Abstract
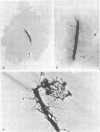
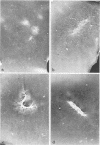
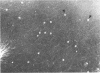
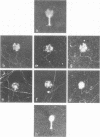
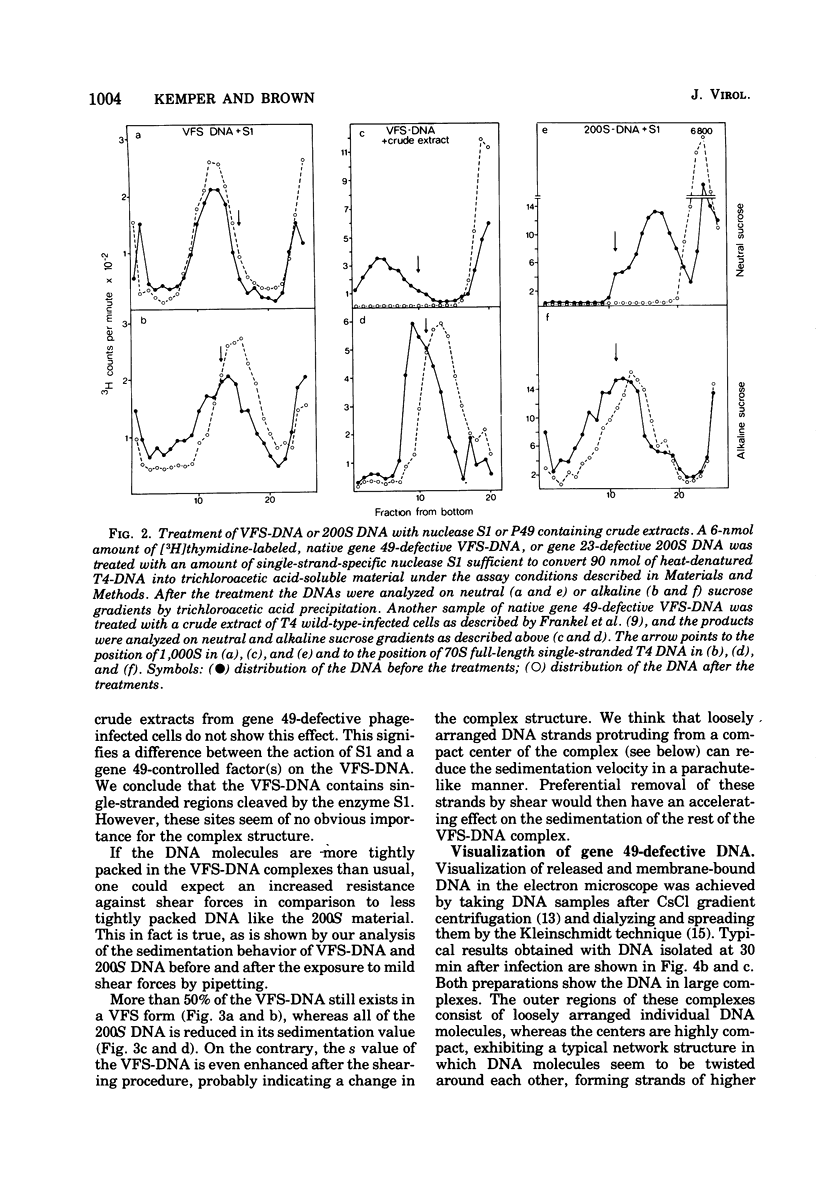
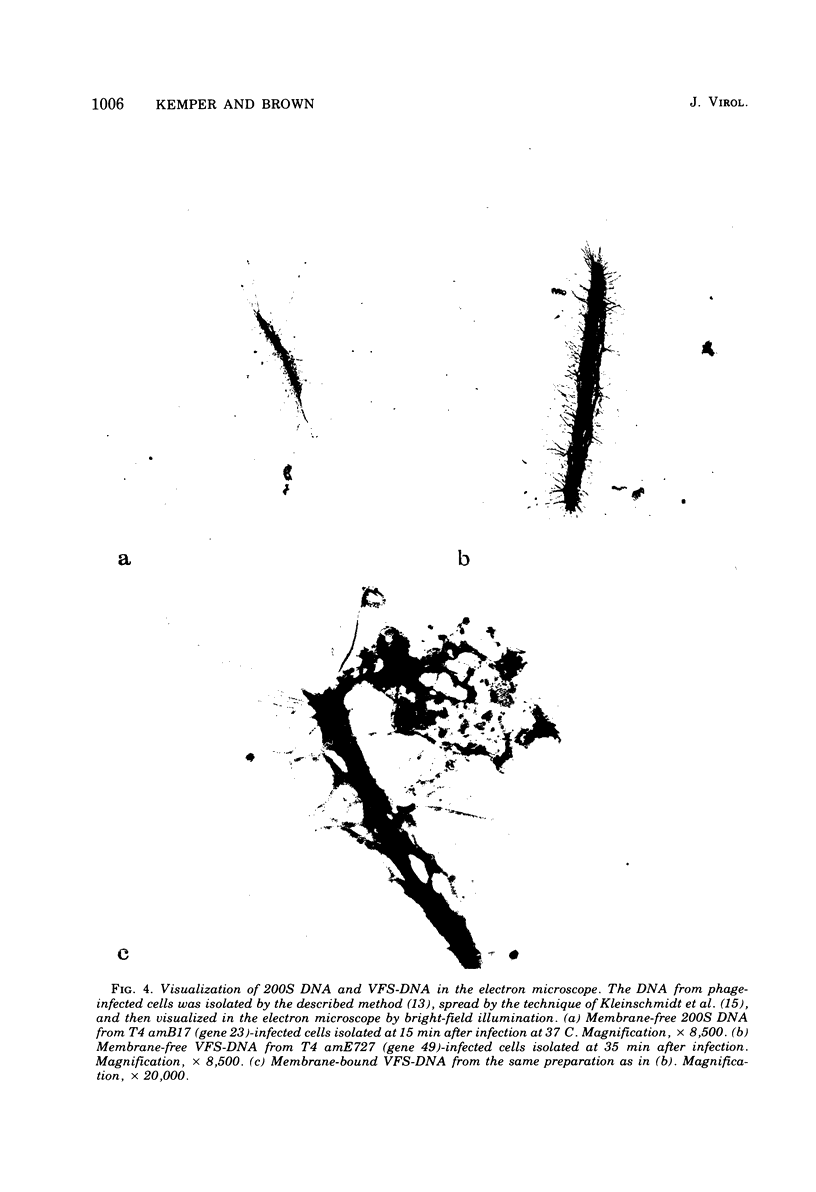
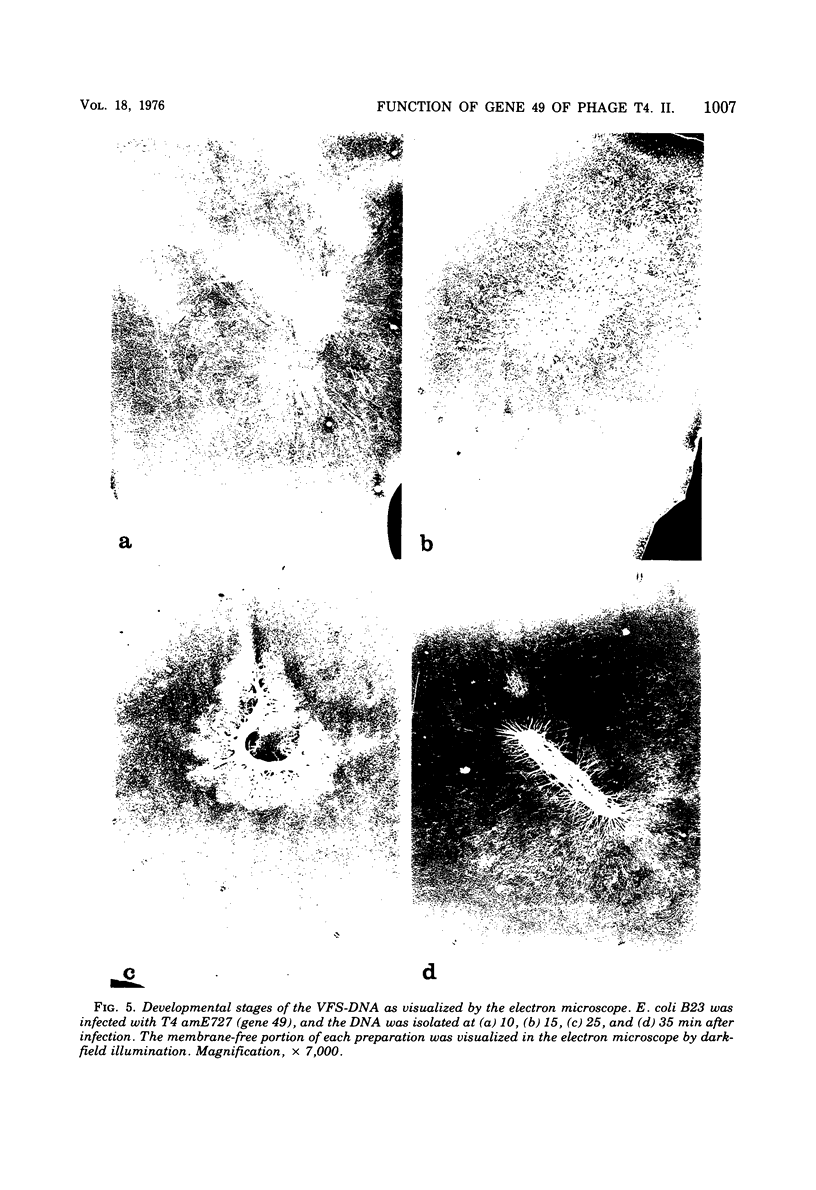
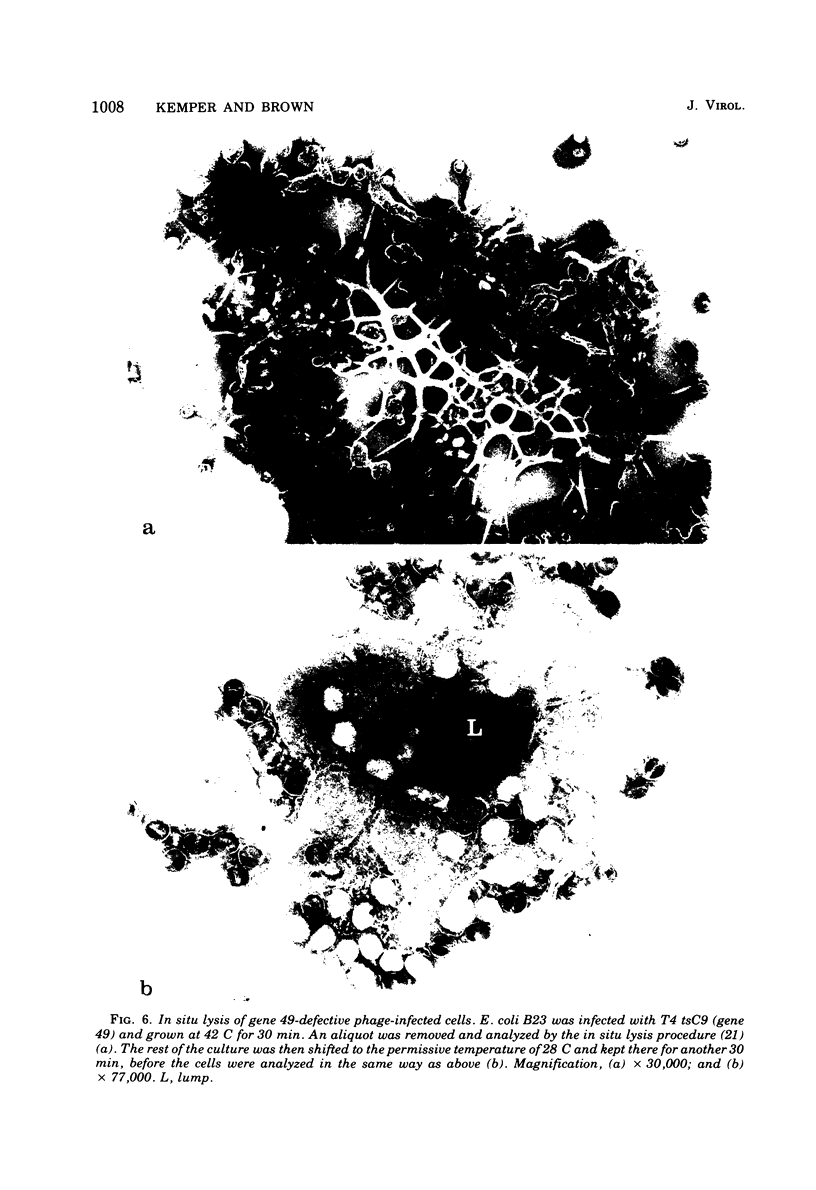
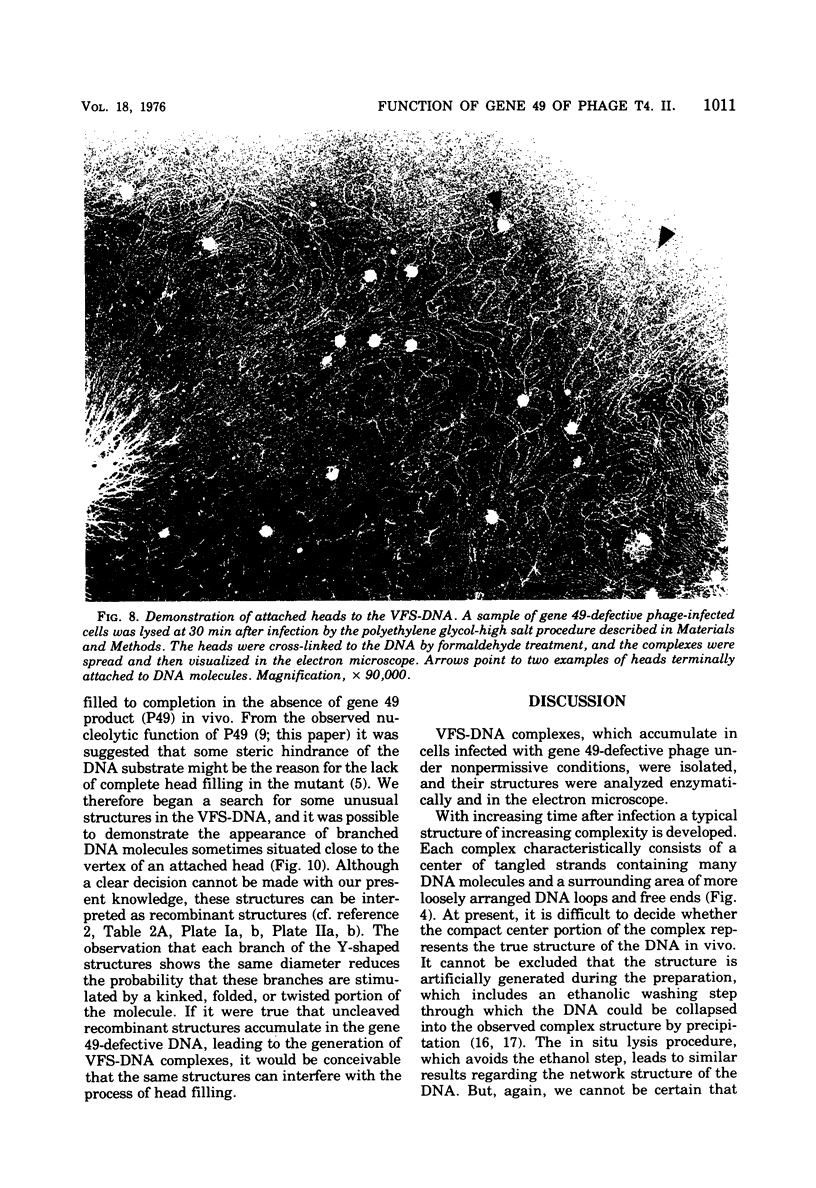
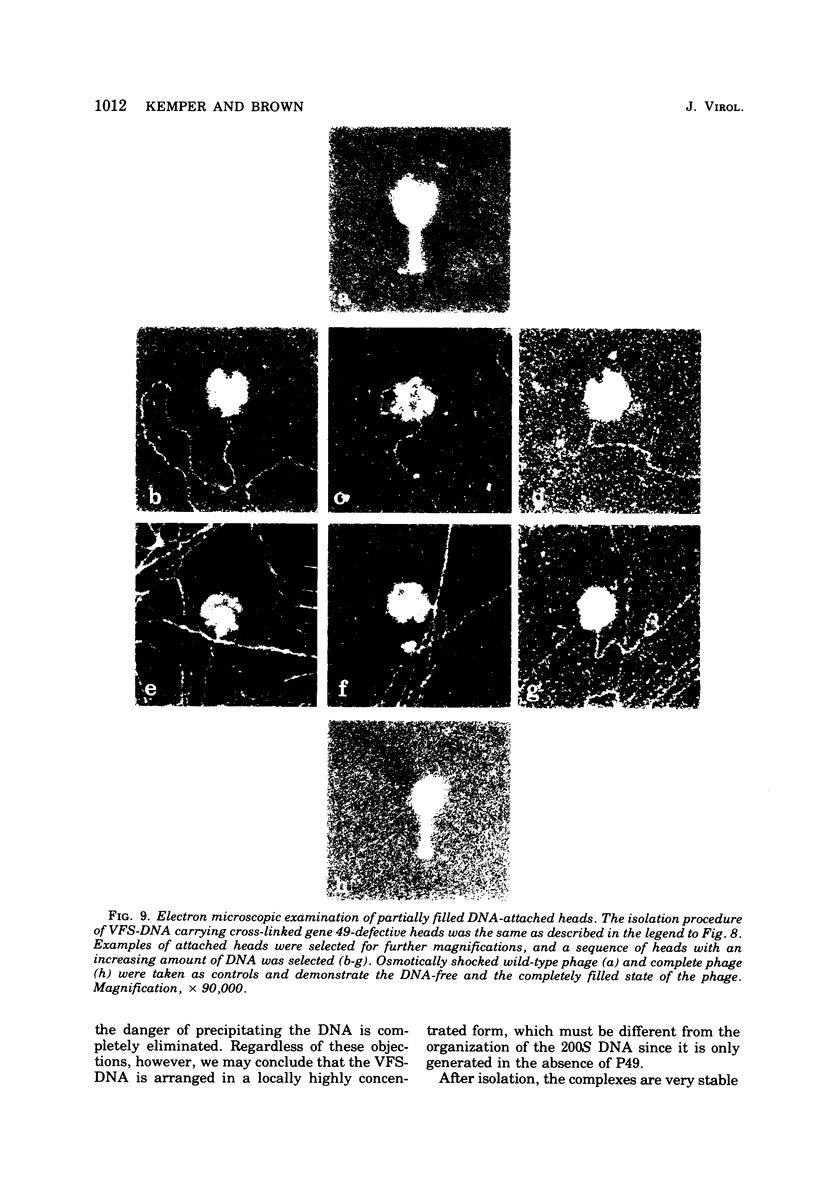
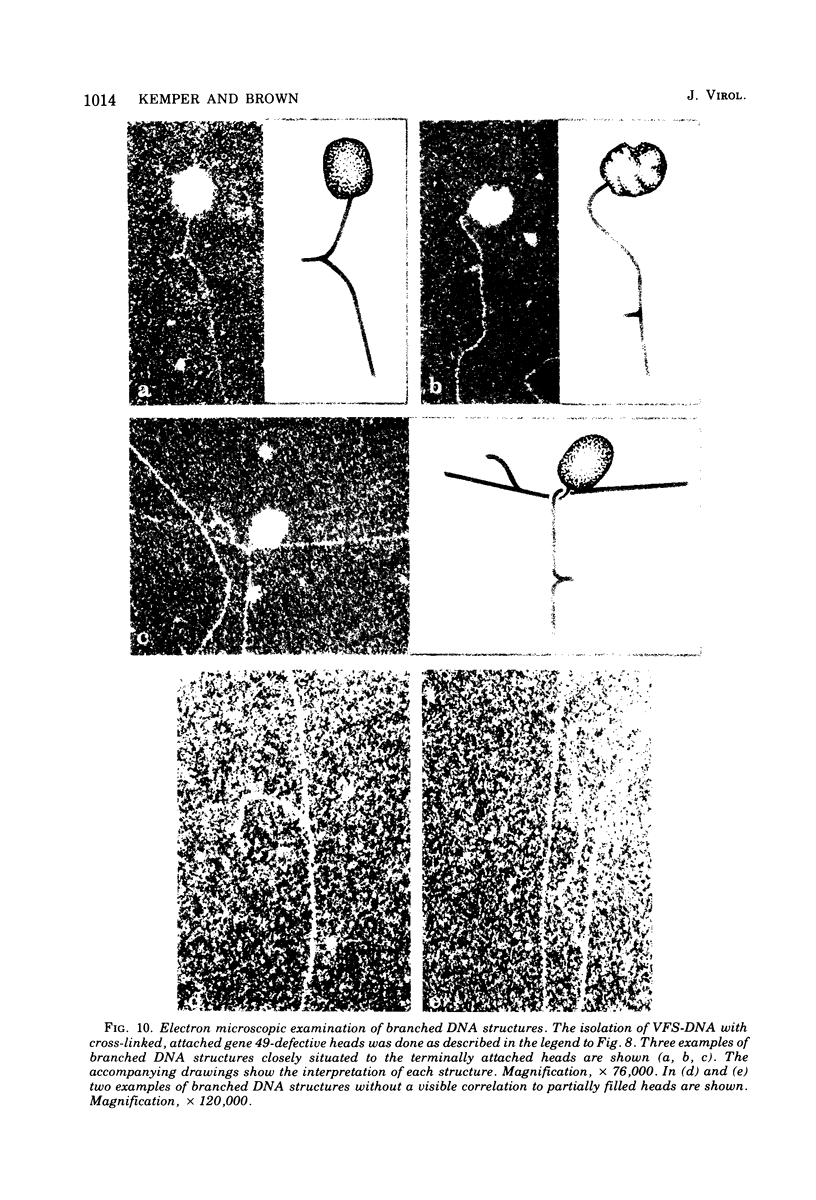
With the exception of mutants in gene 49, all mutants in phage T4 defective in the process of head filling accumulate a normal replicative DNA intermediate of 200S. Mutants in gene 49 produce a very fast-sedimenting (VFS) DNA with s values of greater than 1,000S. The intracellular development of the VFS-DNA generated in gene 49-defective phage-infected cells was followed by sedimentation analysis of crude lysates on neutral sucrose gradients. It was observed that the production of a 200S replicative intermediate is one step in the development of VFS-DNA. After restoring permissive conditions the development of the VFS-DNA can be reversed, but the 200S form is not regenerated under these conditions. The process of head filling can take place from the VFS-DNA under permissive conditions. From the absence of other components in the VFS-DNA complexes, its high resistance to shearing, its resistance against the attack of the single-strand-specific nuclease S1, and from its appearance in the electron microscope, a complex structure of tightly packed DNA is inferred. The demonstration by the electron microscope of branched DNA structures sometimes closely related to partially filled heads is taken in support of the idea that the process of head filling in gene 49-defective phage-infected cells is blocked by some steric hindrance in the DNA. In light of these results, the role of gene 49 is discussed as a control function for the clearance of these structures. A fixation procedure for cross-linking of gene 49-defective heads to the VFS-DNA allowed us to study progressive stages in the process of head filling. Electron microscopic evidence is presented which suggests that during the initial events the DNA accumulates in the vertexes of the head.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broker T. R. An electron microscopic analysis of pathways for bacteriophage T4 DNA recombination. J Mol Biol. 1973 Nov 25;81(1):1–16. doi: 10.1016/0022-2836(73)90243-x. [DOI] [PubMed] [Google Scholar]
- Broker T. R., Lehman I. R. Branched DNA molecules: intermediates in T4 recombination. J Mol Biol. 1971 Aug 28;60(1):131–149. doi: 10.1016/0022-2836(71)90453-0. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Westphal M., Burlingham B. T., Winterhoff U., Doerfler W. Structure and composition of the adenovirus type 2 core. J Virol. 1975 Aug;16(2):366–387. doi: 10.1128/jvi.16.2.366-387.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgi A. W., Robinton J., Carlson C. L. Studies on the folded chromosome of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1974;38:43–51. doi: 10.1101/sqb.1974.038.01.007. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Champoux J. J., Dulbecco R. An activity from mammalian cells that untwists superhelical DNA--a possible swivel for DNA replication (polyoma-ethidium bromide-mouse-embryo cells-dye binding assay). Proc Natl Acad Sci U S A. 1972 Jan;69(1):143–146. doi: 10.1073/pnas.69.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Worcel A. Electron microscopic studies on the folded chromosome of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1974;38:53–58. doi: 10.1101/sqb.1974.038.01.008. [DOI] [PubMed] [Google Scholar]
- Frankel F. R., Batcheler M. L., Clark C. K. The role of gene 49 in DNA replication and head morphogenesis in bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):439–463. doi: 10.1016/0022-2836(71)90147-1. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. DNA replication after T4 infection. Cold Spring Harb Symp Quant Biol. 1968;33:485–493. doi: 10.1101/sqb.1968.033.01.056. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. Studies on the nature of replicating DNA in T4-infected Escherichia coli. J Mol Biol. 1966 Jun;18(1):127–143. doi: 10.1016/s0022-2836(66)80081-5. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Minagawa T. Genetic control of the DNA maturation in the process of phage morphogenesis. Virology. 1971 Jul;45(1):280–291. [PubMed] [Google Scholar]
- Harrison D. P., Brown D. T., Bode V. C. The lambda head-tail joining reaction: purification, properties and structure of biologically active heads and tails. J Mol Biol. 1973 Sep 25;79(3):437–449. doi: 10.1016/0022-2836(73)90397-5. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. Visualization of replicating mammalian and T4 bacteriophage DNA. Cold Spring Harb Symp Quant Biol. 1968;33:509–524. doi: 10.1101/sqb.1968.033.01.059. [DOI] [PubMed] [Google Scholar]
- Kemper B., Janz E. Function of gene 49 of bacteriophage T4. I. Isolation and biochemical characterization of very fast-sedimenting DNA. J Virol. 1976 Jun;18(3):992–999. doi: 10.1128/jvi.18.3.992-999.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Beguin F., Gujer-Kellenberger G. A factor preventing the major head protein of bacteriophage T4 from random aggregation. J Mol Biol. 1970 Jan 14;47(1):69–85. doi: 10.1016/0022-2836(70)90402-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Johnson R. A. Maturation of the head of bacteriophage T4. II. Head-related, aberrant tau-particles. J Mol Biol. 1973 Nov 15;80(4):601–611. doi: 10.1016/0022-2836(73)90199-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Mölbert E., Showe M., Kellenberger E. Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J Mol Biol. 1970 Apr 14;49(1):99–113. doi: 10.1016/0022-2836(70)90379-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Teaff N., D'Ambrosia J. Maturation of the head of bacteriophage T4. III. DNA packaging into preformed heads. J Mol Biol. 1974 Oct 5;88(4):749–765. doi: 10.1016/0022-2836(74)90397-0. [DOI] [PubMed] [Google Scholar]
- Lang D. Collapse of single DNA molecules in ethanol. J Mol Biol. 1969 Nov 28;46(1):209–209. doi: 10.1016/0022-2836(69)90069-2. [DOI] [PubMed] [Google Scholar]
- Lang D. Regular superstructures of purified DNA in ethanolic solutions. J Mol Biol. 1973 Aug 5;78(2):247–254. doi: 10.1016/0022-2836(73)90113-7. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Ganz C. Bacteriophage T4 head morphogenesis. II. Studies on the maturation of gene 49-defective head intermediates. J Virol. 1972 Feb;9(2):377–389. doi: 10.1128/jvi.9.2.377-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Ganz C. Bacteriophage T4 head morphogenesis. IV. Comparison of gene 16-, 17-, and 49-defective head structures. J Virol. 1972 Sep;10(3):545–554. doi: 10.1128/jvi.10.3.545-554.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Wood W. B., Okinaka R. Bacteriophage T4 head morphogenesis. On the nature of gene 49-defective heads and their role as intermediates. J Mol Biol. 1971 May 14;57(3):555–573. doi: 10.1016/0022-2836(71)90109-4. [DOI] [PubMed] [Google Scholar]
- Onorato L., Showe M. K. Gene 21 protein-dependent proteolysis in vitro of purified gene 22 product of bacteriophage T4. J Mol Biol. 1975 Mar 5;92(3):395–412. doi: 10.1016/0022-2836(75)90288-0. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E., Hecht R. RNA molecules bound to the folded bacterial genome stabilize DNA folds and segregate domains of supercoiling. Cold Spring Harb Symp Quant Biol. 1974;38:31–41. doi: 10.1101/sqb.1974.038.01.006. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Black L. W. Assembly core of bacteriophage T4: an intermediate in head formation. Nat New Biol. 1973 Mar 21;242(116):70–75. doi: 10.1038/newbio242070a0. [DOI] [PubMed] [Google Scholar]
- Vanderslice R. W., Yegian C. D. The identification of late bacteriophage T4 proteins on sodium dodecyl sulfate polyacrylamide gels. Virology. 1974 Jul;60(1):265–275. doi: 10.1016/0042-6822(74)90384-5. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Interaction between DNA and an Escherichia coli protein omega. J Mol Biol. 1971 Feb 14;55(3):523–533. doi: 10.1016/0022-2836(71)90334-2. [DOI] [PubMed] [Google Scholar]