Abstract
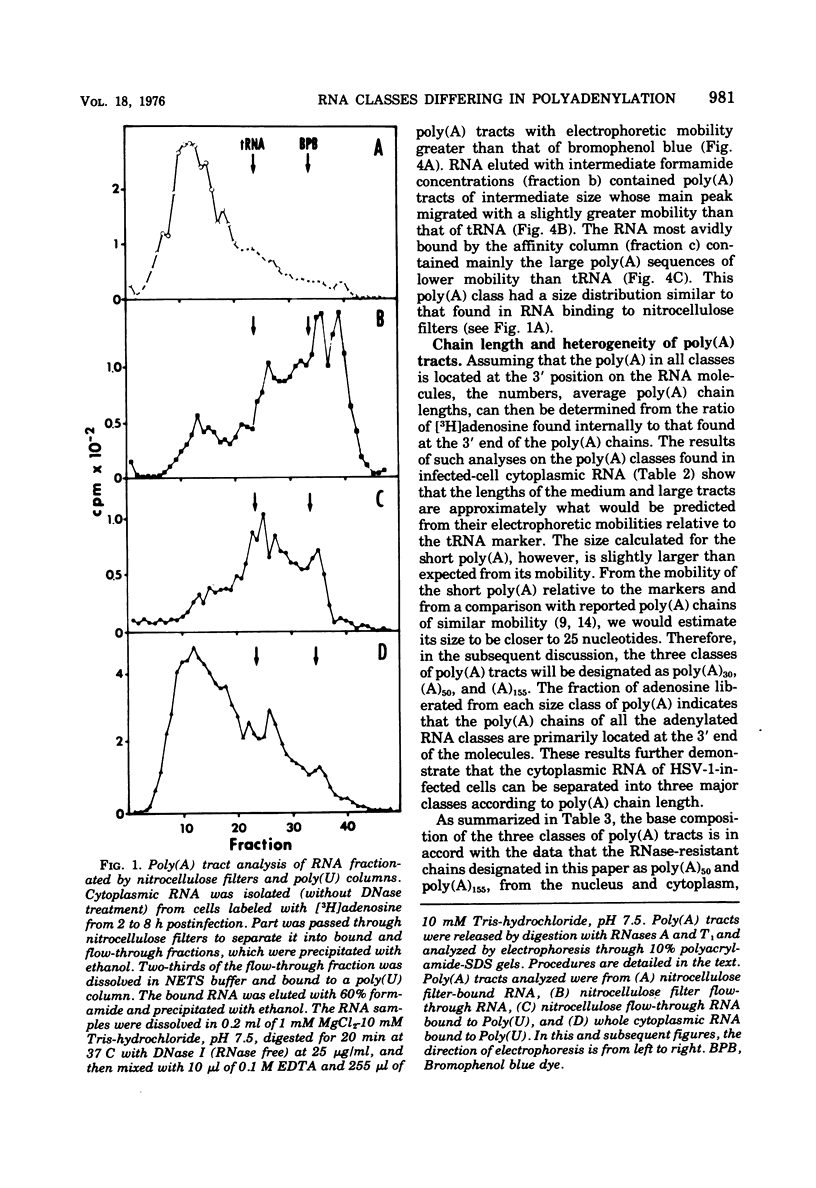
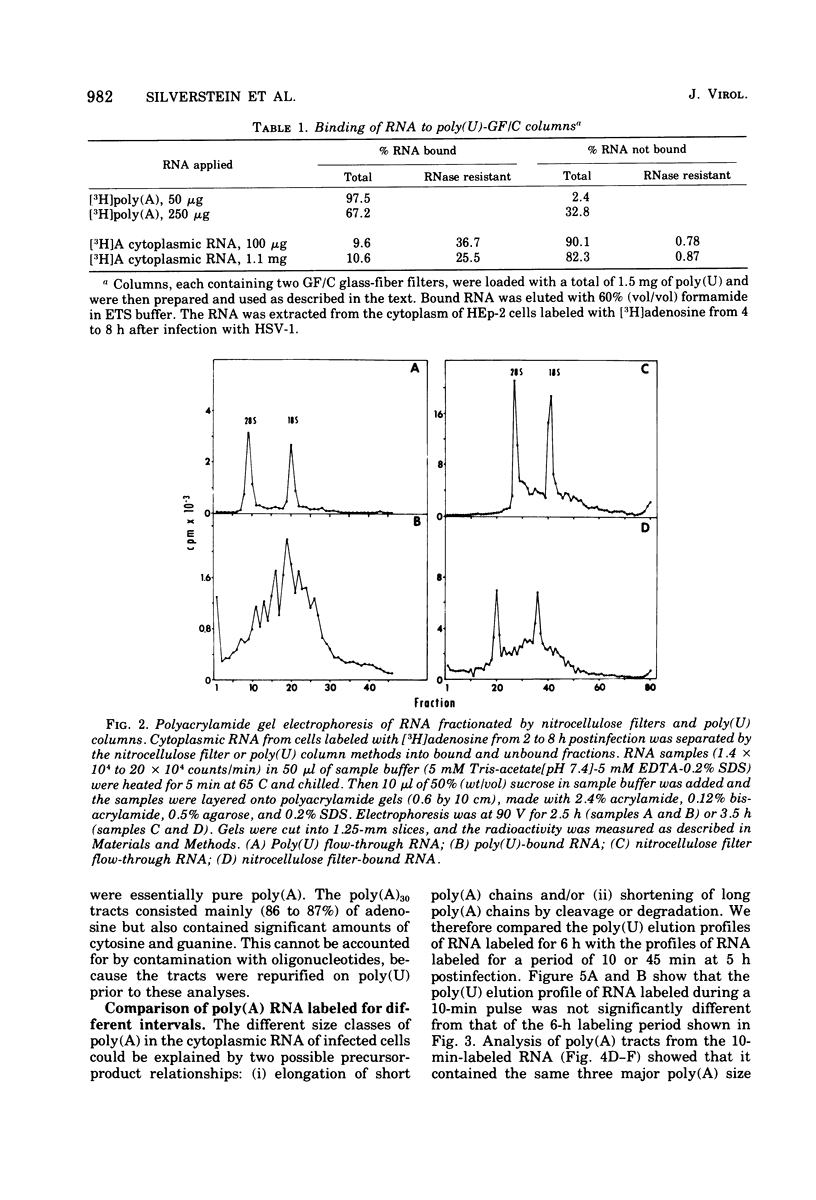
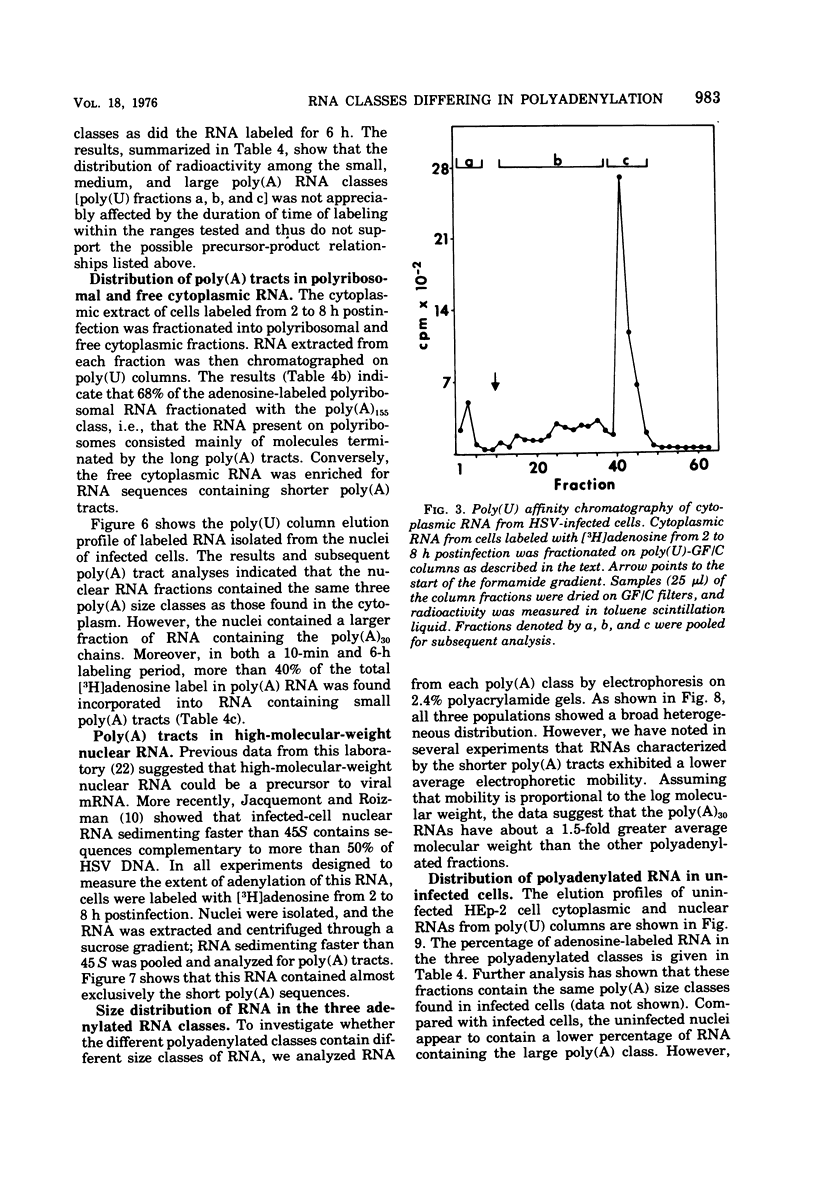
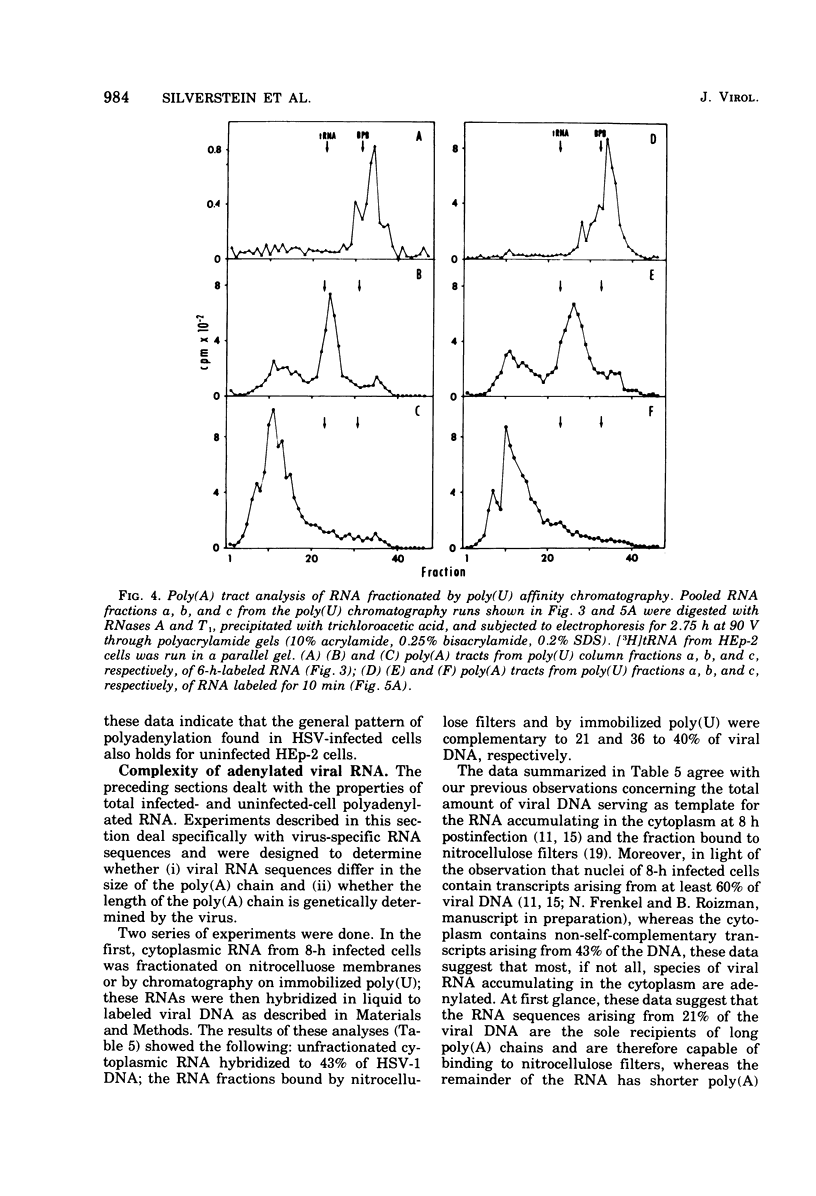
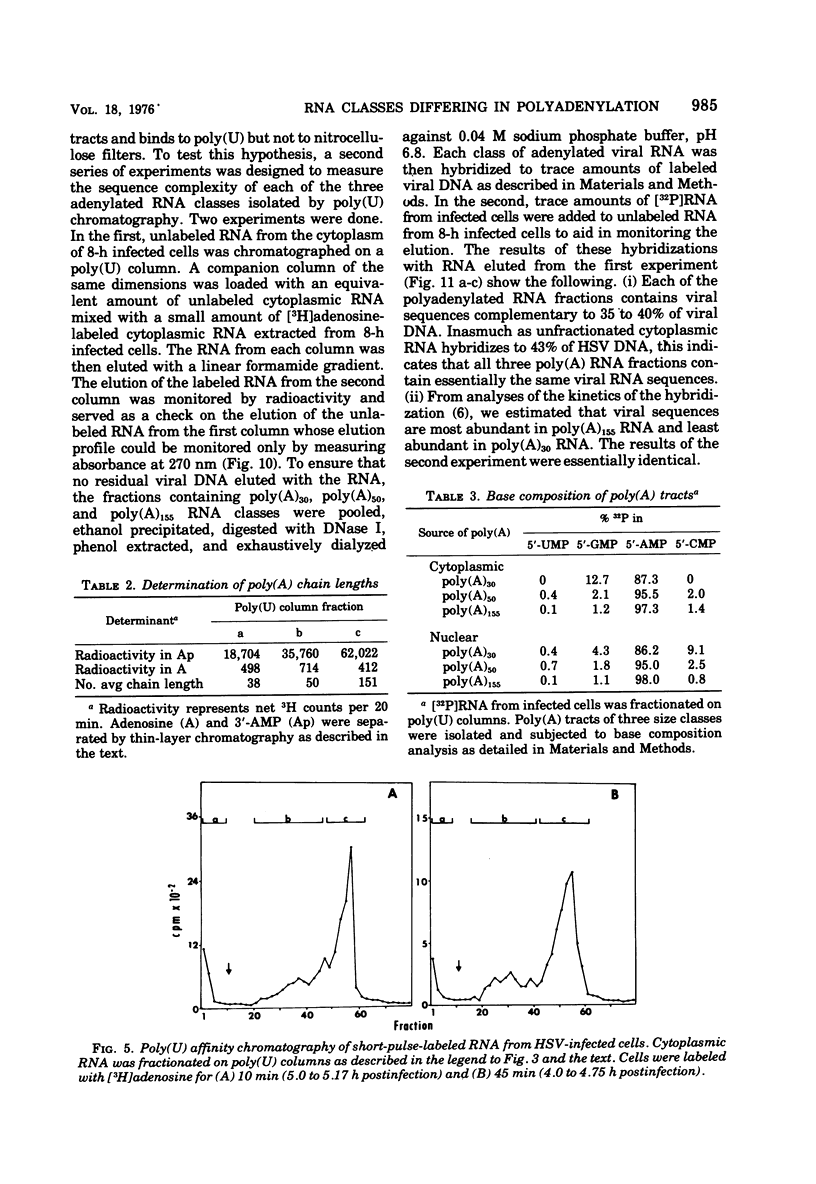
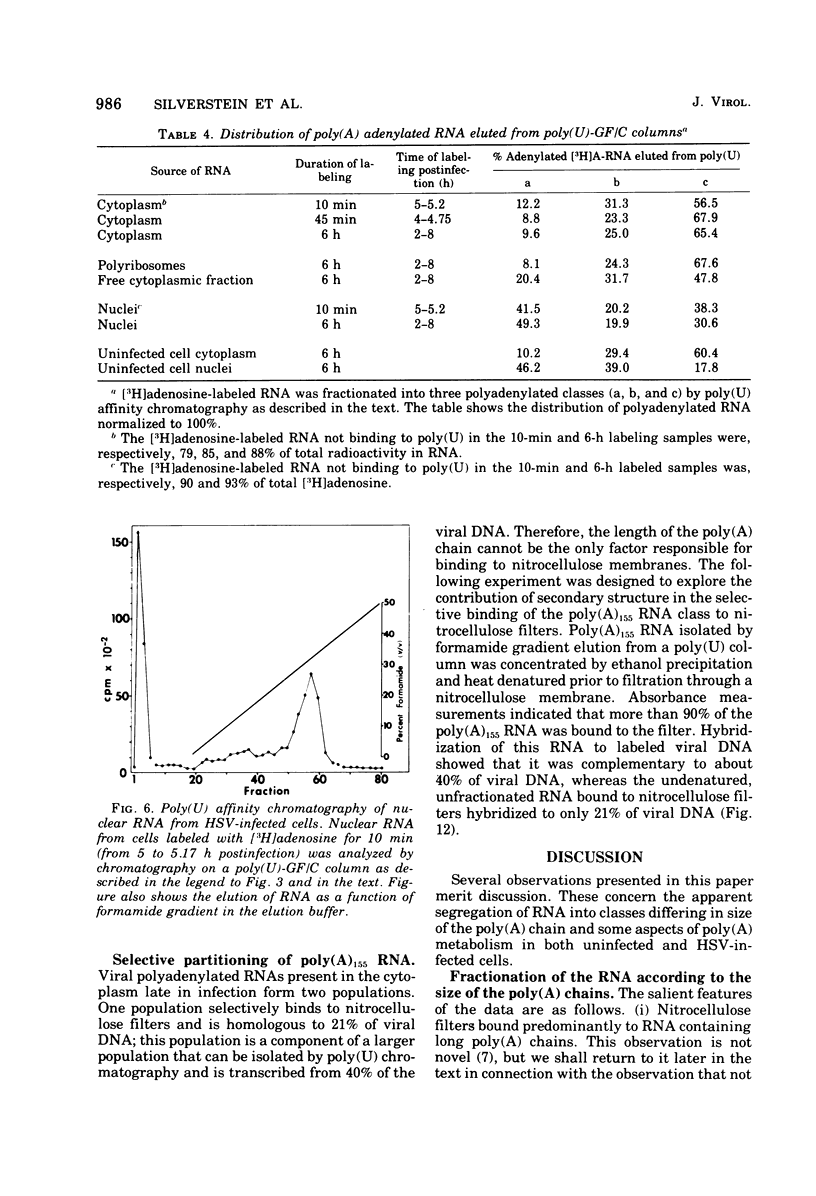
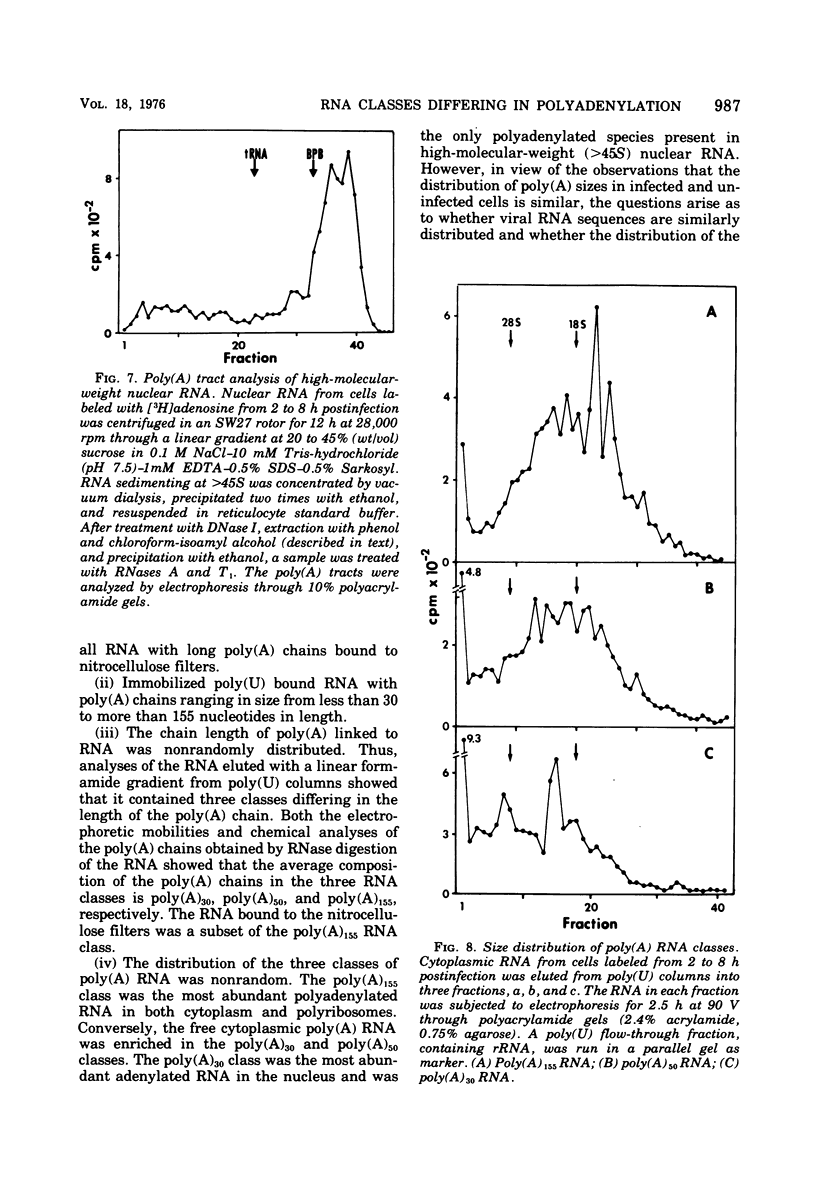
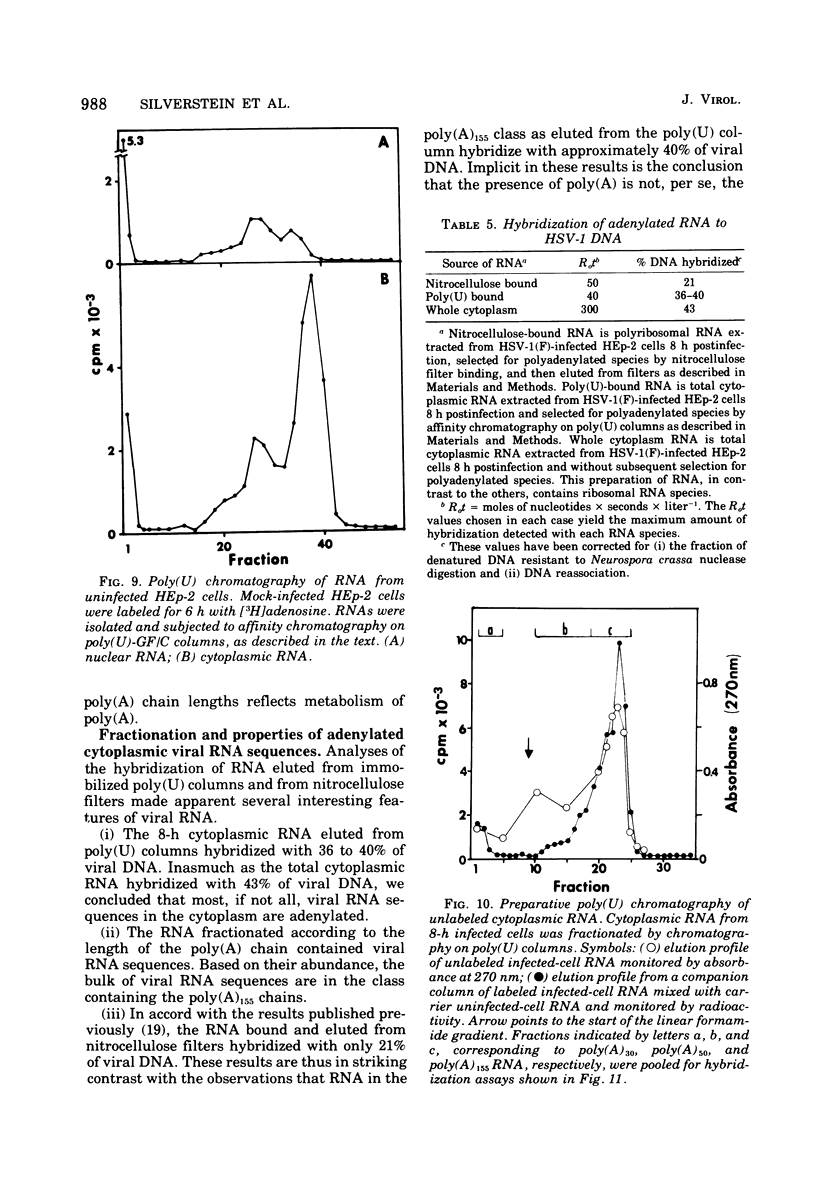
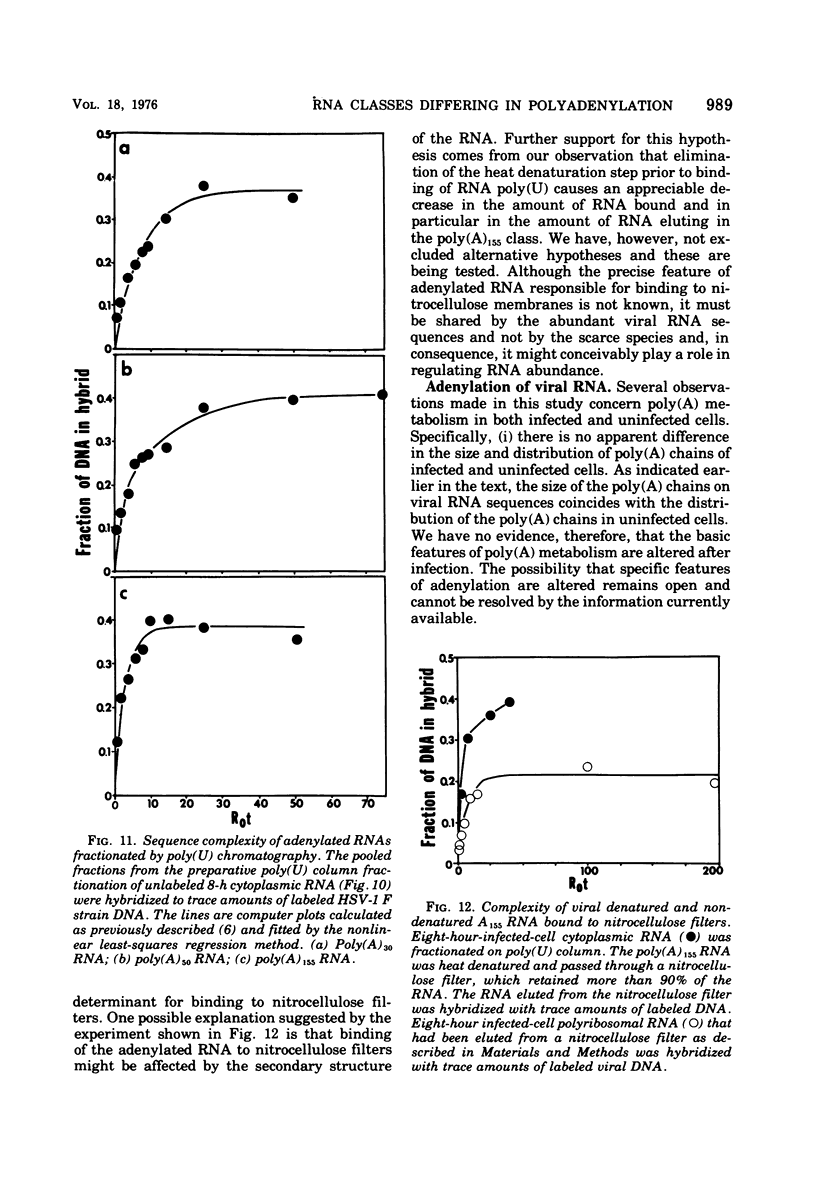
Fractionation of polyadenylated RNA from cells infected with herpes simplex virus by affinity chromatography on columns of poly (U) immobilized on glass-fiber filters yielded three major classes of RNA-containing poly(A) chains with average lengths of 30, 50, and 155 adenylate residues [poly(A)30, poly(A)50, poly(A)155]. In contrast, nitrocellulose membranes bound predominantly a fraction of RNA containing poly(A)155. The distribution of cytoplasmic RNA in the three classes was found to be independent of the labeling interval, ranging from 10 min to 6 h. Cytoplasmic poly(A) RNA consisted mainly (57 to 68%) of the poly(A)155 class; this was also the major class (68%) of polyadenylated RNA found in polyribosomes. Nuclear poly(A) RNA consisted largely (42 to 50%) of poly(A)30 class, whereas high-molecular-weight nuclear RNA sedimenting at greater than 45S contained almost exclusively the poly(A)30 tracts. Hybridization experiments involving unlabeled RNA and labeled viral DNA demonstrated the presence of viral RNA sequences complementary to approximately 40% of viral DNA in all polyadenylated RNA classes. Inasmuch as unfractionated cytoplasmic RNA arises from approximately 40% of the viral DNA, we conclude that most, if not all, viral RNA species present in the cytoplasm are adenylated. In contrast to these results, only a fraction of poly(A)155 RNA, complementary to 21% of viral DNA, bound to nitrocellulose filters. The selective binding of poly(A)155 sequences to nitrocellulose filters might be related to its secondary structure, since transcripts homologous to 40% of viral DNA bind to nitrocellulose membranes, provided the RNA is denatured prior to filtration. The data suggest that poly(A) tracts arise by at least two separate steps. The first involves the appearance of poly(A)30 tracts in the high-molecular-weight nuclear transcripts. The second involves polyadenylation to ply(A)50 and poly(A)155 RNA classes concomitant with processing and transport to the cytoplasm.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachenheimer S. L., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus. VI. Polyadenylic acid sequences in viral messenger ribonucleic acid. J Virol. 1972 Oct;10(4):875–879. doi: 10.1128/jvi.10.4.875-879.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Brawerman G., Mendecki J., Lee S. Y. A procedure for the isolation of mammalian messenger ribonucleic acid. Biochemistry. 1972 Feb 15;11(4):637–641. doi: 10.1021/bi00754a027. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus: controls of transcription and of RNA abundance. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2654–2658. doi: 10.1073/pnas.69.9.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Morrison M. R., Merkel C. G., Lingrel J. B. Poly(A) size class distribution in globin mRNAs as a function of time. Nature. 1975 Feb 27;253(5494):749–751. doi: 10.1038/253749a0. [DOI] [PubMed] [Google Scholar]
- Gorski J., Morrison M. R., Merkel C. G., Lingrel J. B. Size heterogeneity of polyadenylate sequences in mouse globin messenger RNA. J Mol Biol. 1974 Jun 25;86(2):363–371. doi: 10.1016/0022-2836(74)90025-4. [DOI] [PubMed] [Google Scholar]
- Jacobson A., Firtel R. A., Lodish H. Transcription of polydeoxythymidylate sequences in the genome of the cellular slime mold, Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1974 May;71(5):1607–1611. doi: 10.1073/pnas.71.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont B., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus: characterization of viral high molecular weight nuclear RNA. J Gen Virol. 1975 Nov;29(2):155–165. doi: 10.1099/0022-1317-29-2-155. [DOI] [PubMed] [Google Scholar]
- Kozak M., Roizman B. Regulation of herpesvirus macromolecular synthesis: nuclear retention of nontranslated viral RNA sequences. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4322–4326. doi: 10.1073/pnas.71.11.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Firtel R. A., Jacobson A. Transcription and structure of the genome of the cellular slime mold Dictyostelium discoideum. Cold Spring Harb Symp Quant Biol. 1974;38:899–914. doi: 10.1101/sqb.1974.038.01.092. [DOI] [PubMed] [Google Scholar]
- Nakazato H., Edmonds M., Kopp D. W. Differential metabolism of large and small poly(A) sequences in the heterogeneous nuclear RNA of HeLa cells. Proc Natl Acad Sci U S A. 1974 Jan;71(1):200–204. doi: 10.1073/pnas.71.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Kozak M., Honess R. W., Hayward G. Regulation of herpesvirus macromolecular synthesis: evidence for multilevel regulation of herpes simplex 1 RNA and protein synthesis. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):687–701. doi: 10.1101/sqb.1974.039.01.083. [DOI] [PubMed] [Google Scholar]
- Roizman B., Spear P. G. Preparation of herpes simplex virus of high titer. J Virol. 1968 Jan;2(1):83–84. doi: 10.1128/jvi.2.1.83-84.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Jurale C., Kates J. Detection of polyadenylic acid sequences in viral and eukaryotic RNA(polu(U)-cellulose columns-poly(U) filters-fiberglass-HeLa cells-bacteriophage T4). Proc Natl Acad Sci U S A. 1972 Feb;69(2):417–421. doi: 10.1073/pnas.69.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S., Bachenheimer S. L., Frenkel N., Roizman B. Relationship between post-transcriptional adenylation of herpes virus RNA and messenger RNA abundance. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2101–2104. doi: 10.1073/pnas.70.7.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Roizman B. RNA synthesis in cells infected with herpes simplex virus. II. Evidence that a class of viral mRNA is derived from a high molecular weight precursor synthesized in the nucleus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):626–633. doi: 10.1073/pnas.64.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus. I. Patterns of ribonucleic acid synthesis in productively infected cells. J Virol. 1969 Jul;4(1):36–46. doi: 10.1128/jvi.4.1.36-46.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]



