Abstract
Objective
Adult survivors of childhood cancer adhere poorly to recommended medical surveillance. We sought to identify modifiable factors that contribute to non-adherence.
Methods
Latent class analysis categorized survivors (ages 18–52 years) at risk of cardiac, breast, or bone late sequelae on the basis of their health-related concerns, fears, and motivation. These classifications were compared at two time points for self-reported adherence to recommended echocardiography, mammography, and bone densitometry screening.
Results
Three classes (worried, collaborative, self-controlling) characterized survivors in each of the 3 risk groups: cardiac (N=564; BIC=10,824.66; LRMLRT P=.002), breast (N=584; BIC=11,779.97, LRMLRT P<.001), and bone (N=613; BIC=11,773.56; LMRLRT P=.028). Only 9% of at-risk survivors in the self-controlling class reported undergoing bone density screening in 2005, compared to 17.2% in the collaborative class (P=.034). Thirteen percent of the self-controlling, 24% of the collaborative (P=.025), and 34% of the worried (P=.010) classes reported undergoing bone densitometry in 2009. While 73% of at-risk survivors in the worried class reported having had an echocardiogram in 2009, only 57% of the collaborative (P=.040) and 43% of the self-controlling (P<.001) classes did. In 2005 and 2009, respectively, fewer survivors in the self-controlling class (37% and 53%) than in the collaborative (51%, P=.038 and 70%, P=.01) and worried (58%, P=0.002 and 69%, P=0.025) classes reported undergoing mammograms.
Conclusions
Modifiable intrapersonal characteristics associated with these 3 classes predict self-reported participation in medical surveillance. Continued observation and validation of these survivor profiles may inform tailored interventions to enhance survivors’ screening participation.
Keywords: childhood cancer, screening, late effects, pediatric oncology
INTRODUCTION
As childhood cancer survival rates rise,[1, 2] late treatment-related morbidity [3–6] is of increasing concern. Follow-up screening can modify the likelihood and severity of late effects [7–8], but many survivors do not adhere to these recommendations [9–15]. For example, despite female survivors’ increased risk of early breast cancer,[16–18]recent studies found that only 41%– 55% of those with a history of chest radiation underwent mammography;[12, 19] among survivors <40 years, 47.3% had never had a mammogram, and only 52.6% of those 40–50 years were regularly screened (2 mammograms within 4 years)[19].
Treatment of childhood cancer with anthracyclines and/or chest radiation incurs a risk of late cardiotoxicity [6, 20–24]. Thirty years after diagnosis, survivors of childhood cancer have a rate of cardiac death 7 times the age- and sex-matched national average [25]. However, only 28% of at-risk participants in the Childhood Cancer Survivor Study (CCSS) had received the recommended cardiac screening [12, 26]. Cranial radiotherapy, glucocorticoids, methotrexate, and prolonged corticosteroid therapy increase the risk of low bone density and osteonecrosis, [27] but only approximately 25% of survivors at greatest risk had recently undergone bone densitometry [28].
We recently identified 3 distinctive profiles that predicted adult survivors’ intent to undergo routine and/or cancer-related check-ups [29]. These profiles were defined by survivors’ health-related motivation, worry, and concern, -- established modifiable mediators and moderators of health outcomes [30–36]. In this study, we replicated the distinctive survivor profiles in different samples, identified specific profile covariates, and used the profiles to predict self-reported participation in echocardiography, mammography, and bone densitometry screening at two different time points.
METHODS
Data Source
The CCSS is an IRB-approved multi-institutional retrospective cohort study initiated in 1994 to examine late effects in survivors of pediatric cancers diagnosed and treated between 1970 and 1986. Eligible participants had survived 5 or more years after completion of treatment for a malignant disease diagnosed before age 21 years. Survivors completed a baseline questionnaire at study entry and they respond to follow-up questionnaires (available at http://ccss.stjude.org) at regular intervals [37–38]. Participants provided informed consent for study procedures and release of their medical records.
Sample
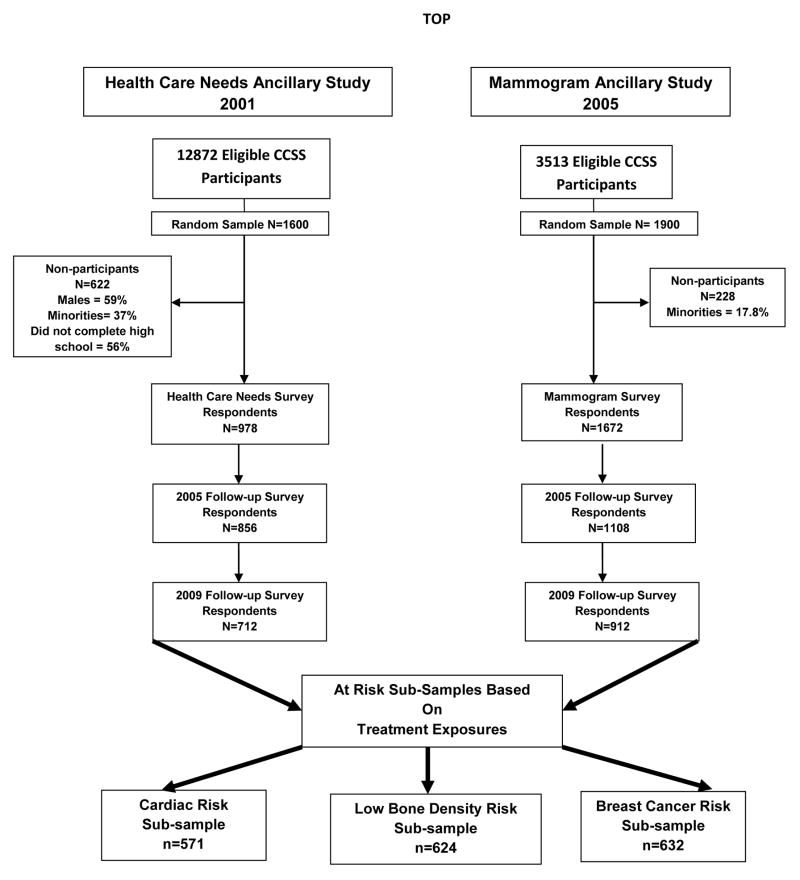
Our study sample was drawn from CCSS participants who: a) had responded to two CCSS follow-up surveys (2005 and 2009) containing the screening outcome measures; b) participated in at least one of two CCSS ancillary studies containing the psychological latent class indicators (Health Care Barriers [HCB] in 2001, Mammogram Survey [MS]) in 2005; and c) were at high risk of treatment exposure–based late effects, including cardiac (anthracycline and/or chest or total body radiation), bone density (cranial radiotherapy, glucocorticoids, methotrexate, and/or prolonged corticosteroid use), and breast cancer (chest or spinal radiation) (Figure 1). The cardiac and bone risk groups had 387/808 survivors in common; the bone and breast-cancer risk groups shared 30/1226 survivors; the cardiac and breast-cancer risk groups shared 62/1141; and 28/1376 survivors belonged to all 3 groups. The overlap in the groups is related to multi-agent chemotherapy and/or multimodal therapy (e.g., chemotherapy, radiation, surgery). Each agent or modality confers excess risk for a spectrum of adverse treatment effects, some of which are overlapping. All survivors were age =18 years at the time of data collection.
Figure 1.
Description of study data sources
Measures
Class indicators (established by confirmatory factor analysis) were fears, health concerns, intrinsic motivation, and extrinsic motivation. These measures were identical and derived from either the Health Care Barriers (2001) or Mammogram (2005) ancillary CCSS studies and were antecedent to the surveys containing the screening outcomes. Three summed items defined survivors’ fears about future health, cancer recurrence, and discovery of a problem at a check-up visit (1=not at all; 5=extremely). Three items were summed to define survivors’ health concerns about general health, the chance of illness or health problems related to previous cancer, and the importance of a check-up visit (1=not at all; 5=extremely). Five summed items from the Multidimensional Health Locus of Control Scale [39] (e.g., “I am in control of my health”) defined survivors’ intrinsic motivation (1=moderately/strongly disagree; 4=moderately/strongly agree). Four summed items from the Multidimensional Health Locus of Control Scale [39] (e.g., “Health professionals control my health”) defined survivors’ extrinsic motivation (1=moderately/strongly disagree; 4=moderately/strongly agree).
Covariates included disease and treatment variables (diagnosis, age at follow-up, age at cancer diagnosis, years since cancer diagnosis), sex, education, race, total personal income, current health insurance status, perceived severity of late effects, and self-reported health status (1=excellent, 5=poor). Survivors indicated whether they had experienced chronic health problems lasting longer than 6 months and rated the severity of their main chronic health problem. These responses were categorized as moderate, severe, or life-threatening chronic problems vs. mild or no chronic problems.
Medical Surveillance
The Children’s Oncology Group has compiled risk-based, exposure-related guidelines for surveillance and management of late effects of treatment [7]. Echocardiography, mammography, and bone densitometry are recommended at specific intervals based on age at treatment, chemotherapy and/or radiation exposures, and clinical indications at the end of therapy. Recommended screening frequency ranges from as often as yearly to every 5 years based on the established criteria. Given the length of time between follow-up surveys, all patients would have been due for at least one of the three screenings. Survivors indicated the time of the most recent echocardiography (ultrasound or MUGA scan), bone densitometry (DEXA or CT scan), and mammography in both of the CCSS follow-up surveys (1=never, 5=5 or more years ago). Survivors who answered “don’t know” to any of these questions were excluded from analysis. We used a very conservative approach to code the medical surveillance outcome for the logistic regressions. For the echocardiogram and bone densitometry multinomial logistic regressions, “never” and “5 or more years ago” were re-coded as 0 (non-adherent); “more than 2 but less than 5 years ago”, “1–2 years ago” and “less than 1 year ago” were re-coded as 1 (adherent). In keeping with previous reports [19], for the mammogram logistic regressions, “more than 2 but less than 5 years ago” was considered non-adherent. Self-reports of medical screening have been established as valid study measures in the general population [40–44]. Recent unpublished ancillary CCSS studies report 90% agreement between self-reports of screening and medical record review.
Statistical Analysis
Latent class analysis (LCA) enables the identification of a set of mutually exclusive typologies that account for the distribution of individuals in the population; typologies are created by cross-tabulation of observed discrete variables [45]. Unlike typical regression analysis, in which the population is assumed to be homogeneous and a single model holds for all cases, LCA can accommodate multiple populations (i.e., typologies) within the population. As recommended by Nylund and colleagues,[46] a combination of the Bayesian information criterion (BIC), Lo-Mendell-Rubin parametric likelihood ratio (LMRLRT), and bootstrap LMRLRT (BLMRT) tests were applied to determine the number of latent classes. Details regarding the interpretation of these parameters have been previously reported. [29] SAS 9.1 software (SAS Institute Inc, Cary, NC) was used to describe sample characteristics and MPlus Version 6.1 (Muthén and Muthén, Los Angeles, CA) was used to develop the latent class models.
Models that had the fewest substantively meaningful distinct classes and a BLMRT p ≤0.05 were accepted. Covariates that were significantly (p ≤.05) associated with the classes were included in the best-fitting latent class model. Binary outcomes (participation in echocardiography, mammography, and bone densitometry) were tested for equality of proportion to determine class-specific self-reported participation. All analyses were repeated for each risk group.
RESULTS
Latent Class Model Selection
Survivor classes were first identified within each risk group on the basis of our indicator variables, without controlling for covariates (Table 1). Five different models were tested for the cardiac and breast-cancer risk groups (1–5 classes) and 6 models (1–6 classes) for the bone risk group (Table 2). Both the 2- and 3-class models were tested for each risk group with covariates. Significant covariates included self-perceived health status and severity of late effects in all three models, health insurance status in the breast cancer risk group, race in the bone density risk group, and sex in the cardiac and bone density risk groups. Final fit statistics for the three models with covariates were: cardiac (N=564; BIC=10,824.66; LRMLRT P=0.002); bone (N=613; BIC=11,773.56; LMRLRT P=0.028); and breast (N=584; BIC=11, 779.97, LMRLRT P=<0.001). The final models with covariates demonstrated posterior probabilities (showing how well each participant fit the assigned class) of 85%-90%, supporting appropriate class assignment and model fit [47].
Table 1.
Descriptive summary of classes within risk groups before controlling for covariates
| BONE RISK | CARDIAC | BREAST RISK | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Total n=624 |
Self-Control n=163 |
Collab n=353 |
Worried n=108 |
P value | Total n=571 |
Self-Control n=136 |
Collab n=326 |
Worried n=109 |
P value | Total n=632 |
Self-Control n=110 |
Collab n=330 |
Worried N=192 |
P value | |
|
| |||||||||||||||
| 26% | 56% | 17% | 24% | 57% | 19% | 17% | 52% | 30% | |||||||
|
| |||||||||||||||
| Age at survey completion, 2003 | .918 | .219 | .149 | ||||||||||||
| Mean | 30.36 | 30.19 | 30.38 | 30.56 | 31.65 | 30.93 | 32.12 | 31.13 | 38.92 | 38.36 | 38.70 | 39.62 | |||
| SD | 7.2 | 6.8 | 7.3 | 7.3 | 7.5 | 7.1 | 7.5 | 7.95 | 6.18 | 6.09 | 6.35 | 5.89 | |||
|
| |||||||||||||||
| Gender(%) | <.001 | <.001 | NA | ||||||||||||
| Female | 52 | 33 | 57 | 67 | 51 | 38 | 51 | 66 | 100 | 100 | 100 | 100 | |||
| Male | 48 | 67 | 43 | 33 | 49 | 62 | 49 | 34 | |||||||
|
| |||||||||||||||
| Race/Ethnicity (%) | .004 | .015 | .859 | ||||||||||||
| White | 74 | 81 | 74 | 65 | 75 | 79 | 76 | 68 | 89 | 90 | 88 | 89 | |||
| Black | 7 | 4 | 8 | 10 | 8 | 5 | 7 | 12 | 1 | 0 | 2 | 1 | |||
| Hispanic | 12 | 4 | 12 | 20 | 12 | 6 | 13 | 16 | 4 | 3 | 5 | 4 | |||
| Other | 7 | 10 | 6 | 5 | 6 | 10 | 4 | 5 | 6 | 7 | 6 | 6 | |||
|
| |||||||||||||||
| Annual Personal Income (%) | .005 | .092 | .834 | ||||||||||||
| < $40,000 | 67 | 58 | 68 | 80 | 64 | 63 | 61 | 72 | 68 | 65 | 68 | 67 | |||
| >=$40,000 | 25 | 32 | 24 | 17 | 31 | 32 | 33 | 22 | 23 | 21 | 25 | 22 | |||
| Not reported | 8 | 10 | 8 | 4 | 5 | 4 | 6 | 6 | 9 | 14 | 7 | 11 | |||
|
| |||||||||||||||
| Education (%) | .005 | .320 | .084 | ||||||||||||
| Less than HS | 7 | 9 | 7 | 5 | 4 | 4 | 4 | 5 | 2 | 3 | 2 | 2 | |||
| Completed HS | 49 | 38 | 49 | 62 | 46 | 40 | 45 | 53 | 38 | 41 | 33 | 44 | |||
| Completed College | 42 | 50 | 42 | 31 | 49 | 54 | 50 | 40 | 53 | 48 | 58 | 47 | |||
| Not reported | 2 | 3 | 1 | 2 | 1 | 2 | 0 | 2 | 7 | 8 | 6 | 8 | |||
|
| |||||||||||||||
| Chemotherapy (%) | .520 | .571 | .312 | ||||||||||||
| Yes | 93 | 91 | 93 | 94 | 89 | 92 | 89 | 89 | 75 | 81 | 73 | 76 | |||
| No | 7 | 9 | 7 | 6 | 11 | 8 | 11 | 11 | 24 | 19 | 26 | 24 | |||
| Not reported | 1 | 1 | |||||||||||||
|
| |||||||||||||||
| Radiation% | .478 | .499 | NA | ||||||||||||
| Yes | 70 | 66 | 71 | 73 | 76 | 71 | 77 | 76 | 100 | 100 | 100 | 100 | |||
| No | 30 | 33 | 29 | 27 | 24 | 28 | 23 | 24 | |||||||
| Not reported | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | |||||||
|
| |||||||||||||||
| Chemotherapy and radiation (%) | .218 | .924 | .312 | ||||||||||||
| Yes | 63 | 57 | 64 | 67 | 65 | 63 | 66 | 65 | 75 | 81 | 73 | 76 | |||
| No | 37 | 42 | 36 | 33 | 35 | 36 | 34 | 35 | 24 | 19 | 26 | 24 | |||
| Not reported | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | |||||
|
| |||||||||||||||
| Age at cancer diagnosis | .723 | .371 | .213 | ||||||||||||
| Mean | 9.02 | 9.31 | 8.95 | 8.81 | 10.11 | 9.65 | 10.41 | 9.77 | 11.54 | 10.78 | 11.56 | 11.95 | |||
| SD | 5.5 | 5.1 | 5.7 | 5.7 | 5.94 | 5.7 | 6.0 | 6.1 | 5.57 | 5.34 | 5.60 | 5.63 | |||
|
| |||||||||||||||
| Years since cancer diagnosis | .238 | .577 | .373 | ||||||||||||
| Mean | 21.34 | 20.89 | 21.43 | 21.75 | 21.54 | 21.27 | 21.71 | 21.37 | 27.38 | 27.58 | 27.14 | 27.67 | |||
| SD | 4.4 | 4.2 | 4.4 | 4.3 | 4.5 | 4.3 | 4.7 | 4.3 | 4.46 | 4.53 | 4.42 | 4.50 | |||
|
| |||||||||||||||
| Cancer type (%) | .210 | .786 | .060 | ||||||||||||
| Leukemia | 50 | 48 | 53 | 44 | 29 | 31 | 29 | 26 | 9 | 9 | 11 | 5 | |||
| Central nervous system | 9 | 12 | 7 | 9 | 1 | 1 | 1 | 1 | 3 | 4 | 4 | 2 | |||
| Hodgkin lymphoma | 11 | 10 | 10 | 17 | 23 | 18 | 23 | 30 | 54 | 46 | 52 | 63 | |||
| Non-Hodgkin lymphoma | 14 | 15 | 15 | 11 | 14 | 17 | 13 | 13 | 7 | 6 | 8 | 7 | |||
| Wilms tumor | 0 | 0 | 0 | 0 | 8 | 8 | 9 | 7 | 12 | 18 | 11 | 10 | |||
| Neuroblastoma | 0 | 0 | 0 | 2 | 5 | 5 | 5 | 3 | 5 | 2 | 6 | 6 | |||
| Soft tissue sarcoma | 7 | 7 | 7 | 7 | 8 | 10 | 6 | 7 | 4 | 6 | 5 | 3 | |||
| Bone cancer | 8 | 7 | 8 | 10 | 13 | 10 | 14 | 13 | 5 | 8 | 5 | 4 | |||
|
| |||||||||||||||
| Chronic late effects (%) | <.001 | <.001 | .003 | ||||||||||||
| Moderate, severe or life- threatening | 20 | 7 | 20 | 38 | 22 | 10 | 21 | 41 | 57 | 47 | 55 | 67 | |||
| Mild or none | 79 | 93 | 78 | 58 | 77 | 90 | 78 | 56 | 43 | 53 | 45 | 53 | |||
| Not reported | 1 | 0 | 1 | 4 | 1 | 1 | 1 | 3 | |||||||
|
| |||||||||||||||
| Self-rated health (%) | <.001 | <.001 | <.001 | ||||||||||||
| Excellent/very good/good | 84 | 96 | 88 | 55 | 83 | 96 | 86 | 59 | 78 | 83 | 83 | 68 | |||
| Fair/poor | 15 | 4 | 12 | 43 | 16 | 2 | 13 | 39 | 15 | 9 | 11 | 24 | |||
| Not reported | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 2 | 7 | 8 | 6 | 8 | |||
|
| |||||||||||||||
| Limited ability to participate in vigorous activities (%) | .002 | .014 | .041 | ||||||||||||
| Yes | 29 | 18 | 33 | 37 | 30 | 21 | 33 | 34 | 38 | 32 | 43 | 34 | |||
| No | 69 | 82 | 67 | 58 | 69 | 79 | 66 | 64 | 54 | 60 | 50 | 58 | |||
| Not reported | 1 | 0 | 1 | 5 | 1 | 0 | 1 | 2 | 7 | 8 | 6 | 8 | |||
|
| |||||||||||||||
| Current health insurance (%) | .381 | .181 | .003 | ||||||||||||
| Yes | 87 | 83 | 88 | 89 | 90 | 90 | 90 | 87 | 87 | 79 | 89 | 88 | |||
| No | 12 | 17 | 11 | 10 | 10 | 10 | 10 | 11 | 6 | 13 | 4 | 5 | |||
| Not reported | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 7 | 8 | 7 | 8 | |||
Abbreviations: SD, standard deviation; HS, high school; self-control, self-controlling; collab, collaborative
Table 2.
Fit statistics of the risk LCA models, not controlled for covariates
| Cardiac(N=571) | |||
|---|---|---|---|
| Classes | Log-Likelihood | BIC | LMR Adjusted LRT P Value |
| 1 | −5597.15 | 11,245.07 | |
| 2 | −5489.75 | 11,062.02 | P<.001 |
| 3 | −5445.57 | 11,005.38 | P=.01 |
| 4 | −5431.99 | 11,009.97 | P=.526 |
| 5 | −5426.30 | 11,030.33 | P=.332 |
| Breast (N=632) | |||
| Classes | Log-Likelihood | BIC | LMR Adjusted LRT P Value |
| 1 | −6482.38 | 13,016.36 | |
| 2 | −6372.91 | 12,829.65 | P<.001 |
| 3 | −6321.50 | 12,759.08 | P<.001 |
| 4 | −6301.62 | 12,751.56 | P=.269 |
| 5 | −6288.56 | 12,757.69 | P=.234 |
| Bone (N=624) | |||
| Classes | Log-Likelihood | BIC | LMR Adjusted LRT P Value |
| 1 | −6157.20 | 12,365.88 | |
| 2 | −6027.78 | 12,139.23 | P<.001 |
| 3 | −5978.91 | 12,073.66 | P =.166 |
| 4 | −5962.22 | 12,072.46 | P=.228 |
| 5 | −5930.69 | 12,042.59 | P=.352 |
| 6 | −5915.41 | 12,043.22 | P=.275 |
Abbreviations: BIC, Bayesian information criterion; LMRLRT, Lo-Mendell-Rubin parametric likelihood ratio test; BLMRT, bootstrap LMRLRT
Interpreting Class Profiles
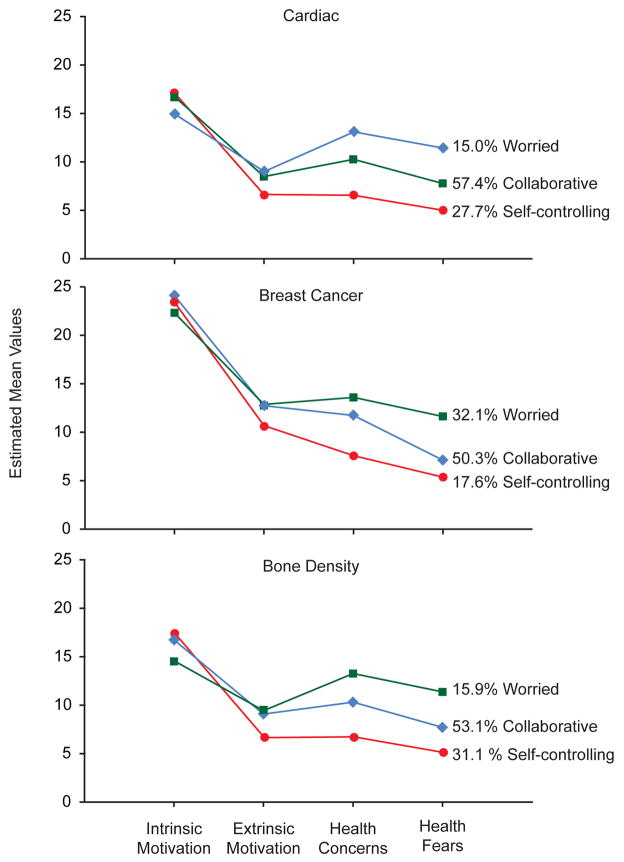
Figure 2 and Table 3 show the class-specific means of the indicator variables. This estimate divided by the standard error (EST/SE) indicates the strength of the relationship between the indicator and the latent class variable. In each risk group, one class reported poor perceived health status and moderate to life-threatening chronic illness more frequently than did the other two classes; these survivors also reported the greatest worries and health concerns, the lowest level of intrinsic motivation, and the highest level of extrinsic motivation (Table 3). We labeled this group “worried.” This class was much larger in the breast-cancer risk group (30%) than in the bone (17%) or cardiac (19%) risk groups.
Figure 2.
Estimated mean values of the latent class indicators by risk group.
Table 3.
Latent class indicators within risk groups, controlled for significant covariates
| Worried | Self-Controlling | Collaborative | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Estimate (95% CI) | S.E. | EST/SE | P | Mean Estimate (95% CI) | S.E. | EST/SE | P | Mean Estimate (95% CI) | S.E. | EST/SE | P | |
| Health Fears | ||||||||||||
| Cardiac(n=564) | 11.66 (10.50–12.83) | 0.594 | 19.63 | <.001 | 5.12 (4.67–5.58) | 0.233 | 22.00 | <.001 | 7.77 (7.23–8.30) | 0.273 | 28.43 | <.001 |
| Breast(n=584) | 11.68 (10.96–12.40) | 0.366 | 31.92 | <.001 | 5.37 (4.84–5.89) | 0.267 | 20.12 | <.001 | 7.01 (6.57–7.45) | 0.227 | 30.93 | <.001 |
| Bone (n=613) | 11.36 (10.32–12.41) | 0.535 | 21.25 | <.001 | 5.12 (4.69–5.55) | 0.219 | 23.31 | <.001 | 7.66 (7.10–8.22) | 0.287 | 26.73 | <.001 |
| Health Concerns | ||||||||||||
| Cardiac(n=564) | 13.35 (12.88–13.83) | 0.242 | 55.15 | <.001 | 6.69 (5.92–7.45) | 0.388 | 17.22 | <.001 | 10.26 (9.70–10.82) | 0.285 | 36.06 | <.001 |
| Breast(n=584) | 13.65 (13.35–13.95) | 0.152 | 89.97 | <.001 | 7.58 (6.88–8.28) | 0.358 | 21.16 | <.001 | 11.64 (11.12–12.15) | 0.261 | 44.54 | <.001 |
| Bone(n=613) | 13.26 (12.66–13.87) | 0.309 | 42.91 | <.001 | 6.73 (5.99–7.47) | 0.378 | 17.81 | <.001 | 10.25 (9.65–10.85) | 0.307 | 33.40 | <.001 |
| Extrinsic Motivation | ||||||||||||
| Cardiac(n=564) | 9.26 (8.36–10.17) | 0.462 | 20.05 | <.001 | 6.76 (6.25–7.27) | 0.259 | 26.08 | <.001 | 8.48 (8.04–8.93) | 0.228 | 37.25 | <.001 |
| Breast(n=584) | 12.92 (12.30–13.54) | 0.317 | 40.73 | <.001 | 10.68 (9.69–11.66) | 0.503 | 21.22 | <.001 | 12.64 (12.11–13.17) | 0.269 | 46.99 | <.001 |
| Bone(n=613) | 9.43 (8.29–10.57) | 0.582 | 16.21 | <.001 | 6.65 (6.21–7.09) | 0.224 | 29.74 | <.001 | 9.03 (8.41–9.66) | 0.317 | 28.50 | <.001 |
| Intrinsic Motivation | ||||||||||||
| Cardiac(n=564) | 15.17 (14.05–16.29) | 0.571 | 26.55 | <.001 | 17.23 (16.67–17.78) | 0.285 | 60.49 | <.001 | 16.73 (16.29–17.17) | 0.225 | 74.49 | <.001 |
| Breast(n=584) | 22.36 (21.52–23.21) | 0.433 | 51.70 | <.001 | 23.42 (22.43–24.42) | 0.509 | 46.02 | <.001 | 23.99 (23.43–24.56) | 0.288 | 83.29 | <.001 |
| Bone(n=613) | 14.57 (13.13–15.78) | 0.735 | 19.82 | <.001 | 17.40 (16.98–17.83) | 0.218 | 79.85 | <.001 | 16.69 (16.22–17.16) | 0.238 | 70.14 | <.001 |
A second class of survivors demonstrated class indicators and health status perceptions markedly opposite to those of the ‘worried’ class. Across all 3 risk groups, survivors in this class reported good/excellent health and little concern about health history or future health problems, and they placed little value on medical check-ups. While strongly intrinsically motivated for self-care, this group was minimally extrinsically motivated to involve health professionals in their long-term care management. We labeled this group “self-controlling.” In the cardiac and bone risk groups, this class was predominantly male; it had the lowest percentages of black and Hispanic survivors, the highest percentage of college graduates, and the highest reported incomes.
A third class, the largest class in each risk group, showed intermediate scores on the class indicators and on self-reported health status, was balanced in sex distribution, and endorsed moderate fears and health concerns. Members were highly intrinsically motivated to manage their health but also willing to work with health providers, as evidenced by their greater extrinsic motivation than the self-controlling group. We labeled this group “collaborative.”
Survivor Participation in Recommended Screening
Self-reported participation in echocardiography, bone densitometry, and mammography in two different follow-up surveys was compared within risk groups by class, controlling for significant covariates in each group (Table 4).
Table 4.
Comparison of participation in screening (2005 and 2009) according to class after adjustment for covariates
| 2005 | 2009 | |||||
|---|---|---|---|---|---|---|
| Class | Worried vs. Self-Controlling | Collaborative vs. Self-Controlling | Worried vs. Collaborative | Worried vs. Self-Controlling | Collaborative vs. Self-Controlling | Worried vs. Collaborative |
| Type of screening | % (p value) | % (p value) | % (p value) | % (p value) | % (p value) | % (p value) |
| Echocardiography (N=564) | 54% vs. 40% (P=0.082) | 50% vs. 40% (P=0.092) | 54% vs. 50% (P=0.550) | 73% vs. 43% (P=<0.001) | 57% vs. 43% (P=0.036) | 73% vs. 57% (P=0.040) |
| Mammography (N=584) | 58% vs. 37% (P=0.002) | 51% vs. 37% (P=0.038) | 58% vs. 51% (P=0.243) | 69% vs. 53% (P=0.025) | 70% vs. 53% (P=0.013) | 69% vs. 70% (P=0.861) |
| Bone densitometry(N=613) | 17.4% vs. 9% (P=0.155) | 17.2% vs. 9% (P=0.034) | 17.4% vs.17.2% (P=0.976) | 34% vs. 13% (P=0.010) | 24% vs. 13% (P=0.025) | 34% vs. 24% (P=0.232) |
Comparison used chi-square equality tests of means across classes with posterior probability-based multiple imputations (2 degrees of freedom for the overall test and 1 for the pair wise tests).
(2005)
While the classes did not differ in echocardiography, a significantly larger proportion of survivors in the collaborative than in the self-controlling group underwent bone densitometry. Similarly, significantly larger proportions of the collaborative and worried classes than of the self-controlling class underwent recommended mammography (Table 4).
(2009)
A greater proportion of survivors reported echocardiography in 2009 than in 2005 across all 3 classes, but the proportions within each class differed significantly (Table 4). Similarly, the proportion of survivors participating in bone densitometry increased in all classes in 2009. A significantly larger proportion of survivors in the worried and collaborative classes than in the self-controlling class participated in both bone density and mammography.
DISCUSSION
We found 3 distinctive groups of survivors—worried, self-controlling, and collaborative—defined by indicators of health-related concerns, motivation, and affect, as in our previous work. This multiple nominal classification, rather than a single linear descriptive model, is supported by the fact that each survivor has a most likely class, the classes make up different proportions of the sample, and the classes have no ordered interrelation [48]. With the exception of the worried class in the breast-cancer risk group, the distribution of survivors across the 3 classes in each risk group was similar. The exclusively female sex of the breast-cancer risk group and the tendency toward female predominance in the worried class is likely to explain this difference.
Because of their self-reported good health and low levels of health concerns and worries, survivors in the self-controlling group may see no need for cancer-related follow-up and screening [49]. Their high levels of intrinsic motivation and low levels of extrinsic motivation indicate that they are unlikely to initiate medical follow-up. This typology may reflect survivors’ unawareness of their long-term risks [50]. Based on our collective and cumulative clinical experience and on-going clinical trials, an optimal first intervention for this group might be distance-based strategies (e.g., web and/or print media detailing treatment-related risks and surveillance recommendations). Because of their strong intrinsic motivation, they may be more likely to initiate cancer-related follow-up and screening after being informed of their risks. Because these survivors report the least worry about their future health, the information provided should be detailed and graphic in explaining the probability and nature of their treatment-related risks [10, 50].
The worried group’s demographic profile suggests the most difficulty in obtaining risk-based health care (Table 1). Survivors who are African-American, older at interview, or uninsured were reported as less likely to receive risk-based, survivor-focused care [12]. The worried group was also distinguished by greater cancer-related fears. Fear and worry exert both positive and negative influences on health-related behaviors. For example worried or health-anxious survivors may avoid screening to moderate their fear of bad news [51, 52]. However, some excess worry can make the individual more sensitive to potential threats or more vigilant about lifestyle and surveillance behaviors [51–56]. Misconceptions and lack of specific risk information can exacerbate fear or contribute to denial of the possibility of significant health problems [57–62]. While our analysis does not specifically consider which particular worries were prominent in each risk group, it is highly likely that worries will differ from one diagnostic group to another, as well as across survivors with different co-morbidities and late effects experiences. Face-to-face encounters, where the clinician could identify specific worry targets, would likely provide the best care for this group; however, individualized print summaries together with supportive telephone interactions detailing long-term risks and ways to reduce those risks would potentially be useful. The worried group should be offered information in a manner that avoids exacerbating fears and concerns.
Survivors in the collaborative group were both receptive to professional care (extrinsically motivated) and highly intrinsically motivated to maintain their health. However, they may not fully understand their risks or the need for periodic screening, as reported among childhood cancer survivors in general [50, 57–63]. Collaborators may be highly motivated to follow screening recommendations if they are provided risk-based information and specific recommendations. Print summaries of their treatment, including exposure risks and recommendations for medical follow-up and screening, would likely be sufficient to motivate collaborators to initiate care and work with their providers to minimize their risks.
In a previous report [29], the worried and collaborative classes differed markedly in that survivors in the worried class were more likely to obtain routine and cancer-related medical care. In this study, the two groups differed significantly only on echocardiography in the 2009 survey. While a larger proportion of the worried class than of the collaborative class participated in screening, statistical power may have been lacking in this small sample.
Limitations
While the CCSS population is a large and heterogeneous cohort of 5-year survivors, our results may not be generalizable to all childhood cancer survivors. Although observation of the same class structure in the risk groups could reflect non-independence of groups, the overlap across all 3 risk groups was only 2%, and the same class structure was previously determined in different samples [29]. Finally, there may be variables that differ across classes but were unavailable in our data set.
Conclusions
Our findings suggest that survivors’ participation in medical screening varies predictably across survivor typologies derived from personal endorsement of indicators of health-related concerns, motivation, and affect. Our current clinical trials are assessing the tailoring of interventions to these profiles to support physical activity among patients undergoing treatment and medical surveillance among adult survivors of childhood cancer. Future studies will examine to what extent these profiles predict other behavior-related health outcomes in other survivor samples.
Acknowledgments
Support: NIH grants R03 NR009203 (CL Cox, PI), U24 CA55727 (LL Robison, PI), and P30 CA 21765; the Robert Wood Johnson Generalist Physician Faculty Scholar award (Oeffinger KC, PI); and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
The authors declare no conflicts of interest and no competing financial interests.
References
- 1.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–48. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, et al., editors. SEER Cancer Statistics Review, 1975–2004. National Cancer Institute; Bethesda, MD: 2007. [accessed April 2012]. Vol. based on November 2006 SEER data Submission, Posted to the SEER Web Site. Available from: http://seer.cancer.gov/csr/1975_2004. [Google Scholar]
- 3.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290:1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 4.Mattano LA, Sather HN, Trigg ME, et al. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the children’s cancer group. Clin Oncol. 2000;18:3262–3272. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 5.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood Cancer Survivor Study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 6.Simbre VC, Duffy SA, Dadlani GH, et al. Cardiotoxicity of cancer chemotherapy: implications for children. Paediatr Drugs. 2005;7:187–202. doi: 10.2165/00148581-200507030-00005. [DOI] [PubMed] [Google Scholar]
- 7.Children’s Oncology Group. [accessed April 2012];The Children’s Oncology Group Long-Term Follow-up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. 2006 Available from: http://www.survivorshipguidelines.org.
- 8.American Academy of Pediatrics Section on Hematology/Oncology Children’s Oncology Group. Long-term follow-up care for pediatric cancer survivors. Pediatrics. 2009;123:906–915. doi: 10.1542/peds.2008-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellizzi KM, Rowland JH, Jeffery DD, et al. Health behaviors of cancer survivors: Examining opportunities for cancer control intervention. J Clin Oncol. 2005;23:8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 10.Cox CL, McLaughlin RA, Steen BD, et al. Predicting and modifying substance use in childhood cancer survivors: application of a conceptual model. Oncol Nurs Forum. 2006;33:51–60. doi: 10.1188/06.ONF.51-60. [DOI] [PubMed] [Google Scholar]
- 11.Klosky JL, Cash DK, Buscemi J, et al. Factors influencing long-term follow-up clinic attendance among survivors of childhood cancer. J Cancer Surviv. 2008;2:225–232. doi: 10.1007/s11764-008-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26:4401–4409. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheen V, Tucker MA, Abramson DH, et al. Cancer screening practices of adult survivors of retinoblastoma at risk of second cancers. Cancer. 2008;113:434–441. doi: 10.1002/cncr.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tercyak KP, Donze JR, Prahlad S, et al. Multiple behavioral risk factors among adolescent survivors of childhood cancer in the Survivor Health and Resilience Education (SHARE) program. Pediatr Blood Cancer. 2006;47:825–830. doi: 10.1002/pbc.20602. [DOI] [PubMed] [Google Scholar]
- 15.Yeazel MW, Oeffinger KC, Gurney JG, et al. The cancer screening practices of adult survivors of childhood cancer. Cancer. 2004;100:631–640. doi: 10.1002/cncr.20008. [DOI] [PubMed] [Google Scholar]
- 16.Kenney LB, Yasui Y, Inskip PD, et al. Breast cancer after childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Intern Med. 2004;141:590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 17.Taylor AJ, Winter DL, Stiller CA, et al. Risk of breast cancer in female survivors of childhood Hodgkin’s disease in Britain: A population-based study. Int J Cancer. 2006;120:384–391. doi: 10.1002/ijc.22261. [DOI] [PubMed] [Google Scholar]
- 18.Diller L, Medeiros Nancarrow C, Shaffer K, et al. Breast cancer screening in women previously treated for Hodgkin’s disease: a prospective cohort study. J Clin Oncol. 2002;20:2085–2091. doi: 10.1200/JCO.2002.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA. 2009;301:404–414. doi: 10.1001/jama.2008.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipshultz S, Colan S. Cardiovascular trials in long-term survivors of childhood cancer. J Clin Oncol. 2004;22:769–773. doi: 10.1200/JCO.2004.12.937. [DOI] [PubMed] [Google Scholar]
- 21.Pinarli FG, Oguz A, Tunaouglu FS, et al. Late cardiac evaluation of children with solid tumors after anthracycline chemotherapy. Pediatr Blood Cancer. 2005;44:370–377. doi: 10.1002/pbc.20281. [DOI] [PubMed] [Google Scholar]
- 22.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scully RE, Lipshultz SE. Anthracycline cardiotoxicity in long-term survivors of childhood cancer. Cardiovasc Toxicol. 2007;7:122–128. doi: 10.1007/s12012-007-0006-4. [DOI] [PubMed] [Google Scholar]
- 24.Kremer LC, Vander Pal HJ, Offringa M, et al. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol. 2002;13:819–829. doi: 10.1093/annonc/mdf167. [DOI] [PubMed] [Google Scholar]
- 25.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathan PC, Greenberg ML, Ness KK, et al. Risk-based care in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study (CCSS) J Clin Oncol. 2002;20:2085–2091. [Google Scholar]
- 27.National Cancer Institute. PDQ Late Effects of Treatment for Childhood Cancer. Bethesda, MD: 2011. [accessed April 2012]. Available from: http://cancer.gov/cancertopics/pdq/treatment/lateeffects/HealthProfessional. [Google Scholar]
- 28.Cox CL, Hudson MM, Mertens AC, et al. Medical screening participation in the childhood cancer survivor study. Arch Intern Med. 2009;169:454–462. doi: 10.1001/archinternmed.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox CL, Zhu L, Finnegan L, et al. Survivor profiles predict health behavior intent: the Childhood cancer survivor study. Psycho-oncology. 2012;21 (5):469–78. doi: 10.1002/pon.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox CL. A model of health behavior to guide studies of childhood cancer survivors. Oncol Nurs Forum. 2003;30:E92–99. doi: 10.1188/03.ONF.E92-E99. [DOI] [PubMed] [Google Scholar]
- 31.Cox CL, Montgomery M, Rai SN, et al. Supporting breast self-examination in female childhood cancer survivors: A secondary analysis of a behavioral intervention. Oncol Nurs Forum. 2008;35:423–430. doi: 10.1188/08.ONF.423-430. [DOI] [PubMed] [Google Scholar]
- 32.Cox CL, Montgomery M, Oeffinger, et al. Promoting physical activity in childhood cancer survivors: Results from the Childhood Cancer Survivor Study. Cancer. 2009;115:642–654. doi: 10.1002/cncr.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox CL, Oeffinger KC, Montgomery M, et al. Determinants of mammography screening participation in adult childhood cancer survivors: Results from the Childhood Cancer Survivor Study. Oncol Nurs Forum. 2009;36:335–344. doi: 10.1188/09.ONF.335-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauman AE, Sallis JF, Dzewaltowski DA, et al. Toward a better understanding of the influences on physical activity: the role of determinants, correlates, causal variables, mediators, moderators, and confounders. Am J Prev Med. 2002;23:5–14. doi: 10.1016/s0749-3797(02)00469-5. [DOI] [PubMed] [Google Scholar]
- 35.Breslow L, Lloyd D, Shumaker SA. Disease prevention research at NIH: An agenda for all. Workshop B: Health behaviors--predictors, mediators, and endpoints. Prev Med. 1994;23:552–553. doi: 10.1006/pmed.1994.1079. [DOI] [PubMed] [Google Scholar]
- 36.Jeffery RW. How can health behavior theory be made more useful for intervention research? Int J Behav Nutr Phys Act. 2004;1:10. doi: 10.1186/1479-5868-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 38.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) Health Educ Monogr. 1978;6:160–170. doi: 10.1177/109019817800600107. [DOI] [PubMed] [Google Scholar]
- 40.Cadarette SM, Beaton DE, Gignac MA, et al. Minimal error in self-report of having had DXA, but self-report of results was poor. J Clin Epidemiol. 2007;60:1306–1311. doi: 10.1016/j.jclinepi.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Caplan LS, McQueen DV, Qualters JR, et al. Validity of women’s self-reports of cancer screening test utilization in a managed care population. Cancer Epidemiol Biomarkers Prev. 2003;12:1182–1187. [PubMed] [Google Scholar]
- 42.Coughlin SS, Uhler RJ, Bobo JK, et al. Breast cancer screening practices among women in the United States, 2000. Cancer Causes Control. 2004;15:159–170. doi: 10.1023/B:CACO.0000019496.30145.62. [DOI] [PubMed] [Google Scholar]
- 43.King ES, Rimer BK, Trock B, et al. How valid are mammography self-reports? Am J Public Health. 1990;80:1386–1388. doi: 10.2105/ajph.80.11.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pijpe A, Mulder RL, Manders P, et al. Validation study suggested no differential misclassification of self-reported mammography history in BRCA 1/2 mutation carriers. J Clin Epidemiol. 2011;64:1434–1443. doi: 10.1016/j.jclinepi.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 45.McCuthcheon AL. Quantitative applications in the social sciences series No 64. Sage Publications, Inc; Newberry Park, CA: 1987. Latent class analysis. [Google Scholar]
- 46.Nylund KL, Asparouhov T, Muthen B. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Struct Equ Modeling. 2007;14:535–569. [Google Scholar]
- 47.Muthen B. Latent variable mixture modeling. In: Marcoulides GA, Schumacker RE, editors. New Developments and Techniques in Structural Equation Modeling. Lawrence Erlbaum Associates; Florence, Kentucky: 2001. pp. 1–33. [Google Scholar]
- 48.Silvia PJ, Kaufman JC, Pretz JE. Is creativity domain-specific? Latent class models of creative accomplishments and creative self-descriptions. Psychology of Aesthetics, Creativity, and the Arts. 2009;3:139–148. [Google Scholar]
- 49.Maeda N, Hortibe K, Kato K, et al. Survey of childhood cancer survivors who stopped follow-up physician visits. Pediatr Int. 2010;52:806–812. doi: 10.1111/j.1442-200X.2010.03158.x. [DOI] [PubMed] [Google Scholar]
- 50.Kadan-Lottick NS, Robison LL, Gurney JG, et al. Childhood cancer survivors’ knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA. 2002;287:1832–1839. doi: 10.1001/jama.287.14.1832. [DOI] [PubMed] [Google Scholar]
- 51.Mullens AB, McCaul KD, Erickson SC, Sandgren AK. Coping after cancer: risk perceptions, worry, and health behaviors among colorectal cancer survivors. Pyschooncology. 2004;13:367–376. doi: 10.1002/pon.751. [DOI] [PubMed] [Google Scholar]
- 52.Leventhal H, Leventhal EA, Cameron L. Representations, procedures, and affect in illness self-regulation: A perceptual-cognitive model. In: Baum A, Revenson TA, Singer JE, editors. Handbook of Health Psychology. Lawrence Erlbaum Associates; Mahwah, New Jersey: 2001. pp. 19–47. [Google Scholar]
- 53.Davey GCL. A comparison of three cognitive appraisal strategies: The role of threat in devaluation of problem-focused coping. Per Individ Dif. 1993b;14:535–546. [Google Scholar]
- 54.Edwards N, Jones D. Uptake of breast cancer screening in older women. Age Ageing. 2000;29:131–5. doi: 10.1093/ageing/29.2.131. [DOI] [PubMed] [Google Scholar]
- 55.McCaul KD, Branstetter AD, O’Donnell SM, Jacobson K, Quinlan KB. A descriptive study of breast cancer worry. J Behav Med. 1998;21:565–579. doi: 10.1023/a:1018748712987. [DOI] [PubMed] [Google Scholar]
- 56.Caplan LS, Helzlsouer KJ, Shapiro S, Wesley MN, Edwards BK. Reasons for delay in breast cancer diagnosis. Prev Med. 1996;25:218–224. doi: 10.1006/pmed.1996.0049. [DOI] [PubMed] [Google Scholar]
- 57.Millar MG, Millar K. Negative affective consequences of thinking about disease detection behaviors. Health Psychol. 1995;14:141–146. doi: 10.1037//0278-6133.14.2.141. [DOI] [PubMed] [Google Scholar]
- 58.Hopwood P. Breast cancer risk perception: what do we know and understand? Breast Cancer Res. 2000;2:387–391. doi: 10.1186/bcr83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahdy N, Fatohy IM, Mounir GM, et al. Assessment of students’ knowledge, attitude, and practice concerning cancer and its prevention. Part I. J Egypt Public Health Assoc. 1998;73:399–431. [PubMed] [Google Scholar]
- 60.Pohls UG, Renner SP, Fasching PA, et al. Awareness of breast cancer incidence and risk factors among healthy women. Eur J Cancer Prev. 2004;13:249–256. doi: 10.1097/01.cej.0000136718.03089.a5. [DOI] [PubMed] [Google Scholar]
- 61.Bashore L. Childhood and adolescent cancer survivors’ knowledge of their disease and effects of treatment. J Pediatr Oncol Nurs. 2004;21:98–102. doi: 10.1177/1043454203262754. [DOI] [PubMed] [Google Scholar]
- 62.Byrne J, Lewis S, Halamek L, et al. Childhood cancer survivors’ knowledge of their diagnosis and treatment. Ann Intern Med. 1989;110:400–3. doi: 10.7326/0003-4819-110-5-400. [DOI] [PubMed] [Google Scholar]
- 63.Caprino D, Wiley TJ, Massimo L. Childhood cancer survivors in the dark. J Clin Oncol. 2004;22:2748–50. doi: 10.1200/JCO.2004.07.153. [DOI] [PubMed] [Google Scholar]