Abstract
Anti-angiogenic vascular endothelial growth factor A (VEGF)165b and pro-angiogenic VEGF165 are generated from the same transcript, and their relative amounts are dependent on alternative splicing. The role of VEGF165b has not been investigated in as much detail as VEGF165, although it appears to be highly expressed in non-angiogenic tissues and, in contrast with VEGF165, is downregulated in tumors and other pathologies associated with abnormal neovascularization such as diabetic retinopathy or Denys Drash syndrome. VEGF165b inhibits VEGFR2 signaling by inducing differential phosphorylation, and it can be used to block angiogenesis in in vivo models of tumorigenesis and angiogenesis-related eye disease. Recent reports have identified three serine/arginine-rich proteins, SRSF1, SRSF2 and SRSF6, and studied their role in regulating terminal splice-site selection. Since the balance of VEGF isoforms is lost in cancer and angiogenesis-related conditions, control of VEGF splicing could also be used as a basis for therapy in these diseases.
Keywords: angiogenesis, VEGF165b, alternative splicing, cancer, age-related macular degeneration, diabetic retinopathy, Denys Drash syndrome, pre-eclampsia, systemic sclerosis
Cells of pluricellular organisms require access to the circulation to subsist. Blood vessels carry the nutrients and oxygen necessary for the survival of these cells, and remove metabolic waste and carbon dioxide away from them. In the adult, the formation of new blood vessels is a tightly regulated phenomenon, becoming activated during brief periods of time such as wound healing or the female reproductive cycle, and subsequently inhibited.1 In a physiological context, stimulators and inhibitors apply positive and negative influences on blood vessel formation to ensure that it is limited to the demands of the growing tissue. This balance is lost in many pathological conditions, leading to an angiogenic phenotype. Vascular endothelial growth factor (VEGF) is a key contributor to this process in health and disease. The VEGF family of growth factors includes VEGF-A, placental growth factor (PGF), VEGF-B, VEGF-C and VEGF-D, being VEGF-A the most important member of the family.
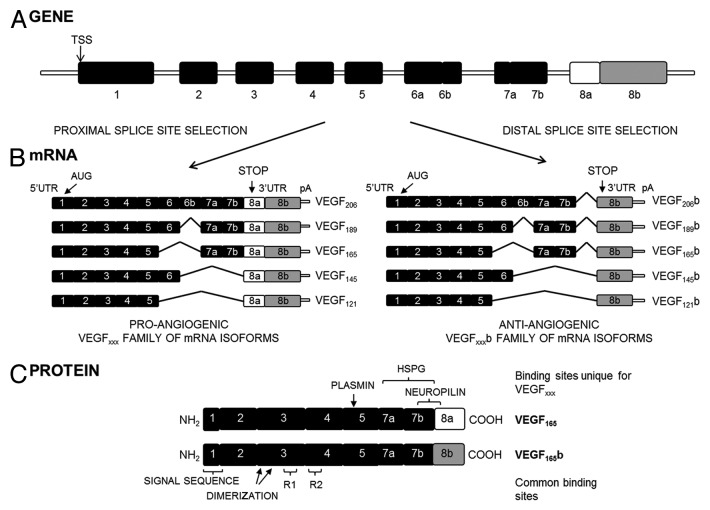
The human vegf-a gene contains eight exons and seven introns.2 Alternative splicing and proteolytic processing of VEGF-A produces various isoforms with distinct biological activities (Fig. 1A).3-8 The particular splicing event in the terminal exon creates two whole families of isoforms: the classic pro-angiogenic VEGFxxx isoforms, generated by proximal splice-site (PSS) selection, and the more recently described anti-angiogenic VEGFxxxb isoforms, formed by distal splice-site (DSS) choice, 66 bases downstream (Fig. 1B).9 This process gives rise to two families of polypeptides with the same size but different sequence at the C-terminus, maintaining the two of them the dimerization and receptor binding domains unchanged.3 Exon 8a encodes Cys-Asp-Lys-Pro-Arg-Arg instead of the Ser-Leu-Thr-Arg-Lys-Asp sequence encoded by exon 8b. Therefore, the basic properties conferred by the two arginines present in 8a are altered, resulting in the neutral charge conferred by the residues lysine and aspartic acid, and loss of one disulfide bond. These conformational changes result in the inability of VEGFxxxb to bind the VEGFR2 co-receptor neuropilin 1 (NRP1), and have been proposed as the cause for the differences in downstream signaling (Fig. 1C).10,11
Figure 1.vegf-a. (A) Gene structure. TSS is the transcriptional start site. (B) mRNA species. Alternative splicing of the vegf-a gene in the terminal exon results in two families of isoforms: the pro-angiogenic VEGFxxx and the anti-angiogenic VEGFxxxb isoforms. AUG, the start site for translation; UTR, the untranslated region; pA, the polyadenylation site. (C) Protein structure of the two major isoforms of each family, VEGF165 and VEGF165b. C-terminal splicing leads to an alternative last six amino acids (CDKPRR or SLTRKD). The isoforms are termed according to the amino acid number of the resulting protein (xxx). HSPG, heparin sulfate proteoglycan; R1, VEGF Receptor 1; R2, VEGF Receptor 2 (from ref. 29).
VEGF165b Physiological Expression and Function
VEGF165b, the major anti-angiogenic isoform, was the first member of the VEGFxxxb family to be described3 and is the most studied member so far. VEGF165b mRNA was first isolated and cloned from human renal cortex tissue, and subsequently identified in other human tissues. In fact, anti-angiogenic VEGF isoforms have been reported to represent a predominant proportion of the total VEGF protein found in normal human tissues such as vitreous fluid, circulating plasma, urine, renal cortex and glomeruli, colonic epithelium, bladder, smooth muscle, lung and pancreatic islets.9,12,13 On the contrary, in placenta, a tissue where angiogenesis takes place under physiological conditions, VEGFxxxb represents only 1.5% of total VEGF.12 In vitro, VEGFxxxb also predominates in primary cultured cells, such as differentiated podocytes or retinal pigmented epithelial cells. VEGFxxxb isoforms have also been reported in other species including pig,14 rabbit, mouse15 and rat,16,17 although its relative expression has been found to be decreased from humans to lower mammals, suggesting that the inclusion level of exon 8b, as well as the complexity of the regulatory mechanism of its splicing, increases through the evolutionary process from mouse to human.15 The splice site is not conserved in lower vertebrates,3 and there is no information on the existence of VEGF165b outside mammals.
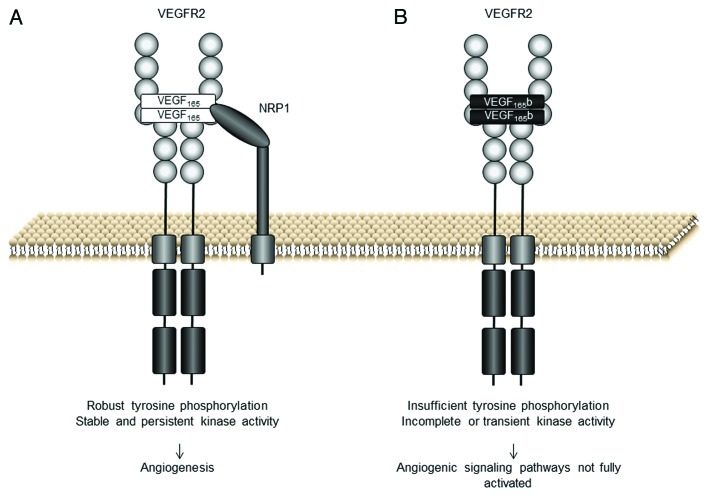
VEGF165 binding to VEGFR2 and NRP1 results in dimerization of the receptor and rotation of its intracellular domain, leading to its autophosphorylation (Fig. 2A).18 By contrast, this full rotational change is predicted to not occur when VEGF165b binds VEGFR2, resulting in inefficient autophosphorylation of the receptor (Fig. 2B). In fact, the preference of VEGF165b toward the various tyrosine phosphorylation sites of the receptor is different than that observed after VEGF165 activation. Particularly, tyrosine 105418 in the kinase regulatory site is not phosphorylated following VEGF165b-VEGFR2 binding. Once VEGF165b has bound the receptor, what happens downstream? Little is known about the mechanisms of VEGF165b signaling. VEGF165b stimulates VEGFR2, ERK1/2 and Akt phosphorylation in endothelial cells.8,19 However, VEGF165b-induced phosphorylation of ERK1/2 has been observed to be more transient and weaker than that promoted by VEGF165.4,8,18,19
Figure 2. VEGF165b and VEGF165 interaction with VEGF receptor 2 (VEGFR2). (A) The VEGFR2 binding site of VEGF165 interacts with the VEGFR2 extracellular domain. VEGF165 functions as a dimer and promotes the formation of VEGFR2 dimers resulting in activation of the kinase domains via the phosphorylation of its tyrosine residues. Robust tyrosine phosphorylation results in the activation of angiogenic signaling pathways. (B) VEGF165b binds the VEGFR2 binding site with equal affinity to VEGF165 but does not bind neuropilin 1 (NRP1) co-receptor. The C-terminus of VEGF165b is neutral and there is insufficient torsional rotation for complete tyrosine phosphorylation, although weak phosphorylation can occur (adapted from ref. 11)
VEGF165 and VEGF165b appear to have contrasting physiological roles. The functions of VEGF165b are summarized in Table 1.
Table 1. VEGF165b physiological functions.
| Function | Effect | References |
|---|---|---|
|
Proliferation and migration |
VEGF165b does not stimulate endothelial cell proliferation, and inhibited proliferation in the presence of VEGF165 and inhibits VEGF165-stimulated migration of endothelial cells |
3 and 35 |
| Endogenous overexpression of VEGF165b inhibits migration and proliferation of endothelial cells |
8 |
|
|
Angiogenesis |
VEGF165b inhibited endothelial cell tube formation in vitro |
30 |
| VEGF165b inhibited physiological angiogenesis in mammary tissue in transgenic mice and rabbit cornea |
8 and 45 |
|
| VEGF165b can specifically inhibit VEGF165-induced angiogenesis in mouse skin, rat mesentery, rabbit cornea, and chick chorioallantoic membrane |
4, 8, 28, 30 and 31 |
|
| VEGF165b prevents angiogenesis and helps maintaining the established vascular system in the fetal human eye |
54 |
|
|
Survival and cytoprotection |
VEGF165b acts as a survival factor in different cell types including endothelial cells, podocytes, retinal pigmented cells or colonic adenoma cells, but not in cone photoreceptors |
12, 22, 28, 35 and 55 |
| VEGF165b could protect the retina from ischemic damage |
35 |
|
|
Permeability and vasodilatation |
VEGF165b does not increase chronic microvascular permeability |
56 |
| Inhibition of VEGFxxxb reduces glomerular endothelial and VEGF165-induced permeability in vitro |
12 |
|
| VEGF165b did not stimulate vasodilatation ex vivo, and inhibited that mediated by VEGF165 |
57 |
|
| Gonadal development | Inhibition of VEGFxxxb in developing testis stimulates vascular development similarly to VEGF164 addition |
58 |
| Inhibition of VEGFxxxb in developing ovary results in follicle progression similarly to VEGF164 stimulation, and abnormal ovarigenesis due to enhanced neovascularization | 16 |
Regulation of VEGF165b Expression
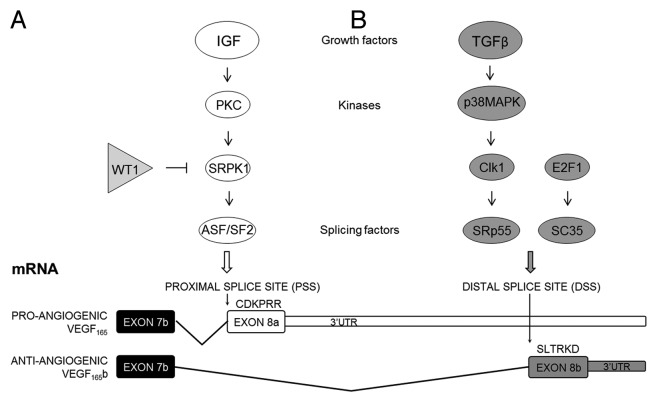
VEGF165b expression can be regulated by growth factors, including insulin-like growth factor 1 (IGF-1), tumor necrosis factor α (TNFα) and transforming growth factor β (TGFβ) in retinal pigmented epithelial cells, podocytes and dermal microvascular endothelial cells (MVECs). IGF-1 and TNFα have been shown to modulate VEGF splicing preferentially at the PSS increasing the expression of pro-angiogenic VEGF isoforms. In podocytes, IGF-mediated VEGFxxx upregulation occurs via stimulation of protein kinase C (PKC), which in turn activates the serine/arginine protein kinase 1 (SRPK1). Finally, SRPK1 phosphorylates the serine/arginine-rich splicing factor 1 (SRSF1), also known as ASF/SF2. Once activated, SRSF1, previously identified as an oncogene due to its transforming capacity in vitro and in vivo,20 is able to bind VEGF pre-mRNA in the area adjacent to the PSS promoting VEGFxxx expression (Fig. 3A).21,22
Figure 3. Regulation of VEGF alternative splicing in the terminal exon.
On the other hand, TGFβ upregulates anti-angiogenic VEGF isoforms most likely through stimulation of the p38 MAPK pathway and successive downstream activation of Clk/sty kinases, which results in phosphorylation of the serine/arginine-rich splicing factor 6 (SRSF6), also known as SRp55. SRSF6 binds directly to VEGF pre-mRNA in the exon 8b region favoring DSS selection and therefore, VEGFxxxb expression (Fig. 3B).22,23 Finally, transcription factor 1 (E2F1) also favors the expression of the anti-angiogenic isoforms in human lung carcinoma cell lines through upregulation of another serine/arginine-rich splicing factor, SRSFR2, also known as SC35 (Fig. 3B).24 SRSF2 overexpression is able to mimic the E2F1-induced VEGF165b expression in vitro, and decreases tumor neovascularization in vivo. In contrast, a direct correlation between VEGFxxx and E2F1 has been observed in colorectal cancer specimens25 suggesting that the regulation of vegf-a splicing by E2F1, and in general by SR splicing factors in human tumor cells, could be more complex and might depend on different factors such as the cell type, the amount of the different SR proteins, or other upstream signals.
VEGF165b in Pathology
The role of VEGF165b in cancer
Vasculature not only provides tumors with an adequate blood supply, but it offers a route for tumor cells to metastasize.26 An upregulation of the pro-angiogenic VEGFxxx variants has been widely reported in human tumors. This upregulation brings about a loss in the balance of isoforms, which causes a drop in the proportion of VEGFxxxb levels. Indeed, VEGFxxxb is downregulated in several adult epithelial cancer types including melanoma,27 renal8 or colorectal carcinoma.25,28 An imbalance of the expression of the two VEGF families of isoforms has also been observed in the pediatric cancer neuroblastoma.29 In contrast, VEGFxxxb is upregulated in intraductal breast carcinoma.19
Overexpression of VEGF165b has been reported to delay the growth of melanoma,8 kidney,30 colon,28 prostate and Ewing sarcoma tumors in mice.31 Recombinant human VEGF165b (rhVEGF165b) treatment in vivo also has a growth-inhibitory effect in nude mice xenograft models of melanoma,8 prostate cancer, renal cell carcinoma, Ewing’s sarcoma,31 colorectal carcinoma28 and neuroblastoma.29 Likewise, rhVEGF165b parenteral administration reduces the growth of melanoma metastases.13 These studies highlight the potential role of VEGF165b as an anti-cancer agent. However, a proper selection of patients that can benefit from this treatment is essential, as rhVEGF165b-based therapies are likely to be effective only in tumors with high VEGF expression vs. those with low VEGF levels, which must rely on other pro-angiogenic factors for their growth.19
Finally, it has been shown that the VEGFxxx/VEGFxxxb ratio has an effect on the sensitivity of tumors to bevacizumab, an anti-VEGF antibody licensed for use in cancer treatment: as both VEGF165 and VEGF165b can bind bevacizumab with a similar affinity, the presence of VEGFxxxb can inhibit the effect of this drug through reducing the amounts of antibody available to bind VEGFxxx.28 Hence, despite having a slower growing rate, tumors with high concentrations of VEGFxxxb might be more resistant to this particular therapy. One alternative treatment option for these tumors would be to use VEGFxxx-specific antibodies, which would inhibit exclusively the pro-angiogenic isoforms. Another possibility would be to develop molecules able to favor the DSS selection, and thus increase the endogenous expression of VEGFxxxb. Indeed, it has been shown that knocking down SRPK1 in a colorectal tumor cell line sensitive to the anti-angiogenic actions of VEGF165b, decreases tumor growth in vivo.32
The role of VEGF165b in eye disease
A high expression of VEGF165b has been reported in human eye tissues, such as the retina or vitreous fluid,9 and in the rodent eye.17,33 However, VEGF165b is relatively downregulated in vitreous fluid of diabetic retinopathy patients, resulting in a switch to an angiogenic phenotype.9 In fact, proliferative diabetic retinopathy is characterized by an abnormal growth of new blood vessels into the vitreous fluid of the eye, stimulated by hypoxia-induced VEGF expression. In a mouse model of oxygen-induced retinopathy (OIR), the development of neovascularization in the retina correlated with an increase of total VEGF, and a specific decrease of VEGF165b, indicating a pro-angiogenic shift.33 In this OIR mouse model, intraocular injection of rhVEGF165b also diminished pre-retinal neovascularization yet permitting the physiological angiogenesis in the inner retina to proceed normally.34 This lack of intra-retinal neovascularization could be a consequence of the cytoprotective effects of VEGF165b on endothelial cells.35 Thus, treatments with VEGF165b would not only inhibit neovascularization but still protect existing endothelial cells in a disease in which angiogenesis is frequently a consequence of endothelial cell loss and ischemia.35 The VEGF balance is also altered in other angiogenic-associated eye conditions, including retinal vein occlusion36 or neovascular age-related macular degeneration (AMD), and rhVEGF165b acted as a powerful anti-angiogenic agent when injected either subcutaneously or intraocularly in a mouse model of AMD, where choroidal neovascularization was laser-induced.37 These results, together with the cytoprotection that VEGF165b confers to cultured retinal epithelial cells,35 point to this anti-angiogenic isoform as a potential therapeutic agent in angiogenesis-associated eye diseases.
In contrast, in avascular eye diseases, including glaucoma,17 rhegmatogenous retinal detachment and proliferative vitreoretinopathy,38 VEGFxxxb has been found to be upregulated suggesting that, besides impeding neovascularization, VEGF165b might play a role in the pathogenesis of these conditions that are not angiogenesis-related.
The role of VEGF165b in renal disease
VEGF165b expression has been reported in the glomeruli of human kidneys.13 However, whereas overexpression of VEGF164 in mouse glomeruli leads to proteinuria, glomerular dysfunction and renal failure,39 a transgenic mouse model where VEGF165b was overexpressed in podocytes, did not have consequences on renal function. Nevertheless, closer observations of these podocytes showed decreased glomerular water permeability, which was dose-dependent.40 In addition, ex vivo incubation of wild-type mouse glomeruli with VEGF165b, decreased permeability to water,41 and constitutive overexpression of VEGF165b could prevent the observed VEGF164-driven increased water permeability and the changes in glomerular ultrastructure in vivo,42 indicating that maintaining an appropriate VEGF isoform balance is also important in the glomerular function of the kidney.
Changes in the balance of VEGF-A variants were also found to be important in Denys Drash syndrome, a urogenital disorder associated with nephropathy and high risk for Wilms tumors. While pro-angiogenic VEGF165 was upregulated, VEGF165b was completely abrogated in glomeruli of patients with Denys Drash syndrome (DDS) suggesting a switch in splicing.43 The authors pointed out that aberrant expression of the WT1 gene may be involved in regulating the vegf-a gene expression, and is the cause of glomerulopathy in DDS. In fact, WT1 in wild-type podocytes could bind the SRPK1 promoter inhibiting its expression (Fig. 3B), whereas the mutated form of WT1 in podocytes isolated from a DDS patient did not bind this site, and SRPK1 expression was upregulated. Wild-type WT1 restoration decreased SRPK1 expression in these podocytes.22,32 Nevertheless, WT1 is known for being able to both repress and activate its target genes, hence its transcription function is highly context-specific and can be modulated by several cofactors.44 Thus, modifying vegf-a splicing by preventing SRPK1 activity may be therapeutically effective in DDS.
The role of VEGF165b in fertility control and pregnancy-related conditions
A transgenic mouse model, whereby overexpression of VEGF165b is driven by the mouse mammary tumor virus promoter, shows expression of VEGF165b in tissues such as the ovary or the mammary tissue during mammary development of female mice. These mice had fewer blood vessels in the mammary tissue, an impaired alveolar vascular coverage of the fat pad and a reduced production of milk for nourishment of their pups, which died of starvation shortly after birth.45 Whereas the litter size of wild-type females mated with transgenic males was normal, when transgenic females were mated with wild-type mice, significantly fewer pups were born. However, no developmental defects in the embryos were detected in culture which, together with the normal birth weight of the pups, indicated that the reduction in litter size could depend on ovarian function, as follicular development in the ovary is angiogenesis-dependent. Indeed, VEGF165b overexpression in the mouse ovary caused an increase in the ovulatory cycle length and a delayed follicular development, resulting in fertility defects in these mice.45 Additionally, VEGF165b neutralization with an antibody specific for VEGFxxxb, as well as treatment with the pro-angiogenic isoform VEGF164, showed an enhanced vascular and follicular development in perinatal rat ovaries.16
Total VEGF levels are upregulated and VEGFxxxb isoforms are expressed at even lower levels than usual in the placenta of patients with pre-eclampsia,46 a condition in which the vasculature of pregnant women is vasoconstricted and shows higher permeability.47 Circulating VEGF165b levels are downregulated during the first trimester in pregnancies that will develop pre-eclampsia, making it a useful clinical marker for high risk of pre-eclampsia.48 Although after the first trimester circulating VEGFxxxb levels are normal in pre-eclamptic patients, pre-eclamptic plasma has been shown to increase vascular permeability. This increase could be inhibited by incubating the pre-eclamptic plasma with an antibody specific for VEGFxxxb, suggesting that VEGFxxxb is responsible for the enhanced vascular permeability in pre-eclamptic plasma.47 Therefore, VEGF165b plays a key role not only in reproductive biology, but also in the control of fertility and pre-eclampsia.
The role of VEGF165b in systemic sclerosis
VEGF165b upregulation has been reported in the skin and circulation of patients with systemic sclerosis (SSc), a chronic disease characterized by impaired angiogenesis and vascular repair that affects the skin and internal organs.49,50 Cultured dermal microvascular endothelial cells (MVECs) isolated from SSc patients expressed higher levels of VEGF165b and VEGFR2 than those from healthy individuals. In spite of this, VEGFR2 phosphorylation was reduced and angiogenesis, defective as a consequence. Furthermore, treatment with rhVEGF165b, as well as treatment with conditioned media from SSc MVECs, inhibited VEGF165-induced VEGFR2 phosphorylation and angiogenesis in healthy MVECs, and these anti-angiogenic effects could be neutralized by treatment with a VEGF165b-blocking antibody.23 Thus, neutralization of the elevated levels of VEGF165b might represent a potential therapeutical approach to stimulate a proper angiogenic response in SSc patients.
In addition, the high VEGF165b levels in SSc skin correlated with increased expression of TGFβ and SRSF6, and expression of VEGF165b and SRSF6 was found to be increased and regulated by TGFβ in cultered SSc MVECs, indicating that in SSc, and other non-angiogenic disorders, favoring PSS rather than DSS selection might represent a potential therapeutic strategy to stimulate adequate angiogenesis.
Perspective
Every year there are more and more studies on VEGF165b, and new conditions where VEGF165b is identified as altered or contributory—for instance a polymorphisim in intron 7 that appears to regulate VEGF165b expression predicts susceptibility to asthma.51 Since its discovery, over 50 publications have described VEGF165b as an anti-angiogenic agent that is downregulated in cancer and other angiogenesis-related conditions such as diabetic retinopathy, and plays an important role in permeability regulation and renal disease, pre-eclampsia and fertility control. Nevertheless, there are researchers who are still skeptical about VEGF165b predominance in human or even existence in mouse tissue.52 Indeed, despite being discovered in 2002, few reports on the splicing mechanism that gives rise to VEGFxxxb and its effects on angiogenesis-related diseases have been published during the last decade, and its downstream signaling effects remain practically unreported. One of the reasons that could explain this lack of research is the difficulty of quantifying the absolute amounts of VEGFxxx and VEGFxxxb mRNA and protein.52,53 The 3′UTR secondary structure of VEGF165 and VEGF165b transcripts is likely to be different, and result in differential reverse transcription efficiency. In fact, VEGF165 has been shown to be preferentially amplified by RT-PCR when mixed at equal amounts or even in excess of VEGF165b cDNA.53 When VEGF was first identified, the importance of ruling out misamplification of VEGF165 by VEGF165b primers was highlighted.3 A recent publication reiterated the importance of primer design in avoiding annealing of the reverse primer to VEGFxxx and amplification of a misprimed product,52 although in that report, the authors did not use positive control DNA to ensure that the primers act specifically, and amplify the VEGFxxxb products efficiently. Mispriming is easily prevented by using primers that can simultaneously amplify both VEGFxxx and VEGFxxxb transcripts, and by subsequent sequencing of the obtained amplicons to confirm their nature, while using control DNA to account for differential amplification. Moreover, there is no commercially available VEGFxxx-specific antibody, and the sensitivity of the currently existing VEGFxxxb ELISA for the different isoforms and heterodimers of these isoforms has not been tested. The total VEGF ELISA, for example, underestimates the total VEGF levels as it has less affinity for VEGF165b than for VEGF165.28 Therefore, further efforts need to be made to develop appropriate and reliable methods that allow the accurate quantification of both VEGFxxx and VEGFxxxb isoforms.
Growing evidence shows that vegf-a alternative splicing can be differentially regulated in conditions characterized by an excess of angiogenesis, such as cancer, or a deficit, such as SSc, but the control of this splicing event is still not well understood, and determining how different key splicing factors regulate an angiogenic splicing switch should be studied much more in depth over the next few years. The cellular machinery underlying the control of splice-site selection, and consequently which isoforms are being expressed to the detriment of others, represents a promising field for continuing investigations, taking into consideration that regulation of this splicing is not likely to be restricted to VEGF-A.
Acknowledgments
I apologize to all those colleagues whose work could not be discussed due to space limitations. I would also like to thank Dave Bates for his valuable comments on the manuscript.
Glossary
Abbreviations:
- AMD
age-related macular degeneration
- DDS
Denys-Drash syndrome
- DSS
distal splice site
- IGF-1
insulin-like growth factor 1
- MVECs
microvascular endothelial cells
- NRP1
neuropilin 1
- OIR
oxygen-induced retinopathy
- PSS
proximal splice site
- SRPK1
serine/arginine protein kinase 1
- SRSF1
serine/arginine-rich splicing factor 1
- SRSF2
serine/arginine-rich splicing factor 2
- SRSF6
serine/arginine-rich splicing factor 6
- SSc
systemic sclerosis
- TGFβ
transforming growth factor beta
- TNFα
tumor necrosis factor alpha
- UTR
untranslated region
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
- WT1
Wilms tumor 1
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/22439
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 3.Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–31. [PubMed] [Google Scholar]
- 4.Cébe Suarez S, Pieren M, Cariolato L, Arn S, Hoffmann U, Bogucki A, et al. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci. 2006;63:2067–77. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 6.Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114:853–65. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- 7.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–54. [PubMed] [Google Scholar]
- 8.Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–35. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 9.Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia. 2005;48:2422–7. doi: 10.1007/s00125-005-1951-8. [DOI] [PubMed] [Google Scholar]
- 10.Cébe-Suarez S, Grünewald FS, Jaussi R, Li X, Claesson-Welsh L, Spillmann D, et al. Orf virus VEGF-E NZ2 promotes paracellular NRP-1/VEGFR-2 coreceptor assembly via the peptide RPPR. FASEB J. 2008;22:3078–86. doi: 10.1096/fj.08-107219. [DOI] [PubMed] [Google Scholar]
- 11.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8:880–7. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bevan HS, van den Akker NM, Qiu Y, Polman JA, Foster RR, Yem J, et al. The alternatively spliced anti-angiogenic family of VEGF isoforms VEGFxxxb in human kidney development. Nephron, Physiol. 2008;110:57–67. doi: 10.1159/000177614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolard J, Bevan HS, Harper SJ, Bates DO. Molecular diversity of VEGF-A as a regulator of its biological activity. Microcirculation. 2009;16:572–92. doi: 10.1080/10739680902997333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribeiro LA, Bacci ML, Seren E, Tamanini C, Forni M. Characterization and differential expression of vascular endothelial growth factor isoforms and receptors in swine corpus luteum throughout estrous cycle. Mol Reprod Dev. 2007;74:163–71. doi: 10.1002/mrd.20589. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Dou T, Liu C, Fu M, Huang Y, Gu S, et al. The evolution of alternative splicing exons in vascular endothelial growth factor A. Gene. 2011;487:143–50. doi: 10.1016/j.gene.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Artac RA, McFee RM, Smith RA, Baltes-Breitwisch MM, Clopton DT, Cupp AS. Neutralization of vascular endothelial growth factor antiangiogenic isoforms is more effective than treatment with proangiogenic isoforms in stimulating vascular development and follicle progression in the perinatal rat ovary. Biol Reprod. 2009;81:978–88. doi: 10.1095/biolreprod.109.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ergorul C, Ray A, Huang W, Darland D, Luo ZK, Grosskreutz CL. Levels of vascular endothelial growth factor-A165b (VEGF-A165b) are elevated in experimental glaucoma. Mol Vis. 2008;14:1517–24. [PMC free article] [PubMed] [Google Scholar]
- 18.Kawamura H, Li X, Harper SJ, Bates DO, Claesson-Welsh L. Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer Res. 2008;68:4683–92. doi: 10.1158/0008-5472.CAN-07-6577. [DOI] [PubMed] [Google Scholar]
- 19.Catena R, Larzabal L, Larrayoz M, Molina E, Hermida J, Agorreta J, et al. VEGF₁₂₁b and VEGF₁₆₅b are weakly angiogenic isoforms of VEGF-A. Mol Cancer. 2010;9:320. doi: 10.1186/1476-4598-9-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007;14:185–93. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowak DG, Amin EM, Rennel ES, Hoareau-Aveilla C, Gammons M, Damodoran G, et al. Regulation of vascular endothelial growth factor (VEGF) splicing from pro-angiogenic to anti-angiogenic isoforms: a novel therapeutic strategy for angiogenesis. J Biol Chem. 2010;285:5532–40. doi: 10.1074/jbc.M109.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121:3487–95. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manetti M, Guiducci S, Romano E, Ceccarelli C, Bellando-Randone S, Conforti ML, et al. Overexpression of VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, leads to insufficient angiogenesis in patients with systemic sclerosis. Circ Res. 2011;109:e14–26. doi: 10.1161/CIRCRESAHA.111.242057. [DOI] [PubMed] [Google Scholar]
- 24.Merdzhanova G, Gout S, Keramidas M, Edmond V, Coll JL, Brambilla C, et al. The transcription factor E2F1 and the SR protein SC35 control the ratio of pro-angiogenic versus antiangiogenic isoforms of vascular endothelial growth factor-A to inhibit neovascularization in vivo. Oncogene. 2010;29:5392–403. doi: 10.1038/onc.2010.281. [DOI] [PubMed] [Google Scholar]
- 25.Díaz R, Peña C, Silva J, Lorenzo Y, García V, García JM, et al. p73 Isoforms affect VEGF, VEGF165b and PEDF expression in human colorectal tumors: VEGF165b downregulation as a marker of poor prognosis. Int J Cancer. 2008;123:1060–7. doi: 10.1002/ijc.23619. [DOI] [PubMed] [Google Scholar]
- 26.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 27.Pritchard-Jones RO, Dunn DB, Qiu Y, Varey AH, Orlando A, Rigby H, et al. Expression of VEGF(xxx)b, the inhibitory isoforms of VEGF, in malignant melanoma. Br J Cancer. 2007;97:223–30. doi: 10.1038/sj.bjc.6603839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, et al. VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer. 2008;98:1366–79. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peiris-Pagès M, Harper SJ, Bates DO, Ramani P. Balance of pro- versus anti-angiogenic splice isoforms of vascular endothelial growth factor as a regulator of neuroblastoma growth. J Pathol. 2010;222:138–47. doi: 10.1002/path.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rennel ES, Hamdollah-Zadeh MA, Wheatley ER, Magnussen A, Schüler Y, Kelly SP, et al. Recombinant human VEGF165b protein is an effective anti-cancer agent in mice. Eur J Cancer. 2008;44:1883–94. doi: 10.1016/j.ejca.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rennel E, Waine E, Guan H, Schüler Y, Leenders W, Woolard J, et al. The endogenous anti-angiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br J Cancer. 2008;98:1250–7. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amin EM, Oltean S, Hua J, Gammons MV, Hamdollah-Zadeh M, Welsh GI, et al. WT1 mutants reveal SRPK1 to be a downstream angiogenesis target by altering VEGF splicing. Cancer Cell. 2011;20:768–80. doi: 10.1016/j.ccr.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao M, Shi X, Liang J, Miao Y, Xie W, Zhang Y, et al. Expression of pro- and anti-angiogenic isoforms of VEGF in the mouse model of oxygen-induced retinopathy. Exp Eye Res. 2011;93:921–6. doi: 10.1016/j.exer.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Konopatskaya O, Churchill AJ, Harper SJ, Bates DO, Gardiner TA. VEGF165b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularization in mice. Mol Vis. 2006;12:626–32. [PubMed] [Google Scholar]
- 35.Magnussen AL, Rennel ES, Hua J, Bevan HS, Beazley Long N, Lehrling C, et al. VEGF-A165b is cytoprotective and antiangiogenic in the retina. Invest Ophthalmol Vis Sci. 2010;51:4273–81. doi: 10.1167/iovs.09-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehlken C, Rennel ES, Michels D, Grundel B, Pielen A, Junker B, et al. Levels of VEGF but not VEGF(165b) are increased in the vitreous of patients with retinal vein occlusion. Am J Ophthalmol. 2011;152:298–303, e1. doi: 10.1016/j.ajo.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 37.Hua J, Spee C, Kase S, Rennel ES, Magnussen AL, Qiu Y, et al. Recombinant human VEGF165b inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2010;51:4282–8. doi: 10.1167/iovs.09-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ricker LJ, Kijlstra A, Kessels AG, de Jager W, Liem AT, Hendrikse F, et al. Interleukin and growth factor levels in subretinal fluid in rhegmatogenous retinal detachment: a case-control study. PLoS One. 2011;6:e19141. doi: 10.1371/journal.pone.0019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–16. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson J, Qiu Y, Sage LM, Neal CR, Bates DO, Harper SJ, et al. Ultrafiltration co-efficient in isolated intact glomeruli from podocyte-specific VEGF165b over-expressing transgenic mice. Microcirculation 14, 643 (Abstract) 2007. [Google Scholar]
- 41.Qiu Y, Ferguson J, Oltean S, Neal CR, Kaura A, Bevan H, et al. Overexpression of VEGF165b in podocytes reduces glomerular permeability. J Am Soc Nephrol. 2010;21:1498–509. doi: 10.1681/ASN.2009060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oltean S, Neal CR, Mavrou A, Patel P, Ahad T, Alsop C, et al. VEGF165b overexpression restores normal glomerular water permeability in VEGF164-overexpressing adult mice. Am J Physiol Renal Physiol. 2012;303:F1026–36. doi: 10.1152/ajprenal.00410.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schumacher VA, Jeruschke S, Eitner F, Becker JU, Pitschke G, Ince Y, et al. Impaired glomerular maturation and lack of VEGF165b in Denys-Drash syndrome. J Am Soc Nephrol. 2007;18:719–29. doi: 10.1681/ASN.2006020124. [DOI] [PubMed] [Google Scholar]
- 44.Reddy JC, Morris JC, Wang J, English MA, Haber DA, Shi Y, et al. WT1-mediated transcriptional activation is inhibited by dominant negative mutant proteins. J Biol Chem. 1995;270:10878–84. doi: 10.1074/jbc.270.18.10878. [DOI] [PubMed] [Google Scholar]
- 45.Qiu Y, Bevan H, Weeraperuma S, Wratting D, Murphy D, Neal CR, et al. Mammary alveolar development during lactation is inhibited by the endogenous antiangiogenic growth factor isoform, VEGF165b. FASEB J. 2008;22:1104–12. doi: 10.1096/fj.07-9718com. [DOI] [PubMed] [Google Scholar]
- 46.Bates DO, MacMillan PP, Manjaly JG, Qiu Y, Hudson SJ, Bevan HS, et al. The endogenous anti-angiogenic family of splice variants of VEGF, VEGFxxxb, are down-regulated in pre-eclamptic placentae at term. Clin Sci (Lond) 2006;110:575–85. doi: 10.1042/CS20050292. [DOI] [PubMed] [Google Scholar]
- 47.Bills VL, Salmon AH, Harper SJ, Overton TG, Neal CR, Jeffery B, et al. Impaired vascular permeability regulation caused by the VEGF₁₆₅b splice variant in pre-eclampsia. BJOG. 2011;118:1253–61. doi: 10.1111/j.1471-0528.2011.02925.x. [DOI] [PubMed] [Google Scholar]
- 48.Bills VL, Varet J, Millar A, Harper SJ, Soothill PW, Bates DO. Failure to up-regulate VEGF165b in maternal plasma is a first trimester predictive marker for pre-eclampsia. Clin Sci (Lond) 2009;116:265–72. doi: 10.1042/CS20080270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LeRoy EC. Systemic sclerosis. A vascular perspective. Rheum Dis Clin North Am. 1996;22:675–94. doi: 10.1016/S0889-857X(05)70295-7. [DOI] [PubMed] [Google Scholar]
- 50.Manetti M, Guiducci S, Ibba-Manneschi L, Matucci-Cerinic M. Mechanisms in the loss of capillaries in systemic sclerosis: angiogenesis versus vasculogenesis. J Cell Mol Med. 2010;14(6A):1241–54. doi: 10.1111/j.1582-4934.2010.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simpson A, Custovic A, Tepper R, Graves P, Stern DA, Jones M, et al. Genetic variation in vascular endothelial growth factor-a and lung function. Am J Respir Crit Care Med. 2012;185:1197–204. doi: 10.1164/rccm.201112-2191OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris S, Craze M, Newton J, Fisher M, Shima DT, Tozer GM, et al. Do anti-angiogenic VEGF (VEGFxxxb) isoforms exist? A cautionary tale. PLoS One. 2012;7:e35231. doi: 10.1371/journal.pone.0035231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiu Y, Hoareau-Aveilla C, Oltean S, Harper SJ, Bates DO. The anti-angiogenic isoforms of VEGF in health and disease. Biochem Soc Trans. 2009;37:1207–13. doi: 10.1042/BST0371207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baba T, McLeod DS, Edwards MM, Merges C, Sen T, Sinha D, et al. VEGF 165 b in the developing vasculatures of the fetal human eye. Dev Dyn. 2012;241:595–607. doi: 10.1002/dvdy.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lipinski DM, Singh MS, MacLaren RE. Assessment of cone survival in response to CNTF, GDNF, and VEGF165b in a novel ex vivo model of end-stage retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011;52:7340–6. doi: 10.1167/iovs.11-7996. [DOI] [PubMed] [Google Scholar]
- 56.Glass CA, Harper SJ, Bates DO. The anti-angiogenic VEGF isoform VEGF165b transiently increases hydraulic conductivity, probably through VEGF receptor 1 in vivo. J Physiol. 2006;572:243–57. doi: 10.1113/jphysiol.2005.103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bates DO, Hillman NJ, Williams B, Neal CR, Pocock TM. Regulation of microvascular permeability by vascular endothelial growth factors. J Anat. 2002;200:581–97. doi: 10.1046/j.1469-7580.2002.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baltes-Breitwisch MM, Artac RA, Bott RC, McFee RM, Kerl JG, Clopton DT, et al. Neutralization of vascular endothelial growth factor antiangiogenic isoforms or administration of proangiogenic isoforms stimulates vascular development in the rat testis. Reproduction. 2010;140:319–29. doi: 10.1530/REP-09-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]