other receptors and a transmembrane domain that has recently been shown to be important for homodimerization of neuropilins. The b1 domain of NRP1 is an essential binding site for the VEGF165 ligand (Fig. 1), the b2 domain being required for optimal binding. In addition, VEGF-164 binds 50-fold more strongly to NRP1 than NRP2.52 Differences in the amino acid composition of NRP L1 loop are responsible for the different affinities of VEGF for NRP1/NRP2; direct repulsion between the electronegative exon 7-encoded residues of the heparin binding domain and the electronegative L1 loop found only in NRP2 significantly contributes to the observed selectivity.52 Following the characterization of NRP as a VEGF receptor (Fig. 2B), it was found that NRPs are also able to function as receptors for the VEGF family members placental growth factor 2 (PlGF2), VEGF-B or VEGF-E.
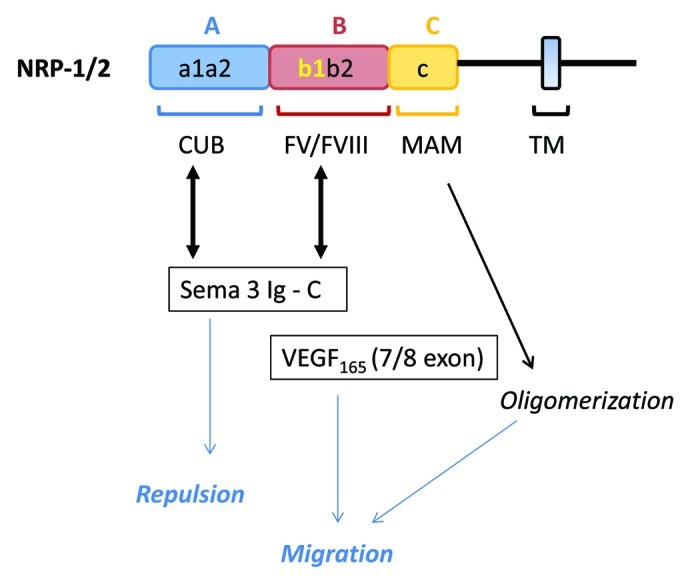
Figure 1. Potential complexes of VEGF and SEMA class 3 with NRPs. Electronegative charges in the B domain interact with both basic region in C and exon 7/8 domains of Sema 3 and VEGF, respectively. Semaphorins class 3 also interact with the a1/a2 domain via the Sema 3 and Ig sequences. The MAM domain induces oligomerization that is important to induce biological activities of both Sema and VEGF.
Figure 2. Schematic representation of molecular complexes involving neuropilins of the plasma membrane of cancer and/or endothelial cells. (A) Interaction between VEGF and neuropilin/VEGF receptor complexes; (B) interaction between class3 semaphorin and neuropilin/plexin complexes; (D) interaction between VEGF and neuropilin/integrin complexes. Oligomerization of NRPs molecule is induced by VEGF or SEMA3A as mentioned in Figure 1 via MAM domain (yellow box). NRPs forms complex with VEGFR (A) or plexin (B) to induce angiogenesis or repulsion and apoptosis. (C) Competition between VEGF and class3 semaphorin for neuropilin binding and activation of signaling pathway. Competition of VEGF and SEMA3A occurs at the level of b1 domain in NRP1. This mechanism is particularly relevant in tumor cell migration. (D) NRPs also participate in cell adhesion via α5β1, αvβ5 and α6β1 integrins. NRPs can also induce independent activities via interaction of the SEA domain with NIP/GIPS.
NRP1 as a major regulator of VEGF and class 3 semaphorin (SEMA3) signaling
As previously mentioned, VEGF165 exerts an autocrine effect, including survival and chemotaxis, on MDA-MB-231 breast cancer cells through neuropilin-1. It has been shown that competing autocrine pathway involving alternative neuropilin-1 ligands regulates chemotaxis or survival of carcinoma cells. Secreted semaphorins can exert either inhibitory or stimulatory functions in multiple tumor cell types.53,54 For example, increasing VEGF165 could modulate SEMA 3A signaling pathway, leading to increased chemotaxis.35 Reducing SEMA 3A and NRP1 expression by RNA interference signaling enhanced migration.35 In another study, SEMA 3B has been reported to induce apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect.55
Class 3 semaphorins are the only secreted semaphorins, while the others are membrane bound. The main receptors for semaphorins are the plexins. However, a subset of the secreted semaphorins requires the presence of neuropilins to bind to Plexin receptors (Fig. 2A). Semaphorins class 3 molecules interact with the A domain (containing a1/a2) of NRP-1 and NRP-2 (see review in ref. 48). In addition, the b1 domain mediates the high affinity of NRPs to the basic domain of Sema3 proteins.56-59 SEMA 3A and VEGF (165) have a common binding region in the b1 domain of NRP-1 via their C-terminal basic sequences (Ig domain for SEMA, c.f. Fig. 1). These two ligands can compete for their binding to the b1 domain of NRPs60,61 (Fig. 2C). While VEGF-A preferentially binds NRP1 rather than NRP2,52 semaphorins do not display the NRP-specific binding to the b1 domain. Furthermore, the proteolytic protein furin processes SEMA class 3 molecules to liberate the basic region recognized by NRP-1/2 binding domains. The furin-dependent proteolytic processing of Sema3 is important to allow the exposure of the NRP1-binding C-terminal sequence of Sema3 and to regulate the chemorepulsive activity of secreted semaphorins.62,63 Cell and tissue-specific proteolytic processing of semaphorin family members may thus represent an important mechanism controlling the production of repellent migration and anti-angiogenic activity of semaphorins.
NRP-integrin signaling
NRP was originally identified as a surface protein mediating cell adhesion.64 The possibility that NRP1/2 influences the activation and function of specific integrins [α5β1 (fibronectin), αvβ5 (vitronectin) and α6β1(laminin)] to contribute to tumor adhesion has been mentioned above. Autocrine VEGF is also necessary for the survival of serum-deprived cells through α6β131 (Fig. 2D).
NRP co-receptors for other receptors
NRP forms complexes with additional cell surface receptors. As an example, NRP1 binds latent and active transforming growth factor (TGF)-β1, and activates the latent form latency-associated peptide (LAP)-TGF-β1 in some breast cancer cells.65 Since TGF-β promotes metastasis, this finding is highly relevant to cancer biology.
NRP-synectin binding
There is evidence that the neuropilins may function independently of tyrosine kinase VEGF receptors. NRP have short intracellular domains that were considered to be too short to support independent signal transduction. Indeed, NRP1 possesses an active cytoplasmic motif (C-terminal three amino-acid), known as SEA, which promotes its association by bridging to an adaptator protein, the PDZ protein of synectin (also known as GIPC1/NIP).66-69 This interaction was first shown to mediate VEGF/NRP1-dependent endothelial migration in angiogenesis. Neuropilin is not only subjected to endocytosis upon ligand binding, but is also implicated to regulate the trafficking of associated molecules at the cell surface, such as the vesicular trafficking of integrin α5β170,71 (Fig. 2D). The SEA motif also promotes fibronectin assembly in vitro. Altogether, these findings support the hypothesis that the activation state of the VEGF-NRP signaling is tightly regulated by autocrine and paracrine factors present in the tumor environment.
Conclusions and Perspectives
In conclusion, in vitro data and transgenic models have enabled an evaluation of the various effects of VEGF-A on mammary tumor development and metastasis. VEGF-A(164/189) involves both blood vessels and tumor cells to increase neovascularization and vasodilation/vessel maturation, and acts by selective autocrine effects to stimulate tumor cell proliferation, survival, adhesion and chemotaxis. The production of VEGF by breast tumor cells, and the activation of VEGF receptors at the surface of cancer cells indicate the existence of a distinct autocrine signaling loop that enables breast cancer cells to promote their own growth, survival and migration by phosphorylation and activation of VEGFR-1/2 or VEGF-induced NRP signaling.
Understanding the mechanisms that mediate autocrine and paracrine VEGF/VEGFR/NRP signaling is of primary importance due to the growing therapeutic use of cancer inhibitors.72 The monoclonal anti-VEGF antibody bevacizumab prevents VEGF binding to the VEGFR-1/2 receptor with successful inhibition of tumor angiogenesis and shows improvement in some metastatic cancers, but not in others. The negative response to angiogenic therapies might be due to an acquired resistance of tumor cells themselves to anti-VEGF, or by recruitment of circulating endothelial progenitors. Cancer stem cells (CSCs) have been described in various cancers. Using a mouse model of skin tumors, Beck et al.41 have investigated the impact of the vascular niche and VEGF signaling on controlling the ‘stemness’ (the ability to self-renew and differentiate) of cancer cells (CSCs) during the early stages of tumor progression. CSCs of skin papillomas are localized in a perivascular niche, in the immediate vicinity of endothelial cells.41 Furthermore, blocking VEGF-R2 induces tumor regression not only by decreasing the microvascular density, but also by reducing CSC pool size and impairing CSC renewal properties. Thus, a vascular niche and a VEGF-NRP1 loop regulate the initiation and stemness of skin tumors. A direct autocrine effect of VEGF on tumor cells may account for the efficiency of VEGF-blockade therapies. However, the effects of targeting specific VEGF isoforms in vivo have received little attention in the clinical setting. The recent identification of novel splice variants of VEGF with anti-angiogenic properties (VEGFb) provided some insight on the lack of current treatment efficacy.73,74 The selective effects of VEGFb isoforms on breast cancer cells, as compared with pro-angiogenic isoforms, are unknown. However, another type of resistance could be caused by increased VEGF/VEGF-R2 signaling or VEGF/NRP signaling. An increased autocrine VEGF/VEGFR2 signaling through p38 mitogen-activated kinase has been reported in tamoxifen-resistant MCF7 cells.25 High levels of NRP1 were significantly associated with chemoresistance and a poor outcome in patients with breast cancers,49 and NRP2 expression correlates with poor prognosis and lymph node metastasis formation. In conclusion, the acknowledgment of the VEGF autocrine pathway provides a new insight in cancer therapy and has to be investigated in the future. This autocrine loop represents an attractive therapeutic target.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/23332
References
- 1.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/er.18.1.4. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–54. [PubMed] [Google Scholar]
- 4.Gitay-Goren H, Soker S, Vlodavsky I, Neufeld G. The binding of vascular endothelial growth factor to its receptors is dependent on cell surface-associated heparin-like molecules. J Biol Chem. 1992;267:6093–8. [PubMed] [Google Scholar]
- 5.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–26. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267:26031–7. [PubMed] [Google Scholar]
- 7.Plouët J, Moro F, Bertagnolli S, Coldeboeuf N, Mazarguil H, Clamens S, et al. Extracellular cleavage of the vascular endothelial growth factor 189-amino acid form by urokinase is required for its mitogenic effect. J Biol Chem. 1997;272:13390–6. doi: 10.1074/jbc.272.20.13390. [DOI] [PubMed] [Google Scholar]
- 8.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–91. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 10.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 11.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–78. doi: 10.5483/BMBRep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 12.Kappas NC, Zeng G, Chappell JC, Kearney JB, Hazarika S, Kallianos KG, et al. The VEGF receptor Flt-1 spatially modulates Flk-1 signaling and blood vessel branching. J Cell Biol. 2008;181:847–58. doi: 10.1083/jcb.200709114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hervé M-A, Buteau-Lozano H, Vassy R, Bieche I, Velasco G, Pla M, et al. Overexpression of vascular endothelial growth factor 189 in breast cancer cells leads to delayed tumor uptake with dilated intratumoral vessels. Am J Pathol. 2008;172:167–78. doi: 10.2353/ajpath.2008.070181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ancelin M, Buteau-Lozano H, Meduri G, Osborne-Pellegrin M, Sordello S, Plouët J, et al. A dynamic shift of VEGF isoforms with a transient and selective progesterone-induced expression of VEGF189 regulates angiogenesis and vascular permeability in human uterus. Proc Natl Acad Sci U S A. 2002;99:6023–8. doi: 10.1073/pnas.082110999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, et al. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–98. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tozer GM, Akerman S, Cross NA, Barber PR, Björndahl MA, Greco O, et al. Blood vessel maturation and response to vascular-disrupting therapy in single vascular endothelial growth factor-A isoform-producing tumors. Cancer Res. 2008;68:2301–11. doi: 10.1158/0008-5472.CAN-07-2011. [DOI] [PubMed] [Google Scholar]
- 17.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–45. doi: 10.1016/S0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 18.Price DJ, Miralem T, Jiang S, Steinberg R, Avraham H. Role of vascular endothelial growth factor in the stimulation of cellular invasion and signaling of breast cancer cells. Cell Growth Differ. 2001;12:129–35. [PubMed] [Google Scholar]
- 19.Weigand M, Hantel P, Kreienberg R, Waltenberger J. Autocrine vascular endothelial growth factor signalling in breast cancer. Evidence from cell lines and primary breast cancer cultures in vitro. Angiogenesis. 2005;8:197–204. doi: 10.1007/s10456-005-9010-0. [DOI] [PubMed] [Google Scholar]
- 20.Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, et al. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001;61:5736–40. [PubMed] [Google Scholar]
- 21.Miralem T, Steinberg R, Price D, Avraham H. VEGF(165) requires extracellular matrix components to induce mitogenic effects and migratory response in breast cancer cells. Oncogene. 2001;20:5511–24. doi: 10.1038/sj.onc.1204753. [DOI] [PubMed] [Google Scholar]
- 22.Yasuoka H, Kodama R, Tsujimoto M, Yoshidome K, Akamatsu H, Nakahara M, et al. Neuropilin-2 expression in breast cancer: correlation with lymph node metastasis, poor prognosis, and regulation of CXCR4 expression. BMC Cancer. 2009;9:220. doi: 10.1186/1471-2407-9-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoeffner DJ, Matheny SL, Akahane T, Factor V, Berry A, Merlino G, et al. VEGF contributes to mammary tumor growth in transgenic mice through paracrine and autocrine mechanisms. Lab Invest. 2005;85:608–23. doi: 10.1038/labinvest.3700258. [DOI] [PubMed] [Google Scholar]
- 24.Buteau-Lozano H, Ancelin M, Lardeux B, Milanini J, Perrot-Applanat M. Transcriptional regulation of VEGF by estradiol and tamoxifen in breast cancer cells: a complex interplay between estrogen receptors alpha and beta. Cancer Res. 2002;62:4977–84. [PubMed] [Google Scholar]
- 25.Aesoy R, Sanchez BC, Norum JH, Lewensohn R, Viktorsson K, Linderholm B. An autocrine VEGF/VEGFR2 and p38 signaling loop confers resistance to 4-hydroxytamoxifen in MCF-7 breast cancer cells. Mol Cancer Res. 2008;6:1630–8. doi: 10.1158/1541-7786.MCR-07-2172. [DOI] [PubMed] [Google Scholar]
- 26.Barr MP, Bouchier-Hayes DJ, Harmey JJ. Vascular endothelial growth factor is an autocrine survival factor for breast tumour cells under hypoxia. Int J Oncol. 2008;32:41–8. [PubMed] [Google Scholar]
- 27.Pidgeon GP, Barr MP, Harmey JH, Foley DA, Bouchier-Hayes DJ. Vascular endothelial growth factor (VEGF) upregulates BCL-2 and inhibits apoptosis in human and murine mammary adenocarcinoma cells. Br J Cancer. 2001;85:273–8. doi: 10.1054/bjoc.2001.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mercurio AM, Lipscomb EA, Bachelder RE. Non-angiogenic functions of VEGF in breast cancer. J Mammary Gland Biol Neoplasia. 2005;10:283–90. doi: 10.1007/s10911-006-9001-9. [DOI] [PubMed] [Google Scholar]
- 29.Lee T-H, Seng S, Sekine M, Hinton C, Fu Y, Avraham HK, et al. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4:e186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vintonenko N, Pelaez-Garavito I, Buteau-Lozano H, Toullec A, Lidereau R, Perret GY, et al. Overexpression of VEGF189 in breast cancer cells induces apoptosis via NRP1 under stress conditions. Cell Adh Migr. 2011;5:332–43. doi: 10.4161/cam.5.4.17287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung J, Yoon S, Datta K, Bachelder RE, Mercurio AM. Hypoxia-induced vascular endothelial growth factor transcription and protection from apoptosis are dependent on alpha6beta1 integrin in breast carcinoma cells. Cancer Res. 2004;64:4711–6. doi: 10.1158/0008-5472.CAN-04-0347. [DOI] [PubMed] [Google Scholar]
- 32.Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, et al. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–35. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchings H, Ortega N, Plouët J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. FASEB J. 2003;17:1520–2. doi: 10.1096/fj.02-0691fje. [DOI] [PubMed] [Google Scholar]
- 34.Goel HL, Pursell B, Standley C, Fogarty K, Mercurio AM. Neuropilin-2 regulates α6β1 integrin in the formation of focal adhesions and signaling. J Cell Sci. 2012;125:497–506. doi: 10.1242/jcs.094433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, et al. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63:5230–3. [PubMed] [Google Scholar]
- 36.Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002;62:7203–6. [PubMed] [Google Scholar]
- 37.Abdelkarim M, Vintonenko N, Starzec A, Robles A, Aubert J, Martin ML, et al. Invading basement membrane matrix is sufficient for MDA-MB-231 breast cancer cells to develop a stable in vivo metastatic phenotype. PLoS One. 2011;6:e23334. doi: 10.1371/journal.pone.0023334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wanami LS, Chen HY, Peiró S, García de Herreros A, Bachelder RE. Vascular endothelial growth factor-A stimulates Snail expression in breast tumor cells: implications for tumor progression. Exp Cell Res. 2008;314:2448–53. doi: 10.1016/j.yexcr.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darrington E, Zhong M, Vo BH, Khan SA. Vascular endothelial growth factor A, secreted in response to transforming growth factor-β1 under hypoxic conditions, induces autocrine effects on migration of prostate cancer cells. Asian J Androl. 2012;14:745–51. doi: 10.1038/aja.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu W, Huang JJ, Cheung PC. Extract of Pleurotus pulmonarius suppresses liver cancer development and progression through inhibition of VEGF-induced PI3K/AKT signaling pathway. PLoS One. 2012;7:e34406. doi: 10.1371/journal.pone.0034406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. [DOI] [PubMed] [Google Scholar]
- 42.Soker S, Miao HQ, Nomi M, Takashima S, Klagsbrun M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem. 2002;85:357–68. doi: 10.1002/jcb.10140. [DOI] [PubMed] [Google Scholar]
- 43.Whitaker GB, Limberg BJ, Rosenbaum JS. Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF(165) and VEGF(121) J Biol Chem. 2001;276:25520–31. doi: 10.1074/jbc.M102315200. [DOI] [PubMed] [Google Scholar]
- 44.Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Favier B, Alam A, Barron P, Bonnin J, Laboudie P, Fons P, et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108:1243–50. doi: 10.1182/blood-2005-11-4447. [DOI] [PubMed] [Google Scholar]
- 46.Caunt M, Mak J, Liang WC, Stawicki S, Pan Q, Tong RK, et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13:331–42. doi: 10.1016/j.ccr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 47.Ellis LM. The role of neuropilins in cancer. Mol Cancer Ther. 2006;5:1099–107. doi: 10.1158/1535-7163.MCT-05-0538. [DOI] [PubMed] [Google Scholar]
- 48.Rizzolio S, Tamagnone L. Multifaceted role of neuropilins in cancer. Curr Med Chem. 2011;18:3563–75. doi: 10.2174/092986711796642544. [DOI] [PubMed] [Google Scholar]
- 49.Wey JS, Gray MJ, Fan F, Belcheva A, McCarty MF, Stoeltzing O, et al. Overexpression of neuropilin-1 promotes constitutive MAPK signalling and chemoresistance in pancreatic cancer cells. Br J Cancer. 2005;93:233–41. doi: 10.1038/sj.bjc.6602663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–62. doi: 10.1016/S0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 51.Parker MW, Xu P, Li X, Vander Kooi CW. Structural basis for selective vascular endothelial growth factor-A (VEGF-A) binding to neuropilin-1. J Biol Chem. 2012;287:11082–9. doi: 10.1074/jbc.M111.331140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parker MW, Xu P, Guo HF, Vander Kooi CW. Mechanism of Selective VEGF-A Binding by Neuropilin-1 Reveals a Basis for Specific Ligand Inhibition. PLoS One. 2012;7:e49177. doi: 10.1371/journal.pone.0049177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8:632–45. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- 54.Tamagnone L. Emerging role of semaphorins as major regulatory signals and potential therapeutic targets in cancer. Cancer Cell. 2012;22:145–52. doi: 10.1016/j.ccr.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 55.Castro-Rivera E, Ran S, Thorpe P, Minna JD. Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect. Proc Natl Acad Sci U S A. 2004;101:11432–7. doi: 10.1073/pnas.0403969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giger RJ, Urquhart ER, Gillespie SK, Levengood DV, Ginty DD, Kolodkin AL. Neuropilin-2 is a receptor for semaphorin IV: insight into the structural basis of receptor function and specificity. Neuron. 1998;21:1079–92. doi: 10.1016/S0896-6273(00)80625-X. [DOI] [PubMed] [Google Scholar]
- 57.Renzi MJ, Feiner L, Koppel AM, Raper JA. A dominant negative receptor for specific secreted semaphorins is generated by deleting an extracellular domain from neuropilin-1. J Neurosci. 1999;19:7870–80. doi: 10.1523/JNEUROSCI.19-18-07870.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen H, He Z, Bagri A, Tessier-Lavigne M. Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron. 1998;21:1283–90. doi: 10.1016/S0896-6273(00)80648-0. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura F, Tanaka M, Takahashi T, Kalb RG, Strittmatter SM. Neuropilin-1 extracellular domains mediate semaphorin D/III-induced growth cone collapse. Neuron. 1998;21:1093–100. doi: 10.1016/S0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- 60.Geretti E, Shimizu A, Kurschat P, Klagsbrun M. Site-directed mutagenesis in the B-neuropilin-2 domain selectively enhances its affinity to VEGF165, but not to semaphorin 3F. J Biol Chem. 2007;282:25698–707. doi: 10.1074/jbc.M702942200. [DOI] [PubMed] [Google Scholar]
- 61.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–42. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adams RH, Lohrum M, Klostermann A, Betz H, Püschel AW. The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J. 1997;16:6077–86. doi: 10.1093/emboj/16.20.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parker MW, Hellman LM, Xu P, Fried MG, Vander Kooi CW. Furin processing of semaphorin 3F determines its anti-angiogenic activity by regulating direct binding and competition for neuropilin. Biochemistry. 2010;49:4068–75. doi: 10.1021/bi100327r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shimizu M, Murakami Y, Suto F, Fujisawa H. Determination of cell adhesion sites of neuropilin-1. J Cell Biol. 2000;148:1283–93. doi: 10.1083/jcb.148.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glinka Y, Stoilova S, Mohammed N, Prud’homme GJ. Neuropilin-1 exerts co-receptor function for TGF-beta-1 on the membrane of cancer cells and enhances responses to both latent and active TGF-beta. Carcinogenesis. 2011;32:613–21. doi: 10.1093/carcin/bgq281. [DOI] [PubMed] [Google Scholar]
- 66.Cai H, Reed RR. Cloning and characterization of neuropilin-1-interacting protein: a PSD-95/Dlg/ZO-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. J Neurosci. 1999;19:6519–27. doi: 10.1523/JNEUROSCI.19-15-06519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao Y, Li M, Chen W, Simons M. Synectin, syndecan-4 cytoplasmic domain binding PDZ protein, inhibits cell migration. J Cell Physiol. 2000;184:373–9. doi: 10.1002/1097-4652(200009)184:3<373::AID-JCP12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 68.Wang L, Mukhopadhyay D, Xu X. C terminus of RGS-GAIP-interacting protein conveys neuropilin-1-mediated signaling during angiogenesis. FASEB J. 2006;20:1513–5. doi: 10.1096/fj.05-5504fje. [DOI] [PubMed] [Google Scholar]
- 69.Prahst C, Héroult M, Lanahan AA, Uziel N, Kessler O, Shraga-Heled N, et al. Neuropilin-1-VEGFR-2 complexing requires the PDZ-binding domain of neuropilin-1. J Biol Chem. 2008;283:25110–4. doi: 10.1074/jbc.C800137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valdembri D, Caswell PT, Anderson KI, Schwarz JP, König I, Astanina E, et al. Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol. 2009;7:e25. doi: 10.1371/journal.pbio.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang L, Lau JS, Patra CR, Cao Y, Bhattacharya S, Dutta S, et al. RGS-GAIP-interacting protein controls breast cancer progression. Mol Cancer Res. 2010;8:1591–600. doi: 10.1158/1541-7786.MCR-10-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8:210–21. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8:880–7. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hilmi C, Guyot M, Pagès G. VEGF spliced variants: possible role of anti-angiogenesis therapy. J Nucleic Acids. 2012;2012:162692–8. doi: 10.1155/2012/162692. [DOI] [PMC free article] [PubMed] [Google Scholar]