Synopsis
A host’s defensive response to a pathogen is a phylogenetically ancient reaction that consists of a CNS-mediated series of autonomic, hormonal and behavioral responses that combine to combat infection. The absence of such defense results in greater morbidity and mortality and thus, these responses are essential for survival. The postnatal period represents a malleable phase in which the long-term behavior and physiology of the developing organism, including its immune responses, can be influenced. Postnatal challenge of the immune system by introduction of live replicating infections, or administration of bacterial and viral mimetics, can result in a multidomain alteration to the defenses of the adult host. Findings from our laboratory and others’ indicate that the postnatal administration of lipopolysaccharide (LPS) or polyinosinic:polycytidylic acid (PolyI:C), which mimic bacterial and viral infections respectively, can influence the neuroimmune response (generation of fever and production of cytokines) to a second challenge to the immune system in adulthood. This long-lasting alteration in the innate immune response is associated with myriad other effects on the animal’s physiology and appears to be primarily mediated by a sensitized hypothalamic-pituitary-adrenal axis. Thus, a transient immunological perturbation to a developing animal may program the organism for subsequent health complications as an adult. In this review we discuss some of the potential mechanisms for these phenomena.
Introduction
The perinatal environment can be critical in programming many aspects of adult physiology. For example, smoking during pregnancy increases the likelihood of childhood asthma (Carlsen and Lodrup Carlsen 2005), emotional neglect during early life may lead to anxiety and depression in adulthood (Heim and Nemeroff 2001; Gilmer and McKinney 2003; McEwen 2003) and neonatal overfeeding may affect anxiety and stress responses in the long-term (Spencer and Tilbrook 2009). In rodents, Meaney and colleagues have demonstrated that parenting style, i.e. either high- or low-intensity nursing, can predict responses of the hypothalamic-pituitary-adrenal (HPA) axis to stress, and the performance by the offspring of cognitive tasks (Liu et al. 1997, 2000; Caldji et al. 1998). It can also produce long-term changes in their subsequent reproductive and parenting behavior (Francis et al. 1999; Champagne and Meaney 2001). Even an experience as subtle as a brief daily period of separation from the mother during postnatal life can lead to anxiety-like behaviors in adulthood, such as reduced exploration of novel environments (Kalinichev et al. 2002; Brake et al. 2004; Daniels et al. 2004; Marin and Planeta 2004). The literature on long-term effects of psychological stress has been extensively and insightfully reviewed elsewhere (Weaver et al. 2004; Zhang et al. 2006). However, increasing evidence now also suggests that neuroimmune stress resulting from exposure to a bacterial or viral challenge at crucial times during development can permanently alter some aspects of the innate immune response, and this phenomenon will be the focus of the present review.
The febrile response: an important component of the innate immune response
Whereas an acquired immune response requires prior exposure to an antigen in order to mitigate the pathogenic effects of a subsequent infection, an innate immune response is activated in response to an initial exposure to a pathogen (reviewed in Arancibia et al. 2007). An important component of the innate immune response is the generation of fever, and pathogens that cause fever are often called pyrogens, derived from the Greek word pyros, meaning “to heat up”. The innate immune response is activated when molecular components of the invading pathogen bind to Toll-like receptors (TLRs) on circulating monocytes and tissue macrophages, such as the Kupffer cells in the liver. For example, lipopolysaccharide (LPS), the pyrogenic moiety of Gram-negative bacterial cell walls, mimics bacterial fever and inflammation by activating TLR4, while polyinosinic:polycytidylic acid (PolyI:C) is a synthetic double strand RNA that mimics a viral fever and immune response by activating TLR3. Activation of TLR4 stimulates a myeloid differentiation primary response gene (MyD88)-dependant pathway leading to the phosphorylation of inhibitory κB, which releases nuclear factor (NF)κB from its complex. This NFκB is then translocated to the nucleus of Kupffer cells (using the liver as an example) and of circulating immune cells where it initiates transcription of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)α, as well as anti-inflammatory cytokines such as IL-1 receptor antagonist and IL-10 (Cartmell et al. 2003; Conti et al. 2004). Cytokines are released into the blood stream and bind to receptors on endothelial cells lining brain capillaries (Matsumura and Kobayashi 2004), and on cells of the circumventricular organ to stimulate cyclo-oxygenase (COX)-2, the rate-limiting enzyme in the conversion of arachidonic acid to prostaglandin (PG) E2. Compounds like LPS also initiate a complement cascade involving the local release of PGE2 which acts directly on peripheral nerves (the vagus nerve in particular) and other tissues to signal the brain (Blatteis et al. 2004; Blatteis 2007). In the brain, PGE2 then acts principally in the ventromedial preoptic area of the anterior hypothalamus, where it binds to EP3 receptors (Lazarus et al. 2007) to inhibit a population of gamma-aminobutyric acid inhibitory neurons and thus disinhibit downstream neural pathways that stimulate conservation of heat (reviewed in Morrison et al. 2008). Heat conservation occurs through cutaneous vasoconstriction and through reduction of sweating or panting. In addition, pathways that drive heat production are activated, stimulating metabolism in brown adipose tissue. The outcome of these actions is fever, a regulated increase in body temperature (Blatteis et al. 2000). Concurrent with the activation of these pro-inflammatory responses is an activation of an anti-inflammatory response mediated by activation of the HPA axis to release corticosterone and allow a more precise control of the inflammation (reviewed in Beishuizen and Thijs 2003).
Fever is part of the acute phase response that also includes sickness behaviors such as hyperalgesia, anhedonia, social withdrawal, anorexia, amnesia, inactivity and activation of the HPA axis (Dantzer and Kelley 2007). Peripheral immune activation also causes synthesis of cytokines and transcription factors within the brain (Laye et al. 1994; Rivest 2001). Therefore peripheral inflammation can result in a secondary, cytokine-mediated “mirror response” in the CNS, even in the absence of overt CNS infection. Pro-inflammatory cytokines like IL-1β, IL-6 and TNFα in the brain cause fever, but are also responsible for many of the ancillary sickness behaviors (Dantzer et al. 2008) through interactions with fundamental neurotransmitters, receptors and biosynthetic enzymes (Kent et al. 1992; Luheshi et al. 1996; Cartmell et al. 2001; Nadeau and Rivest 2001; Rivest 2001; Thibeault et al. 2001; Dunn 2006; O’Connor et al. 2009; Schafers and Sorkin 2008).
The ability to develop fever may be important for survival (Hart 1988). When animals of numerous species ranging from lizards and goldfish to mammals are prevented from developing a fever, they have higher pathogen loads and are more likely to die from the infection (Hart 1988; Kluger et al. 1998; Jiang et al. 2000). Fever is thought to increase survival ability by increasing body temperature enough to impair bacterial and viral proliferation and survival (Jiang et al. 2000). It also enhances some of the existing immune defense mechanisms of the host (reviewed by Roberts Jr 1991; Blatteis 2003). All mammals, including humans, are constantly challenged by pathogens and infectious agents from the environment, which are often neutralized without obvious detection. When we get sick, we usually recover without incident. Our acquired immune system is activated and produces antibodies that rapidly detect invading pyrogens and eliminates them, and our innate immune system causes fever generation and other sickness behaviors to further combat the invading pathogen. From our data and those of others, it now appears that an infection experienced during the perinatal period can impose permanent alterations to this innate immune response that may compromise our ability to fight infection.
Postnatal programming of the innate immune response
Several subtly different methodologies have been used by different groups to examine neonatal programming with an immune challenge. In general, these variations include the number of times and the ages at which the postnatal animals are exposed to LPS, or the use of a live bacterial infection (which can replicate and therefore has a much longer duration of action) rather than the LPS molecule. Thus, the severity of the stress, as well as the maturational state of both the immune and nervous systems will be very different and could account for a wide range of results. In terms of comparison to the human, a rodent pup of 3–5 days in age is probably developmentally equivalent to a third-trimester human fetus, whereas a more mature two-week-old pup may be more similar in development to a 6-months to 1- to 2-year-old child (Gottlieb et al. 1977; Avishai-Eliner et al. 2002).
Our experimental paradigm involves the administration of small amounts of the bacterial endotoxin LPS (E. coli; 100 μg/kg), or pyrogen-free saline for control, to postnatal day-old (P)14 Sprague-Dawley rats (Fig. 1A). This dose of LPS is sufficient to cause a mild fever (about 1–1.5°C) under the appropriate thermoneutral environmental conditions and resolves within 8 h as determined by measurement of core body temperature (Heida et al. 2004). Pups treated with LPS in this way do not show significant differences in weight gain during (Spencer et al. 2006c) or after (Spencer et al. 2007b) the postnatal period, or in maternal attention received compared to that of saline-treated littermates (Spencer et al. 2006c, 2007b). This is important because if the infant rats that received LPS were to vocalize or otherwise behave differently than their saline-treated litter-mates, this could lead to differences in quality or quantity of maternal attention, which could affect long-term physiology independently of the effects of an immune challenge (Meaney 2001). It should be noted that at least one group has seen acute reductions in body weight when LPS is given at younger ages (P3 and P5) (Walker et al. 2004). In other cases involving LPS administration at much younger ages to mice or to different strains of rats, differences in maternal attention have also been found (Hood et al. 2003; Walker et al. 2004); as these authors did not employ cross-fostering, it is difficult to differentiate between the effects of the immune stimulus and those of the altered maternal attention.
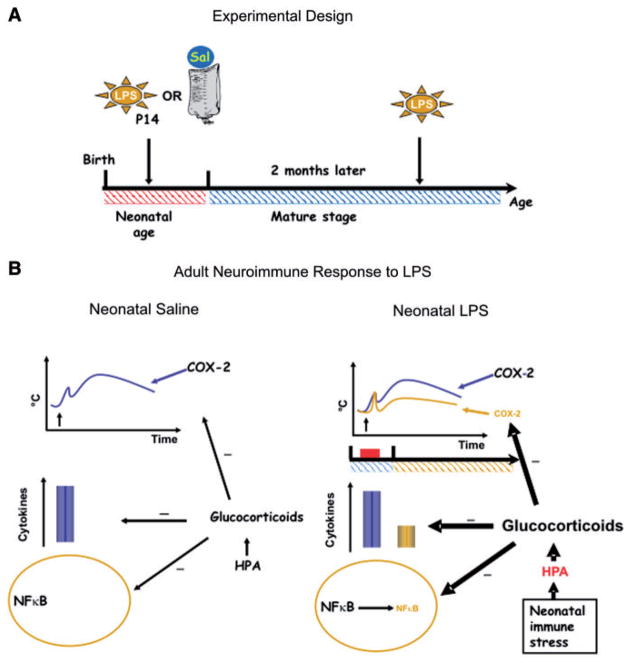
Fig. 1.
(A) Schematic of the experimental protocol, wherein LPS or saline is administered to postnatal day (P)14 pups. As adults, both groups are treated with LPS. (B) On the left is a schematic displaying important components of the innate immune response to LPS, which includes an elevation of body temperature mediated by COX-2, an elevation of circulating cytokines and an activation of NFκB. There is a tonic brake on various aspects of this pro-inflammatory response mediated by glucocorticoids. On the right is a depiction of how these responses are altered in adult rats that had been exposed at P14 to LPS. Fever, COX-2 induction, pro-inflammatory cytokines and activation of NFκB are all reduced due to an action of an amplified HPA axis leading to higher levels of glucocorticoids.
After weaning, pups receive standard specific pathogen-free husbandry until they are experimental subjects again at 8–10 weeks of age (i.e., as adults). Results from our laboratory have shown that a single peripheral injection of LPS on P14 results in an attenuated febrile response to the same LPS challenge later in life. This phenomenon has been seen by our group and others in both male and female rats of different strains (Boisse et al. 2004; Spencer et al. 2006b; Walker et al. 2006), as well as in mice (unpublished results). This attenuation can even be evoked in the absence of an elevation of temperature (fever) in the P14 pup (Boisse et al. 2004).
This programming of an attenuated neuroimmune response in adult rats occurs only when LPS is given at P14 or P21, but not at P7 or P28, and indicates a vulnerable developmental period when subtle immunological responses can permanently alter the innate immune system (Spencer et al. 2006c). The finding that there are “critical windows” during maturation when the organism is susceptible to programming by LPS has been seen in other paradigms (Galic et al. 2008; Spencer et al. 2008). It is interesting that in very young pups (P3 and P5) treated with LPS, a similar reduction in the adult febrile response was seen, suggesting either that there is another critical period at this (or even younger) ages or that it is the altered maternal behavior seen after LPS treatments at this young age that is responsible for the programming effect (Walker et al. 2006). Whatever the age tested, the phenomenon cannot be attributed merely to an extended “tolerance” phase that sometimes follows an immune challenge. The same attenuated fever is not seen if the initial LPS exposure is given in adulthood (or even as early as P28). Clearly there is something specific to the developmental period that is being affected by the LPS. This finding is also not attributable to an antibody-mediated effect. Antibodies to LPS are raised against the O-antigen portion, which is not conserved between species. When we repeated the experiment on postnatal-immune-challenge but used S. enteritidis LPS, which has a different O-antigen sequence, attenuated fever to E. coli LPS in adulthood was still seen (Boisse et al. 2004). Overall, there clearly are demarcated periods when LPS can modify innate immunity.
Possible mechanisms underlying programming
Reduced febrile responses in adulthood could be due to an inability to engage in heat production mechanisms, an alteration in the responsiveness to a central pyrogen such as PGE2 or an alteration in the interaction of LPS with peripheral macrophages and the subsequent elaboration of cytokines. CNS responses appear unchanged in the neonatally programmed animals; responses to either central injection of PGE2 or to peripherally injected IL-1β were unaltered (Boisse et al. 2004). Therefore these neonatally LPS-treated rats are capable of mounting robust fevers, but do not do so in response to LPS. However, postnatal LPS does affect circulating pro-inflammatory cytokine responses to a further LPS challenge and it is this difference that is likely responsible for the attenuated fever. Plasma concentrations of the pro-inflammatory cytokines, IL-1β, IL-6 and TNFα were attenuated after a second LPS challenge, in adulthood, in postnatally LPS-treated rats compared to saline-treated controls (Ellis et al. 2005). Interestingly, reduced cytokine levels after adult LPS were not seen if neonates had received LPS at P3 and P5 (Walker et al. 2006). In the P14-treated rats, the resulting reduction in circulating cytokine production is probably directly responsible for reduced activation in PGE2-catalysing COX-2 that is seen in the brain after LPS (Boisse et al. 2004).
Consideration was given to the possibility that this dampening of the response to LPS could be due to altered negative feedback by corticosterone. Glucocorticoids are known to modify cytokine expression, chiefly via their actions on NFκB activity (Conti et al. 2004; Turrin and Rivest 2004), and have previously been shown to be altered by postnatal immune challenge, albeit under different conditions (Shanks et al. 1995, 2000), making them good candidates for underlying altered cytokine expression and febrile responses. In support of this hypothesis, we observed that the corticosterone elevation within the first hour after the second, adult treatment of LPS is strongly enhanced in postnatally LPS-treated rats. Furthermore, removal of this corticosterone response by adrenalectomy or by the glucocorticoid receptor antagonist RU-486, completely restores normal febrile responses and plasma cytokine concentrations (Ellis et al. 2005). Therefore, the role of increased corticosterone during adults’ response to LPS appears as the essential mediator of the attenuated febrile response in adulthood. The mechanisms underlying this amplified corticosterone response to LPS are currently under investigation. Possible candidates include alterations in TLR4 and/or changes in neurally mediated corticosterone feedback. Figure 1B summarizes the collective findings and the known mechanisms by which neonatal LPS at P14 alters adult febrile responses. Interestingly, the Fischer 344 rats, that also show reduced febrile responses in adulthood after neonatal LPS at P3 and P5, did not show this early corticosterone surge. In these experiments, however, the first measurements were not made until 90 min post-LPS raising the possibility that the time at which the samples were collected may have been after the peak response had occurred (Walker et al. 2006).
Although the precise mechanisms of how post-natal programming occurs is unknown, some have hypothesized that long-term changes in physiological responses are governed through epigenetic modifications (Meaney and Szyf 2005a, 2005b; Meaney et al. 2007). Epigenetic information can be conveyed through an interaction between heritable patterns of DNA methylation and chromatin structure (Rakyan et al. 2001). Specific patterns of methylation are established in early development and are propagated during DNA replication by DNA-methyltransferase enzymes. Epigenetic modification can cause changes in gene expression mediated by DNA methylation or histone acetylation which promotes gene silencing by affecting chromatin structure. To our knowledge, no studies have been conducted that explore the role of methylation in programming of the neuroimmune response, despite the evidence that genes coding for COX-2 and TLR4 can exhibit epigenetic regulation (Akhtar et al. 2001; Zampetaki et al. 2006). Future studies should investigate if such epigenetic alterations occur after LPS injection during early development.
Complimentary aspects of postnatal programming
Developmental windows
As indicated above, an attenuated febrile response to LPS injection in adulthood happens with a single 100 μg/kg exposure to LPS only if LPS exposure occurs between P14 and P21. Although we have not tested extensively outside of this window (P7 and P28), changes in COX-2 induction, at least, show a similar LPS-induced sensitivity window, and we are assuming that the changes in cytokine levels, and corticosterone secretion will also (Spencer et al. 2006c). In a test of this assumption, Walker and colleagues gave similar doses of LPS to Fischer rats on P3 and P5. In adulthood, despite similar reduced fevers, there were no changes in major circulating pro-inflammatory cytokines or in concentrations of plasma corticosterone (Walker et al. 2006). Thus, it is apparent that exposure to LPS at this earlier developmental age (or possibly in this different strain of rats) has very different effects, and the mechanisms are still obscure. In other species, such as newborn lambs, there is a short period immediately after birth when no fevers are observed despite competent thermoregulatory capabilities (Pittman et al. 1973, 1974). There are also substantial alterations in the innate immune response in dams during late gestation (reviewed by Spencer et al. 2008) that may have implications for both prenatal and immediate postnatal responses in the offspring. Likewise, it has been shown that administration of LPS to pregnant dams can produce both sex-dependant and age-dependent changes in innate immune function in the offspring (Hodyl et al. 2007). It will be important to more clearly define the innate immune status of the prenatal and postnatal animal to further our understanding of the various developmental windows that exist.
Viral pyrogens
It could be argued that, particularly in the developed world, viral infectious diseases are more prevalent than are bacterial diseases. Is there similar programming in response to viruses? We have asked this question using the TLR3 ligand PolyI:C (Ellis et al. 2006). As seen with LPS, PolyI:C treatment at P14 was associated with reduced fevers and with an exaggerated corticosterone response to PolyI:C in adulthood. An equally interesting question is whether or not the postnatal animal treated with a viral mimetic will show an altered response to LPS as an adult. In contrast to what was seen with the homotypic challenge (LPS-LPS or PolyI:C-PolyI:C), there was no crossover between the two different TLR activators. That is, P14 PolyI:C, followed by adult LPS (and vice versa) neither attenuated fever nor altered the circulating-corticosterone response in the adult. These findings highlight the specificity of the programming capacity of early-life immune stimulation and suggest that these long-lasting changes to fever and corticosterone secretion may be tied to the individual TLR being activated. Still, the curious part of the story is that most downstream signaling pathways between TLR3 and TLR4 are similar (Beutler 2004).
Related host-defense responses
Fever is not the only host-defense response activated by LPS. Other responses include sickness behaviors associated with hyperalgesia, anhedonia, social withdrawal, anorexia, amnesia, and inactivity (Hart 1988; Konsman et al. 2002). Similar to the profound alterations in pro-inflammatory cytokine and febrile responses, the hyperalgesia and induction of spinal cord COX-2 seen after LPS was also attenuated (Boisse et al. 2005). Interestingly, some other responses were affected much less. Adults rats with a history of LPS treatment at P14 displayed similar reductions in locomotion (Boisse et al. 2004) and anorexia (Spencer et al. 2007b) compared to saline controls after injection of LPS. Similarly, postnatal LPS causes only subtle differences in various aspects of learning and memory in adulthood, under basal conditions (Spencer et al. 2005; Harre et al. 2008). However, using a somewhat different model in which young rats (P4) are given a live E. coli infection, Bilbo and colleagues observed that, whereas basal memory indices were unchanged, LPS–induced impairment of memory in adulthood was greater in rats that were exposed neonatally to E. coli infection (Bilbo et al. 2005a, 2005b, 2006). Thus it appears that the programming effect is not apparent in the noninflamed adult, but rather in response to a subsequent neuroimmune exposure.
Similar neonatal inflammation paradigms have also demonstrated a variety of long-term alterations in the acquired immune response including increased periodontal disease (Breivik et al. 2002), increased tumor colonization, impaired activity of natural killer cells, and antigen-stimulated production of antibodies (Hodgson et al. 2001; Hodgson and Knott 2002; Walker et al. 2009). Neonatal inflammatory processes can also suppress allergic responses to environmental allergens (Wang and McCusker 2006) and increase resistance to adjuvant-induced arthritis (Shanks et al. 2000), despite minimal changes to T helper cell activity (Walker et al. 2009) in adulthood. In summary, the diversity of permanent changes caused by postnatal infections and inflammatory processes is great, yet the understanding of the underlying mechanisms is poor and creates difficulty in translating these experimental findings to clinical relevance.
Implications
Given that the febrile response is thought to synergize with other aspects of host-defense to confer survival value, one might anticipate that post-natally exposed rats would show greater morbidity and mortality in response to a live bacterial infection in adulthood. This remains to be tested. However, postnatal LPS may lead to permanent alterations in the ability to combat some disorders with inflammatory components. For instance, postnatal LPS leads to a strong exacerbation of colonic damage and weight loss in a model of ulcerative colitis (Spencer et al. 2007a). Although the mechanisms for this are as yet unknown, they appear to involve altered TNFα production and it is possible that altered TLR4 expression in the gut may exacerbate responses to inflammatory challenges.
Another disorder with a large inflammatory component is cerebral ischaemia. Although the underlying pathophysiology is uncertain, concurrent inflammation in both the brain and periphery generally exacerbates damage following cerebral ischaemia (Spencer et al. 2007c; Thornhill and Asselin 1998). We therefore hypothesized that there should be less damage after cerebral ischaemia in animals postnatally treated with LPS if their inflammatory responses are dampened. This did not prove to be the case (Spencer et al. 2006a). In fact cell loss in some areas of the brain was increased. It is possible that postnatal LPS had effects on the brain that are independent from its effects on innate immunity and glucocorticoid responses. For example, postnatal immune challenge may affect neuronal excitability (Galic et al. 2008), which may be manifest as increased susceptibility to insults such as ischaemia. In support of this, mRNA of some of the excitatory glutamate receptor subunits are expressed differently in postnatally LPS-treated rats (Harre et al. 2008). It is important to note that these changes can be very specific, and not all systems are equally affected by postnatal challenge with LPS.
In conclusion, a variety of challenges to the immune system during development can exert powerful long-lasting effects that are deserving of further attention. Unraveling the mechanisms that mediate immunologically sensitive developmental periods that allow for programming of adults’ responses will permit prediction as well as the possibility for intervention. The ubiquitous nature of microbes in the environment and their capacity for pathogenicity emphasizes the need to recognize whether an organism is less capable of surviving or combating an infection due to some ostensibly benign perinatal immune challenge.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (CIHR), Heart and Stroke Foundation of Canada (HSF), and personnel awards from CIHR, HSF, Astra Zeneca, the Alberta Heritage Foundation for Medical Research, the Natural Sciences and Engineering Research Council of Canada and the Killam Trust.
Footnotes
From the symposium “Psychoneuroimmunology Meets Integrative Biology” presented at the annual meeting of the Society for Integrative and Comparative Biology, January 3–7, 2009, at Boston, Massachusetts.
For permissions please email: journals.permissions@oxfordjournals.org.
References
- Akhtar M, Cheng Y, Magno RM, Ashktorab H, Smoot DT, Meltzer SJ, Wilson KT. Promoter methylation regulates Helicobacter pylori-stimulated cyclooxygenase-2 expression in gastric epithelial cells. Cancer Res. 2001;61:2399–403. [PubMed] [Google Scholar]
- Arancibia SA, Beltran CJ, Aguirre IM, Silva P, Peralta AL, Malinarich F, Hermoso MA. Toll-like receptors are key participants in innate immune responses. Biol Res. 2007;40:97–112. doi: 10.4067/s0716-97602007000200001. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–24. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beishuizen A, Thijs LG. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J Endotoxin Res. 2003;9:3–24. doi: 10.1179/096805103125001298. [DOI] [PubMed] [Google Scholar]
- Beutler B. Inferences, questions and possibilities in toll-like receptor signalling. Nature. 2004;430:257–63. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005a;25:8000–9. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci. 2005b;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Rudy JW, Watkins LR, Maier SF. A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behav Brain Res. 2006;169:39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Blatteis CM. Fever: pathological or physiological, injurious or beneficial? J Therm Biol. 2003;28:1–13. [Google Scholar]
- Blatteis CM. The onset of fever: new insights into its mechanism. Prog Brain Res. 2007;162:3–14. doi: 10.1016/S0079-6123(06)62001-3. [DOI] [PubMed] [Google Scholar]
- Blatteis CM, Li S, Li Z, Perlik V, Feleder C. Signaling the brain in systemic inflammation: the role of complement. Front Biosci. 2004;9:915–31. doi: 10.2741/1297. [DOI] [PubMed] [Google Scholar]
- Blatteis CM, Sehic E, Li S. Pyrogen sensing and signaling: old views and new concepts. Clin Infect Dis. 2000;31(Suppl 5):S168–77. doi: 10.1086/317522. [DOI] [PubMed] [Google Scholar]
- Boisse L, Mouihate A, Ellis S, Pittman QJ. Long-term alterations in neuroimmune responses after neonatal exposure to lipopolysaccharide. J Neurosci. 2004;24:4928–34. doi: 10.1523/JNEUROSCI.1077-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisse L, Spencer SJ, Mouihate A, Vergnolle N, Pittman QJ. Neonatal immune challenge alters nociception in the adult rat. Pain. 2005;119:133–41. doi: 10.1016/j.pain.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on meso-corticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19:1863–74. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Breivik T, Stephan M, Brabant GE, Straub RH, Pabst R, von Horsten S. Postnatal lipopolysaccharide-induced illness predisposes to periodontal disease in adulthood. Brain Behav Immun. 2002;16:421–38. doi: 10.1006/brbi.2001.0642. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA. 1998;95:5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen KH, Lodrup Carlsen KC. Parental smoking and childhood asthma: clinical implications. Treat Respir Med. 2005;4:337–46. doi: 10.2165/00151829-200504050-00005. [DOI] [PubMed] [Google Scholar]
- Cartmell T, Ball C, Bristow AF, Mitchell D, Poole S. Endogenous interleukin-10 is required for the defervescence of fever evoked by local lipopolysaccharide-induced and Staphylococcus aureus-induced inflammation in rats. J Physiol. 2003;549:653–64. doi: 10.1113/jphysiol.2002.037291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell T, Luheshi GN, Hopkins SJ, Rothwell NJ, Poole S. Role of endogenous interleukin-1 receptor antagonist in regulating fever induced by localised inflammation in the rat. J Physiol. 2001;531:171–80. doi: 10.1111/j.1469-7793.2001.0171j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Front Biosci. 2004;9:1433–49. doi: 10.2741/1341. [DOI] [PubMed] [Google Scholar]
- Daniels WM, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 2004;19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–60. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ. Effects of cytokines and infections on brain neurochemistry. Clin Neurosci Res. 2006;6:52–68. doi: 10.1016/j.cnr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S, Mouihate A, Pittman QJ. Early life immune challenge alters innate immune responses to lipopolysaccharide: implications for host defense as adults. FASEB J. 2005;19:1519–21. doi: 10.1096/fj.04-3569fje. [DOI] [PubMed] [Google Scholar]
- Ellis S, Mouihate A, Pittman QJ. Neonatal programming of the rat neuroimmune response: stimulus specific changes elicited by bacterial and viral mimetics. J Physiol. 2006;571:695–701. doi: 10.1113/jphysiol.2005.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–8. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Galic MA, Riazi K, Heida JG, Mouihate A, Fournier NM, Spencer SJ, Kalynchuk LE, Teskey GC, Pittman QJ. Postnatal inflammation increases seizure susceptibility in adult rats. J Neurosci. 2008;28:6904–13. doi: 10.1523/JNEUROSCI.1901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer WS, McKinney WT. Early experience and depressive disorders: human and non-human primate studies. J Affect Disord. 2003;75:97–113. doi: 10.1016/s0165-0327(03)00046-6. [DOI] [PubMed] [Google Scholar]
- Gottlieb A, Keydar I, Epstein HT. Rodent brain growth stages: an analytical review. Biol Neonate. 1977;32:166–76. doi: 10.1159/000241012. [DOI] [PubMed] [Google Scholar]
- Harre EM, Galic MA, Mouihate A, Noorbakhsh F, Pittman QJ. Neonatal inflammation produces selective behavioural deficits and alters N-methyl-D-aspartate receptor subunit mRNA in the adult rat brain. Eur J Neurosci. 2008;27:644–53. doi: 10.1111/j.1460-9568.2008.06031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–37. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Heida JG, Boisse L, Pittman QJ. Lipopolysaccharide-induced febrile convulsions in the rat: short-term sequelae. Epilepsia. 2004;45:1317–29. doi: 10.1111/j.0013-9580.2004.13704.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hodgson DM, Knott B. Potentiation of tumor metastasis in adulthood by neonatal endotoxin exposure: sex differences. Psychoneuroendocrinology. 2002;27:791–804. doi: 10.1016/s0306-4530(01)00080-4. [DOI] [PubMed] [Google Scholar]
- Hodgson DM, Knott B, Walker FR. Neonatal endotoxin exposure influences HPA responsivity and impairs tumor immunity in Fischer 344 rats in adulthood. Pediatr Res. 2001;50:750–5. doi: 10.1203/00006450-200112000-00020. [DOI] [PubMed] [Google Scholar]
- Hodyl NA, Krivanek KM, Lawrence E, Clifton VL, Hodgson DM. Prenatal exposure to a pro-inflammatory stimulus causes delays in the development of the innate immune response to LPS in the offspring. J Neuroimmunol. 2007;190:61–71. doi: 10.1016/j.jneuroim.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Hood KE, Dreschel NA, Granger DA. Maternal behavior changes after immune challenge of neonates with developmental effects on adult social behavior. Dev Psychobiol. 2003;42:17–34. doi: 10.1002/dev.10076. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Cross AS, Singh IS, Chen TT, Viscardi RM, Hasday JD. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun. 2000;68:1265–70. doi: 10.1128/iai.68.3.1265-1270.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav. 2002;73:131–40. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci USA. 1992;89:9117–20. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. Role of fever in disease. Ann N Y Acad Sci. 1998;856:224–33. doi: 10.1111/j.1749-6632.1998.tb08329.x. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–9. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Laye S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. 1994;27:157–62. doi: 10.1016/0169-328x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10:1131–3. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Luheshi G, Miller AJ, Brouwer S, Dascombe MJ, Rothwell NJ, Hopkins SJ. Interleukin-1 receptor antagonist inhibits endotoxin fever and systemic interleukin-6 induction in the rat. Am J Physiol. 1996;270:E91–5. doi: 10.1152/ajpendo.1996.270.1.E91. [DOI] [PubMed] [Google Scholar]
- Marin MT, Planeta CS. Maternal separation affects cocaine-induced locomotion and response to novelty in adolescent, but not in adult rats. Brain Res. 2004;1013:83–90. doi: 10.1016/j.brainres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Kobayashi S. Signaling the brain in inflammation: the role of endothelial cells. Front Biosci. 2004;9:2819–26. doi: 10.2741/1439. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. 2003;9:149–54. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–92. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005a;7:103–23. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci. 2005b;28:456–63. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13:269–77. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–97. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. The complement system is an integrated part of the natural innate immune response in the brain. FASEB J. 2001;15:1410–2. doi: 10.1096/fj.00-0709fje. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–22. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman QJ, Cooper KE, Veale WL, Van Petten GR. Fever in newborn lambs. Can J Physiol Pharmacol. 1973;51:868–72. doi: 10.1139/y73-133. [DOI] [PubMed] [Google Scholar]
- Pittman QJ, Cooper KE, Veale WL, Van Petten GR. Observations on the development of the febrile response to pyrogens in sheep. Clin Sci Mol Med. 1974;46:591–602. doi: 10.1042/cs0460591. [DOI] [PubMed] [Google Scholar]
- Rakyan VK, Preis J, Morgan HD, Whitelaw E. The marks, mechanisms and memory of epigenetic states in mammals. Biochem J. 2001;356:1–10. doi: 10.1042/0264-6021:3560001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. How circulating cytokines trigger the neural circuits that control the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 2001;26:761–88. doi: 10.1016/s0306-4530(01)00064-6. [DOI] [PubMed] [Google Scholar]
- Roberts NJ., Jr Impact of temperature elevation on immunologic defenses. Rev Infect Dis. 1991;13:462–72. doi: 10.1093/clinids/13.3.462. [DOI] [PubMed] [Google Scholar]
- Schafers M, Sorkin L. Effect of cytokines on neuronal excitability. Neurosci Lett. 2008;437:188–93. doi: 10.1016/j.neulet.2008.03.052. [DOI] [PubMed] [Google Scholar]
- Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J Neurosci. 1995;15:376–84. doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci USA. 2000;97:5645–50. doi: 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Auer RN, Pittman QJ. Rat neonatal immune challenge alters adult responses to cerebral ischaemia. J Cereb Blood Flow Metab. 2006a;26:456–67. doi: 10.1038/sj.jcbfm.9600206. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Boisse L, Mouihate A, Pittman QJ. Long term alterations in neuroimmune responses of female rats after neonatal exposure to lipopolysaccharide. Brain Behav Immun. 2006b;20:325–30. doi: 10.1016/j.bbi.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Heida JG, Pittman QJ. Early life immune challenge–effects on behavioural indices of adult rat fear and anxiety. Behav Brain Res. 2005;164:231–8. doi: 10.1016/j.bbr.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Hyland NP, Sharkey KA, Pittman QJ. Neonatal immune challenge exacerbates experimental colitis in adult rats: potential role for TNF-alpha. Am J Physiol Regul Integr Comp Physiol. 2007a;292:R308–15. doi: 10.1152/ajpregu.00398.2006. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Martin S, Mouihate A, Pittman QJ. Early-life immune challenge: defining a critical window for effects on adult responses to immune challenge. Neuropsychopharmacology. 2006c;31:1910–8. doi: 10.1038/sj.npp.1301004. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Mouihate A, Galic MA, Ellis SL, Pittman QJ. Neonatal immune challenge does not affect body weight regulation in rats. Am J Physiol Regul Integr Comp Physiol. 2007b;293:R581–89. doi: 10.1152/ajpregu.00262.2007. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Mouihate A, Galic MA, Pittman QJ. Central and peripheral neuroimmune responses: hyporesponsiveness during pregnancy. J Physiol. 2008;586:399–406. doi: 10.1113/jphysiol.2007.144006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Mouihate A, Pittman QJ. Peripheral inflammation exacerbates damage after global ischemia independently of temperature and acute brain inflammation. Stroke. 2007c;38:1570–7. doi: 10.1161/STROKEAHA.106.476507. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Tilbrook A. Neonatal overfeeding alters adult anxiety and stress responsiveness. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibeault I, Laflamme N, Rivest S. Regulation of the gene encoding the monocyte chemoattractant protein 1 (MCP-1) in the mouse and rat brain in response to circulating LPS and proinflammatory cytokines. J Comp Neurol. 2001;434:461–77. doi: 10.1002/cne.1187. [DOI] [PubMed] [Google Scholar]
- Thornhill J, Asselin J. Increased neural damage to global hemispheric hypoxic ischemia (GHHI) in febrile but not nonfebrile lipopolysaccharide Escherichia coli injected rats. Can J Physiol Pharmacol. 1998;76:1008–16. doi: 10.1139/cjpp-76-10-11-1008. [DOI] [PubMed] [Google Scholar]
- Turrin NP, Rivest S. Unraveling the molecular details involved in the intimate link between the immune and neuroendocrine systems. Exp Biol Med (Maywood) 2004;229:996–1006. doi: 10.1177/153537020422901003. [DOI] [PubMed] [Google Scholar]
- Walker FR, Brogan A, Smith R, Hodgson DM. A profile of the immediate endocrine, metabolic and behavioural responses following a dual exposure to endotoxin in early life. Physiol Behav. 2004;83:495–504. doi: 10.1016/j.physbeh.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Walker FR, Hodyl NA, Hodgson DM. Neonatal bacterial endotoxin challenge interacts with stress in the adult male rat to modify KLH specific antibody production but not KLH stimulated ex vivo cytokine release. J Neuroimmunol. 2009;207:57–65. doi: 10.1016/j.jneuroim.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Walker FR, Hodyl NA, Krivanek KM, Hodgson DM. Early life host-bacteria relations and development: long-term individual differences in neuroimmune function following neonatal endotoxin challenge. Physiol Behav. 2006;87:126–34. doi: 10.1016/j.physbeh.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Wang Y, McCusker C. Neonatal exposure with LPS and/or allergen prevents experimental allergic airways disease: development of tolerance using environmental antigens. J Allergy Clin Immunol. 2006;118:143–51. doi: 10.1016/j.jaci.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Zampetaki A, Xiao Q, Zeng L, Hu Y, Xu Q. TLR4 expression in mouse embryonic stem cells and in stem cell-derived vascular cells is regulated by epigenetic modifications. Biochem Biophys Res Commun. 2006;347:89–99. doi: 10.1016/j.bbrc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Bagot R, Parent C, Nesbitt C, Bredy TW, Caldji C, Fish E, Anisman H, Szyf M, Meaney MJ. Maternal programming of defensive responses through sustained effects on gene expression. Biol Psychol. 2006;73:72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]