Abstract
Using top predators as sentinels of the marine environment, Hg contamination was investigated within the large subantarctic seabird community of Kerguelen Islands, a remote area from the poorly known Southern Indian Ocean. Chicks of 21 sympatric seabirds presented a wide range of Hg concentrations, with the highest contaminated species containing ∼102 times more feather Hg than the less contaminated species. Hence, Kerguelen seabirds encompass the whole range of chick feather Hg values that were previously collected worldwide in poorly industrialized localities. Using stable isotopes, the effects of foraging habitats (reflected by δ13C) and trophic positions (reflected by δ15N) on Hg concentrations were investigated. Species-related Hg variations were highly and positively linked to feather δ15N values, thus highlighting the occurrence of efficient Hg biomagnification processes within subantarctic marine trophic webs. By contrast, Hg contamination overall correlated poorly with feeding habitats, because of the pooling of species foraging within different isotopic gradients corresponding to distinct seabird habitats (benthic, pelagic, neritic and oceanic). However, when focusing on oceanic seabirds, Hg concentration was related to feather δ13C values, with species feeding in colder waters (lower δ13C values) south of Kerguelen Islands being less prone to be contaminated than species feeding in northern warmer waters (higher δ13C values). Within the context of continuous increase in global Hg emissions, Kerguelen Islands that are located far away from anthropogenic sources can be considered as an ideal study site to monitor the temporal trend of global Hg contamination. The present work helps selecting some seabird species as sentinels of environmental pollution according to their high Hg concentrations and their contrasted foraging ecology.
Introduction
Mercury (Hg) is a highly toxic non-essential metal that negatively influences humans and wildlife [1], [2]. In birds, mercury’s adverse effects are far-ranging, with impacts on reproduction being usually the end-point of more direct effects on behaviour, neurology, endocrinology and development [2]. Overall, Hg derives from both natural and anthropogenic sources, but human activities have increased the global amount of Hg cycling around the world by a factor of three to five [3]. Owing to its high volatility and long atmospheric residence time, Hg reaches remote areas through long-range atmospheric transport, thus contaminating oceanic islands and polar and sub-polar regions [4], [5]. Once deposited in aquatic ecosystems, inorganic Hg is subject to biotic reaction (methylation) carried out by microorganisms [6]. Thereafter, methylmercury (Me-Hg), the persistent and highly toxic form of Hg, is assimilated by living organisms via food intake, bioaccumulates in individuals and biomagnifies within food webs from lower to higher trophic levels [7]. Hence, top predators have been used to monitor Hg contamination in various aquatic ecosystems [8], with still limited existing information for significant parts of the world ocean, including the southern Indian Ocean [9].
Seabirds are useful bio-indicators of environmental pollution, including Hg contamination because they are long-lived animals that prey at the top of the marine food webs [8], [10]. Feathers are the most attractive avian tissue to sample because, in many cases, they contain most of the Hg body burden and are considered as the main route for Hg elimination [8]. Hg accumulated and stored within soft tissues between moults is transferred and sequestered in the growing feathers and cannot thus be re-incorporated into living tissues. Feather sampling has also the benefit to be non-destructive.
Here, we investigated feather Hg concentrations within the large community of seabirds that breed in Kerguelen Islands, a remote subantarctic archipelago from the Southern Ocean [11]. The seabird assemblage is mainly composed of penguins and Procellariiformes, including the very long-lived and slow-moulting albatrosses that are amongst the most Hg contaminated vertebrate species [12]. Kerguelen seabirds feed on a large diversity of prey (crustaceans, cephalopods and fish) and use contrasted foraging strategies that include benthic and pelagic feeding as well as foraging in neritic and oceanic waters, with the oceanic birds ranging from the warmer subtropical to the colder Antarctic waters (Table 1). The distribution of Hg species in the main oceanic water masses is still poorly documented, with a complete lack of information from Kerguelen waters. However, a recent investigation on Hg speciation within the Southern Ocean revealed a poleward increase in the concentrations of total Hg and Me-Hg in surface waters to some of the highest Me-Hg concentrations so far observed in the open ocean [13]. This Me-Hg increase most likely results from a strong net Hg methylation in the hypoxic zone of the water column in Antarctic waters [13].
Table 1. Species, foraging habitats and diets during the chick-rearing period, and durations of the chick rearing period of seabirds at Kerguelen Islands.
| Species | Chick rearing | Main foraging habitats | Main prey classes | References | ||
| period (days) | Horizontal | Vertical | ||||
| Spheniscidae | ||||||
| King penguin (Aptenodytes patagonicus) | KP | 315 | oceanic | mesopelagic | mesopelagic fish | [58] |
| Gentoo penguin (Pygosce lis papua) | GP | 72 | neritic (open sea) | benthic | benthic fish (crustaceans) | [59] |
| Macaroni penguin (Eudyptes chrysolophus) | MP | 65 | neritic (open sea)/oceanic | epipelagic | crustaceans and fish | Unpublished data |
| Southern rockhopper penguin (Eudyptes chrysocome) | SRP | 71 | neritic (closed sea) | epipelagic | crustaceans | [60] |
| Diomedeidae | ||||||
| Wandering albatross (Diomedea exulans) | WA | 275 | oceanic | sea surface | benthopelagic fish and cephalopods | Unpublished data |
| Black-browed albatross (Thalassarche melanophrys) | BBA | 125 | neritic (open sea) | sea surface | benthopelagic fish (cephalopods) | [19] |
| Light-mantled sooty albatross (Phoebetria palpebrata) | LMSA | 154 | oceanic | sea surface | cephalopods (crustaceans, carrion) | [61] a |
| Procellariidae | ||||||
| Northern giant petrel (Macronectes halli) | NGP | 113 | on land and at sea | on land, sea surface | carrion/seabirds | [61] a |
| Grey petrel (Procellaria cinerea) | GrP | 128 | oceanic | sea surface | fish (cephalopods) | Unpublished data |
| White-chinned petrel (Procellaria aequinoctialis) | WCP | 96 | oceanic | sea surface | fish (cephalopods, crustaceans) | [62] |
| Great-winged petrel (Pterodroma macroptera) | GWP | 126 | oceanic | sea surface | cephalopods (crustaceans) | [61] a |
| White-headed petrel (Pterodroma lessonii) | WHP | 101 | oceanic | sea surface | fish (cephalopods) | Unpublished data |
| Kerguelen petrel (Aphrodroma brevirostris) | KeP | 60 | oceanic | sea surface | crustaceans | [61] a |
| Blue petrel (Halobaena caerulea) | BP | 55 | oceanic | sea surface | crustaceans (mesopelagic fish) | [49] |
| Antarctic prion (Pachyptila desolata) | AP | 50 | oceanic | sea surface | crustaceans | [63] |
| Thin-billed prion (Pachyptila belcheri) | TBP | 50 | oceanic | sea surface | crustaceans | [63] |
| Pelecanoididae | ||||||
| Common diving petrel (Pelecanoides urinatrix) | CDP | 54 | neritic (closed sea) | epipelagic | crustaceans | [64] |
| South-Georgian diving petrel (Pelecanoides georgicus) | SGDP | 55 | oceanic | epipelagic | crustaceans | [64] |
| Phalacrocoracidae | ||||||
| Kerguelen shag (Phalacrocorax verrucosus) | KS | 56 | neritic (open sea) | benthic | benthic fish | [65] |
| Stercorariidae | ||||||
| Subantarctic skua (Catharacta antarctica lönnbergi) | SS | 45 | on land and at sea | on land, sea surface | small petrels | [47] |
| Laridae | ||||||
| Kelp gull (Larus dominicanus) | KG | 49 | on land and at sea | on land, sea surface | carrion/seabirds (limpets) | [66] a |
Stahl and Mougin (1986) and Ridoux (1994) refer to the related Crozet Islands.
In a first descriptive step, Hg was determined in 21 seabird species to assess contamination levels of marine ecosystems within the poorly known southern Indian Ocean. In a second explanatory step, the effect of differences of seabird foraging ecology on feather Hg concentration was tested, because ingestion of food is the main route of Hg exposure in birds [10]. The respective roles of habitats and diets were tested by using the isotopic niche as a proxy of the trophic niche of the species, with the ratios of stable isotopes of carbon (δ13C) and nitrogen (δ15N) reflecting their foraging habitats and trophic positions, respectively [14]. The isotopic method was already validated in the area, with seabird δ13C values indicating their latitudinal foraging grounds and depicting offshore versus inshore consumers [15], [16] and their δ15N values increasing with trophic level [17]. Taking into account the species’ foraging ecology, we make the following predictions. Firstly, feeding habitat (δ13C) should shape seabird Hg contamination, because Hg is not homogeneously distributed in marine ecosystems. For example, (i) benthic foragers should have relatively high feather Hg concentrations in relation to the substantial production of Me-Hg in coastal marine sediments [6], and (ii) oceanic foragers should be more contaminated in cold than in warm waters, in relation to the latitudinal gradient in the bioavailability of Me-Hg in the Southern Ocean [13]. Secondly, Hg should magnify within subantarctic food webs, which means that seabirds with the highest trophic positions (δ15N) should show the highest feather Hg concentrations. Finally, the longer the chick rearing period, the higher the chick feather Hg concentrations should be, because much of the dietary Hg accumulated in soft tissues is mobilized and excreted into growing feathers [18].
Most previous investigations on pollutants in birds were conducted on adults that present some disadvantages related to their foraging areas, moult schedule and migration patterns. Instead, we focused on chicks as indicators and sentinels of contamination [10]. Firstly, young birds that have not yet fledged have obtained all of their food from their parents who forage in the vicinity of the breeding colonies at that time. The chick Hg concentrations and stable isotope signatures thus reflect those of the adult foraging ecology during the chick-rearing period, a period during which feeding information (chick diet and adult foraging grounds) were previously collected using complementary methods (i.e. dietary analysis and bio-logging [19]). Secondly, the integration period is almost identical for Hg and stable isotopes in body feathers of chicks, because (i) Hg accumulated during the chick-rearing period is excreted during the pre-fledging moult, and (ii) feather isotope ratios reflect diet at the time of feather synthesis [20]. Thirdly, since all chick feathers were grown almost simultaneously, both Hg and stable isotopes are fairly homogeneous in the plumage of pre-fledglings [8], [21], while sequential moult in adults induces larger Hg concentrations in the first than in the late growing feathers [22].
Materials and Methods
Ethics Statement
Animals were cared for in accordance with the guidelines of the ethics committee of the Institut Polaire Français Paul Emile Victor that approved fieldwork in the present study (Program no. 109, H. Weimerskirch).
Study Area, Species and Sample Collection
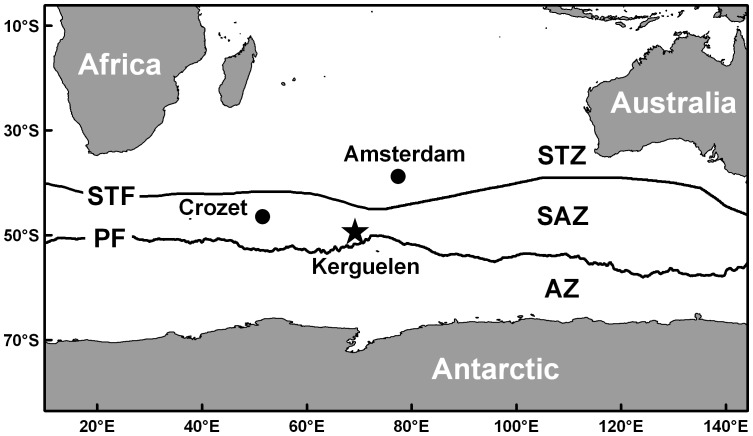
Fieldwork was carried out from 2003 to 2012 on Kerguelen archipelago (49°21′S, 70°18′E), which is located in the southern part of the Subantarctic Zone, in the immediate vicinity of the Polar Front [23]. The Southern Ocean was defined as the ocean south the Subtropical Front and the Subantarctic and Antarctic Zones, as the zones between the Subtropical and Polar Fronts, and between the Polar Front and Antarctica, respectively (Fig. 1). Chicks from 21 seabird species belonging to 4 orders and 7 families were sampled, totalizing 280 individuals (n = 7–22 per species). Sampling was conducted at different locations of the archipelago, depending on the species’ breeding sites. Most neritic seabirds were studied in colonies close to the open sea, but two species (the southern rockhopper penguin and common diving petrel) were sampled at Mayes Island that is located within a large bay (closed sea) where the parent birds feed. Body feathers were collected from the lower back of large chicks at fledging, i.e. at the end of the breeding season. Once collected, feathers were stored dry in sealed plastic bags and analysed at the University of La Rochelle, France.
Figure 1. Location of the Kerguelen Islands and of the main oceanic fronts and zones in the southern Indian Ocean.
Abbreviations: STF, Subtropical Front; PF, Polar Front; STZ, Subtropical Zone; SAZ, Subantarctic Zone; AZ, Antarctic Zone [23].
Sample Analyses
Prior to chemical analysis, all body feathers were cleaned of surface lipids and contaminants using a 2∶1 chloroform: methanol solution for 2 min followed by two successive methanol rinses. They were then cut with scissors into small fragments. A first sub-sample of homogenized feathers was oven dried for 48 hr at 50°C and then analyzed in an advanced Hg analyzer spectrophotometer (Altec AMA 254). Hg determination involved evaporation of Hg by progressive heating until 800°C under oxygen atmosphere for 2′30 min and subsequent amalgamation on a gold trap. The net was heated to liberate the collected Hg that was measured by UV atomic absorption spectrophotometry. Samples were analysed for total Hg, which approximates the amount of Me-Hg since essentially all Hg in feathers is under organic form [24], [25], [26]. All analyses were repeated 2–3 times until having a relative standard deviation <10%. Accuracy was checked using certified reference material (Tort-2 Lobster Hepatopancreas, NRC, Canada; mean 0.27±0.06 µg•g−1 dry mass). Our measured values were 0.24±0.01 µg•g−1 dry mass, n = 56. Blanks were analysed at the beginning of each set of samples and the detection limit of the method was 0.005 µg•g−1dry mass.
A second sub-sample of homogenized feathers was weighed (∼0.3 mg) with a microbalance and packed into a tin container. Relative abundance of C and N isotopes were determined with a continuous flow mass spectrometer (Thermo Scientific Delta V Advantage) coupled to an elemental analyzer (Thermo Scientific Flash EA 1112). Results are presented in the δ notation relative to Vienna PeeDee Belemnite and atmospheric N2 for δ13C and δ15N, respectively. Replicate measurements of internal laboratory standards (acetanilide) indicate measurement errors <0.10‰ for both δ13C and δ15N values.
Statistical Analyses
Statistical tests were performed using R 2.7.1 [27]. All samples submitted to statistical tests were first checked for normality and homogeneity of variances by means of Shapiro-Wilk and Fisher tests, respectively. Depending on the results, parametric or non-parametric tests were used. A significance level of α <0.05 was used for all tests. Values are means ± SD.
In univariate analyses, correlations between Hg and continuous explanatory variables (feather δ13C and δ15N values, and the duration of the chick rearing period) were tested using Pearson correlations. In multivariate analyses, the influence of species and continuous explanatory variables on feather Hg concentrations were investigated using General Linear Models (GLMs). Feather Hg concentrations were log transformed and the models were constructed with a normal distribution and an identity link function. Explanatory variables were defined as follows: species as a factor, and feather δ15N, δ13C values, and the duration of the chick rearing period as covariates. The influence of the sampling year on Hg concentrations was not incorporated in model selection, since, as most species were sampled in only one year (Table 2), the year effect is confounded by the species effect. Noticeably however, two species (the Kerguelen petrel and subantarctic skua) that were sampled twice did not present significant inter-annual differences in feather Hg concentrations (data not shown).
Table 2. Chick feather Hg, δ13C and δ15N values of Kerguelen seabirds.
| Species | Years of sampling | n | Total Hg (µg•g−1 dry mass) | δ13C (‰) | δ15N (‰) |
| South Georgian diving petrel | 2012 | 16 | 0.05±0.01 (0.04–0.08) | −21.3±0.3 | 8.8±0.3 |
| Common diving petrel | 2003 | 17 | 0.11±0.02 (0.07–0.15) | −17.0±0.5 | 12.1±0.4 |
| Antarctic prion | 2008 | 10 | 0.21±0.05 (0.16–0.31) | −21.5±0.5 | 9.3±0.4 |
| Thin-billed prion | 2003 | 9 | 0.22±0.09 (0.12–0.40) | −21.5±0.5 | 9.1±0.4 |
| Southern rockhopper penguin | 2007 | 12 | 0.27±0.06 (0.20–0.37) | −15.3±0.4 | 11.5±0.4 |
| Macaroni penguin | 2007 | 12 | 0.36±0.07 (0.25–0.52) | −18.3±0.5 | 10.0±0.5 |
| Kelp gull | 2011 | 7 | 0.73±0.38 (0.40–1.38) | −12.8±0.7 | 13.4±1.0 |
| Kerguelen petrel | 2009, 2010 | 18 | 0.78±0.17 (0.51–1.20) | −22.1±0.5 | 11.7±0.5 |
| Blue petrel | 2003 | 13 | 0.84±0.18 (0.58–1.14) | −21.8±0.5 | 9.8±0.5 |
| King penguin | 2006 | 12 | 1.12±0.16 (0.83–1.50) | −21.6±0.3 | 10.6±0.3 |
| White-headed petrel | 2003 | 10 | 1.54±0.34 (1.07–1.99) | −22.0±0.5 | 12.2±0.2 |
| Great-winged petrel | 2005 | 10 | 1.64±0.48 (0.96–2.68) | −20.0±0.4 | 12.9±0.4 |
| White-chinned petrel | 2005 | 14 | 1.82±0.51 (1.13–2.76) | −22.2±0.7 | 11.3±0.8 |
| Kerguelen shag | 2006 | 10 | 2.21±1.06 (1.35–4.64) | −13.8±1.0 | 14.0±0.6 |
| Gentoo penguin | 2006 | 12 | 2.45±0.67 (1.14–3.66) | −16.5±1.2 | 12.4±0.8 |
| Light-mantled sooty albatross | 2005 | 15 | 2.46±0.67 (1.56–3.69) | −21.0±0.4 | 12.6±0.4 |
| Black-browed albatross | 2005 | 18 | 2.58±0.59 (1.54–3.70) | −18.5±0.8 | 12.9±0.5 |
| Grey petrel | 2005 | 16 | 3.16±1.21 (1.59–5.70) | −19.9±0.6 | 13.6±0.4 |
| Wandering albatross | 2005 | 15 | 4.45±1.60 (2.19–8.43) | −19.3±0.4 | 14.2±0.4 |
| Subantarctic skua | 2005, 2010 | 22 | 5.15±1.56 (2.40–7.93) | −21.8±0.4 | 10.8±0.3 |
| Northern giant petrel | 2005 | 12 | 5.31±1.12 (4.06–7.94) | −19.2±1.2 | 13.4±0.8 |
Species were deliberately ranked by increasing Hg concentrations and not in taxonomic order. Values are means ± SD with ranges in parentheses for Hg.
Biologically relevant models were constructed by incorporating the different variables and their interactions. Continuous variables that were significantly correlated were not included in the same models. The most parsimonious models were selected according to the bias-adjusted Akaïke’s Information Criterion (AICc), which is a small-sample bias adjustment [28], [29]. As a general guideline, if AICc values differ by more than 2, the model with the lowest AICc value is the most accurate, whereas models with AICc values differing by less than 2 are fairly similar in their ability to describe the data, and the model including the least number of parameters (the simplest) is the most accurate [30]. The likelihood of a model, referred to as the Akaïke weight (wi) was estimated following Johnson and Omland [31] (Table 3). The wi can be interpreted as approximate probabilities that the model i is the best one for the observed data, given the candidate set of models. Model fit was assessed by a chi-square goodness-of-fit test [32], and residuals were checked for normality using Shapiro-Wilk test and Q-Q plot. The coefficient of determination R2adj was calculated for each model [31] (Table 3).
Table 3. AICc model ranking for feather Hg concentrations within the Kerguelen seabird community (see text for details).
| Models | AICc | ΔAICca | wi b | R2adj | gdf |
| species+δ15N | 78.79 | 0.00 | 0.999 | 0.96 | 1.00 |
| species+δ13C+species* δ13C | 97.08 | 18.29 | <0.001 | 0.96 | 1.00 |
| species+δ15N+species* δ15N | 99.36 | 20.57 | <0.001 | 0.96 | 1.00 |
| species+CRP+ species* CRP | 110.12 | 31.33 | <0.001 | 0.96 | 1.00 |
| species+CRP | 110.12 | 31.33 | <0.001 | 0.96 | 1.00 |
| species | 110.12 | 31.33 | <0.001 | 0.96 | 1.00 |
| species+δ13C | 110.20 | 31.41 | <0.001 | 0.96 | 1.00 |
| δ15N | 841.16 | 762.37 | <0.001 | 0.37 | 0.03 |
| CRP | 930.02 | 851.23 | <0.001 | 0.14 | <0.001 |
| null | 971.35 | 892.56 | <0.001 | 0.00 | <0.001 |
| δ13C | 972.11 | 893.32 | <0.001 | <0.01 | <0.001 |
Abbreviations: AICc, bias-adjusted Akaike’s Information Criteria values;
wi, AICc weights; R2adj, R-squared adjusted; gdf, goodness-of-fit; CRP, duration of the chick rearing period.
Scaled ΔAICc; ΔAICc = 0.00 is interpreted as the best fit to the data among the models.
Weight of evidence interpreted as a proportion. Weights across all models sum to 1.00.
Results
Univariate analysis showed that total Hg contamination varied widely within the seabird community, with chick feather Hg concentrations differing significantly between species (Kruskal-Wallis, H = 260.23, P<0.001, n = 21). Mean Hg concentrations ranged from 0.05±0.01 to 5.31±1.12 µg•g−1 dry mass in South-Georgian diving-petrels and northern giant petrels, respectively (Table 2). The lowest chick feather Hg concentration occurred in a common diving-petrel and the highest in a wandering albatross (0.04 and 8.43 µg•g−1, respectively).
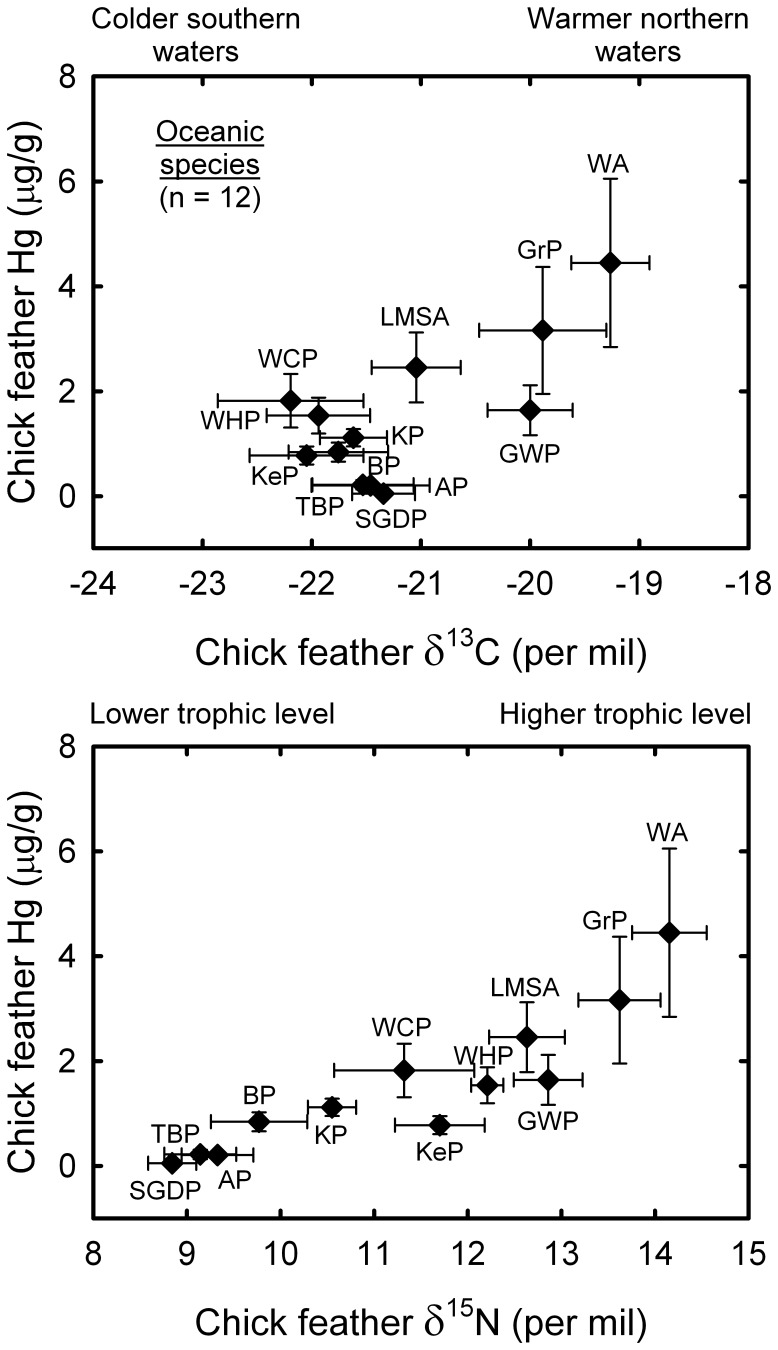
No overall significant correlation between feather Hg and δ13C values was found (Pearson correlation, r = −0.08, t = −1.31, P = 0.19, n = 280), while feather Hg concentration was highly significantly and positively correlated with δ15N values (r = 0.48, t = 9.19, P<0.001). Feather Hg concentration was also significantly and positively related to the duration of the chick rearing period (r = 0.26, t = 4.54, P<0.001). Furthermore, feather δ15N and δ13C values were positively correlated (r = 0.63, t = 10.24, P<0.001), and they were significantly related to the duration of the chick rearing period (r = −0.12 and 0.35, t = −2.10 and 6.33, p = 0.037 and <0.001 for δ13C and δ15N values, respectively). When focusing on oceanic seabirds (12 species and 158 individuals) a highly significant and positive correlation was found between feather Hg and both δ13C (r = 0.61, t = 9.68, p<0.001) and δ15N values (r = 0.80, t = 16.90, P<0.001) (Fig. 2).
Figure 2. Relationship between chick feather Hg concentrations (means ± SD; µg•g−1 dry mass) and (a) foraging habitat (chick feather δ13C) and (b) trophic position (chick feather δ15N) of oceanic species.
Filled diamonds and empty circles refer to oceanic and other species, respectively. See Table 1 for species abbreviations.
In multivariate analyses, the most parsimonious GLM model selected by AICc values included the effects of species and δ15N (Table 3) in explaining Hg concentrations in body feathers. Indeed, Hg concentrations differed significantly among species (F20, 258 = 217, P<0.001) and were significantly and positively related to their δ15N values (F1, 278 = 2776, P<0.001). The second-ranked model included an effect of species, δ13C and their interaction, but it had a low likelihood when compared to the first-ranked model (Table 3). Models including the duration of the chick rearing period had also a low likelihood, thus explaining poorly feather Hg concentrations when compared to species and feather δ15N values.
Discussion
To the best of our knowledge, this study is the first to investigate Hg contamination in such a large number of sympatric seabirds. It completes the few works previously conducted in the southern Atlantic [33], [34] and the southern Pacific Oceans [35], [36], thus partly filling the gap of knowledge from the southern Indian Ocean sector [9]. The study adds a substantial number of seabird chicks that were either not previously investigated (n = 16) or inadequately sampled (n = 4) [35], with only data from wandering albatross [37] being available in the scientific literature (Table 4).
Table 4. An overall synthesis of Hg concentrations (means ± SD with ranges in parentheses; µg•g−1 dry mass) in body feathers of seabird chicks.
| Species | Location | n | Total Hg | References |
| Spheniscidae | ||||
| Adelie penguin (Pygoscelis adeliae) | Terra Nova Bay (Antarctica) | 11 | 0.37±0.15 | [67] |
| Diomedeidae | ||||
| Wandering albatross (Diomedea exulans) | Bird Island (South Georgia) | 10 | 3.31±0.68 | [37] |
| Black-footed albatross (Phoebastria nigripes) | Midway Atoll | 17 | 5.57±0.36a | [40], [68] |
| Laysan albatross (Phoebastria immutabilis) | Midway Atoll | 35 | 2.15±0.12a | [40], [68] |
| 15 | 1.95±0.15a | [69] | ||
| Procellariidae | ||||
| Barau’s petrel (Pterodroma baraui) | La Réunion | 32 | 0.30±0.07 | [41] |
| Bonin petrel (Pterodroma hypoleuca) | Midway Atoll | 20 | 3.87±0.32a | [40], [70] |
| Audubon’s shearwater (Puffinus lherminieri) | La Réunion | 38 | 0.07±0.01 | [41] |
| Aride Island (Seychelles) | 10, 8 | 0.15±0.03, 0.27±0.06 | [38] | |
| Pink-footed shearwater (Puffinus creatopus) | Mocha Island (Chile) | 8 | 0.36±0.10 | [71] |
| Sooty shearwater (Puffinus griseus) | Humboldt Current (Peru) | 14 | 0.72±0.35 | [72] |
| New Zealand Region | 4 | 0.8±0.8 | [35] | |
| Wedge-tailed shearwater (Puffinus pacificus) | Aride Island (Seychelles) | 10 | 0.39±0.05 | [38] |
| Cory’s shearwater (Calonectris diomedea) | Azores | 14 | 0.87±0.10a (0.33–1.5) | [73] |
| 30 | 0.7±0.2 | [74] | ||
| Berlengas (Portugal) | 25 | 1.1±0.3 | [74] | |
| Hydrobatidae | ||||
| Leach’s storm-petrel (Oceanodroma leucorhoa) | New Brunswick (Canada) | 20 | 1.42 (0.90–2.22)b | [75] |
| Phaethontidae | ||||
| Red-tailed tropicbird (Phaethon rubricauda) | Midway Atoll | 12 | 2.51±0.28a | [40], [70] |
| White-tailed tropicbird (Phaethon lepturus) | La Réunion | 16 | 0.29±0.02 | [41] |
| Aride Island (Seychelles) | 10, 10 | 0.52±0.11, 0.70±0.10 | [38] | |
| Phalacrocoracidae | ||||
| European shag (Phalacrocorax aristotelis) | Atlantic sector (Spain) | 20, 12 | from 0.54±0.19 to 1.07±0.38 | [76] |
| Cantabrian sector (Spain) | 15, 10 | 3.09±1.40, 5.09±1.82 | [76] | |
| Stercorariidae | ||||
| Great skua (Catharacta skua) | Foula (Shetland) | 40 | 1.3±0.4 | [54] |
| 28 | 1.22±0.38 | [77] | ||
| 29 | 2.16±1.15 | [53] | ||
| St Kilda (Outer Hebrides) | 22 | 5.37±1.29 | [53] | |
| Arctic skua (Stercorarius parasiticus) | Foula (Shetland) | 30 | 0.46±0.22 | [77] |
| Laridae | ||||
| Audoin’s gull (Larus audouinii) | Dodecanese (Greece) | 20, 10 | from 0.94±0.27 (0.61–1.46) to 1.71±0.48 (0.32–2.55) | [78] |
| Cyclade (Greece) | 20, 10 | from 1.42±0.29 (0.88–2.04) to 2.02±0.38 (1.13–2.45) | [78] | |
| Kythera (Greece) | 8 | 1.20±0.33 (0.76–1.77) | [78] | |
| Ebro Delta (Western Mediterranean) | 39 | 5.09 (4.68–5.54)d | [79] | |
| Alboran Island (Western Mediterranean) | 15 | 3.87 (3.28–4.57)d | [79] | |
| Chafarinas Islands (Western Mediterranean) | 12 | 3.17 (2.31–4.35)d | [79] | |
| Black-headed gull (Larus ridibundus) | German North Sea | 36 | 0.88±0.53 (0.14–2.11), 0.94±0.45 (0.10–2.07) | [80] |
| Common gull (Larus canus) | Elbe estuary (German North Sea) | 12 | 2.24±1.84 | [81] |
| Jade Bay (German North Sea) | 11 | 1.40±0.37 | [81] | |
| Franklin’s gull (Larus pipixcan) | Interior U.S.A. | ≥79 | 0.80±0.06a | [82] |
| Minnesota | 15 | 0.31±0.11a | [83] | |
| Glaucous-winged gull (Larus glaucescens) | Aleutian Islands | 36 | 1.98±0.18a | [84] |
| Herring gull (Larus argentatus) | New York | 15, 20, 15 | 0.81±0.12, 1.80±0.11, 2.83±0.27a | [85], [86] |
| New Jersey | 14, 15 | 1.76±0.35, 2.58±0.23a | [86] | |
| Virginia | 15 | 0.76±0.11a | [86] | |
| German North Sea | 38 | 5.88±4.90 (0.78–27.14) | [87], [88] | |
| 39 | 1.27±0.60 (0.47–2.98), 1.31±0.62 (0.49–2.89) | [80] | ||
| Shetland | 12 | 2.24±0.83 (1.04–4.12) | [88] | |
| Red-billed gull (Larus novaehollandiae) | Kaikoura Peninsula (New Zealand) | 27 | 2.02±1.16 | [89] |
| Yellow-legged gull (Larus michahellis atlantis) | Azores | 34 | 2.3±1.0 | [74] |
| Madeira | 22 | 2.6±0.8 | [74] | |
| Berlengas (Portugal) | 28 | 2.4±0.5 | [74] | |
| Kittiwake (Rissa tridactyla) | Foula (Shetland) | 26 | 0.37±0.12 | [77] |
| Shetland | 9 | 0.49±0.28 (0.26–1.03) | [88] | |
| German North Sea | 13 | 2.65±0.61 (1.61–3.64) | [88] | |
| Northeast Norway | 27 | 0.55±0.10 | [89] | |
| Sternidae | ||||
| Arctic tern (Sterna paradisaea) | Foula (Shetland) | 15 | 0.69±0.14 | [77] |
| Common tern (Sterna hirundo) | Bird island (Massachusetts) | 21 | 3.1±0.2a | [90] |
| 15 | 4.2±3.1 | [91] | ||
| Long Island (New York) | 16 | 1.4±0.6 (0.6–2.6) | [92] | |
| 14, 21 | 2.01±0.25, 2.61±2.55a | [93] | ||
| German North Sea | 21 | 6.14±4.33 (1.51–18.40) | [87] | |
| 13 | 3.00±0.50 (1.97–3.74), 3.26±0.70 (2.41–4.91) | [80] | ||
| 27 | 12.89±6.90 (1.51–70.00) | [88] | ||
| Elbe estuary (German North Sea) | 4 | 36.4±18.9 (21.7–62.9) | [88] | |
| Jadebusen (German North Sea) | 9 | 3.8±0.7 (2.9–5.1) | [88] | |
| East Scotland | 19 | 1.80±0.79 (0.92–3.11) | [88] | |
| Shetland | 12 | 1.40±0.72 (0.84–2.95) | [88] | |
| Azores | 10, 19 | from 1.1±0.4 to 1.5±0.4 | [74] | |
| Forster’s tern (Sterna forsteri) | San Francisco Bay | 89 | 6.44±0.28d | [94] |
| Little tern (Sterna albifrons) | Portugal | 168 | 4.07±1.42 | [95] |
| Vaia (Portugal) | 12, 10 | 4.40±1.31, 4.67±1.38 | [96] | |
| Roseate tern (Sterna dougallii) | Azores | 19, 14 | 0.8±0.2, 1.1±0.2 | [74] |
| Aride Island (Seychelles) | 12 | 0.69±0.32 | [97] | |
| White tern (Gygis alba) | Aride Island (Seychelles) | 10, 10 | 0.21±0.03, 0.40±0.05 | [38] |
| Midway Atoll | 7 | 1.65±0.18a | [40], [98] | |
| Sooty tern (Onychoprion fuscata) | Lys (Glorieuses) | 32 | 0.05±0.03 | [41] |
| Aride Island (Seychelles) | 10 | 0.26±0.05 | [38] | |
| Hawaii | 16 | 0.16±0.02a | [98] | |
| Brown noddy (Anous stolidus) | Aride Island (Seychelles) | 10, 10 | 0.27±0.05, 0.37±0.06 | [38] |
| Hawaii | 20 | 0.07±0.003a | [99], [100] | |
| Lesser noddy (Anous tenuirostris) | Aride Island (Seychelles) | 10, 5 | 0.17±0.03, 0.41±0.17 | [38] |
| Alcidae | ||||
| Razorbill (Alca torda) | New Brunswick (Canada) | 16 | 1.40 (0.86–2.29) | [75] |
| Common murre (Uria aalge) | New Brunswick (Canada) | 9 | 1.14 (0.59–2.18)b | [75] |
| Atlantic puffin (Fratercula arctica) | New Brunswick (Canada) | 17 | 1.00 (0.27–0.73)b | [75] |
Down Hg values and studies with too low numbers of sampled chicks (n <4) were excluded.
Values are means ± SE.
Values are estimated marginal means with 95% confidence limits in parentheses.
Median value.
Values are geometric means with 95% confidence limits in parentheses.
Statistical analyses pointed out the important effect of species, feeding habits (δ15N) and foraging habitats (δ13C) on chick feather Hg concentrations, which, by contrast, are little explained by the duration of the chick rearing period. However, univariate analysis showed a positive relationship between Hg contamination and the duration of the chick- rearing period, the most likely explanation being that assimilated Hg accumulates during chick growth over weeks and months and is ultimately excreted in newly grown feathers at the end of the period [18]. The positive co-variation between the duration of the chick rearing period and δ15N likely results from the longer chick rearing period of large seabirds (e.g. albatrosses) that feed at higher trophic positions than smaller species with shorter growth period (e.g. petrels).
Feather Hg Concentrations: Comparison with Other Species and Areas
Kerguelen seabird chicks presented a wide range of Hg concentrations, with the highest contaminated species containing ∼102 times (two orders of magnitude) more feather Hg than the less contaminated species. Both the lowest and highest Hg values occurred in flying birds, with contamination levels of the flightless penguins ranging from low to intermediate values. No other feather Hg concentrations are available from Kerguelen seabirds, but a preliminary analysis conducted on internal tissues of adults of five species of zooplankton-eating petrels [9] ranked the species in the same decreasing order than in the present study, with blue petrels containing more Hg than prions and diving petrels. In the same way, the decreasing order is roughly the same in the only other comparable investigations that were conducted on seabirds breeding in the southern Atlantic Ocean [33], [37]. Feather Hg was higher in albatrosses and giant petrels, intermediate in the white-chinned petrel and lower in the blue petrel, prions and diving petrels. Hg concentrations were however much higher in south Atlantic Procellariiformes than in the present study [33], with the most likely explanation being that work was conducted on adult birds, not on chicks. Indeed, adult birds have consistently higher feather Hg concentrations than their chicks [38]. Adults have a longer period to assimilate and accumulate metals from their food between two successive moults, whereas chicks only have the several weeks (to months) of the chick-rearing period [39].
A review of the existing literature on seabird chicks (Table 4) shows that mean feather Hg concentration can reach very high values in acutely polluted areas (up to 36.4 µg•g−1 for common terns in the German North Sea). Elsewhere, however, Hg concentration ranges from 0.05 to 5.6 µg•g−1 (the sooty tern and black-footed albatross, respectively). This range is remarkably similar to that from the present investigation, indicating that seabirds from only one location (Kerguelen) encompass the whole range of values that were collected worldwide in poorly industrialized areas. Consequently, comparison of Hg levels between distant locations using seabirds as sentinels of environmental contamination necessitates collecting feathers from many species, because a small subset could not be fully representative of the whole seabird assemblage, and thus of the surrounding marine environment.
At the species level, chick feather Hg concentrations from Kerguelen seabirds fall within the concentration range reported for similar species elsewhere. The wandering albatross from South Georgia [37], presented identical Hg concentrations than chicks from Kerguelen, and the taxonomically closely-related but spatially distant Scottish great skua and Kerguelen subantarctic skua showed almost similar feather Hg concentrations, depending on breeding colonies (Table 4). At the community level, only two previous investigations on chick Hg contamination included more than five sympatric species and both were conducted in tropical waters of the Pacific (Midway Atoll, six species [40]) and the Indian Oceans (the Seychelles, seven species [38]). Based on feather Hg concentrations, the Kerguelen seabird community compares well with the Midway Atoll assemblage (0.34–5.57 µg•g−1) that also includes albatrosses, i.e. the species group that contains the highest feather Hg concentrations among seabirds [12] (Table 4). By contrast (and excluding albatross data), chick feather Hg concentrations were overall higher in Kerguelen species than in seabirds from the Seychelles (0.15–0.70 µg•g−1) and La Réunion (0.07–0.42 µg•g−1 [41]), thus suggesting higher Me-Hg bioavailability in subantarctic than in tropical waters of the Indian Ocean. The hypothesis remains to be investigated because there is a paucity of information on mercury speciations in marine waters, and more specifically, on the sources of Me-Hg to marine consumers, including seabirds and their prey [6], [13].
Potential Adverse Effect
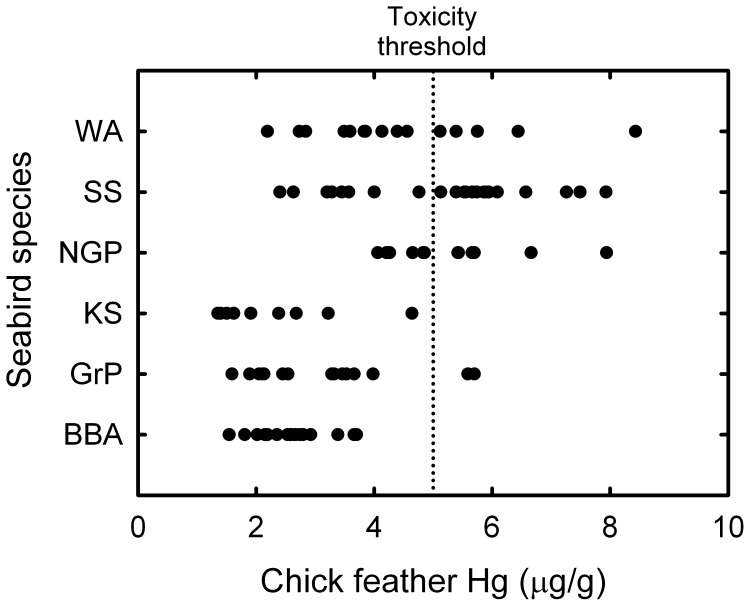
One of the key problems in environmental toxicology is to interpret the impact of observed contaminant concentrations. In general, Hg levels of 1.5–18 µg•g−1 dry mass in eggs are sufficient to cause decreased egg mass, embryo malformations, lower hatchability, decreased chick growth, and lowered chick survival [42]. The threshold levels in feathers of negative effects to chicks is currently unknown, but laboratory and field studies on adult birds indicate that feather Hg concentrations of 2.4 to 40 µg•g−1 are associated with adverse effects, with the commonest used toxicity threshold being 5 µg•g−1 [42], [43]. Feather Hg concentrations of most Kerguelen seabirds are below this threshold, but some individuals from four species showed higher levels. Namely, 64%, 50%, 33% and 13% of chicks of subantarctic skuas, northern giant petrels, wandering albatrosses and grey petrels, respectively, exceeded the threshold value and were thus potentially threatened by Hg (Fig. 3). However, those levels are difficult to interpret because chicks showed no observed obvious ill effects. Moreover, seabirds cope efficiently with high Hg concentrations in their prey through efficient detoxification processes and, hence, they are expected to have higher toxicity thresholds than terrestrial birds [36]. Nonetheless, such comparisons are invaluable to use seabirds as sentinels of ecosystem health, because it provides ways to identify not only the species the more at risk, but also the species that would be useful as bio-indicators [10].
Figure 3. Feather Hg concentrations (µg•g−1 dry mass) of individual chicks from the six most contaminated species from the Kerguelen seabird community.
See text for toxicity threshold and Table 1 for species abbreviations. BBA illustrates the most contaminated species of the assemblage with all individual values being below the threshold value.
Feather Hg Concentrations, Diet and Trophic Levels
As expected, the overall statistical analysis indicated a δ15N effect on feather Hg concentrations within the Kerguelen seabird community. The positive correlation verifies our hypothesis stating that Hg concentration should increase with trophic level, because δ15N is a proxy of consumers’ trophic position [44]. Noticeably, the relationship was partially hindered by pooling species that forage in distinct habitats (neritic vs. oceanic and benthic vs. pelagic) marked by different isotopic baselines. Within this context, the positive correlation between Hg and δ15N is particularly relevant. Indeed, the relationship was even stronger when looking at oceanic seabirds only (Fig. 2). The feather δ15N values of the 12 oceanic species ranged from 8.8‰ (the crustacean-eater South Georgian diving petrel) to 14.2‰ (the squid-eater wandering albatross), which, assuming a trophic enrichment factor of 2.7‰ for carnivorous organisms [45], corresponds to ∼3 trophic levels. The corresponding feather Hg values indicated an 86-fold Hg enrichment within the oceanic seabird assemblage. Accordingly, Hg contents of pelagic organisms from the Southern Ocean (including Kerguelen Islands) increase in the order crustaceans <fish ≤ squids < seabirds [9], [33], [46]. Hence, while patterns of Hg accumulation in food webs of the open ocean are largely unknown [6], the present work highlights the occurrence of efficient Hg biomagnification processes in subantarctic waters of the southern Indian Ocean that merit further oceanographic investigations.
The subantarctic skua was clearly an outlier species within the Kerguelen seabird assemblage, with chick Hg concentration being high when compared to feather δ15N, and, to a lesser extent, δ13C values (Table 2). At the study site, adult skuas forage on land where they feed their chicks almost exclusively with small seabirds, mainly blue petrels [47]. Feather δ15N value of skua chicks agrees with a blue petrel-based diet, being 2.7‰ 15N-enriched when compared to blood of their prey (author’s unpublished data). The skua Hg content is also in agreement with feeding on blue petrels, because petrel muscle contains disproportionately more total Hg than fish and crustaceans [9], [46]. High petrel Hg contamination is likely to result from the combination of two life-history traits of the species: (i) blue petrels are long-lived animals [48] and are thus prone to Hg bioaccumulation over the long-term, and (ii) they prey on crustaceans and mesopelagic fish [49], [50], with the latter containing high Hg concentrations [46], [51]. The trophic explanation of the high Hg levels of subantarctic skua chicks therefore suggests that seabirds feeding on other seabirds are at risk to accumulate critical pollutant loads. Indeed, Scottish great skuas feeding predominantly on other seabirds contain more Hg than those feeding on fish [52], [53], but with no negative effects on breeding performance and survival [54].
Feather Hg Concentrations and Foraging Habitats
Univariate analysis indicated no δ13C effects on feather Hg concentrations within the Kerguelen seabird community, and, in multivariate analysis, species and δ15N produced the best model. This result seems to contradict our hypothesis that foraging habitat should play an important role in shaping seabird Hg contamination. However, the large range of seabird δ13C values indicates that, again, species that forage within different isoscapes were pooled, thus resulting in a confounding effect [15] for the interpretation of feather Hg concentrations. For example, the five seabirds with δ13C values ≥ −18‰ were all neritic species feeding either along the shoreline (the kelp gull) or on pelagic (the southern rockhopper penguin and common diving petrel) or benthic prey (the gentoo penguin and Kerguelen shag) (Table 1). The relatively high feather Hg concentrations in feathers of the two latter species are in agreement with the Me-Hg-enrichment of coastal benthic areas [6]. Noticeably, the two species feeding in the closed sea (the southern rockhopper penguin and common diving petrel) and the sibling species foraging in the open sea (the macaroni penguin and South Georgian diving petrel, respectively) have low feather Hg concentrations. Such low levels can be related to a crustacean-based diet and suggest that no significant inshore/offshore gradient occurs in the availability of Me-Hg in pelagic waters surrounding the Kerguelen Islands.
At a larger spatial scale, the importance of foraging habitat is exemplified by the positive correlation between feather Hg concentrations and δ13C values in oceanic seabirds. Taken into account the latitudinal δ13C gradient within the Southern Ocean [15], [16], the relationship indicates that species foraging in cold waters south of Kerguelen Islands are less prone to be contaminated than species feeding in northern warmer waters. This finding does not fit well with the only study on Hg speciation in the Southern Ocean showing higher Me-Hg concentrations in Antarctic than in subantarctic and subtropical waters [13]. This mismatch reinforces the need to better document the bioavailability of Me-Hg in the main oceanic and neritic water masses and to determine the levels of Hg contamination of seabirds breeding in the north (e.g. Amsterdam Islands) and south (e.g. Adélie Land) of the Kerguelen Archipelago to confirm or not this latitudinal trend both at the bottom and at the top of the marine trophic webs.
Conclusions
The pattern of Hg contamination of Kerguelen seabirds is remarkable due to its wide range of values, but the circumpolar annular structure of the Southern Ocean [55] suggests it may be generalized to other subantarctic localities. The source of Hg in subantarctic waters is still poorly known, but it ultimately derives mostly from anthropogenic contamination, because (i) human activities have increased emissions to the atmosphere by approximately a factor of 3, and (ii) atmospheric deposition is the dominant input term in the world ocean [6], including the Southern Ocean [13]. Since global Hg emissions will increase in the future, Hg contamination will increase as well in remote areas [56]. Hence, Kerguelen Archipelago, together with other isolated islands located far away from anthropogenic sources, can be considered as ideal study sites to monitor the temporal trend of global Hg contamination. Our study allows selecting chicks of some seabirds as sentinels of environmental pollution according to their high Hg concentrations with relatively low variances and to their contrasted foraging ecology. Three representative species are the gentoo penguin (benthic neritic forager), black-browed albatross (pelagic neritic forager) and light-mantled sooty albatross (southern oceanic forager). Despite its larger variance in Hg concentrations, the wandering albatross (northern oceanic forager) must also be included, because this iconic seabird is known to be among the most Hg contaminated vertebrate species [36], [57].
Acknowledgments
The authors thank the numerous fieldworkers who helped with collecting seabird feathers at the different breeding sites, and C. Churlaud for her assistance during Hg analyses. F. Capoulun, M. Connan, T. Cook and A. Maglio prepared some isotopic samples and G. Guillou run all of them.
Funding Statement
Financial support was provided by the Agence Nationale de la Recherche (program POLARTOP, O. Chastel), the Institut Polaire Français Paul Emile Victor (IPEV, program no. 109, H. Weimerskirch), and the Terres Australes et Antarctiques Françaises (TAAF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW (2007) Effects of environmental methylmercury on the health of wild birds, mammals, and fish. AMBIO 36: 12–19. [DOI] [PubMed] [Google Scholar]
- 2. Grandjean P, Satoh H, Murata K, Eto K (2010) Adverse effects of methylmercury: environmental health research implications. Environ Health Persp 118: 1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Selin NE (2009) Global biogeochemical cycling of mercury: a review. Annu Rev Environ Resourc 34: 43–63. [Google Scholar]
- 4. Fitzgerald WF, Engstrom DR, Mason RP, Nater EA (1998) The case for atmospheric mercury contamination in remote areas. Environ Sci Technol 32: 1–7. [Google Scholar]
- 5. Ebinghaus R, Kock HH, Temme C, Einax JW, Löwe AG, et al. (2002) Antarctic springtime depletion of atmospheric mercury. Environ Sci Technol 36: 1238–1244. [DOI] [PubMed] [Google Scholar]
- 6. Fitzgerald WF, Lamborg CH, Hammerschmidt CR (2007) Marine biogeochemical cycling of mercury. Chem Rev 107: 641–662. [DOI] [PubMed] [Google Scholar]
- 7. Morel FMM, Kraepiel AML, Amyot M (1998) The chemical cycle and bioaccumulation of mercury. Annu Rev Ecol Syst 29: 543–566. [Google Scholar]
- 8. Monteiro LR, Furness RW (1995) Seabirds as monitors of mercury in the marine environment. Water Air Soil Pollut 80: 851–870. [Google Scholar]
- 9. Bocher P, Caurant F, Miramand P, Cherel Y, Bustamante P (2003) Influence of the diet on the bioaccumulation of heavy metals in zooplankton-eating petrels at Kerguelen archipelago, Southern Indian Ocean. Polar Biol 26: 759–767. [Google Scholar]
- 10. Burger J, Gochfeld M (2004) Marine birds as sentinels of environmental pollution. EcoHealth 1: 263–274. [Google Scholar]
- 11. Weimerskirch H, Zotier R, Jouventin P (1989) The avifauna of the Kerguelen Islands. Emu 89: 15–29. [Google Scholar]
- 12. Thompson DR, Furness RW, Lewis SA (1993) Temporal and spatial variation in mercury concentrations in some albatrosses and petrels from the sub-Antarctic. Polar Biol 13: 239–244. [Google Scholar]
- 13. Cossa D, Heimbürger LE, Lannuzel D, Rintoul SR, Butler ECV, et al. (2011) Mercury in the Southern Ocean. Geochim Cosmochim Acta 75: 4037–4052. [Google Scholar]
- 14. Newsome SD, Martinez del Rio C, Bearhop S, Phillips DL (2007) A niche for isotopic ecology. Front Ecol Environ 5: 429–436. [Google Scholar]
- 15. Cherel Y, Hobson KA (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar Ecol Prog Ser 329: 281–287. [Google Scholar]
- 16. Jaeger A, Lecomte VJ, Weimerskirch H, Richard P, Cherel Y (2010) Seabird satellite tracking validates the use of latitudinal isoscapes to depict predators’ foraging areas in the Southern Ocean. Rapid Comm Mass Spectr 24: 3456–3460. [DOI] [PubMed] [Google Scholar]
- 17. Cherel Y, Fontaine C, Richard P, Labat JP (2010) Isotopic niches and trophic levels of myctophid fishes and their predators in the Southern Ocean. Limnol Oceanogr 55: 324–332. [Google Scholar]
- 18. Lewis SA, Furness RW (1991) Mercury accumulation and excretion in laboratory reared black-headed gull Larus ridibundus chicks. Arch Environ Contam Toxicol 21: 316–320. [Google Scholar]
- 19. Cherel Y, Weimerskirch H, Trouvé C (2000) Food and feeding ecology of the neritic-slope forager black-browed albatross and its relationships with commercial fisheries in Kerguelen waters. Mar Ecol Prog Ser 207: 183–199. [Google Scholar]
- 20. Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes I: turnover of 13C in tissues. Condor 94: 181–188. [Google Scholar]
- 21. Jaeger A, Connan M, Richard P, Cherel Y (2010) Use of stable isotopes to quantify seasonal changes of trophic niche and levels of population and individual specialisation in seabirds. Mar Ecol Prog Ser 40: 269–277. [Google Scholar]
- 22. Furness RW, Muirhead SJ, Woodburn M (1986) Using bird feathers to measure mercury in the environment: relationships between mercury content and moult. Mar Pollut Bull 17: 27–30. [Google Scholar]
- 23. Park YH, Gambéroni L (1997) Cross-frontal exchange of Antarctic intermediate water and Antarctic bottom water in the Crozet Basin. Deep-Sea Res II 44: 963–986. [Google Scholar]
- 24. Thompson DR, Furness RW (1989) Comparison of the levels of total and organic mercury in seabird feathers. Mar Pollut Bull 20: 577–579. [Google Scholar]
- 25. Kim EY, Murakami T, Saeki K, Tatsukawa R (1996) Mercury levels and its chemical form in tissues and organs of seabirds. Arch Environ Contam Toxicol 30: 259–266. [Google Scholar]
- 26. Bond AL, Diamond AW (2009) Total and methyl mercury concentrations in seabird feathers and eggs. Arch. Environ. Contam Toxicol 56: 286–291. [DOI] [PubMed] [Google Scholar]
- 27.R Development Core Team (2008) A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna.
- 28.Akaike H (1973) Information theory and an extension of the maximum likelihood principle. Proceedings of the 2nd international symposium on information theory (eds B.N Petrov & F. Csaki), vol 1, 267–281. Akademiai Kiado, Budapest.
- 29.Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd ed. Springer, New York.
- 30. Lebreton JD, Burnham KP, Clobert J, Anderson DR (1992) Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol Monogr 62: 67–118. [Google Scholar]
- 31. Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19: 101–108. [DOI] [PubMed] [Google Scholar]
- 32.Lancelot R, Lesnoff M (2005) Sélection de modèles avec l’AIC et critères d’information dérivés. ftp://ftp.cirad.fr/pub/group-r/groupe-r/Fiches/AIC_v3.pdf.
- 33. Anderson ORJ, Phillips RA, McDonald RA, Shore RF, McGill RAR, et al. (2009) Influence of trophic position and foraging range on mercury levels within a seabird community. Mar Ecol Prog Ser 375: 277–288. [Google Scholar]
- 34. Muirhead SJ, Furness RW (1988) Heavy metal concentrations in the tissues of seabirds from Gough Island, South Atlantic Ocean. Mar Pollut Bull 19: 278–283. [Google Scholar]
- 35. Lock JW, Thompson DR, Furness RW, Bartle JA (1992) Metal concentrations in seabirds of the New Zealand region. Environ Pollut 75: 289–300. [DOI] [PubMed] [Google Scholar]
- 36. Stewart FM, Phillips RA, Bartle JA, Craig J, Shooter D (1999) Influence of phylogeny, diet, moult schedule and sex on heavy metal concentrations in New Zealand Procellariiformes. Mar Ecol Prog Ser 178: 295–305. [Google Scholar]
- 37. Becker PH, González-Solís J, Behrends B, Croxall J (2002) Feather mercury levels in seabirds at South Georgia: influence of trophic position, sex and age. Mar Ecol Prog Ser 243: 261–269. [Google Scholar]
- 38. Catry T, Ramos JA, Le Corre M, Kojadinovic J, Bustamante P (2008) The role of stable isotopes and mercury concentrations to describe seabird foraging ecology in tropical environments. Mar Biol 155: 637–647. [Google Scholar]
- 39. Burger J (1993) Metals in avian feathers: bioindicators of environmental pollution. Rev Environ Toxicol 5: 203–311. [Google Scholar]
- 40. Burger J, Gochfeld M (2000) Metal levels in feathers of 12 species of seabirds from Midway Atoll in the northern Pacific Ocean. Sci Total Environ 257: 37–52. [DOI] [PubMed] [Google Scholar]
- 41. Kojadinovic J, Bustamante P, Churlaud C, Cosson RP, Le Corre M (2007) Mercury in seabird feathers: Insight on dietary habits and evidence for exposure levels in the western Indian Ocean. Sci Total Environ 384: 194–204. [DOI] [PubMed] [Google Scholar]
- 42. Burger J, Gochfeld M (1997) Risk, mercury levels, and birds: relating adverse laboratory effects to field biomonitoring. Environ Res 75: 160–172. [DOI] [PubMed] [Google Scholar]
- 43. Evers DC, Savoy LJ, DeSorbo CR, Yates DE, Hanson W, et al. (2008) Adverse effects from environmental mercury loads on breeding common loons. Ecotoxicology 17: 69–81. [DOI] [PubMed] [Google Scholar]
- 44. Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718. [Google Scholar]
- 45. Vanderklift MA, Ponsard S (2003) Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136: 169–182. [DOI] [PubMed] [Google Scholar]
- 46. Bustamante P, Bocher P, Cherel Y, Miramand P, Caurant F (2003) Distribution of trace elements in the tissues of benthic and pelagic fish from the Kerguelen Islands. Sci Total Environ 313: 25–39. [DOI] [PubMed] [Google Scholar]
- 47. Mougeot F, Genevois F, Bretagnolle V (1998) Predation on burrowing petrels by the brown skua (Catharacta skua lönnbergi) at Mayes Island, Kerguelen. J Zool 244: 429–438. [Google Scholar]
- 48. Barbraud C, Weimerskirch H (2003) Climate and density shape population dynamics of a marine top predator. Proc R Soc B 270: 2111–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cherel Y, Bocher P, Trouvé C, Weimerskirch H (2002) Diet and feeding ecology of blue petrels Halobaena caerulea at Iles Kerguelen, Southern Indian Ocean. Mar Ecol Prog Ser 228: 283–299. [Google Scholar]
- 50. Connan M, Mayzaud P, Trouvé C, Barbraud C, Cherel Y (2008) Interannual dietary changes and demographic consequences in breeding blue petrels from Kerguelen Islands. Mar Ecol Prog Ser 373: 123–135. [Google Scholar]
- 51. Monteiro LR, Costa V, Furness RW, Santos RS (1996) Mercury concentrations in prey fish indicate enhanced bioaccumulation in mesopelagic environments. Mar Ecol Prog Ser 141: 21–25. [Google Scholar]
- 52. Bearhop S, Phillips RA, Thompson DR, Waldron S, Furness RW (2000) Variability in mercury concentrations of great skuas Catharacta skua: the influence of colony, diet and trophic status inferred from stable isotope signatures. Mar Ecol Prog Ser 195: 261–268. [Google Scholar]
- 53. Bearhop S, Waldron S, Thompson D, Furness R (2000) Bioamplification of mercury in great skua Catharacta skua chicks: the influence of trophic status as determined by stable isotope signatures of blood and feathers. Mar Pollut Bull 40: 181–185. [Google Scholar]
- 54. Thompson DR, Hamer KC, Furness RW (1991) Mercury accumulation in great skuas Catharacta skua of known age and sex, and its effects upon breeding and survival. J Appl Ecol 28: 672–684. [Google Scholar]
- 55. Orsi AH, Whitworth III T, Nowlin Jr WD (1995) On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep-Sea Res I 42: 641–673. [Google Scholar]
- 56. Streets DG, Zhang Q, Wu Y (2009) Projections of global mercury emissions in 2050. Environ Sci Technol 43: 2983–2988. [DOI] [PubMed] [Google Scholar]
- 57. Hindell MA, Brothers N, Gales R (1999) Mercury and cadmium concentrations in the tissues of three species of southern albatrosses. Polar Biol 22: 102–108. [Google Scholar]
- 58. Bost CA, Zorn T, Le Maho Y, Duhamel G (2002) Feeding of diving predators and diel vertical migration of prey: king penguins’ diet versus trawl sampling at Kerguelen Islands. Mar Ecol Prog Ser 227: 51–61. [Google Scholar]
- 59. Lescroel A, Bost CA (2005) Foraging under contrasting oceanographic conditions: the gentoo penguin at Kerguelen Archipelago. Mar Ecol Prog Ser 302: 245–261. [Google Scholar]
- 60. Tremblay Y, Cherel Y (2000) Benthic and pelagic dives: a new foraging behaviour in rockhopper penguins. Mar Ecol Prog Ser 204: 257–267. [Google Scholar]
- 61. Ridoux V (1994) The diets and dietary segregation of seabirds at the subantarctic Crozet Islands. Mar. Ornithol. 22: 1–192. [Google Scholar]
- 62. Delord K, Cotté C, Péron C, Marteau C, Pruvost P, et al. (2010) At-sea distribution and diet of an endangered top predator: relationship between white-chinned petrels and commercial longline fisheries. Endang Species Res 13: 1–16. [Google Scholar]
- 63. Cherel Y, Bocher P, De Broyer C, Hobson KA (2002) Food and feeding ecology of the sympatric thin-billed Pachyptila belcheri and Antarctic P. desolata prions at Iles Kerguelen, Southern Indian Ocean. Mar Ecol Prog Ser 228: 263–281. [Google Scholar]
- 64. Bocher P, Cherel Y, Hobson KA (2000) Complete trophic segregation between South Georgian and common diving petrels during breeding at Iles Kerguelen. Mar Ecol Prog Ser 208: 249–264. [Google Scholar]
- 65. Watanabe YY, Takahashi A, Sato K, Viviant M, Bost CA (2011) Poor flight performance in deep-diving cormorants. J Exp Biol 214: 412–421. [DOI] [PubMed] [Google Scholar]
- 66. Stahl JC, Mougin JL (1986) Le régime alimentaire du goéland dominicain Larus dominicanus de l’île de la Possession, archipel Crozet (46°25′S, 51°45′E). L’Oiseau et la RFO 56: 287–291. [Google Scholar]
- 67. Bargagli R, Monaci F, Sanchez-Hernandez JC, Cateni D (1998) Biomagnification of mercury in an Antarctic marine coastal food web. Mar Ecol Prog Ser 169: 65–75. [Google Scholar]
- 68. Burger J, Gochfeld M (2000) Metals in albatross feathers from Midway Atoll: influence of species, age, and nest location. Environ Res A 82: 207–221. [DOI] [PubMed] [Google Scholar]
- 69. Burger J, Gochfeld M (2000) Metals in Laysan albatrosses from Midway Atoll. Arch Environ Contam Toxicol 38: 254–259. [DOI] [PubMed] [Google Scholar]
- 70. Gochfeld M, Gochfeld DJ, Minton D, Murray Jr BG, Pyle P, et al. (1999) Metals in feathers of Bonin petrel, Christmas shearwater, wedge-tailed shearwater, and red-tailed tropicbird in the Hawaiian Islands, northern Pacific. Environ Monit Assess 59: 343–358. [Google Scholar]
- 71. Becker PH (2000) Mercury levels in pink-footed shearwaters (Puffinus creatopus) breeding on Mocha Island, Chile. Ornitol Neotrop 11: 165–168. [Google Scholar]
- 72. Gochfeld M (1980) Mercury levels in some seabirds of the Humboldt Current, Peru. Environ Pollut A 22: 197–205. [Google Scholar]
- 73. Monteiro LR, Furness RW, del Nevo AJ (1995) Mercury levels in seabirds from the Azores, mid-north Atlantic Ocean. Arch Environ Contam Toxicol 28: 304–309. [Google Scholar]
- 74. Monteiro LR, Granadeiro JP, Furness RW, Oliveira P (1999) Contemporary patterns of mercury contamination in the Portuguese Atlantic inferred from mercury concentrations in seabird tissues. Mar Environ Res 47: 137–156. [Google Scholar]
- 75. Bond AL, Diamond AW (2009) Mercury concentrations in seabird tissues from Machias Seal Island, New Brunswick, Canada. Sci Total Environ 407: 4340–4347. [DOI] [PubMed] [Google Scholar]
- 76. Moreno R, Jover L, Velando A, Munilla I, Sanpera C (2011) Influence of trophic ecology and spatial variation on the isotopic fingerprints of seabirds. Mar Ecol Prog Ser 442: 229–239. [Google Scholar]
- 77. Stewart FM, Phillips RA, Catry P, Furness RW (1997) Influence of species, age and diet on mercury concentrations in Shetland seabirds. Mar Ecol Prog Ser 151: 237–244. [Google Scholar]
- 78. Goutner V, Furness RW, Papakonstantinou K (2000) Mercury in feathers of Audoin’s gull (Larus audouinii) chicks from northeastern Mediterranean colonies. Arch Environ Contam Toxicol 39: 200–204. [DOI] [PubMed] [Google Scholar]
- 79. Sanpera C, Ruiz X, Moreno R, Jover L, Waldron S (2007) Mercury and stable isotopes in feathers of Audoin’s gulls as indicators of feeding habits and migratory connectivity. Condor 109: 268–275. [Google Scholar]
- 80. Becker PH, Henning D, Furness RW (1994) Differences in mercury contamination and elimination during feather development in gull and tern broods. Arch Environ Contam Toxicol 27: 162–167. [DOI] [PubMed] [Google Scholar]
- 81. Kahle S, Becker PH (1999) Bird blood as bioindicator for mercury in the environment. Chemosphere 39: 2451–2457. [DOI] [PubMed] [Google Scholar]
- 82. Burger J (1996) Heavy metal and selenium levels in feathers of Franklin’s gulls in interior north America. Auk 113: 399–407. [Google Scholar]
- 83. Burger J, Gochfeld M (1999) Heavy metals in Franklin’s gull tissues: age and tissue differences. Environ Toxicol Chem 18: 673–678. [Google Scholar]
- 84. Burger J, Gochfeld M, Jeitner C, Burke S, Volz CD, et al. (2009) Mercury and other metals in eggs and feathers of glaucous-winged gulls (Larus glaucescens) in the Aleutians. Environ Monit Assess 152: 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Burger J (1995) Heavy metal and selenium levels in feathers of herring gulls (Larus argentatus): differences due to year, gender, and age at Captree, Long Island. Environ Monit Assess 38: 37–50. [DOI] [PubMed] [Google Scholar]
- 86. Burger J (1997) Heavy metals and selenium in herring gulls (Larus argentatus) nesting in colonies from eastern Long Island to Virginia. Environ Monit Assess 48: 285–296. [Google Scholar]
- 87. Thompson DR, Becker PH, Furness RW (1993) Long-term changes in mercury concentrations in herring gulls Larus argentatus and common terns Sterna hirundo from the German North Sea coast. J Appl Ecol 30: 316–320. [Google Scholar]
- 88. Furness RW, Thompson DR, Becker PH (1995) Spatial and temporal variation in mercury contamination of seabirds in the North Sea. Helgoländer Meeresun 49: 605–615. [Google Scholar]
- 89. Wenzel C, Gabrielsen GW (1995) Trace element accumulation in three seabird species from Hornøya, Norway. Arch Environ Contam Toxicol 29: 198–206. [Google Scholar]
- 90. Burger J, Nisbet ICT, Gochfeld M (1994) Heavy metal and selenium levels in feathers of known-aged common terns (Sterna hirundo). Arch Environ Contam Toxicol 26: 351–355. [DOI] [PubMed] [Google Scholar]
- 91. Nisbet ICT, Montoya JP, Burger J, Hatch JJ (2002) Use of stable isotopes to investigate individual differences in diets and mercury exposures among common terns Sterna hirundo in breeding and wintering grounds. Mar Ecol Prog Ser 242: 267–274. [Google Scholar]
- 92. Gochfeld M (1980) Tissue distribution of mercury in normal and abnormal young common terns. Mar Pollut Bull 11: 362–377. [Google Scholar]
- 93. Burger J, Gochfeld M (1992) Trace element distribution in growing feathers: Additional excretion in feather sheaths. Arch Environ Contam Toxicol 23: 105–108. [DOI] [PubMed] [Google Scholar]
- 94. Ackerman JT, Eagles-Smith CA, Takekawa JY, Iverson SA (2008) Survival of postfledging Forster’s terns in relation to mercury exposure in San Francisco Bay. Ecotoxicol 17: 789–801. [DOI] [PubMed] [Google Scholar]
- 95. Tavarez PC, Monteiro LR, Lopes RJ, Pereira ME, Duarte AC, et al. (2005) Variation of mercury contamination in chicks of little tern Sterna albifrons in southwest Europe: brood, age, and colony related effects. Bull. Environ. Contam Toxicol 74: 177–183. [DOI] [PubMed] [Google Scholar]
- 96. Tavarez PC, Kelly A, Lopes RJ, Pereira ME, Duarte AC, et al. (2007) The influence of dietary specialization and trophic status on mercury levels in two species using common coastal wetlands, Himantopus himantopus and Sterna albifrons . Ardeola 54: 275–288. [Google Scholar]
- 97. Monticelli D, Ramos JA, Tavares PC, Bataille B, Lepoint G, et al. (2008) Diet and foraging ecology of roseate terns and lesser noddies breeding sympatrically on Aride Island, Seychelles. Waterbirds 31: 231–240. [Google Scholar]
- 98. Burger J, Shukla T, Dixon C, Shukla S, McMahon MJ, et al. (2001) Metals in feathers of sooty tern, white tern, gray-backed tern, and brown noddy from islands in the north Pacific. Environ Monit Assess 71: 71–89. [DOI] [PubMed] [Google Scholar]
- 99. Burger J (1993) Metals in feathers of brown noddy (Anous stolidus): evidence for bioaccumulation or exposure levels? Environ Monit Assess 24: 181–187. [DOI] [PubMed] [Google Scholar]
- 100. Burger J, Gochfeld M (1995) Biomonitoring of heavy metals in the Pacific Basin using avian feathers. Environ Toxicol Chem 14: 1233–1239. [Google Scholar]