Abstract
Low Frequency (≪100Hz) applied electric fields have been shown to modulate neuronal activity both In Vitro and in acute whole animal studies [1–3]. We have been working to apply this technology for seizure control in chronically implanted animals. We have developed electronics for simultaneously recording neural activity while stimulating with low frequency fields. We have observed transient entrainment of spike and wave activity during spontaneous seizures with open loop sinusoidal stimulation with frequencies between 9–15 Hz. This is the first demonstration of low frequency field modulation of neural activity in chronically implanted mammalian brain.
I. Introduction
Low frequency (≪100 Hz) applied electric fields have been shown to modulate neuronal activity both In Vitro [1,3] and in acute whole animal studies [2]. In contrast to pulse stimulation, this mode of interacting with neuronal activity has two advantages for development of neural prosthetics. First, it has a modulatory effect on neuron activity proportional to the field amplitude and polarity. Second, when instrumented correctly, neural recordings can be done simultaneously with stimulation with minimal artifact. Therefore, this mode of interaction is ideal for use in continuous feedback controllers.
In brain slices, we have demonstrated feedback control of epileptiform activity [1] and control of traveling wave propagation [2], and in acutely instrumented animals, we demonstrated modulation of neural activity [3].
We have been working to implement this method for control of seizures in chronically implanted animals. We have now developed instrumentation for simultaneously stimulating and recording in chronically implanted animals for extended periods (days to weeks). Neural activity can be recorded from either large surface electrodes or bipolar depth pairs with high fidelity and minimal stimulation artifact during stimulation. Direct modulation with electric fields was observed in response to periodic low frequency sinusoidal stimulation (9–15 Hz). Clear entrainment of activity was transiently observed in recordings of hippocampal activity both at seizure onset and at termination.
II. Methods
Electrode Construction
Stimulation electrodes were fabricated from 250μm diameter 316L or 304L stainless steel wire, with polyimide insulation. 3mm of the wire is exposed to a final geometrical area of 2.5mm2. A modification of the Meyer et al (2001) technique for electrochemical deposition of iridium oxide film (EIROF) is used [4].
The deposition solution is prepared with iridium tetrachloride (4mM) and oxalic acid (40mM), and adjusted to a pH of 10.3 with the addition of high molarity potassium carbonate (500mM).
Iridium oxide is electrochemically deposited onto stainless steel substrates by sweeping from 0 to 0.55V and back at 50mV/s for 50 cycles, followed by 1600 cycles of a 1Hz square wave, between 0 and 0.55V, versus a silver/silver chloride pellet. The sequence formed by the triangular and square waves constitutes one deposition cycle, and takes 45 minutes to complete. Electrodes are deposited for typically 32 cycles (approximately 24 hours).
Bipolar recording electrodes are formed from pairs of stainless steel wire (125μm diameter), glued together and cut with a staggered distance of 300–1000μm, depending on their targets.
Surgical Procedures
All procedures were carried out under George Mason University Institutional Animal Care and Use Committee (GMU IACUC) approval. Male Sprague-Dawley rats (250 to 350g) were implanted with stimulating electrodes in the right ventral hippocampus at a maximum insertion speed of 1.2 mm/min. The target for the electrode tip is in the ventral hippocampus (AP=−5.15; ML=+5.35; DV=−7.6). The exposed length of 3mm is aligned within the arc defined by the CA3 region of the hippocampus, and therefore inserted diagonally, with the exposed area of the electrode extending from AP=−4.1; ML=+4.96; DV=−4.6 to the target. The resulting electric field extends radially out from the electrode, and is aligned with the dendrite-soma axis of much of the CA3 pyramidal neurons, as described in [3]. A matching stimulation electrode was implanted parallel and 2 mm anterior to the hippocampal electrode. In addition, bipolar pairs of recording electrodes were placed in the dorsal hippocampus both on the right and left sides, at positions: AP=−2.5; ML=+/−2.0; DV=−3.3 and AP=−3.5; ML=−3.0; DV=−3.3. All stereotaxic coordinates are given in millimeters, and referred to bregma, as defined in the Rat Brain Atlas by Paxinos and Watson [5].
Seizures were induced by microinjection of tetanus toxin into the ventral hippocampus [6]. Specifically, 1μl of tetanus toxin, mixed at a concentration of 5ng/μl in 2% bovine serum albumen in phosphate buffered saline, was injected over a period of 5 minutes at a target position of AP=−4.65; ML=5.144, DV=−6.1.
Electronics and Recording Technique
Stimulation and recording electronics were built in house. Stimulation was performed with a voltage controlled isolation amplifier between the pair of stimulation electrodes. Stimulation isolation was used to prevent current passage through other electrodes. Voltage programming across the electrode pairs limits the individual junction potentials at each electrode within the water window to prevent harmful electrochemical damage.
Recording electronics were designed with differential amplifiers with high common mode rejection and low bias current in order to accommodate the large common-mode signal introduced during electric field stimulation.
Field potentials were filtered from 0.1–1000 Hz and further amplified. EEG, stimulation amplitude and stimulation current were acquired at 2000 samples per second. Animals were cabled and recording begun 3–5 days post surgery, and monitored continuously for up to 3 weeks. In addition, behavior was recorded on digital video at a rate of 1 or 3 frames per seconds, with infrared illumination during night.
Stimulation Protocol
Various open loop stimulation protocols were applied, including on/off cycles of sinusoidal, broadband noise, and mixed sinusoid plus noise. We report here results on only presentations of one-hour 9, 11, or 15 Hz stimulation, in which each presentation was followed by one hour free of stimulation.
Data processing
We expect some stimulus artifact equal to the line integral of the applied electric field between the measurement points. Under the assumption that the local field is proportional to total current applied to the stimulation electrodes, the artifact should be proportional to a scale factor times the stimulation current. We estimate this proportionality factor from the correlation coefficient between the stimulus current and the EEG channel outside the seizures.
Seizures were detected from the EEG dynamics based on sustained high RMS power in the range 5–25 Hz from depth electrodes.
III. Results
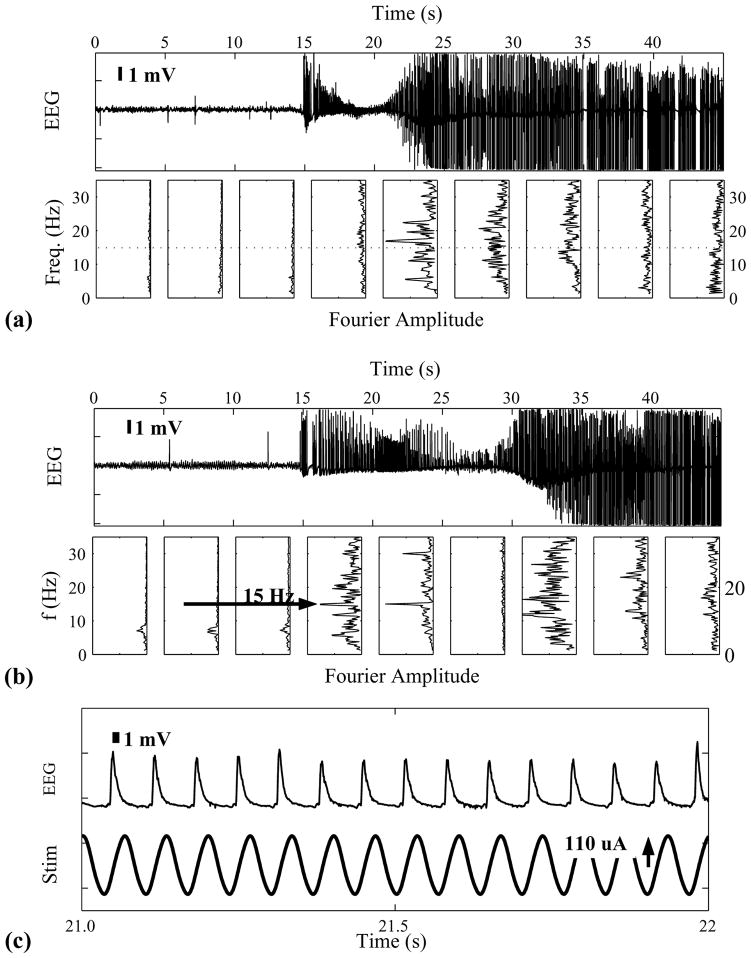
Two example seizures from a single animal are shown in the figure. Epochs 45 s long, beginning 15 s prior to seizure onset, are shown in (a) and (b) of the figure measured from a depth electrode pair in the right hand ventral hippocampus. Fourier spectral amplitudes, measured in 5 s windows, are shown below. In (a) is shown a typical seizure without stimulation. As reported elsewhere [7], hippocampal seizures in the tetanus toxin model begin with low frequency (9–15 Hz) spike and wave behavior.
In part B of the figure is shown a typical seizure observed during stimulation with a 15 Hz voltage controlled sinusoid of amplitude 800 mV and a maximal current density of ~44 μA/mm2. Based on a bulk conductance of 150 Ωcm, this corresponds to a field of roughly 66 mV/mm at the electrode surface, and 20 mV/mm at the CA3 cell body layer. In this case, the seizure occurred hundreds of seconds after initiation of this stimulation epoch.
A sharp prominent peak is observed at 15 Hz in the spectra throughout the first 10 s of the seizure. We note that this is not simply an increase in stimulation artifact. In part C of the figure, we show one second of EEG from B at an expanded scale (upper trace), highlighting the spike and wave dynamics, along with the stimulus voltage (lower trace). It is the timing of the spikes that is shifted by the stimulus. Phase analysis (not shown) of the spike times reveal significant entrainment by the stimulation. Therefore, the sharp spectral peak indicates a neural response to the stimulation.
Similar entrainment was observed in 4 of 4 animals which were stimulated with sinusoidal field in the range of 9–15 Hz. For example, in one animal entrainment was observed in 4 of 5 seizures with 15 Hz stimulation as evidenced by sharp spectral peaks. In contrast, when no stimulation was applied, only 16 of 52 seizures had spectral peaks near 15 Hz. With 9 Hz stimulation, 2 of 5 seizures demonstrated sharp responses at 9 Hz, and with 11 Hz stimulation, 7 of 11 seizures demonstrated sharp responses at 11 Hz. We are currently working on better quantification of these results.
IV. Discussion and Conclusion
This is the first demonstration of neural modulation with low frequency nonpulsatile electric field stimulation in chronically implanted animals, as evidenced by transient entrainment on seizure activity.
To achieve this, we have addressed a number of difficult technical issues in both electronics and electrode materials, the solutions of which will be detailed elsewhere.
In terms of electronics, we have designed stimulation electronics to apply low frequency fields without leaking current to the recording system. We have also designed recording electronics that can measure neural signals with amplitudes of order hundreds of microvolts in the presence of very high – hundreds of millivolts - common mode signals. The current versions of these electronics are small enough to encase in head-mounted preamplifiers. Despite the high common mode rejection, some stimulation artifact is still recorded from the applied field due to the spacing of the recording electrodes in the applied field. We are currently working on methods for subtracting this component in hardware, as well as further miniaturizing these electronics for implantation use.
In electrode technology, we have improved the stability and charge passing capacity of EIROF electrodes. Preliminary work on stimulation safety demonstrated no detectable damage due to current application. Unfortunately, in long term In Vivo studies these electrodes suffer from biofouling (data not shown). This reduces their capacity by a factor of 10 from their preimplantation capacity. Clinical application of this technology will require much higher charge passing capacity than we observe under chronic use, and we are pursuing a number of methods of addressing the biofouling through both additional surface treatments and charge passing materials.
In conclusion, we have demonstrated transient entrainment of spike and wave activity during seizure with low frequency (9–15 Hz) sinusoidal stimulation in chronically implanted animals. This is the first demonstration of this mode of interaction in chronically implanted mammals, and represents a major step in creating a broad range of neural prostheses based on field modulation. Because we are able to simultaneously record neural activity with high fidelity while stimulating, this system is ready for use for a broad range of applications including those requiring feedback. Our next steps include applying various control algorithms for controlling seizure and seizure spread.
Figure.
Traces and Fourier Amplitude of EEG during Seizure. (a) and (b) 45 seconds of data from right hippocampal depth electrode without (a) and with (b) 15 Hz stimulation. Below are the Fourier amplitude measured in 5 seconds windows. During stimulation (b) a strong 15 Hz response is transiently observed. (c) Expanded view of one second from (b), along with the stimulation current.
Acknowledgments
Work Supported under NIH grants R01EB001507, R01MH50006, and K02MH01493.
Contributor Information
S. Sunderam, Department of Engineering Science and Mechanics, The Pennsylvania State University, University Park, PA
N. Chernyy, Department of Engineering Science and Mechanics, The Pennsylvania State University, University Park, PA
J. Mason, Department of Engineering Science and Mechanics, The Pennsylvania State University, University Park, PA
N. Peixoto, Department of Electrical Engineering, George Mason University, Fairfax, VA 22201
S.L. Weinstein, Department of Neurology, Children’s National Medical Center and George Washington University School of Medicine
S.J. Schiff, Department of Neurosurgery and Department of Engineering Science and Mechanics, The Pennsylvania State University, University Park, PA
B.J. Gluckman, Email: bjg18@psu.edu, Department of Engineering Science and Mechanics, and Department of Neurosurgery, The Pennsylvania State University, University Park, PA. (phone: 814-865-4523; fax: 814-863-7967;.
References
- 1.Gluckman BJ, Nguyen H, Weinstein SL, Schiff SJ. Adaptive Electric Field Control of Epileptic Seizures. J Neuroscience. 2001;21:590–600. doi: 10.1523/JNEUROSCI.21-02-00590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson KA, Schiff SJ, Gluckman BJ. Control of traveling waves in the Mammalian cortex. Phys Rev Lett. 2005;94(2):028103. doi: 10.1103/PhysRevLett.94.028103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson KA, Gluckman BJ, Weinstein SL, Glosch CE, Moon JB, Gwinn RP, Gale K, Schiff SJ. In vivo modulation of hippocampal epileptiform activity with radial electric fields. Epilepsia. 2003;44(6):768–77. doi: 10.1046/j.1528-1157.2003.35402.x. [DOI] [PubMed] [Google Scholar]
- 4.Meyer RD, Cogan SF, Nguyen TH, Rauth RD. Electrodeposited iridium oxide for neural stimulation and recording electrodes. IEEE Trans Neural Syst and Rehab Eng. 2001;9(1):2–11. doi: 10.1109/7333.918271. [DOI] [PubMed] [Google Scholar]
- 5.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates – The New Coronal Set. 5. Elsevier; 2004. [Google Scholar]
- 6.Jefferys JGR, Walker MC. Tetanus Toxin Model of Focal Epilepsy. In: Pitkänen A, Schwartzkroin PA, Moshé LS, editors. Models of Seizures and Epilepsy. Academic Press; 2005. [Google Scholar]
- 7.Finnerty GT, Jefferys JG. 9–16 Hz oscillation precedes secondary generalization of seizures in the rat tetanus toxin model of epilepsy. J Neurophysiol. 2000;83(4):2217–26. doi: 10.1152/jn.2000.83.4.2217. [DOI] [PubMed] [Google Scholar]