Abstract
Background
Despite the availability of clinical practice guidelines (CPGs), optimal hypertension control is not achieved in many parts of the world; one of the challenges is the volume of guidelines on this topic and their variable quality. To systematically review the quality, methodology, and consistency of recommendations of recently-developed national CPGs on the diagnosis, assessment and the management of hypertension.
Methodology/Principal Findings
MEDLINE, EMBASE, guidelines' websites and Google were searched for CPGs written in English on the general management of hypertension in any clinical setting published between January 2006 and September 2011. Four raters independently appraised each CPG using the AGREE-II instrument and 2 reviewers independently extracted the data. Conflicts were resolved by discussion or the involvement of an additional reviewer. Eleven CPGs were identified. The overall quality ranged from 2.5 to 6 out of 7 on the AGREE-II tool. The highest scores were for “clarity of presentation” (44.4% −88.9%) and the lowest were for “rigour of development” (8.3%–30% for 9 CGPs). None of them clearly reported being newly developed or adapted. Only one reported having a patient representative in its development team. Systematic reviews were not consistently used and only 2 up-to-date Cochrane reviews were cited. Two CPGs graded some recommendations and related that to levels (but not quality) of evidence. The CPGs' recommendations on assessment and non-pharmacological management were fairly consistent. Guidelines varied in the selection of first-line treatment, adjustment of therapy and drug combinations. Important specific aspects of care (e.g. resistant hypertension) were ignored by 6/11 CPGs. The CPGs varied in methodological quality, suggesting that their implementation might not result in less variation of care or in better health-related outcomes.
Conclusions/Significance
More efforts are needed to promote the realistic approach of localization or local adaptation of existing high-quality CPGs to the national context.
Introduction
Globally, the prevalence of hypertension among adults aged 25 and over was approximately 40% in 2008 [1] and the total economic burden of hypertension in the United States was estimated at $73.4 billion in 2009 [2].
Better hypertension management leads to improved health outcomes. A large systematic review of 147 trial reports on the management of hypertension has shown that a reduction of 10 mm Hg in systolic blood pressure and 5 mm Hg in diastolic was associated with a 20% reduction of coronary heart disease and 32% reduction in stroke in one year [3]. And, the management of hypertension is cost-effective; treatment with medication results in improved health outcomes (higher quality-adjusted life-years; QALYs) [4]. However, awareness of hypertension, its treatment and control are far from adequate worldwide [5]–[7]. The variation in the multiple CPGs on hypertension published between 1997 and 2005 has been addressed in an earlier study [8] and it is clear that variation in the quality of guidelines exists for other conditions and is not unique to hypertension [9]–[13]. Of the CPGs used in 235 studies assessing the effectiveness and efficiency dissemination and implementation strategies, only 3% of guidelines used were based on good evidence [14].
The aim of this systematic review was to assess the quality and consistency of recommendations of recently-developed national and international CPGs on the diagnosis, assessment and the management of hypertension and, to determine the extent to which these CPGs are informed by Cochrane and non-Cochrane systematic reviews.
Methods
This systematic review was completed based on a protocol with input from experts in hypertension and systematic review methodology, as recommended in the PRISMA Statement [15] (Table S1). The institutional review board was not obtained because there was no direct involvement with patients or bodily samples.
Eligibility criteria
Multi-disciplinary CPGs endorsed by a national governmental or provider organization related to the diagnosis, assessment and management of hypertension were included. All subgroups of the population had to be examined to ensure that the CPGs cater for the needs of those with comorbidities in different settings; CPGs focused exclusively on hypertension among special groups (e.g. pregnancy, children, elderly, blacks or diabetes) or specific settings (e.g. primary care only or emergency management only) were excluded. To ensure that the most up-to-date CPGs were included, inclusion was limited to January 2006 onwards. Furthermore, only CPGs written in English were included.
Information sources
Medical Subject Headings and text words related to hypertension and guidelines were used to search MEDLINE and EMBASE using the OVID interface from January 2006 to September 2011. The electronic database search was supplemented by searching websites and Google, as CPGs are not always cited in such databases. Specifically, the following websites were searched: Guidelines International Network (G-I-N; www.g-i-n.net), National Guidelines Clearinghouse (www.guideline.gov), Australia National Health and Medical Research Council (www.nhmrc.gov.au/guidelines/index.htm), National Institute for Health and Clinical Excellence (www.nice.org.uk) and Scottish Intercollegiate Guidelines Network (SIGN; www.sign.ac.uk), The word ‘hypertension’ was entered into the website search utility and the first 30 results were reviewed. Google was also searched using the keywords ‘hypertension’ and ‘guideline’ in a similar manner. To ensure all potentially relevant guidelines were identified, targeted searching by country was conducted in Google, the reference lists of included CPGs were scanned, and a list of the included guidelines were emailed to experts in the field to identify additional CPGs.
Search
An experienced information specialist (LP) conducted all of the literature searches. The search strategy for the main electronic search (MEDLINE) is presented in Box S1; details on the EMBASE search are available upon request.
Study selection
To ensure reliability, a training exercise was conducted prior to commencing the study selection process using a random sample of 25 citations. Two reviewers independently screened the search results for inclusion using a pre-defined relevance criteria form. The full-text article was obtained for potentially relevant CPGs and these were subsequently screened by two independent reviewers. Discrepancies at any stage were resolved by discussion or the involvement of a third reviewer.
Data collection process and data items
A draft data extraction form was developed, piloted, and modified as necessary. Two reviewers independently extracted all of the data using the standardized data extraction form. Discrepancies were resolved by discussion or the involvement of a third reviewer.
All the relevant documents and websites of the selected CPGs were examined. The extracted data included CPG characteristics (e.g., year of dissemination, country/region, development team, funding organization), recommendations related to the diagnosis and assessment of hypertension, and recommendations related to the management of hypertension. The Appraisal of Guidelines Research and Evaluation (AGREE) II tool [16] was used by 4 reviewers independently to appraise the validity of each included CPGs. The 4 assessors also provided their judgments on the overall assessment, the possible risk of bias and recommendation for future use for each CPG that they appraised. Discrepancies were resolved by discussion or the involvement of a fifth reviewer. The agreement of the 4 raters in the “Rigour of Development” domain was explored using percentage of agreement. To measure inter-rater agreement, values for the eight items were collapsed from 7 to 3 values as follows: 1, 2, 3 as 1 to represent “disagree” and 5, 6 and 7 as 2 to represent “agree” and 4 becomes 3 as “neutral “. This analysis was conducted using the AgreeStat software [17].
The reference list of each of the selected CPGs was reviewed and the number of Cochrane and non-Cochrane systematic reviews in each was recorded. The end of search date for each CPG was checked to determine the available and relevant reviews prepared by the Cochrane Hypertension Group [18] by that date. For CPGs where the end-of-search date was not reported, the principal author was contacted. If there was no response from the author, it was assumed that search ended one year prior to publication of the CPG. Two reviewers independently screened all the abstracts of the reviews prepared by the Cochrane Hypertension Group to assess their relevance to the general management of primary hypertension.
Synthesis of results
The included CPGs were summarized descriptively according to diagnosis, assessment and management recommendations. For each item, we noted whether the CPG recommended it, the level of evidence (which is based on the study design), and the quality of studies supporting/refuting the recommendation (determined when the reviewers critically appraised the studies). For diagnosis and assessment, the following categories were used: identification of cardiovascular risk factors, blood pressure measurement methods, medical history, physical examination, subclinical organ damage, and laboratory investigations. For management, the following categories were used: lifestyle modifications, initiation of therapy, type of therapy, adjustment of therapy, combination therapy, harms associated with the therapy, consideration of special groups (e.g., elderly, diabetics, renal dysfunction, pregnancy), follow-up, compliance, and specialist referral.
Results
Study selection
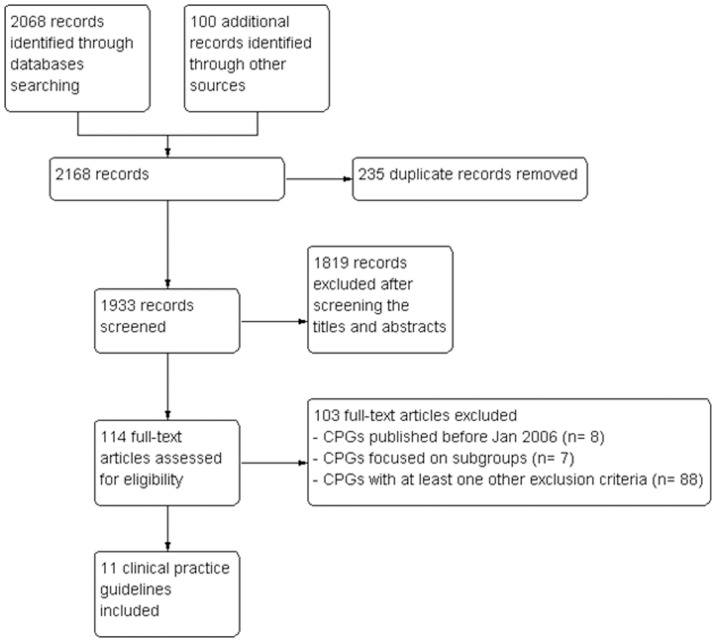
The search strategy retrieved 2168 citations, of which 114 were considered for full-text screening and 11 were included in the review (Figure 1). Two CPGs were multinational efforts to develop a unified hypertension CPG (EUR and LAT) [19]–[21]. The remaining hypertension CPGs were conducted in South Africa (SOA) [22], India (IND) [23], Poland (POL) [24], Malaysia (MAL) [25], Japan (JAP) [26], Australia (AUS) [27], Canada (CAN) [28], Saudi Arabia (SAU) [29] and the United Kingdom (NICE) [30].
Figure 1. Flow chart using the PRISMA statement for the systematic review.
Clinical practice guideline characteristics
Table 1 displays the characteristics and methods related to CPG development. Two CPGs were new (POL and LAT); the rest were updates. All CPGs (except the SAU and IND CPGs) were retrieved through searching the medical literature databases. The SAU and IND CPGs were retrieved through the country-specific Google search.
Table 1. Characteristics and Methods Used For Developing the 11 Clinical Practice Guidelines.
| Characteristics | SOA 2006 | IND 2007 | POL 2007 | MAL 2008 | EUR 2009 | JAP 2009 | LAT 2009 | AUS 2010 | CAN 2011 | SAU 2011 | NICE 2011 |
| Status of the CPG | |||||||||||
| New | No | No | Yes | No | No | No | Yes | No | No | No | No |
| Updated | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Level of development | |||||||||||
| National | Yes | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes |
| Regional | No | No | No | No | Yes | No | Yes | No | No | No | No |
| Organization behind the guideline | |||||||||||
| Professional organization (e.g. Societies) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Government | Yes | No | No | Yes | No | No | No | No | No | Yes | Yes |
| Funding/Sponsorship | No | NR | NR | Industry educational grant | NR | NR | NR | Professional Grants | Professional grants | Industry | NICE |
| Developing team structure & affiliation described | |||||||||||
| Number of members | 7 | 33 | 15 | 17 | 32 | 30 | 14 | 14 | 65 | 19 | 15 |
| Affiliation described? | No | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Specialty described? | No | No | No | Yes | Not clear | No | No | No | Yes | Yes | Yes |
| Searching and selecting references | |||||||||||
| Search Strategy Described? | No | No | No | No | No | No | No | Yes | Yes | No | Yes |
| Total references cited | 110 | 146 | 4 | 251 | 293 | 742 | 157 | 64 | 56 | 53 | 662 |
| Total systematic reviews cited | 6 | 0 | 0 | 19 | 19 | 36 | 5 | 6 | 6 | 8 | 12 |
| Total Cochrane reviews cited | 0 | 0 | 0 | 3 | 1* | 8 | 1 | 0 | 0 | 0 | 3 |
| Total cited/available** relevant Cochrane reviews from the Hypertension group only | 0/11 | 0/15 | 0/15 | 0/17 | Jan-32 | 0/32 | 0/32 | 0/34 | 0/39*** | 0/39*** | 2****/39 |
| Methods of deriving recommendations | |||||||||||
| Evidence linked, Formal consensus method | No | No | No | No | No | No | No | No | Yes | No | Yes |
| Evidence-linked, no description of method | No | No | No | Yes | No | No | No | Yes | No | No | No |
| Consensus method, no detailed description | Yes | Yes | No | No | No | No | Yes | No | No | No | No |
| Not described | No | No | Yes | No | Yes | Yes | No | No | No | Yes | No |
| Implementation strategies described | Yes | No | No | No | Yes, weak | No | No | Yes | Yes | Yes | Yes |
| Year of publication of the previous version of the CPG | 2003 | 2001 | - | 2002 | 2007 | 2004 | - | 2009 | 2010 | 2007 | 2006 |
| Next Update of the CPG | 2012 | NR | NR | 2012 | NR | NR | NR | NR | 2012 | NR | NR |
NR: Not reported. SOA: South Africa; IND: India; POL: Poland; MAL: Malaysia; EUR: Europe; JAP: Japan; LAT: Latin America; AUS: Australia; CAN: Canada, SAU: Saudi Arabia and NICE (The UK's National Institute for Health and Clinical Excellence).
The ESH Reappraisal in 2009 cited only 1 review (4 reviews were cited in 2007).
Produced by the Hypertension Cochrane Review Group calculated for up to one year before the date of publication of the CPGs when the search date was not reported.
The total number of reviews available at that time was 41 but two reviews were excluded because they were judged as irrelevant.
The updated version of Murlow's review was published in 2008 but the 2000 version was the one cited.
The affiliation and/or the specialties of the developing group team members were not described in three CPGs (SOA, IND, LAT); the remaining guidelines provided some or a detailed description of the team. Two CPGs were funded by drug companies (SAU, MAL), three reported funding from professional organizations and provided a list of members with their declaration of interest (AUS, CAN, NICE), and the remainder did not disclose a funding source. In the CAN guideline, members with conflict of interest for certain recommendations were “recused” from voting. The size of the guideline development team varied from 7 to 65 members. Most of the CPGs (7/10) provided information on the affiliation of these members but only 3 (MAL,CAN and SAU) provided information on their specialties. Except for one CPG (NICE), 10/11 CPGs did not report including patient representatives in their guideline development team. Apart from the AUS, CAN and NICE CPGs, none of the guidelines reported a search strategy in their methods section. All CPGs (except for the IND and POL) cited some systematic reviews in the reference section. The number of systematic reviews cited ranged from 5 to 31. Five CPGs (SOA, IND, POL, AUS, CAN) did not refer to reviews from the Cochrane Collaboration developed by the Hypertension Review Group that were available at the time of guideline development. The JAP, SAU, MAL, LAT and NICE cited 8, 5,3, 1 and 3 Cochrane reviews, respectively. Of the reviews from the Cochrane Hypertension Group, one was cited by the EUR CPG [31] and 2 were cited by NICE CPG [32], [33]. Table 1 shows the numbers of available and relevant reviews from the Hypertension Group for each CPG that could have potentially been used by the guidelines' development teams. Some of the guidelines clearly reported that they referred to other international guidelines (SAU, LAT and IND) )but none of them reported being an adaptation of another CPG.
AGREE-II appraisal results
In general, the guidelines received the lowest scores for rigour of development among all 6 AGREE domains (mean 27%, range: 8.3%–86.4%), whereas, they scored highest on clarity of presentation (mean 66.8%, range: 44.4%–88.9%). The CAN CPG scored the highest on rigour of development (Domain 3) and the NICE CPG scored the highest for the scope and purpose and editorial independence (Domains 1 and 6; Tables 2–3). The applicability (Domain 5) and stakeholder involvement (Domain 2) domains were scored consistently low across the CPGs (Tables 2–3). The overall quality of the CPGs ranged from 2.5 to 6 on a 7 point scale. With the exception of the CAN CPG, all guidelines were either not recommended for use or were recommended for use with modifications. The risk of bias (judged by the reviewers as an inverse overall assessment of the rigour of development domain) was lower in the CAN and NICE CPG and higher in the SOA, POL, EUR, LAT and the SAU CPGs. The degree of agreement among reviewers was tested using percentage of agreement for the rigour of development domain. The agreement varied across the guidelines from as high as 88% (CAN, POL), 73%,71%, 69%, 62% (SOA, JAP, LAT, MAL, respectively) to as low as 58%, 56%, 52%, 50%, 46% (IND, MAL, AUS, NICE, SAU, respectively)
Table 2. Domain Scores (%) for the 11 Clinical Practice Guidelines Using the AGREE-II Instrument.
| SOA 2006 | IND 2007 | POL 2007 | MAL 2008 | EUR 2009 | JAP 2009 | LAT 009 | AUS 2010 | CAN 2011 | SAU 2011 | NICE 2011 | |
| DOMAIN 1. SCOPE AND PURPOSE | 47.2 | 44.4 | 25 | 65.3 | 36.1 | 20.8 | 61.1 | 22.2 | 75 | 44 | 83 |
| DOMAIN 2. STAKEHOLDER INVOLVEMENT | 37.5 | 13.9 | 12.5 | 45.8 | 27.7 | 18 | 41.6 | 38.9 | 75 | 49 | 74 |
| DOMAIN 3. RIGOUR OF DEVELOPMENT | 13.5 | 21.8 | 8.3 | 26.5 | 23.4 | 18.75 | 15.6 | 27.1 | 86.4 | 30 | 62 |
| DOMAIN 4. CLARITY OF PRESENTATION | 55.5 | 50 | 75 | 69.4 | 69.4 | 62.5 | 44.4 | 88.9 | 88.9 | 64 | 55 |
| DOMAIN 5. APPLICABILITY | 38.5 | 17.7 | 16.6 | 42.7 | 21.8 | 14.6 | 30.2 | 59.3 | 59.3 | 46 | 72 |
| DOMAIN 6. EDITORIAL INDEPENDENCE | 39.6 | 16.6 | 4.1 | 68.75 | 64.6 | 29.1 | 35.4 | 64.6 | 75 | 38 | 88 |
SOA: South Africa; IND: India; POL: Poland; MAL: Malaysia; EUR: Europe; JAP: Japan; LAT: Latin America; AUS: Australia; CAN: Canada; SAU: Saudi Arabia and NICE: UK's National Institute for Health and Clinical Excellence.
Table 3. Quality of the 11 Hypertension Clinical Practice Guidelines for the six domains of the AGREE-II Instrument (D1–D6) and the Overall Impression of the 4 Assessors.
| D1 | D2 | D3 | D4 | D5 | D6 | Overall* | Risk of Bias** | Recommend CPG for Use*** | ||||||||||||||||||
| Item # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | # | 22 | 23 | |||
| SOA 2006 | 5 | 3 | 4 | 4 | 1 | 5 | 1 | 1 | 1 | 2 | 2 | 2 | 4 | 3 | 5 | 6 | 3 | 3 | 4 | 3 | 3 | 5 | 2 | 3 | +++ | No |
| IND 2007 | 4 | 3 | 4 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 5 | 2 | 4 | 5 | 3 | 2 | 2 | 2 | 2 | 3 | 1 | 2.5 | ++ | No |
| POL 2007 | 2 | 1 | 4 | 3 | 1 | 1 | 1 | 1 | 2 | 1 | 3 | 2 | 1 | 1 | 6 | 6 | 5 | 1 | 2 | 1 | 3 | 1 | 1 | 3 | +++ | No |
| MAL 2008 | 5 | 5 | 5 | 5 | 2 | 5 | 2 | 1 | 1 | 1 | 4 | 4 | 4 | 4 | 5 | 6 | 5 | 3 | 4 | 4 | 4 | 6 | 5 | 3 | ++ | Unsure |
| EUR 2009 | 3 | 3 | 3 | 4 | 1 | 3 | 2 | 3 | 4 | 1 | 4 | 2 | 3 | 1 | 5 | 6 | 5 | 5 | 2 | 2 | 1 | 4 | 6 | 3.5 | +++ | No |
| JAP 2009 | 2 | 2 | 3 | 3 | 1 | 2 | 2 | 2 | 2 | 2 | 4 | 3 | 2 | 1 | 4 | 6 | 5 | 2 | 2 | 2 | 1 | 4 | 2 | 4 | ++ | Unsure |
| LAT 2009 | 6 | 4 | 5 | 3 | 3 | 5 | 2 | 1 | 1 | 2 | 3 | 2 | 3 | 3 | 4 | 4 | 3 | 3 | 2 | 3 | 4 | 4 | 2 | 3 | +++ | Unsure |
| AUS 2010 | 2 | 3 | 3 | 4 | 4 | 2 | 3 | 1 | 1 | 3 | 4 | 2 | 6 | 2 | 6 | 6 | 7 | 5 | 6 | 3 | 5 | 3 | 7 | 4.5 | ++ | Yes, with modifications |
| CAN 2011 | 5 | 6 | 6 | 7 | 5 | 6 | 7 | 7 | 6 | 7 | 6 | 6 | 5 | 7 | 7 | 7 | 6 | 4 | 6 | 4 | 4 | 5 | 6 | 6 | + | Yes |
| SAU 2011 | 4 | 4 | 4 | 5 | 3 | 4 | 3 | 3 | 2 | 3 | 3 | 3 | 2 | 4 | 5 | 6 | 4 | 5 | 4 | 3 | 3 | 4 | 3 | 3.5 | +++ | No |
| NICE 2011 | 6 | 6 | 6 | 6 | 5 | 6 | 6 | 6 | 6 | 6 | 5 | 5 | 4 | 5 | 6 | 6 | 6 | 5 | 6 | 6 | 6 | 7 | 6 | 6 | + | Yes, with modifications |
D1 : Scope & purpose, D2: Stakeholder involvement, D3: Rigor of involvement, D4: Clarity of presentation, D5: Applicability, D6: editorial independence.
All the 23 items of the AGREE-II instrument are rated on a 7-point scale where a score of 1 is given when there is no information that is relevant to the item or if the concept is very poorly reported; a score of 7 is given if the quality of reporting is exceptional and where the full criteria and considerations articulated in the AGREE-II User's Manual have been met; and a score between 2 and 6 is assigned when the reporting of the AGREE II item does not meet the full criteria or considerations. Scores increase as more criteria are met and considerations addressed. In other words, the higher the score, the better the quality of the CPG item.
SOA: South Africa; IND: India; POL: Poland; MAL: Malaysia; EUR: Europe; JAP: Japan; LAT: Latin America; AUS: Australia; CAN: Canada; SAU: Saudi Arabia and NICE: UK's National Institute for Health and Clinical Excellence).
Although the scoring is done in integers, the numbers in this column represent the averages of the scoring done by 4 assessors.
Risk of bias: +++ high, ++ intermediate, + low.
This is based on the subjective assessment made individually by each of the 4 assessors in response to: “Do you recommend this CPG for use?”
Only two guidelines linked their grade of recommendations to the level of evidence (MAL, CAN), yet they did not elaborate on the quality of studies contributing to the recommendations (Table 4). Agreement between these CPGs on the grade of recommendations was not observed. For example, the advice on exercise was graded A in MAL and D in CAN. The NICE CPG provided the evidence tables for their recommendations and the SAU CPG reported the level of evidence for some recommendations but none of them reported the strength of recommendations. The other 7 CPGs did not disclose the level of evidence or how their recommendations were decided upon.
Table 4. Strength of the recommendations stated in the Malaysian and Canadian Clinical Practice Guidelines.* .
| Recommendations | Strength of recommendation | |
| MAL 2008 | CAN 2011 | |
| Recommendations to attain normal body mass index | C | B |
| An intake of <100 mmol of sodium daily | A | B |
| Advice to restrict intake of alcohol | C | B |
| General advice on exercise | A | D |
| Adapting healthy DASH diet | A | B |
| Smoking Cessation | C | Not graded |
| Recommendations to use ACEI in presence of microalbuminuria | A | A |
| Use of ARB if ACEI is not tolerated | A | B |
| Recommendation for diuretics or calcium channel blockers as alternative therapy in diabetic hypertensive patients | A | A |
| Combination of ACEIs and ARBs in patients with hypertension and no diabetic renal disease | A | B |
None of the other CPGs stated their strength of recommendations.
MAL: Malaysia; CAN: Canada.
Clinical practice guideline recommendations
Definition
Most CPGs considered high normal blood pressure to range from 120–129 systolic blood pressure (SBP) or 80–84 for diastolic blood pressure (DBP) (Table 5). The exception was the IND and NICE CPGs, which defined hypertension using a higher cut off points. The CAN CPG did not use cut off points for hypertension.
Table 5. Recommendations from Clinical Practice Guidelines About Diagnosis and Assessment of Patients with Hypertension.
| ITEM | SOA 2006 | IND 2007 | POL 2007 | MAL 2008 | EUR 2009 | JAP 2009 | LAT 2009 | AUS 2010 | CAN 2011 | SAU 2011 | NICE 2011 |
| Definition of Hypertension | |||||||||||
| Normal: SBP (120–129) or DBP(80–84), if different, state | √ | SBP<130 DBP<85 | √ | SBP<120, DBP<80 | √ | <125/80 | √ | √ | NR | SBP<120 and DBP<80 | Clinic <140/90 mmHg or HBPM/ABPM <135/85 |
| High normal: SBP (130–139) or DBP (85–89) if different, state | √ | √ | √ | Pre-HTN SBP 120–139 mmHg, DBP 80–89 mmHg | √ | √ | √ | SBP (120–139) or DBP (80–89) | √ | Pre-HTN 120–139, and/or 80–89 | X |
| Mild (G1): SBP (140–159) or DBP (90–99) | √ | √ | √ | √ | √ | √ | √ | √ | X | √ | Clinic ≥140/90 mmHg and HBPM/ABPM ≥135/85 |
| Moderate (G2): SBP (160–179) or DBP (100–109) | √ | √ | √ | √ | √ | √ | √ | √ | X | √ | Clinic ≥160/100 mmHg and ABPM/HBPM ≥150/95 mmHg |
| Severe (G3): SBP>180 or DBP>110 | √ | √ | √ | √ | √ | √ | √ | √ | X | ≥180 and/or ≥110 | Clinic SBP ≥180/110 mmHg |
| Isolated systolic hypertension: SBP>140 and DBP<90 if different, state | NR | √ | √ | √ | √ | √ | √ | √ | NR | NR | SBP ≥160 mmHg |
| Isolated systolic hypertension+widened pulse pressure: SBP>160 and DBP<70 | NR | √ | √ | √ | √ | √ | √ | √ | NR | NR | NR |
| Cardiovascular Risk Assessment | |||||||||||
| Data elements recommended for cardiovascular risk stratification | |||||||||||
| SBP/DBP | √ | NR | √ | NR | √ | √ | √ | √ | √ | √ | √ |
| Smoking | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Dyslipidaemia | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Diabetes | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Subclinical organ damage | |||||||||||
| LVH on ECG/ECHO | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Microalbuminuria: ECR 3–30 mg/mmol | √ | 1.2–2 mg/dl | Recommended but cut-off not reported | ≥ 2.0 mg/mmol (males) or ≥2.5 mg/mmol (females) on spot urine screening test OR 24-hour urinary albumin excretion rate ≥20 µg/minute | 30–300 mg/24 hours | Recommended but cut-off not reported | Recommended but cut-off not reported | ≥2.0 mg/mmol (males) or ≥2.5 mg/mmol (females) on spot urine screening test OR 24-hour urinary albumin excretion rate ≥20 µg/minute | Recommended but cut-off not reported | NR | Albuminuria stated but no cut-off reported |
| CKD | |||||||||||
| Elevated creatinine: Men 115–133, Women 107–124 µmol/l | √ | elevated serum creatinine 1.2–2.0 mg/dl | Recommended but no cut-off | x | √ | √ | CR>1.3 mg/dL | X | x | √ | Reported with no cut-off stated |
| Proteinurea protein/creatinine ratio ≥30 mg/mmol on spot urine test or urine protein >300 mg/day on timed urine sample | √ | x | Urinary albumin/creatinine ratio but the ratio is not stated | Urinary protein >500 mg/24 hr or albumin to creatinine ratio [ACR] >30 mg/mmol | Albumin-creatinine ratio: >or = 22 (M); or 31 (W) mg/g creatinine | √ | x | √ | √ | NR | Reported with no cut-off stated |
| eGFR <60 mL/minute/1.73 m | × | √ | √ | √ | √ | √ | eGFR <30 ml/min/1.73 m | √ | √ | NR | Reported with no cut-off stated |
| Subclinical organ damage: Vascular disease | |||||||||||
| Atherosclerotic plaque (aorta, carotid, coronary, femoral and iliac arteries) evident on US or radiology | Not stated clearly | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Hypertensive retinopathy (grade II or greater) | √ | √ | × | √ | √ | √ | grade III/IV | √ | √ | √ | √ |
| Stratification: Low; Moderate; High/Very High added risk | normal, high normal, mild, moderate, severe | √ | normal, high normal, Grade 1, 2 and 3) | √ | √ | √ | low, intermediate and high | Low, Mod, High | NR | √ | NR |
| BP measurement | |||||||||||
| Office: 140/90 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Home: 135/85 | √ | √ | × | √ | √ | √ | √ | × | √ | √ | √ |
| Ambulatory: 120/70 (mean night); 135/85 (mean day); 130/80 (24-hour) * Suggested for selected cases | √* | √* | × | √* | √* | √ | √* | √* | √ | √* | √* |
| Reason(s) for Home and self-monitoring | For select groups | For White-Coat HTn only | NR | White coat HTN Monitoring | White-coat HTN Monitoring Dx of resistant HTN | More accurate Dx Masked and white-coat HTN Improves adherence | Masked and white-coat HTN and FU | Masked and white-coat HTN and FU | For white-coat HTN | White-coat HTN Monitoring Dx of resistant HTN | confirm diagnosis, white-coat HTN |
| List of devices provided | √ | NR | NR | √ | √ | √ | √ | NR | NR | √ | √$ |
| Both arms * First visits only | √* | √* | NR | √ | √* | NR | √ | √* | √* | √* | √* |
| Family History | |||||||||||
| Early CVD: Men aged <55 years and Women aged <65 years | √ | √ | √ | √ | √ | √ | Not clear | √ | √ | √ | √ |
| High blood pressure | × | √ | NR | √ | √ | √ | NR | √ | NR | √ | √ |
| Obesity | × | √ | × | √ | × | √ | √ | × | × | √ | × |
| Stroke | × | √ | × | √ | √ | √ | √ | √ | × | √ | × |
| Dyslipidaemia | × | √ | × | √ | √ | x | × | √ | × | × | × |
| Diabetes | × | √ | × | √ | √ | √ | NR | √ | × | × | × |
| Clinical History | |||||||||||
| CAD | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Heart Failure | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| CKD | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Stroke or TIA | √ | √ | NR | √ | √ | √ | √ | √ | √ | √ | √ |
| Peripheral vascular disease | √ | √ | NR | √ | √ | √ | √ | √ | √ | √ | √ |
| Retinopathy | √ | √ | NR | √ | √ | √ | √ | √ | √ | √ | √ |
| Aortic disease | × | √ | NR | √ | √ | √ | √ | √ | × | √ | √ |
| Hypercholesterolaemia: Serum TC>7.5 mmol/L | √ | √ | √ | √ | √ | √ | × | √ | √ | √ | √ |
| Previous medications | × | √ | NR | √ | √ | √ | √ | √ | √ | √ | √ |
| Other significant conditions (asthma, sleep apnea, COPD) | × | × | NR | √ | √ | √ | × | √ | × | √ | √ |
| Modifiable lifestyle risk factors | √ | √ | √ | √ | √ | √ | NR | √ | √ | √ | √ |
| History of hypokalaemia or suggestive symptoms | × | × | NR | √ | × | NR | × | √ | √ | √ | √ |
| Other | - | Smoking, gout, sexual dysfunction, Dietary (Salt, Alcohol, Caffeine) | Smoking and Gout | personal, psychosocial and environmental factors | Smoking, dietary, obesity, physical exercise | - | - | psychosocial and environmental factor | - | Growth retardation | Symptoms of identifiable cause s of HTN |
| Physical Examination | |||||||||||
| Cardiovascular | × | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| ECG | √ | √ | NR | √ | √ | √ | √ | √ | √ | √ | √ |
| Obesity( Waist-to-hip ratio or BMI) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Other physical examination | Body weight | - | - | Abnormalities of optic fundi evidence of abnormalities of the endocrine system (e.g. Cushing's syndrome, thyroid disease) | - | - | associated risk factors and possible complications such as peripheral edema, angina pectoris, dyspnea, headache, ectopic heart beats | ABI | - | Typical cushingoid appearance | Signs of secondary causes |
| Searching for subclinical organ damage | |||||||||||
| Heart: LVH | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Blood vessels: Peripheral arterial disease | √ | √ | NR | √ | √ | √ | √ | √ | √ | √ | √ |
| Blood vessels: Aortic disease | NR | NR | NR | √ | NR | √ | NR | √ | NR | NR | √ |
| Kidney: CKD | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Kidney: Other | elevated creatinine | Albumin/CR Ration | - | - | - | - | ultrasound/Doppler for renal arterial stenosis or kidney alterations | Diabetic nephropathy, Glomerulonephritis, Hypertensive kidney disease. | - | Diabetic nephropathy | - |
| Fundoscopy: Haemorrhages OR Exudates or Papilloedema | √ | √ | NR | √ | √ | √ | NR | √ | √ | √ | √ |
| Brain: Stroke or TIA | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Lab investigations | |||||||||||
| Urine dipstick for blood, protein sugar | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Microalbuminurea | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Blood tests: FBG, random total cholesterol, creatinine, potassium. | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| ECG | √ | √ | NR | √ | √ | √ | √ | √ | √ | √ | √ |
| C-reactive protein > l mg/dl | NR | √ | NR | NR | NR | √ | NR | NR | NR | NR | NR |
| Other Investigations | - | Echo uric acid | Carotid-femoral pulse wave velocity glomerular filtration rate | ABI, ECD, Plasma aldosterone/renin ratio | - | - | Thyroid function test, LFT | ECD, Plasma aldosterone/renin ratio24-H urinary catecholamine, RAU evidence of abnormalities of the endocrine system thyroid disease) | Screening for hyperaldosteronism (hypokalemia) or hypokalaemia Screening pheochromocytoma renovascular hypertension Captopril-enhanced radioisotope renal scan Doppler sonography, magnetic resonance angiography and CT- angiography (for those with normal renal function) | CBC Uric acid TSH, free T4 CXR abdominal US Echo Significantly 24-H urinary catecholamines Overnight dexamethasone suppression testing Plasma aldosterone/renin ratio | total/HDL cholesterol levels, TSH, polysomnograph |
NR: Not reported, √: Recommended, ×: Not Recommended; SOA: South Africa; IND: India; POL: Poland; MAL: Malaysia; EUR: Europe; JAP: Japan; LAT: Latin America; AUS: Australia; CAN: Canada; SAU: Saudi Arabia and NICE (The UK's National Institute for Health and Clinical Excellence).
not endorsed by NICE. ABI: ankle-brachial index ECD: Echo Carotid Doppler, RAU: Renal artery duplex ultrasound.
“White coat syndrome” (i.e., the propensity for patients to have higher blood pressure when measured by a clinician) was addressed using self-measured blood pressure in 9/11 CPGs. The devices used in self-monitoring were described in 7 CPGs (SOA, MAL, EUR, JAP, AUS, CAN and NICE). The distinction between different settings (office, home and ambulatory) for measuring blood pressure and identifying patients as having hypertension was made in all CPGs except two (POL, AUS).
Cardiovascular Risk
All CPGs recommended assessing hypertension in relation to other cardiovascular risk factors during patient assessment (Table 5).
Family and Clinical history
The clinical assessment included asking patients about their family history of hypertension (IND, MAL, EUR, JAP, LAT, SAU, NICE), stroke (IND, MAL, EUR, JAP, LAT, AUS, and SAU), dyslipidemia (IND, MAL, EUR and AUS) and diabetes (IND, MAL, EUR, JAP and AUS). All CPGs recommended inquiring about previous coronary artery disease, heart failure and chronic kidney disease (Table 5). All but one CPG (POL) recommended asking about past history of stroke and existing peripheral artery disease and retinopathy.
Physical examination searching for subclinical organ damage
All CPGs recommended assessing the patient's body mass index. Similarly, all addressed modifiable lifestyle risk factors, except for one CPG (LAT) (Table 5). All except for one CPG (POL) considered ECG as a necessary component of the physical examination. All recommended fundoscopy, except for the POL and LAT CPGs.
Laboratory testing
All CPG suggested assessing fasting blood glucose, fasting blood cholesterol, creatinine, potassium and urine dipstick testing for glucose, blood (hematuria), protein and albumin (Table 5). Only two CPGs (IND and JAP) recommended assessing C-reactive protein as part of the workup for patients with hypertension.
Recommendations for the management of hypertension:
Findings from guidelines about the management of hypertension are presented in Table 6. All guidelines advocated similar life style changes as a cornerstone in the management of hypertension. Minor differences included recommendation of dietary supplements, increase of potassium intake, exercise, and stress and emotional management.
Table 6. Recommendations from Clinical Practice Guidelines about Managing Patients with Hypertension.
| ITEM | SOA 2006 | IND 2007 | POL 2007 | MAL 2008 | EUR 2009 | JAP 2009 | LAT 2009 | AUS 2010 | CAN 2011 | SAU 2011 | NICE 2011 |
| Advice about Lifestyle changes | |||||||||||
| Maintain weight | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Lower sodium intake | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Limit alcohol | √ | √ | √ | √ | √ | √ | √ | √ | √ | NR | √ |
| Follow nutrition guidelines | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Limit sugar intake | √ | NR | √ | NR | NR | NR | NR | √ | √ | NR | NR |
| Lower fat intake | √ | √ | √ | √ | √ | √ | √ | NR | √ | √ | √ |
| moderate-intensity exercise for at least 30 minutes on most or preferably all days of the week | NR | NR | NR | √ | √ | √ | √ | NR | √ | NR | √ |
| Stop smoking | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Other | |||||||||||
| Dietary supplements | NR | NR | NR | √ | NR | NR | NR | NR | NR | NR | NR |
| Increasing K | NR | NR | NR | NR | NR | NR | √ | NR | √ | NR | NR |
| Stress management | NR | NR | NR | √ | NR | √ | NR | NR | √ | NR | √ |
| When to initiate Therapy? | |||||||||||
| Low added risk despite a period of 6–12 months of lifestyle modification and observation | √ | 3 months cut-off | NR | √1 | √ | 3 months cut-off | √ | √ | √2 | √ | NR |
| Moderate added risk despite a period of 3–6 months of lifestyle modification and observation | √ | 2–3 months cut-off | NR | √3 | √ | 1 month cut-off | √ | √ | √4 | √ | NR |
| High or very high added risk | √ | √ | √ | √5 | √ | √ | √ | √ | √ | √ | √ |
| How to initiate drug therapy? | |||||||||||
| Step 1 Use a low-dose diuretic as initial therapy | √ | √ | NR | NR | NR | NR | NR | NR | NR | √ | NR |
| use agent from any of the 5 classes (A,B,C,D) as first line | NR | √ | √ | √ | √ | √ | √ | √ | √ | √ | √6 |
| Step 2: Consider costs, other conditions contraindications and if OK prescribe ACE-Is and CCBs | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Other second-line medication from the 5 classes | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Step 3: Other third-line medication? | Add A or C | A or B+C+D | A diuretic should be one of them | Combination of therapies | √ | Add a third agent | √ | √ | Yes if not controlled | Renin inhibitors | A+C+D |
| Adjustment of therapy | |||||||||||
| Strategies: | |||||||||||
| Increase dose of 1st agent | NR | NR | NR | √ | NR | √ | NR | NR | NR | NR | √ |
| Substitute with another agent | NR | √ | NR | √ | NR | √ | NR | NR | NR | NR | NR |
| Add another agent | √ | √ | NR | √ | √ | √ | NR | NR | NR | NR | √ |
| Other Strategies | NR | intensify life style | long acting mono-therapy | NR | NR | give drug twice daily | NR | NR | Changes in nocturnal BP | NR | NR |
| Choice of anti-hypertension therapy | |||||||||||
| Start with mono-therapy and move to combo therapy | √ | √ | √ | √ | √ | √ | not clear | √ | √7 | √ | √ |
| and/or two drug combination as initial | NR | √ | NR | NR | NR | NR | NR | NR | NR | √7 | NR |
| Recommendations about combination therapy | |||||||||||
| Which drug combination? | D+BB | various | various | various | Various | Various | Not clear | A+C | various | A+C | A+C |
| Considerations for special groups | |||||||||||
| Elderly | √ | √ | NR | √ | √ | √ | √ | √ | NR | √ | √ |
| Diabetics | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | NR |
| Proteinuria | √ | √ | NR | √ | √ | √ | √ | √ | √ | √ | √ |
| Renal insufficiency | √ | NR | NR | √ | NR | √ | √ | NR | √ | √ | NR |
| renal failure | NR | √ | √ | NR | √ | √ | √ | NR | NR | NR | √8 |
| Bilateral artery stenosis | NR | NR | NR | √ | NR | √ | NR | NR | NR | NR | NR |
| Heart Failure | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Post MI | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | NR |
| Angina | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | NR |
| Peripheral vascular disease | √ | √ | √ | NR | √ | √ | √ | NR | NR | √ | NR |
| Carotid atherosclerosis | √ | NR | NR | NR | √ | √ | NR | NR | NR | NR | NR |
| CCB for Supraventricular tachycardia | √ | NR | NR | NR | √ | √ | √ | NR | NR | √ | NR |
| Left ventricular dysfunction/LVH | √ | NR | √ | √ | √ | √ | √ | NR | √ | √ | √ |
| Tachyarrhythmias | √ | √ | NR | √ | √ | NR | √ | √ | √ | √ | NR |
| COPD | √ | √ | √ | √ | √ | √ | NR | √ | NR | √ | NR |
| Pregnancy | √ | √ | √ | √ | √ | √ | √ | √ | NR | √ | NR |
| Metabolic Syndrome | NR | √ | √ | NR | √ | √ | √ | NR | NR | √ | NR |
| Resistant Hypertension | √ | NR | NR | NR | √ | √ | NR | NR | NR | √ | √ |
| HTN Emergencies | |||||||||||
| Hospitalization and IV drugs | √ | √ | NR | √ | NR | √ | √ | NR | NR | √ | NR |
| Recommendations on managing associated risk factors | |||||||||||
| Antiplatelet therapy8 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √13 |
| Lipid Lowering agent8 | NR | √ | √ | √9 | √ | √ | √ | √ | √ | √ | √13 |
| Glycemic control | NR | √10 | NR | NR | √11 | NR | √12 | NR | NR | NR | NR |
| Frequency of follow up | |||||||||||
| Frequency of follow up during stabilization phase | NR | NR | NR | NR | NR | NR | NR | Every 6 weeks or as needed | NR | Monthly or according to risk | NR |
| Frequency of follow up for patients with stabilized hypertension | √14 | √15 | NR | √14 | NR | NR | NR | √16 | NR | √15 | √17 |
| Assessment of compliance discussed | √ | NR | NR | √ | √ | √ | NR | NR | √ | √ | √ |
| Strategies to improve adherence discussed | √ | NR | NR | √ | NR | √ | NR | √ | √ | √ | √ |
| “When to Refer?” discussed | √ | √ | NR | √ | NR | √ | √ | √ | √18 | √ | √ |
SOA: South Africa; IND: India; POL: Poland; MAL: Malaysia; EUR: Europe; JAP: Japan; LAT: Latin America; AUS: Australia; CAN: Canada and SAU: Saudi Arabia and NICE (The UK's National Institute for Health and Clinical Excellence). NR: not reported. A: angiotensin converting enzyme inhibitor (ACEI), or angiotensin receptor blockers (ARB), C: calcium channel blocker (CCB), D: Diuretic.
And if SBP>150 and or DBP>95- treat.
If the SBP> or = 140 mm Hg or DBP> or = 90 mm Hg across 5 visits.
if SBP = 120–159 mmHg AND/OR DBP = 80–99 mmHg.
If, at visit 2 within one month, SBP is > or = 140 mm Hg and/or DBP is > or = 90 mm Hg.
If SBP = 120–159 mmHg AND/OR DBP = 80–99 mmHg with high risk or if SBP 160 mmHg AND/OR DBP 100 mmHg regardless of risk.
NICE CPG favored A for those below 55 years and C, D, for those aged 55 years or older and for black patients.
Yes if SBP>10 mmHg above target.
Recommended in at least certain high risk groups.
Recommended for those with atherosclerotic renal artery stenosis only.
No target level stated.
A1c<6.5 mmol/L.
A1c between 6.5–7% in patients with HTN, DM and nephropathy.
Referred to previous guideline version.
Every 3–6 months;
Every 3 months for high risk patients and every 6 months for low risk patients;
Every 3 months for the first year then 6-monthly thereafter;
Once a year.
For pheochromocytoma cases only.
All CPGs emphasized the need to stop smoking, maintaining weight, following nutritional guidelines, lowering sodium intake, limiting alcohol intake (except for SAU) and lowering fat intake (except for AUS) for hypertensive patients.
Most guidelines recommended the same criteria for initiating drug therapy; minor differences were noted regarding the duration of a life style modification trial before starting drug therapy. All CPGs recommended starting antihypertensive therapy without delay for patients with high blood pressure or high cardiovascular risk defined by most guidelines (except for the AUS CPG) as ≥20% risk of developing a cardiovascular event over 10 years. The AUS CPGs defined high risk as ≥15% risk of developing an event over 5 years.
Most CPGs recommended use of any of the 5 classes of antihypertensive drugs (angiotensin converting enzymes inhibitors, angiotensin receptor blockers, beta-blocker, calcium channel blockers or diuretics) as first line therapy. However, low-dose diuretics were preferred by the IND and SAU CPGs and were exclusively recommended by the SOA CPGs. The CPGs also differed in their strategies of adjustment of therapy. Most recommended adding another drug if the blood pressure is not adequately controlled (10/11); others suggested substituting with another drug (3/11) and/or increasing the dose of the first agent (3/11). Recommendations about drug combinations were variable across guidelines. Selection of therapeutic agents for compelling indications such as established cardiovascular disease or diabetes were similar, yet there were some differences in relative or absolute contraindication definitions. Only five CPGs discussed managing resistant hypertension and five CPGs did not discuss hypertensive emergencies. Controlling associated risk factors by the use of antiplatelet therapy, statins and/or glycemic control were addressed in 11/11, 10/11 and 3/11 CPGs respectively.
Follow up, compliance, adherence strategies and referral
Only two CPGs (AUS and SAU) addressed how often patients should be seen during the stabilization phase. The AUS suggested that this should occur every 6 weeks or as indicated (which could be few days to 2 months). The SAU suggested monthly visits. Six CPGs suggested one of 2 plans for follow up of patients with stable BP; either to follow all patients every 3–6 months or to follow high risk (20% risk or higher) patients three monthly and low risk patients six monthly. The methods for assessing compliance with medication and strategies to improve adherence were discussed in 7/11 CPGs. The indications for referral to other specialties were discussed in 9/11 CPGs
Discussion
Most of the CPGs clearly presented their recommendations. However methodological gaps exist across the guidelines that should be addressed including clarifying the scope and purpose, ensuring representation of all stakeholders including consumers, developing guidelines with scientific rigour, supporting implementation of the recommendations and declaring the presence or absence of editorial independence. These results are similar to a recent review of 42 reviews of guidelines (a total of 626 CPGs on a variety of topics) published between 1980 and 2007, which showed that despite some increase in quality of CPGs over time, the average quality scores as measured with the AGREE Instrument have remained moderate (43% for ‘Rigour of Development’) to low (35% for ‘Stakeholder Involvement’, 30% for ‘Editorial Independence’ and 20% for ‘Applicability’) [34].
In general, the recommendations of the CPGs on diagnosis, assessment and non-pharmacological management were consistent despite scoring poorly in their rigour of development. It is difficult to tell whether this happened because there was no evidence to guide or because the authors did not search and make use of the best available evidence. This finding is similar to that of Burgers, et al, who reviewed 15 CPGs for patients with diabetes from 13 countries [35]. They found an international consensus in the recommendations despite the variation in cited evidence and preferential citation of evidence in each CPG. The influence of professional bodies such as the American Diabetes Association was suggested an important factor in explaining international consensus. He concluded that globalization of recommended management of diabetes was not a simple consequence of the globalization of research evidence.
For example, all the CPGs have embraced the concept of traditional or global cardiovascular risk assessment as a method for stratifying treatment which is presumably an evidence-based move, yet the level of evidence for this recommendation was not reported. Keeping in mind the recent concerns as that the current methods for assessing risk may ignore some patient characteristics [36], [37], that short-term assessment of cardiovascular risk may not translate directly into life-time risk estimates [38], the recent calls for improving cardiovascular risk assessment [39], the finding that externally validated tools for cardiovascular risk assessment may not fit well with certain populations with different baseline risks [40] and that there is no consensus among the guidelines for assessing cardiovascular risk in healthy checks on their approach and screening tests [41], users of the CPGs may decide not to implement this recommendation.
Office blood pressure measurement was recommended as the mainstay for the diagnosis and/or monitoring of hypertension with ambulatory or home self monitoring being recommended for a selected group of patients in 10/11 CPGs. Although this pragmatic approach is attractive and may have many merits, this recommendation was linked to weak evidence in the CAN and MAL CPGs. A recent systematic review found that neither clinic nor home measurement had sufficient sensitivity or specificity (compared to ambulatory-monitoring) to be recommended alone as a diagnostic test. [42].
Across the CPGs, major differences were related to the pharmacologic management of hypertension, namely, the selection of first-line treatment, adjustment of therapy and drug combinations. Even when CPG developers claimed that they related their grade of recommendations to the level of evidence, recommendations were not graded or were inconsistent. This variation may be related to the developers' search strategy, the process of selecting the scientific evidence and the way the recommendations were formulated. [43].
As is the finding of earlier analysis of multiple CPGs on various condition, we found that guideline developers did not consistently use systematic reviews [35]. Only two up-to-date reviews from the Cochrane Hypertension Group [31] [33] were cited in the guidelines we reviewed. This finding is consistent with a recent analysis of 106 NICE guidelines, which showed that one fifth of the CPGs referred to no Cochrane citations and two fifths referred to only 1–5 Cochrane reviews [44] although the majority were felt to directly address guideline questions. It is surprising that despite the increased production of Cochrane reviews, recent CPGs barely referred to relevant reviews. The reasons for this need to be explored and the Cochrane Collaboration need to consider the practical means for increasing the uptake by guidelines developers.
Limitations of this review
First, only CPGs that were written in English were included; high-quality CPGs written exclusively in other languages might have been missed. It has been shown that restricting the search for systematic reviews to English language only did not affect the quality of most reviews [45]
Second, only the AGREE-II instrument was used in assessing the quality of CPGs. Other instruments, such as the recently-published 4-item Global Rating Scale (GRS) may be used in addition to the AGREE-II instrument. A comparison of both instruments has shown that the GRS is less sensitive in detecting differences in guideline quality but it could predict important outcome measures related to guideline adoption [46]. Third, our search was limited to January 2006 to September 2011 because it is believed that CPGs should be assessed for validity every 3 years [47], [48]. As a result, well-known guidelines such as the US Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) [49] and the World Health Organization/International Society of Hypertension guidelines [50] were excluded because they were published in 2003 and are likely to be out-of-date.
Future steps
Despite these limitations, it is clear that more efforts are needed to improve the quality of the developed CPGs at the national or continental levels and to keep them up-to-date. With such variation and deficiencies in the methodological quality of CPGs, there is no guarantee that the recommendations would result in better health-related outcomes for patients with hypertension. Guidelines for developing high-quality evidence-based guidelines' have been established by various organizations [51]–[55].
Given the time-intensive and resource-intensive nature of CPG development, local adaptation of existing high-quality CPGs to the national context might be a more realistic approach to developing national or continental CPGs to avoid duplication of efforts [56]. Use of the ADAPTE framework [57] may be considered by local and national implementation teams and guideline developers and [58] de novo guideline development would only be needed if no high quality guideline exists for a given topic.
Supporting Information
Medline Search Strategy.
(DOCX)
PRISMA 2009 Checklist for the systematic review of 11 recent hypertension clinical practice guidelines.
(DOC)
Acknowledgments
The protocol of this systematic review was initiated as part of an introductory course on systematic reviews and meta-analysis taught by ACT and SES through the Li Ka Shing Knowledge Institute of St Michael's Hospital. Part of the work was presented as a poster presentation at the 19th Cochrane Colloquium in Madrid, Spain (October 2011).
Four authors (LAA, ACT, YAA and GAB) had full access to the original guidelines and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors would like to acknowledge the efforts of Ms Reem Fattouh and Ms Rana Al-Marek, final year medical students at King Saud University, who participated in some parts of level 1 (titles and abstracts) screening and who helped in compiling the extracted data from all the reviewers.
Funding Statement
The whole work was partly funded through research training collaboration between King Saud University and the Li Ka Shing Institute at St Michael's Hospital but the sponsors had no role at all in study design, in the data collection and analysis, interpretation of data, writing of the article or in the decision to submit it for publication.
References
- 1. WHO (2011) Global Atlas on cardiovascular disease prevention and control. Raised blood pressure (hypertension): A major risk factor of CVDs [Google Scholar]
- 2. Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, et al. (2009) Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119: 480–486. [DOI] [PubMed] [Google Scholar]
- 3. Law MR, Morris JK, Wald NJ (2009) Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute for Health and Clinical Excellence (2011) Cost-Effective Analysis - Pharmacological Treatment (Appendix I). The Clinical Management of Primary Hypertension in Adults (CG127). pp.421–445.
- 5. Cai L, Liu A, Zhang L, Li S, Wang P (2011) Prevalence, Awareness, Treatment, and Control of Hypertension among Adults in Beijing, China. Clinical and Experimental Hypertension 0: 1–8. [DOI] [PubMed] [Google Scholar]
- 6. Whelton PK, He J, Muntner P (2004) Prevalence, awareness, treatment and control of hypertension in North America, North Africa and Asia. J Hum Hypertens 18: 545–551. [DOI] [PubMed] [Google Scholar]
- 7. Alsuwaida A, Alghonaim M (2011) Gender Disparities in the Awareness and Control of Hypertension. Clinical and Experimental Hypertension 33: 354–357. [DOI] [PubMed] [Google Scholar]
- 8. Wang YR, Alexander GC, Stafford RS (2007) Outpatient Hypertension Treatment, Treatment Intensification, and Control in Western Europe and the United States. Arch Intern Med 167: 141–147. [DOI] [PubMed] [Google Scholar]
- 9. Fervers B, Burgers JS, Haugh MC, Brouwers M, Browman G, et al. (2005) Predictors of high quality clinical practice guidelines: examples in oncology. International Journal for Quality in Health Care 17: 123–132. [DOI] [PubMed] [Google Scholar]
- 10. Watine J, Friedberg B, Nagy E, Onody R, Oosterhuis W, et al. (2006) Conflict between Guideline Methodologic Quality and Recommendation Validity: A Potential Problem for Practitioners. Clinical Chemistry 52: 65–72. [DOI] [PubMed] [Google Scholar]
- 11. Nagy E, Watine J, Bunting PS, Onody R, Oosterhuis WP, et al. (2008) Do Guidelines for the Diagnosis and Monitoring of Diabetes Mellitus Fulfill the Criteria of Evidence-Based Guideline Development? Clinical Chemistry 54: 1872–1882. [DOI] [PubMed] [Google Scholar]
- 12. Pentheroudakis G, Stahel R, Hansen H, Pavlidis N (2008) Heterogeneity in cancer guidelines: should we eradicate or tolerate? Annals of Oncology 19: 2067–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Watine JC, Bunting PS (2008) Mass colorectal cancer screening: Methodological quality of practice guidelines is not related to their content validity. Clinical Biochemistry 41: 459–466. [DOI] [PubMed] [Google Scholar]
- 14. Grimshaw JM, Thomas RE, G M, C F, C R, et al. (2004) Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 8. [DOI] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The AGREE Research Trust (2009) Appraisal of Guidelines for Research and Evaluation II Instrument. In: Brouwers MC, editor.
- 17.Advanced Analytics L (2011) AgreeStat 2011.2 for MS Excel. In: Excel AfM, editor.
- 18. The Cochrane Collaboration (2011) Cochrane Hypertension Group. [Google Scholar]
- 19. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, et al. (2007) 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 28: 1462–1536. [DOI] [PubMed] [Google Scholar]
- 20. Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, et al. (2009) Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 27: 2121–2158. [DOI] [PubMed] [Google Scholar]
- 21. Sanchez RA, Ayala M, Baglivo H, Velazquez C, Burlando G, et al. (2009) Latin American guidelines on hypertension. Latin American Expert Group. J Hypertens 27: 905–922. [DOI] [PubMed] [Google Scholar]
- 22. Seedat YK, Croasdale MA, Milne FJ, Opie LH, Pinkney-Atkinson VJ, et al. (2006) South African hypertension guideline 2006. S Afr Med J 96: 337–362. [PubMed] [Google Scholar]
- 23. Shah S, Hegde BM, Wander GS, Anand MP, Mukherjee S, et al. (2007) Indian Hypertension Guidelines II. 2007: The Association of Physicians of India. [Google Scholar]
- 24. Tykarski A, Podolec P, Kopec G, Pajak A, Kawecka-Jaszcz K, et al. (2007) Polish Forum for Prevention Guidelines on Arterial Hypertension. Kardiol Pol 65: 1137–1141. [PubMed] [Google Scholar]
- 25. CPG Secretariat HU, Medical Development Division, Ministry of Health of Malaysia (2008) Clinical Practice Guidelines: Management of Hypertension. [Google Scholar]
- 26. Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, et al. (2009) The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009). Hypertens Res 32: 3–107. [PubMed] [Google Scholar]
- 27.Foundation NH (2010) Guide to management of hypertension 2008: Assessing and managing raised blood pressure in adults (Updated December 2010).
- 28. Rabi DM, Daskalopoulou SS, Padwal RS, Khan NA, Grover SA, et al. (2011) The 2011 Canadian Hypertension Education Program recommendations for the management of hypertension: blood pressure measurement, diagnosis, assessment of risk, and therapy. Can J Cardiol 27: 415–412, 415-433, e411-412. [DOI] [PubMed] [Google Scholar]
- 29. Society SHM (2011) Saudi Hypertension Management Society Guidelines: Synopsis 2011. [Google Scholar]
- 30. National Institute for Health and Clinical Excellence (2011) The Clinical Management of Primary Hypertension in Adults (CG127). August 2011: NICE [Google Scholar]
- 31.Wright James M, Musini Vijaya M (2009) First-line drugs for hypertension. Cochrane Database Syst Rev: John Wiley & Sons, Ltd. [DOI] [PubMed]
- 32. Mulrow Cynthia D, Chiquette E, Angel L, Cornell J, Summerbell Carolyn D, et al. (2000) Dieting to reduce body weight for controlling hypertension in adults. Cochrane Database Syst Rev [DOI] [PubMed] [Google Scholar]
- 33.Musini Vijaya M, Tejani Aaron M, Bassett K, Wright James M (2009) Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev: John Wiley & Sons, Ltd. [DOI] [PubMed]
- 34. Alonso-Coello P, Irfan A, Sola I, Gich I, Delgado-Noguera M, et al. (2010) The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. Qual Saf Health Care 19. [DOI] [PubMed] [Google Scholar]
- 35. Burgers JS, Bailey JV, Klazinga NS, Van der Bij AK, Grol R, et al. (2002) Inside Guidelines. Diabetes Care 25: 1933–1939. [DOI] [PubMed] [Google Scholar]
- 36. Ellis CJ, Legget ME, Edwards C, Van Pelt N, Ormiston JA, et al. (2011) High calcium scores in patients with a low Framingham risk of cardiovascular (CVS) disease: implications for more accurate CVS risk assessment in New Zealand. N Z Med J 124: 13–26. [PubMed] [Google Scholar]
- 37. Julius S (2009) Tachycardia in hypertension: a saga of progress despite prejudice, confusion, and inertia. Prog Cardiovasc Dis 52: 26–30. [DOI] [PubMed] [Google Scholar]
- 38. Elward KS, Simpson RJ Jr, Mendys P (2010) Improving cardiovascular risk reduction for primary prevention–utility of lifetime risk assessment. Postgrad Med 122: 192–199. [DOI] [PubMed] [Google Scholar]
- 39. White HD (2011) Improving cardiovascular risk assessment. N Z Med J 124: 5–9. [PubMed] [Google Scholar]
- 40. Matheny M, McPheeters ML, Glasser A, Mercaldo N, Weaver RB, et al. (2011) Systematic Review of Cardiovascular Disease Risk Assessment Tools. Systematic Review of Cardiovascular Disease Risk Assessment Tools. Rockville (MD). [PubMed] [Google Scholar]
- 41. Ferket BS, Colkesen EB, Visser JJ, Spronk S, Kraaijenhagen RA, et al. (2010) Systematic review of guidelines on cardiovascular risk assessment: Which recommendations should clinicians follow for a cardiovascular health check? Arch Intern Med 170: 27–40. [DOI] [PubMed] [Google Scholar]
- 42. Hodgkinson J, Mant J, Martin U, Guo B, Hobbs FD, et al. (2011) Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ 342: d3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Campbell F, Dickinson H, Cook J, Beyer F, Eccles M, et al. (2006) Methods underpinning national clinical guidelines for hypertension: describing the evidence shortfall. BMC Health Serv Res 6: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alderson PTT (2011) Use of Cochrane Reviews in NICE clinical guidelines. The Cochrane Library Aug 10. [Google Scholar]
- 45.Morrison A MK, Clark M, Polisena J, Fiander M, Mierzwinski-Urban M, et al.. (2009) English-Language Restriction When Conducting Systematic Review-based Metaanalyses: Systematic Review of Published Studies. Ottawa: Canadian Agency for Drugs and Technologies in Health.
- 46. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, et al. (2011) The Global Rating Scale complements the AGREE II in advancing the quality of practice guidelines. Journal of Clinical Epidemiology [DOI] [PubMed] [Google Scholar]
- 47. Shekelle P, Eccles MP, Grimshaw JM, Woolf SH (2001) When should clinical guidelines be updated? BMJ 323: 155–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shekelle PG, Ortiz E, Rhodes S, Morton SC, Eccles MP, et al. (2001) Validity of the Agency for Healthcare Research and Quality clinical practice guidelines: how quickly do guidelines become outdated? JAMA 286: 1461–1467. [DOI] [PubMed] [Google Scholar]
- 49. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, et al. (2003) Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252. [DOI] [PubMed] [Google Scholar]
- 50. World Health Organization ISoHWG (2003) 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. Journal of Hypertension 21: 1983–1992. [DOI] [PubMed] [Google Scholar]
- 51. Oxman A, Fretheim A, Schunemann H (2006) SURE (2006) Improving the use of research evidence in guideline development: introduction. Health Research Policy and Systems 4: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Davis D, Goldman J, Palda V (2007) CMA Handbook of Clinical Practice Guidelines. Canadian Medical Association [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. National Institute for Health and Clinical Excellence (2009) The guidelines manual 2009. [Google Scholar]
- 54.Guidelines CoSfDTCP, Medicine Io (2011) Clinical Practice Guidelines We Can Trust; Graham R, Mancher M, Wolman DM, Greenfield S, Steinberg E, editors: The National Academies Press. [PubMed]
- 55. Scottish Intercollegiate Guidelines Network (2011) SIGN 50: A guideline developer's handbook. [Google Scholar]
- 56. Misso ML, Pitt VJ, Jones KM, Barnes HN, Piterman L, et al. (2008) Quality and consistency of clinical practice guidelines for diagnosis and management of osteoarthritis of the hip and knee: a descriptive overview of published guidelines. Med J Aust 189: 394–399. [DOI] [PubMed] [Google Scholar]
- 57.The ADAPTE Collaboration (2009) The ADAPTE Process: Resource Toolkit for Guideline Adaptation. Version 2 ed.
- 58. Fervers B, Burgers JS, Voellinger R, Brouwers M, Browman GP, et al. (2011) Guideline adaptation: an approach to enhance efficiency in guideline development and improve utilisation. Bmj Quality & Safety 20: 228–236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Medline Search Strategy.
(DOCX)
PRISMA 2009 Checklist for the systematic review of 11 recent hypertension clinical practice guidelines.
(DOC)