Abstract
Peripheral inflammation causes production of central cytokines that alter transmission at the N-methyl-D-aspartate receptor (NR). During development, NRs are important for synaptic plasticity and network connectivity. We therefore asked if neonatal inflammation would alter expression of NRs in the brain and behavioural performance in adulthood. We gave lipopolysaccharide (LPS) (100 μg/kg, i.p.) or saline to male rats on postnatal day (P)5, P14, P30 or P77. Subsequently we assessed mRNA levels of the NR1, NR2A, B, C and D subunits in the hippocampus and cortex either acutely (2 h) or in adulthood using real-time reverse transcriptase-polymerase chain reaction. We explored learning and memory behaviours in adult rats using the Morris water maze and contextual fear conditioning paradigms. Hippocampal NR1 mRNA was acutely increased in the P5- and P77-treated rats but was reduced in adults treated with LPS at P5, P30 and P77. P14 LPS-treated rats showed few acute changes but showed pronounced increases in NR2A, B, C and D subunit mRNA later in adulthood. The cortex displayed relatively few acute changes in expression in the neonatal-treated rats; however, it showed robust changes in NR2B, C and D mRNA in all groups given LPS in adulthood. Behavioural deficits were observed specifically in the P5 and P30 LPS-treated groups in the water maze probe trial and fear conditioning tests, consistent with hippocampal NR1 mRNA down-regulation. Thus, a single bout of inflammation during development can programme specific and persistent differences in NR mRNA subunit expression in the hippocampus, which could be associated with behavioural and cognitive deficits in adulthood.
Keywords: hippocampus, learning, lipopolysaccharide, memory, N-methyl-D-aspartate receptor
Introduction
Many neonatal interventions cause long-lasting changes in the behaviour and physiology of rats. In particular, a single episode of inflammation during a sensitive neonatal period may be sufficient to alter the developmental trajectory or organization of the maturing brain. For example, either infection or lipopolysaccharide (LPS) (a component of the outer membrane of Gram-negative bacteria) given to neonatal rats can alter many aspects of adult physiology, including immunological (Boisse et al., 2004; Ellis et al., 2005), behavioural (Bilbo et al., 2005; Spencer et al., 2005), neurochemical (Boisse et al., 2004) and endocrine homeostasis (Shanks et al., 1995, 2000), but only when given during a critical neonatal period (Spencer et al., 2006b). These central changes appear to enhance the vulnerability of the organism to subsequent pathological challenges, as adult rats that received an inflammatory stimulus as neonates display increased sensitivity to ischaemic injury following stroke (Spencer et al., 2006a), to gastrointestinal insult following colitis (Spencer et al., 2007) and to inflammation-induced cognitive impairments (Bilbo et al., 2005).
Excitatory glutamatergic transmission through the N-methyl-D-aspartate receptor (NR) is thought to play a crucial role in neuronal development and plasticity (Shatz, 1990). NRs are tetrameric receptors composed of obligatory NR1 subunits, coupled to a variety of ancillary subunits such as NR2A, B, C, D and NR3, all with unique electrophysiological characteristics (Moriyoshi et al., 1991; Monyer et al., 1992). The various subunit combinations endow NRs with specific kinetic and pharmacological properties (Paoletti & Neyton, 2007) that vary according to anatomical location (Watanabe et al., 1993; Monyer et al., 1994; Cull-Candy & Leszkiewicz, 2004) and developmental time line (Sheng et al., 1994; Zhong et al., 1995; Wenzel et al., 1997). Studies with N-methyl-D-aspartate antagonists in the immature rat brain suggest that disturbances of NR function during development could lead to severe impairment of neuronal circuitry and reorganization of NR expression (Hofer & Constantine-Paton, 1994). Moreover, it has been shown that glutamate (Beas-Zarate et al., 2001) or N-methyl-D-aspartate application (Zhou & Baudry, 2006) can change the NR subunit composition of the neonatal rat brain.
During peripheral inflammation, proinflammatory cytokines such as tumour necrosis factor α, interleukin-1β and interleukin-6 are synthesized and released within the brain (Laye et al., 1994; Rothwell & Luheshi, 2000; Heida & Pittman, 2005). Several of these have been shown to facilitate NR activity (Viviani et al., 2003) as well as to inhibit glutamate uptake (Ye & Sontheimer, 1996; Hu et al., 2000). It is possible that proinflamatory cytokines, through their actions at NRs, may influence the development of glutamatergic synapes in the neonatal brain. Thus, we analysed hippocampal-dependent behaviours, as well as hippocampal NR mRNA, primarily in adult rats after neonatal LPS. Cortex tissue was also analysed to serve as a comparative structure to help to determine if any changes that we observed in the hippocampus were region-specific or simply the result of a global reorganization in NR expression. The mRNA of NR subunits was also measured 2 h after LPS injection to determine whether any acute changes persist into adulthood or become altered over time.
Materials and methods
Animals
Pregnant Sprague-Dawley rats (Charles River Laboratories, QC, Canada) arrived at our facility about 1 week prior to the expected delivery date. At 3 days after birth, male rats from each dam were redistributed among all dams to minimize individual dam-dependent differences and to maintain litter size at a maximum of 12 rats per litter. We used male rats exclusively to minimize any potential variability due to sex-specific effects in NR expression (Gore, 2001) and behavioural performance. After weaning, animals were housed two to three per cage under standard laboratory conditions with a 12/12-h light/dark cycle and free access to food and water. All drugs were obtained from Sigma (MO, USA). All experimental protocols were approved by the University of Calgary Animal Care Committee and carried out in accordance with the Canadian Council on Animal Care guidelines.
Induction of inflammation
On postnatal day (P)5, P14, P30 and P77, male rats (n = 18–20 per group) were injected intraperitoneally with either pyrogen-free saline (SAL) or LPS (Escherichia coli, serotype O26:B6; 100 μg/kg). This dose of LPS has been previously associated with a number of long-term changes in adult physiology and behaviour when administered to neonatal rats (Boisse et al., 2004; Spencer et al., 2005). This single injection procedure was very brief, requiring ≤ 5 min of separation from the dam (P5 and P14), and was of equal duration for those animals injected with SAL or LPS. Prior investigations using an identical injection procedure have found no changes in maternal behaviours of the dams towards their offspring (Spencer et al., 2006b). Because of the diverse range of age-dependent expression of the NR subtypes (Monyer et al., 1994; Wenzel et al., 1997), we chose time points during early (P5), middle (P14) and late (P30) neonatal maturation, as well as an adult (P77) age, to examine the potential impact of LPS on acute and chronic NR expression. Care was taken to ensure that each litter contained equal numbers of both SAL- and LPS-treated rats. Animals were ear clipped for subsequent identification and weaned at 21 days of age. For acute measurements of NRs, groups of rats at all time points (P5, P14, P30 and P77) were killed 2 h after treatment. At P77, a subset of rats injected on or before P30 were subjected to behavioural tests of learning and memory (see below), whereas the remaining rats were killed for mRNA analysis of NRs using real-time reverse transcriptase-polymerase chain reaction (RT-PCR) techniques. Similarly at P144, the rats treated at P77 were either subjected to behavioural testing or killed for real-time RT-PCR.
Real-time RT-PCR for NR mRNA
We performed real-time RT-PCR to assess the NR subunit (NR1, NR2A, B, C and D) mRNA expression. For acute measurements, a subset of animals were anaesthetized with pentobarbital (60 mg/kg) and perfused with cold SAL through the left cardiac ventricle at 2 h after SAL or LPS treatment (n = 5–6 per group). For the chronic measurements of NRs, a subset of rats at P77 (or P144 for the P77 groups) from the P5, P14 and P30 groups that received either SAL or LPS (n = 5–6 per group) were anaesthetized and perfused as above. The hippocampi and cortex (frontal) samples were quickly dissected, immediately frozen on dry ice and stored at −80 °C until further processing. We chose to examine the cortex as well to help identify whether any changes were the result of a non-specific response to LPS or due to a selective alteration in the hippocampus. Tissues were prepared for mRNA extraction using Trizol Reagents (Life Technologies, MD, USA) according to the manufacturer’s instructions. Total cellular RNA was isolated and dissolved in ultra-pure distilled water. Extracted RNA (2 μg) was used to synthesize complementary DNA and real-time RT-PCR was performed. Semi-quantitative analysis was performed by real-time monitoring of the increase in fluorescence of the SYBR-green dye (Molecular Probes, OR, USA), run together with the calibration dye Fluorescein (Bio-Rad, CA, USA), on an i-Cycler (Bio-Rad). A threshold cycle value for each gene of interest was calculated by determining the point at which the fluorescence exceeded a threshold limit (12-fold increase above the SD of the initial baseline). Melt-curves analysis and gel electrophoresis were performed to confirm the specificity of amplification. All data were normalized against the mRNA levels of an endogenous reference gene, glyceraldehyde-3-phosphate dehydrogenase, and expressed relative to SAL-treated controls using the ‘delta-delta threshold cycle’ method (Livak & Schmittgen, 2001). Briefly, each sample’s glycer-aldehyde-3-phosphate dehydrogenase threshold cycle was subtracted from the threshold cycle for the NR of interest, resulting in the ‘delta’ value for the sample. An average of SAL-treated samples ‘delta’ was used as a reference and subtracted from all samples delta, resulting in ‘delta–delta’ values. Considering an approximate amplification efficiency of 2, 2 to the power of minus ‘delta–delta’ represents mRNA ‘relative fold change’ compared with the control group. Gene-specific primer sets (Invitrogen, CA, USA; see Table 1) were designed as described elsewhere (Lai et al., 2000).
Table 1.
RT-PCR primers
| Primer | Sequence | Length (bp) |
|---|---|---|
| GAPDH-for | 5′-GCATGGCCTTCCGTGTTCCTACCC-3′ | 110 |
| GAPDH-rev | 5′-GGCCGCCTGCTTCACCACCTTCT-3′ | |
| NR1-for | 5′-AACCTGCAGAACCGCAAG-3′ | 333 |
| NR1-rev | 5′-GCTTGATGAGCAGGTCTATGC-3′ | |
| NR2A-for | 5′-TCCATTCTTCTGTCATCCTGC-3′ | 224 |
| NR2A-rev | 5′-AAGACCGTCTCTCACTCTTGC-3′ | |
| NR2B-for | 5′-TGCACAATTACTCCTCGACG-3′ | 222 |
| NR2B-rev | 5′-TCCGATTCTTCTTCTGAGCC-3′ | |
| NR2C-for | 5′-TTGAGGACAACGTGGACACC-3′ | 204 |
| NR2C-rev | 5′-TCCAGTCGTATTCCTCCAGC-3′ | |
| NR2D-for | 5′-GCACTTGCATCAGAGACTCG-3′ | 224 |
| NR2D-rev | 5′-CTCACCAATCATGCCATTCC-3′ |
Primers and their forward (for) and reverse (rev) sequences used in the RT-PCR experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Morris water maze
The Morris water maze was used to examine the spatial memory ability of adult rats treated with SAL or LPS (n = 8 per group) during development. The water maze pool measured 200 cm in diameter by 60 cm deep and was filled to a depth of 35 cm. A 20-cm diameter platform was located 2.5 cm below the surface of the water. A non-toxic blue paint was added to the pool to visually obscure the platform location. Spatial cues were present in the testing room, consisting of shelves, tables and other equipment, and their positions were not modified for the duration of the experiment. The pool was divided (virtually) into four equally-sized quadrants with the platform located in the centre of one of the quadrants. The water maze task consisted of six sessions conducted once daily over six successive days. Each session consisted of four trials separated by 30 s. Rats whose history was unknown to the experimenter were placed into the centre of one of the quadrants that did not contain the platform. All rats were trained using the same quadrant release schedule across trials and sessions. This release schedule was randomly generated a priori. The latency to find the platform was recorded to a maximum of 120 s for each trial. Rats that did not find the platform within 120 s were assigned this value and guided onto the platform. On the following day (probe trial) after the last hidden-platform training session, the escape platform was removed from the pool and rats were released into the quadrant opposite to that previously associated with the platform. The time spent swimming in each of the four quadrants of the pool was manually recorded. A single observation per second corresponded to the location of the rat in the pool. Training day results are expressed in seconds to locate the platform, whereas the probe trial score is expressed as percentage of time spent in the target quadrant. We have successfully used this protocol elsewhere (McKay et al., 2002).
Contextual fear conditioning
To examine the operant learning ability of adult rats treated neonatally with SAL or LPS, a contextual fear conditioning task was conducted. At 1 week after water maze testing, rats were placed in a conditioning chamber (Coulbourn Instruments, PA, USA) for a 3-min baseline period and were then given three unsignalled footshocks through a metal grid floor (2 s, 0.5 mA, 60-s interstimulus interval). During the baseline period, spontaneous motor activity (midline chamber crossovers) was recorded for each rat. Every 8 s after each footshock, rats were also scored for defensive freezing, which was defined as the cessation of all movement except for that required for breathing. Subjects were returned to their home cages 60 s after the last footshock and the conditioning apparatus was cleaned with a 10% ethanol solution. Twenty-four hours later, rats were placed back into the conditioning chamber for an extinction trial and scored for defensive freezing every 8 s for 8 min. Both the amount of postshock freezing and extinction-trial freezing scores were converted to a percentage for analysis as previously described (McKay et al., 2002).
Data analysis
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS; v. 13) software. To evaluate the mRNA and behavioural data, age and treatment groups were evaluated as independent units with their individual control groups. Non-parametric Mann–Whitney U-tests were used to compare mRNA values for each LPS-treatment group with the appropriate SAL-control group. For the water maze, repeated-measures ANOVA was used to examine acquisition trial performance by treatment. All other behavioural tests were analysed using independent t-tests. Statistical significance was set at P < 0.05. Data are presented as means ± SEM.
Results
Real-time RT-PCR for NR mRNA
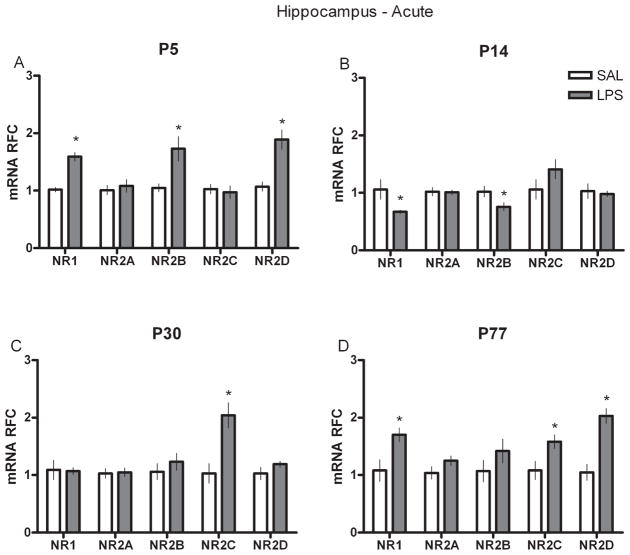
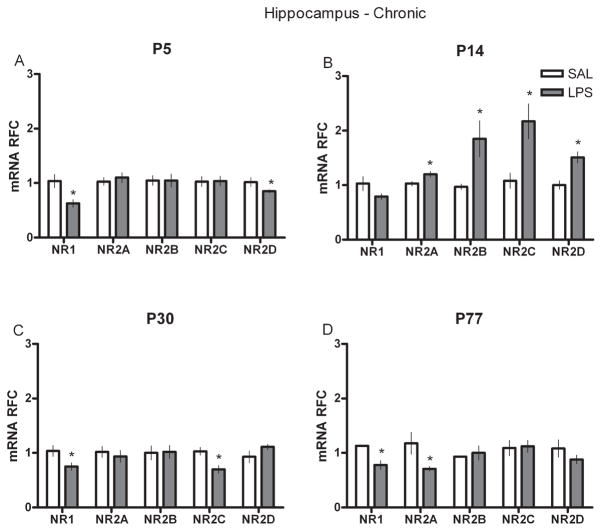
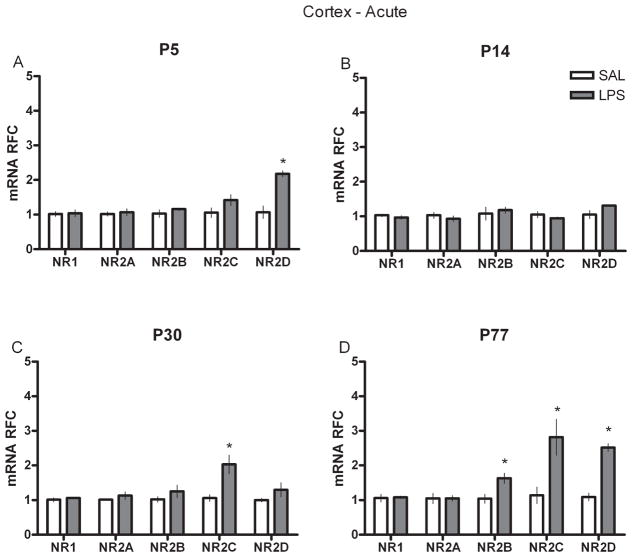
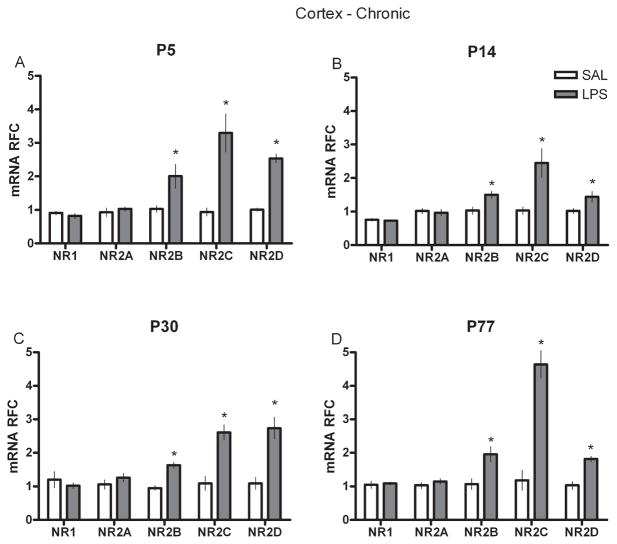
To examine whether a single injection of LPS at various developmental intervals influenced the mRNA of NR subunits in the hippocampus and cortex either acutely, or chronically in adult animals, we performed real-time RT-PCR on neural tissue collected either at 2 h after SAL or LPS injection (acute) or on P77 (P144 for the P77 groups) from animals injected at P5, P14 or P30 (chronic). P5-treated rats showed significantly higher levels of NR1, NR2B and D subunits in the hippocampus 2 h after injection (Fig. 1A) but significantly lower levels of NR1 and NR2D in adulthood (Fig. 2A). In the P14-treated group, hippocampal NR1 and NR2B levels were markedly reduced after LPS (Fig. 1B) but all of the NR2 subunits in adulthood were significantly increased compared with controls (Fig. 2B). Rats given LPS at P30 displayed an increased level of NR2C immediately after LPS (Fig. 1C) but showed significantly less mRNA of the NR1 and NR2C subunits later as adults (Fig. 2C). In the P77 LPS-treated rats, acute changes in mRNA were primarily found in the NR1 and NR2C and D subunits (Fig. 1D), whereas at P144 they showed reductions in the NR1 and NR2A subunits (Fig. 2D). In the cortex, a different pattern of NR subunit mRNA was found. Most neonatal groups displayed only a few changes in NR subunits after LPS (Fig. 3); increased NR2D was observed in the P5-treated group and increased NR2C in the P30-treated group; however, the P77-treated rats showed significantly greater NR2B, C and D expression (Fig. 4). Interestingly, this acute P77-treated pattern of expression was again observed in all groups of rats treated with LPS in adulthood.
Fig. 1.
Real-time RT-PCR data for acute hippocampal NR subunit mRNAs (NR1 and NR2A–D) in relative fold change (RFC) from animals treated with either SAL or LPS (100 μg/kg) at (A) P5, (B) P14, (C) P30 or (D) P77. Values are presented as means ± SEM. *Treatment groups that differ significantly (P < 0.05) from their respective control groups.
Fig. 2.
Real-time RT-PCR data for chronic hippocampal NR subunit mRNAs (NR1 and NR2A–D) in relative fold change (RFC) from animals treated with either SAL or LPS (100 μg/kg) at (A) P5, (B) P14, (C) P30 or (D) P77. Values are presented as means ± SEM. *Treatment groups that differ significantly (P < 0.05) from their respective control groups.
Fig. 3.
Real-time RT-PCR data for acute cortex (frontal) NR subunit mRNAs (NR1 and NR2A–D) in relative fold change (RFC) from animals treated with either SAL or LPS (100 μg/kg) at (A) P5, (B) P14, (C) P30 or (D) P77. Values are presented as means ± SEM. *Treatment groups that differ significantly (P < 0.05) from their respective control groups.
Fig. 4.
Real-time RT-PCR data for chronic cortex (frontal) NR subunit mRNAs (NR1 and NR2A–D) in relative fold change (RFC) from animals treated with either SAL or LPS (100 μg/kg) at (A) P5, (B) P14, (C) P30 or (D) P77. Values are presented as means ± SEM. *Treatment groups that differ significantly (P < 0.05) from their respective control groups.
Morris water maze
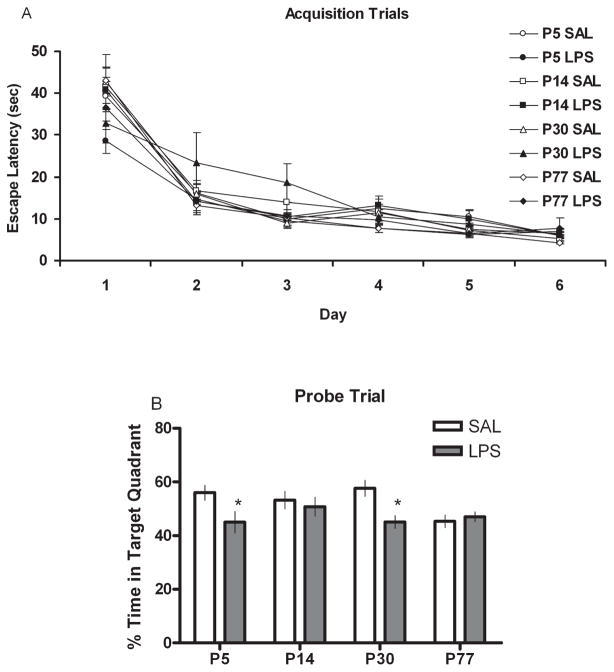
The Morris water maze was used to evaluate the spatial learning ability of adult rats treated with SAL or LPS during development. Repeated-measures ANOVA with one level repeated (days [1–6]) and one level not repeated (treatment [SAL or LPS]) was performed for each age group independently (P5, P14, P30 and P77). In general, all groups showed the expected main effect for days [all F ’s (5,70) ≥ 23.61; P ≤ 0.001] as shown by a robust decrease in escape latency over time that asymptoted around day 4 (Fig. 5A). Repeated-measures ANOVA discerned no statistically meaningful interaction between days and treatment (all P’s > 0.05) for any of the groups, indicating no differences in the performance of any of the treatment groups relative to their respective controls at any age. However, when animals were tested for persistence in searching for the escape platform during the probe trial, t-tests discerned significant reductions in the time spent in the target quadrant for animals treated with LPS at P5 and P30 compared with their respective SAL controls. The remaining groups showed no statistical differences between treatment and time spent in the target quadrant (Fig. 5B).
Fig. 5.
Water maze (A) acquisition and (B) probe trial scores presented as escape latency (s) or percent time in the target quadrant, respectively, for animals treated with either SAL or LPS (100 μg/kg) at P5, P14, P30 or P77. Scores are presented as means ± SEM. *Treatment groups that differ significantly (P < 0.05) from their respective control groups.
Contextual fear conditioning
Contextual fear conditioning was used to assess the ability of adult rats to learn and recall an association between a novel environment (context) and a negative stimulus (footshock). Each age group (P5, P14, P30 and P77) by treatment (SAL or LPS) was analysed separately to determine the relative effects of LPS on learning and memory during various developmental epochs. Analyses determined that P5- and P14-treated animals showed no statistically significant differences in the number of midline chamber crossovers (data not shown), postshock freezing or extinction-trial freezing scores. However, the P5 LPS-treated group did show a marked trend (P = 0.08) towards less freezing during the postshock trial relative to controls. In the P30 group, LPS-treated rats also showed a strong trend towards reduced postshock freezing (P = 0.06) and a statistically significant (P < 0.05) reduction in extinction trial freezing compared with SAL-treated controls (Fig. 6). There were no significant differences in the number of midline chamber crossovers between LPS- and SAL-treated rats at P30 or P77 (data not shown).
Fig. 6.
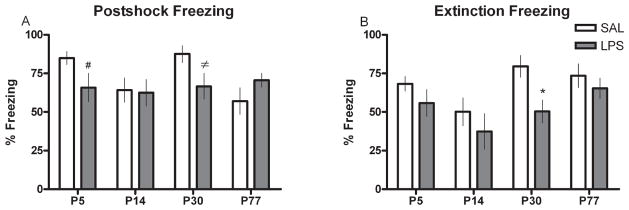
Contextual fear conditioning scores presented as percent freezing during (A) the postshock trial or (B) the extinction trial for animals treated with either SAL or LPS (100 μg/kg) at P5, P14, P30 or P77. Scores are presented as means ± SEM. *Treatment groups that differ significantly (P < 0.05) from their respective control groups. # and ≠ Treatment groups that are approaching statistical significance (P = 0.08 and P = 0.06, respectively) from SAL-treated controls.
Discussion
In the present study, we report acute and long-term changes in the NR subunit mRNA from the hippocampus and cortex of rats treated with LPS at various stages of development. Several findings are of interest in this study. Firstly, acute injection of LPS to mimic an inflammation results in significant, age-dependent changes in mRNA levels of several of the NR subunits. Secondly, a single neonatal LPS injection causes long-lasting (over 2 months) and possibly permanent changes in both hippocampal and cortex NR mRNA. Thirdly, these changes are not identical in the two brain areas. Finally, subtle changes in behaviour are seen in adults of neonatally exposed rats that may be related to the altered NR subunit expression.
We observed a significant reduction in adult hippocampal expression of NR1 mRNA of rats given LPS at P5, P30 and P77. These changes were most strongly associated with deficits in learning and memory performance of rats in the P5 and P30 groups as determined by scores in the water maze and fear conditioning tests. Despite the finding that behavioural differences were not detected between the P14 SAL- and LPS-treated rats, this group showed the greatest chronic differences in all hippocampal NR2 subunits. In the cortex, however, we observed a much more global and consistent set of chronic changes in the mRNA of NR2B, C and D subunits in all rats that received LPS. It is important to note that acute changes in NR expression in the hippocampus and cortex immediately after LPS were largely transient and were not maintained into adulthood, and were therefore less likely to have influenced the adult behavioural tests. In fact, many of the chronic changes in the hippocampus in particular appeared to be the opposite of those observed acutely after LPS. For example, in the P5 and P77 LPS-treated groups, NR1 mRNA was acutely increased but in adulthood it was significantly reduced. These results provide additional evidence of the programming effects of LPS when given during critical periods of development that are sufficient to induce discernible alterations in molecular and behavioural domains well into adulthood.
The NR is considered essential for the proper functioning of the hippocampus and the behavioural roles that it supports (Morris et al., 1986). We therefore asked if adult animals that had previously received an immune challenge, either as neonates or adults, and displayed altered levels of NR mRNA would show functional changes in behaviours known to involve glutamatergic signalling in the hippocampus. One of the most common tests for assessing hippocampal and NR function is the Morris water maze. In general, our results indicated that groups of animals treated with LPS at various developmental stages showed no significant performance deficits as compared with their respective age-matched controls in the acquisition phase of water maze testing. Similarly, Bilbo et al. (2005) have also noted that neonatal infection does not appear to be associated with memory impairments in adulthood unless the animals are re-exposed to a second immune challenge at that time. However, we have observed that, when these rats were tested for spatial preference in the probe trial, we found marked deficits in goal quadrant swimming in rats treated with LPS at P5 and P30 but not at P14 or P77. Such discordance between spatial memory acquisition and retrieval performance has also been reported in other studies (Minetti et al., 1996; Williams et al., 2003; Vorhees et al., 2004). Our results suggest that the memory impairments in these animals could have been primarily reference memory dysfunctions and the most likely change associated with such an effect is the observed NR1 reduction in the hippocampus that was seen in P5 and P30 LPS-treated rats. In contrast, P14 LPS-treated rats did not show behavioural impairment in either the acquisition or probe trials when compared with SAL controls. This group also did not show any differences in NR1 subunit mRNA. Memory studies in rats have concluded that the NR1 subunit in the hippocampus (CA1 and CA3 regions) is critical for the induction and retrieval of the spatial memory (Tsien et al., 1996; Nakazawa et al., 2002). Therefore, LPS-induced suppression of NR1 mRNA may be associated with the observed spatial memory deficits seen in rats treated at P5 and P30.
Optimal functioning of hippocampal NRs has also been demonstrated to be essential in contextual fear conditioning (Bast et al., 2003). We observed a robust tendency towards decreased postshock freezing in both the P5 and P30 LPS-treated groups and a significant reduction in extinction freezing in the P30 LPS group. In contrast, there was no detectable impairment in operant conditional learning in the P14 LPS-treated group relative to the control condition. Such differences in freezing scores between the control and treatment condition suggest a reduction in the effective pairing of the novel context with the aversive stimulus. As a whole, the performance of neonatally LPS-treated rats in the conditioned fear task closely mirrors the pattern of NR1 mRNA reduction in the hippocampus and further contributes to the evidence that NR1 subunits are required for normal hippocampal-regulated behaviours. Therefore, we suggest that LPS-induced NR reduction in the hippocampus may be associated with inferior performance in the fear conditioning task, similar to that observed in the water maze.
An alternative explanation for the poorer performance of the adult rats treated as neonates with LPS could be differences in stress reactivity. There is some evidence that neonatal rats given bacterial endotoxin show increased anxiety-like behaviours due to a chronic heightened stress response. For example, Fischer 344 rats treated with LPS on P3 and P5 demonstrate fewer open arm entries in the elevated-plus maze than control rats, suggesting greater anxiety (Walker et al., 2004). However, experiments from our laboratory did not reveal any differences in elevated-plus maze performance in adult Sprague-Dawley rats treated with LPS at P14 (Spencer et al., 2005).
When we analysed the cortex of adult animals treated with SAL or LPS during development, we observed alterations in subunit distribution that were markedly different from the changes seen in the hippocampus. This finding could reflect an interaction between the morphological developmental characteristics of the two structures, which differ slightly in growth rate (Koop et al., 1986; Kretschmann et al., 1986), spatial NR expression (Goebel & Poosch, 1999) and the functional ontogeny of the NR subunits (Sheng et al., 1994; Wenzel et al., 1997). Although we observed that NR1 and NR2A were quite stable (with the exception of a reduction in the P5-treated animals), there were increases in the NR2B, C and D subunits of the LPS-treated rats in all age groups. Such a uniform response across all ages suggests that LPS can produce reliable long-term up-regulation of NR2B, C and D mRNA following a transient period of inflammation. If we assume that this mRNA up-regulation translates into a functional increase in receptor proteins, and recall that these NR subunits conduct Ca2+ and have the slowest deactivation kinetics when glutamate is sequestered (Cull-Candy & Leszkiewicz, 2004), then the potential for long-lasting changes in synaptic plasticity or sensitivity to Ca2+-induced excitotoxicity may be a prominent feature of the cortex after exposure to LPS. Examination of these potential outcomes may be the objective of subsequent studies with LPS and NRs.
One explanation for why P14-treated rats showed no behavioural deficits or NR1 subunit changes in response to LPS could be due to age-dependent down-regulation of stress-induced adrenocortical response pathways (Sapolsky & Meaney, 1986). Roughly between the first and second postnatal weeks, rats undergo a period of stress hyporesponsiveness during which time they are more resistant to the physiological consequences of stress, including immune stress. Interestingly, the hippocampus is considered essential in the negative feedback regulation of the stress axis (Jacobson & Sapolsky, 1991) due to a high concentration of glucocorticoid receptors (Sapolsky et al., 1983) and also shows high NR concentration (Monaghan & Cotman, 1985). Thus, the potential for an interaction between glucocorticoid activity and NR expression may be viable under normal conditions (Lee et al., 2003), as we have seen here in the P5 and P30 groups, but remains absent during the period when stress responses are blunted (e.g. P14). Interestingly, these P14 LPS-treated rats showed hippocampal NR expression that closely resembled those found in the cortex. This suggests that the mechanism responsible for the NR differences may be preferentially affecting ancillary NR2 subunits more so than the obligatory NR1.
In considering the changes brought about by LPS in the brain, there are number of potential mediators. Central immune system activation is responsible for the induction of fever and sickness behaviours during infection and LPS-induced inflammation (Rothwell & Hopkins, 1995; Konsman et al., 2002). The proinflammatory mediators of this response are primarily cytokines and prostaglandins (Saper, 1998; Luheshi, 1998). Cytokines are synthesized and released in the brain and can facilitate glutamatergic transmission (Ye & Sontheimer, 1996; Kamikawa et al., 1998; Hu et al., 2000). For example, interleukin-1β can cause acute changes in hippocampal glutamate release (Zhu et al., 2006) and also directly alters NR function via tyrosine kinase-mediated phosphorylation of NRs (Viviani et al., 2003). In addition, there is evidence that other neuromodulators may also be affected after peripheral inflammation that may be contributing to NR changes. Serotonin and norepinephrine concentrations have been shown to change in the adult hippocampus after LPS administration (Linthorst et al., 1996; Linthorst & Reul, 1998); however, we can only speculate as to how transient changes in these transmitters would alter the NR subunit expression in the adult rat after a neonatal injection of LPS. Other downstream molecules resulting from cytokine activation could include a variety of transcription factors, as has been reported in the hypothalamus (Reyes et al., 2003) and hippocampus (Srinivasan et al., 2004), and may be responsible for the observed long-lasting changes in NR mRNA that were associated with the reported behavioural deficits. If there were such transcriptional changes, this would suggest that the promoter regions of the genes regulating these subunits are quite different and respond differentially to the intracellular changes brought about by LPS and its signalling products. It is interesting that, even after acute injection, some subunits are very stable (e.g. NR2A), whereas other NR2 subunits are quite labile. It is known that NRs undergo changes during development. NR1, NR2B and NR2D mRNAs occur already prenatally, and NR2A and NR2C occur around birth (Monyer et al., 1994). There is a dramatic alteration in gene expression postpartum (Stead et al., 2006) and, in keeping with this, it is known that all NR transcripts peak around P20, with the exception of NR2D which has its maximum at P7 after which it decreases to adult levels (Zhong et al., 1995). Given these differing developmental profiles, an acute onetime early life challenge with LPS might be expected to have differential effects on subunit mRNA levels. Such changes in NR expression should not be surprising considering that a single injection of LPS has been shown to alter approximately 5% of the total gene expression in the rat brain 3 days later (Eklind et al., 2006). Further research will be required to identify the regulatory sites on the NR genes that could respond to inflammation-induced transcription factors.
The alterations in NR subunits observed here in mature animals may be responsible for the changes in learning and memory that we observed. NR hypofunction has also been proposed as a feature of some human cognitive disorders, making neonatally LPS-exposed animals potential models of some of these disorders (Tsai & Coyle, 2002). However, NRs have also been implicated in neuronal death following acute insults such as stroke and seizures, as well as in some degenerative diseases (Choi, 1988). It is possible that animals exposed to LPS as neonates may display altered susceptibility to such insults as a result of the altered NR subunit expression. In addition to exploring these possibilities, future studies will also have to determine the cellular locus of the changes, as not only neurones but also astrocytes (Gallo & Ghiani, 2000) and oligodendrocytes (Salter & Fern, 2005) express NRs.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR). E.-M.H. was supported by a fellowship from the Alberta Heritage Foundation for Medical Research (AHFMR). M.A.G. was supported by scholarships from the AHFMR, CIHR and the Natural Sciences and Engineering Research Council of Canada. F.N. was supported by fellowships from AHFMR and CIHR. Q.J.P. is an AHFMR Medical Scientist. We would like to thank Dr M. Tsutsui for technical assistance.
Abbreviations
- LPS
lipopolysaccharide
- NR
N-methyl-D-aspartate receptor
- P
postnatal day
- RT-PCR
reverse transcriptase-polymerase chain reaction
- SAL
saline
References
- Bast T, Zhang WN, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: effects of local NMDA-receptor blockade and stimulation. Hippocampus. 2003;13:657–675. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Beas-Zarate C, Rivera-Huizar SV, Martinez-Contreras A, Feria-Velasco A, Armendariz-Borunda J. Changes in NMDA-receptor gene expression are associated with neurotoxicity induced neonatally by glutamate in the rat brain. Neurochem Int. 2001;39:1–10. doi: 10.1016/s0197-0186(01)00008-0. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci. 2005;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- Boisse L, Mouihate A, Ellis S, Pittman QJ. Long-term alterations in neuroimmune responses after neonatal exposure to lipopolysaccharide. J Neurosci. 2004;24:4928–4934. doi: 10.1523/JNEUROSCI.1077-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;255:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Eklind S, Hagberg H, Wang X, Savman K, Leverin AL, Hedtjarn M, Mallard C. Effect of lipopolysaccharide on global gene expression in the immature rat brain. Pediatr Res. 2006;60:161–168. doi: 10.1203/01.pdr.0000228323.32445.7d. [DOI] [PubMed] [Google Scholar]
- Ellis S, Mouihate A, Pittman QJ. Early life immune challenge alters innate immune responses to lipopolysaccharide: implications for host defense as adults. FASEB J. 2005;19:1519–1521. doi: 10.1096/fj.04-3569fje. [DOI] [PubMed] [Google Scholar]
- Gallo V, Ghiani CA. Glutamate receptors in glia: new cells, new inputs and new functions. Trends Pharmacol Sci. 2000;21:252–258. doi: 10.1016/s0165-6147(00)01494-2. [DOI] [PubMed] [Google Scholar]
- Goebel DJ, Poosch MS. NMDA receptor subunit gene expression in the rat brain: a quantitative analysis of endogenous mRNA levels of NR1Com, NR2A, NR2B, NR2C, NR2D and NR3A. Brain Res Mol Brain Res. 1999;69:164–170. doi: 10.1016/s0169-328x(99)00100-x. [DOI] [PubMed] [Google Scholar]
- Gore AC. Gonadotropin-releasing hormone neurons, NMDA receptors, and their regulation by steroid hormones across the reproductive life cycle. Brain Res Brain Res Rev. 2001;37:235–248. doi: 10.1016/s0165-0173(01)00121-7. [DOI] [PubMed] [Google Scholar]
- Heida JG, Pittman QJ. Causal links between brain cytokines and experimental febrile convulsions in the rat. Epilepsia. 2005;46:1906–1913. doi: 10.1111/j.1528-1167.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- Hofer M, Constantine-Paton M. Regulation of N-methyl-D-aspartate (NMDA) receptor function during the rearrangement of developing neuronal connections. Prog Brain Res. 1994;102:277–285. doi: 10.1016/S0079-6123(08)60546-4. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Ehrlich LC, Peterson PK, Chao CC. Cytokine effects on glutamate uptake by human astrocytes. Neuroimmuno-modulation. 2000;7:153–159. doi: 10.1159/000026433. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Kamikawa H, Hori T, Nakane H, Aou S, Tashiro N. IL-1beta increases norepinephrine level in rat frontal cortex: involvement of prostanoids, NO, and glutamate. Am J Physiol. 1998;275:R803–R810. doi: 10.1152/ajpregu.1998.275.3.R803. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Koop M, Rilling G, Herrmann A, Kretschmann HJ. Volumetric development of the fetal telencephalon, cerebral cortex, diencephalon, and rhombencephalon including the cerebellum in man. Bibl Anat. 1986;28:53–78. [PubMed] [Google Scholar]
- Kretschmann HJ, Kammradt G, Krauthausen I, Sauer B, Wingert F. Growth of the hippocampal formation in man. Bibl Anat. 1986;28:27–52. [PubMed] [Google Scholar]
- Lai SK, Wong CK, Yang MS, Yung KK. Changes in expression of N-methyl-D-aspartate receptor subunits in the rat neostriatum after a single dose of antisense oligonucleotide specific for N-methyl-D-aspartate receptor 1 subunit. Neuroscience. 2000;98:493–500. doi: 10.1016/s0306-4522(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Laye S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. 1994;27:157–162. doi: 10.1016/0169-328x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady D, Koenig JI. Corticosterone alters N-methyl-D-aspartate receptor subunit mRNA expression before puberty. Brain Res Mol Brain Res. 2003;115:55–62. doi: 10.1016/s0169-328x(03)00180-3. [DOI] [PubMed] [Google Scholar]
- Linthorst AC, Reul JM. Brain neurotransmission during peripheral inflammation. Ann NY Acad Sci. 1998;840:139–152. doi: 10.1111/j.1749-6632.1998.tb09558.x. [DOI] [PubMed] [Google Scholar]
- Linthorst AC, Flachskamm C, Holsboer F, Reul JM. Activation of serotonergic and noradrenergic neurotransmission in the rat hippocampus after peripheral administration of bacterial endotoxin: involvement of the cyclo-oxygenase pathway. Neuroscience. 1996;72:989–997. doi: 10.1016/0306-4522(95)00604-4. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luheshi GN. Cytokines and fever. Mechanisms and sites of action. Ann NY Acad Sci. 1998;856:83–89. doi: 10.1111/j.1749-6632.1998.tb08316.x. [DOI] [PubMed] [Google Scholar]
- McKay BE, Lado WE, Martin LJ, Galic MA, Fournier NM. Learning and memory in agmatine-treated rats. Pharmacol Biochem Behav. 2002;72:551–557. doi: 10.1016/s0091-3057(02)00724-4. [DOI] [PubMed] [Google Scholar]
- Minetti A, Arolfo MP, Virgolini MB, Brioni JD, Fulginiti S. Spatial learning in rats exposed to acute ethanol intoxication on gestational day 8. Pharmacol Biochem Behav. 1996;53:361–367. doi: 10.1016/0091-3057(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Cotman CW. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985;5:2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci. 2003;23:5607–5616. doi: 10.1523/JNEUROSCI.23-13-05607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ, Hopkins SJ. Cytokines and the nervous system II: Actions and mechanisms of action. Trends Neurosci. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Saper CB. Neurobiological basis of fever. Ann NY Acad Sci. 1998;856:90–94. doi: 10.1111/j.1749-6632.1998.tb08317.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, McEwen BS, Rainbow TC. Quantitative autoradiography of [3H]corticosterone receptors in rat brain. Brain Res. 1983;271:331–334. doi: 10.1016/0006-8993(83)90295-0. [DOI] [PubMed] [Google Scholar]
- Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J Neurosci. 1995;15:376–384. doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci USA. 2000;97:5645–5650. doi: 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Heida JG, Pittman QJ. Early life immune challenge – effects on behavioural indices of adult rat fear and anxiety. Behav Brain Res. 2005;164:231–238. doi: 10.1016/j.bbr.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Auer RN, Pittman QJ. Rat neonatal immune challenge alters adult responses to cerebral ischaemia. J Cereb Blood Flow Metab. 2006a;26:456–467. doi: 10.1038/sj.jcbfm.9600206. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Martin S, Mouihate A, Pittman QJ. Early-life immune challenge: defining a critical window for effects on adult responses to immune challenge. Neuropsychopharmacology. 2006b;31:1910–1918. doi: 10.1038/sj.npp.1301004. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Hyland NP, Sharkey KA, Pittman QJ. Neonatal immune challenge exacerbates experimental colitis in adult rats: potential role for TNF-alpha. Am J Physiol Regul Integr Comp Physiol. 2007;292:R308–R315. doi: 10.1152/ajpregu.00398.2006. [DOI] [PubMed] [Google Scholar]
- Srinivasan D, Yen JH, Joseph DJ, Friedman W. Cell type-specific interleukin-1beta signaling in the CNS. J Neurosci. 2004;24:6482–6488. doi: 10.1523/JNEUROSCI.5712-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead JD, Neal C, Meng F, Wang Y, Evans S, Vazquez DM, Watson SJ, Akil H. Transcriptional profiling of the developing rat brain reveals that the most dramatic regional differentiation in gene expression occurs postpartum. J Neurosci. 2006;26:345–353. doi: 10.1523/JNEUROSCI.2755-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Skelton MR, Williams MT. Exposure to 3,4-methylenedioxymethamphetamine (MDMA) on postnatal days 11–20 induces reference but not working memory deficits in the Morris water maze in rats: implications of prior learning. Int J Dev Neurosci. 2004;22:247–259. doi: 10.1016/j.ijdevneu.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Walker FR, March J, Hodgson DM. Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behav Brain Res. 2004;154:63–69. doi: 10.1016/j.bbr.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Distinct distributions of five N-methyl-D-aspartate receptor channel subunit mRNAs in the forebrain. J Comp Neurol. 1993;338:377–390. doi: 10.1002/cne.903380305. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Fritschy JM, Mohler H, Benke D. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem. 1997;68:469–478. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Wallace TL, Fukumura M, Broening HW, Vorhees CV. Developmental D-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse. 2003;48:138–148. doi: 10.1002/syn.10159. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Sontheimer H. Cytokine modulation of glial glutamate uptake: a possible involvement of nitric oxide. Neuroreport. 1996;7:2181–2185. doi: 10.1097/00001756-199609020-00025. [DOI] [PubMed] [Google Scholar]
- Zhong J, Carrozza DP, Williams K, Pritchett DB, Molinoff PB. Expression of mRNAs encoding subunits of the NMDA receptor in developing rat brain. J Neurochem. 1995;64:531–539. doi: 10.1046/j.1471-4159.1995.64020531.x. [DOI] [PubMed] [Google Scholar]
- Zhou M, Baudry M. Developmental changes in NMDA neurotoxicity reflect developmental changes in subunit composition of NMDA receptors. J Neurosci. 2006;26:2956–2963. doi: 10.1523/JNEUROSCI.4299-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Okada M, Yoshida S, Mori F, Ueno S, Wakabayashi K, Kaneko S. Effects of interleukin-1beta on hippocampal glutamate and GABA releases associated with Ca2+-induced Ca2+ releasing systems. Epilepsy Res. 2006;71:107–116. doi: 10.1016/j.eplepsyres.2006.05.017. [DOI] [PubMed] [Google Scholar]