Abstract
Vitamin D metabolites are important effectors of bone and mineral homeostasis. Human bone marrow stromal cells (hMSCs) are targets of 1α,25-dihydroxyvitamin D [1α, 25(OH)2D] action to promote their differentiation to osteoblasts. Osteoblastogenesis is also stimulated by 25-hydroxyvitamin D [25(OH)D], an effect that requires conversion to 1α, 25(OH)2D3 by 25-hydroxyvitamin D3 1α-hydroxylase (CYP27B1). These findings support an autocrine/paracrine role of vitamin D metabolism in osteoblastogenesis of hMSCs. In this study, we assessed whether and by what mechanisms osteoblastogenesis could be rejuvenated with hMSCs from elders. First, knockdown studies with VDR-siRNA showed that both the pro-differentiation and anti-proliferative effects of 1α, 25(OH)2D3 required VDR. Second, 100 nM 25(OH)D3 (p<0.01 vs. control, ANOVA) and 100 nM PTH1-34 (p<0.05) significantly stimulated alkaline phosphatase activity (a measure of osteoblastogenesis), with a synergistic effect when combined (p<0.001). Scriptaid, an inhibitor of histone deacetylase, blocked the effect of 25(OH)D3 and PTH on osteoblastogenesis. Scriptaid alone downregulated VDR in hMSCs. These data demonstrate that histone deacetylation is required for the synergistic effect of 25(OH)D3 and PTH on osteoblastogenesis in hMSCs. Both VDR siRNA and Scriptaid dowregulated VDR mRNA and inhibited osteoblastogenesis. Thus, epigenetic regulation of the VDR may be central to rejuvenating osteoblastogenesis in hMSCs from elders.
Keywords: Vitamin D, Vitamin D Receptor, Parathyroid Hormone, Epigenetic Regulation, Osteoblastogenesis, Human Marrow Stromal Cells
1. Introduction
Vitamin D metabolites are important effectors of bone and mineral homeostasis. Classically, 1α,25-dihydroxyvitamin D [1α, 25(OH)2D] mediates its actions through activation of the Vitamin D Receptor (VDR), a ligand-dependent transcription factor. Human bone marrow stromal cells (hMSCs) are a target of 1α, 25(OH)2D action to promote their differentiation to osteoblasts [1]. Osteoblastogenesis is also stimulated by 25-hydroxyvitamin D [25(OH)D3] [2], an effect that requires conversion to 1α, 25(OH)2D3 by 25-hydroxyvitamin D3 1α-hydroxylase (CYP27B1) [3]. CYP27B1 in hMSCs is upregulated by 25OHD [2], IGF-I [2], and PTH [4] and is downregulated by 1α, 25(OH)2D [2] and with age of the subject [4]. These findings support an autocrine/paracrine role of vitamin D metabolism in osteoblastogenesis of hMSCs. There are striking age-associated declines in baseline osteoblast differentiation of hMSCs [4–7], as well as in in vitro stimulation of osteoblastogenesis by 1α, 25(OH)2D3 [8], 25(OH)D3 [4], and PTH (1-34) [9]. The combination of 25(OH)D3 and PTH-pretreatment, however, rejuvenated osteoblast differentiation in hMSCs from elders [4]. The studies herein test the hypotheses 1) that VDR is required for actions of 1α, 25(OH)2D3 in hMSCs, 2) that simultaneous treatment with PTH and 1α, 25(OH)2D3 stimulates osteoblastogenesis, and 3) that an epigenetic mechanism mediates the rejuvenation of osteoblastogenesis by a combination of 25(OH)D3 and PTH.
2. Materials and methods
2.1 Human Marrow Stromal Cells (hMSCs)
Bone marrow samples were obtained with IRB approval as femoral tissue discarded during primary hip arthroplasty for osteoarthrosis, with exclusion criteria and isolation methods as described previously [2]. The hMSCs were maintained in phenol red-free α-MEM, 10% fetal bovine serum-heat inactivated (FBS-HI), 100 u/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA).
2.2 RNA interference with VDR-siRNA
Transient tranfection of siRNA into hMSCs was performed by electroporation with the Human MSC Nucleofector Kit (Lonza/Amaxa Biosystems) with either VDR siRNA (Invitrogen) or non-silencing control siRNA (a non-homologous, scrambled sequence equivalent) (Santa Cruz Biotech., CA) according to the manufacturer’s instructions and as described [3]. For proliferation assays, cells were transferred to 12-well plates in growth medium ± 10 nM 1α, 25(OH)2D3 for 3 days. Cells were re-suspended with 0.05% trypsin-EDTA (Invitrogen), and cell number was determined by hemacytometer. For differentiation assays, cells were cultured until confluent in 60-mm dishes and treated ± 10 nM 1,25(OH)2D3 in osteoblastogenic medium (α-MEM with 1% FBS-HI, 100 U/ml penicillin, 100 μg/ml streptomycin, 10−8 M dexamethasone, 5 mM β-glycerophosphate, 50 μg/ml ascorbate-2-phosphate) for 3 days and assayed for ALP gene expression by RT-PCR [3].
2.3 Osteoblast differentiation
Upon confluence of passage 2 hMSCs, medium was changed to osteoblastogenic medium± treatments. As indices of osteoblast differentiation, alkaline phosphatase (ALP) enzyme activity at day 7 was measured as described [6]. Previous studies showed that, with various stimuli, changes in ALP activity corresponded to changes in osteoblastic genes and mineralization [2–4, 9]. Scriptaid (10 μM) was added 45 minutes before the hormone treatments._Reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
2.4 Statistical analysis
Experiments were performed at least in triplicate. Group data are presented as Mean ± SEM, unless otherwise indicated. Quantitative data were analyzed with non-parametric tools; if data allowed, parametric tools were used, either t-test for two group or one-way ANOVA for multiple group comparisons.
3. Results
3.1 VDR is required for anti-proliferative and pro-osteoblastogenic effects of 1,25(OH)2D3 in adult human mesenchymal stem cells
Transfection with VDR-siRNA resulted in reduced VDR expression (Fig. 1A), with no effect on viability (Fig. 1B). In a short-term assay of proliferation of hMSCs that had been transfected with control siRNA (NC), the number of cells after exposure to 1α, 25(OH)2D3 for 3 days was 65.8 ± 3.3% of cells not exposed to 1,25(OH)2D3 (p<0.0001, Fig. 1C). In sharp contrast, the number of cells that had been transfected with VDR-siRNA was not affected by exposure to 1α, 25(OH)2D3 (103.1 ± 1.0%, compared with those not exposed to 1α, 25(OH)2D3) (Fig. 1C). Although cultures looked similar (Fig 1B), there were fewer cells with VDR siRNA compared with control siRNA (Fig. 1C); this may be related to information that short interfering RNAs can sequence-dependently induce unexpected and divergent changes in the levels of untargeted proteins, such as p53 and p21, in mammalian cells [10]. As expected, in control hMSCs, 1α, 25(OH)2D3 stimulated ALP gene expression (160% of untreated, Fig 1D). In contrast, in hMSCs that had been transfected with VDR-siRNA, there was no stimulation of ALP by 1α, 25(OH)2D3 (90% compared with those not exposed to 1α, 25(OH)2D3).
Fig. 1.
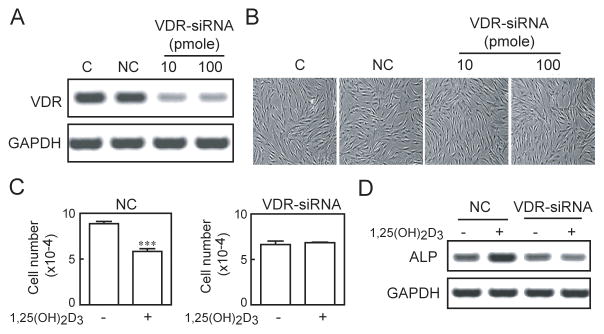
Requirement for VDR in the anti-proliferative and pro-osteoblastogenic effects of 1α, 25(OH)2D3 in hMSCs. A) Knockdown of VDR gene expression was achieved with 10 or 100 pmole of VDR-siRNA, compared with non-silencing control siRNA (NC). B) There was no visible difference in cellularity with the method. C) Proliferation of control hMSCs (NC) was inhibited with 1α, 25(OH)2D3 (10 nM, ***p<0.0001), but there was no inhibitory effect of 1α, 25(OH)2D3 in cells transfected with 100 pmole of VDR-siRNA (p=0.62). D) Gel electrophoretogram shows that stimulation of ALP gene expression by 1α, 25(OH)2D3 (10 nM, 3 days in osteoblastogenic medium) in control hMSCs (NC) was not present in cells transfected with VDR-siRNA (100 pmole).
3.2 CYP27B1/1α-hydroxylase is required for 25(OH)D3 to stimulate osteoblastogenesis in hMSCs
Human MSCs were cultured for 7 days in osteoblastogenic medium ± 25(OH)D3 (10 nM) ± cytochrome P450 inhibitor ketoconazole (10 μM). There was significant stimulation of osteoblastogenesis by 25(OH)D3, but not in the presence of ketoconozole (Fig. 2A). The inhibition of alkaline phosphatase activity by ketoconozole alone may be due to its inhibition of other enzymes [11] or of hydroxylation of endogenous vitamin D in serum [12].
Fig. 2.
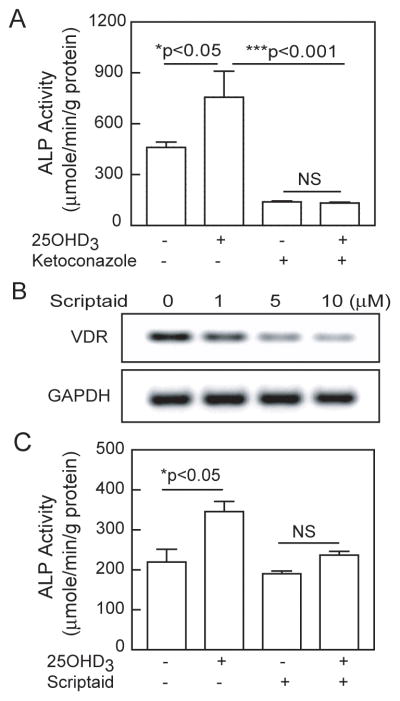
Roles of 1α-hydroxylase and histone deacetylases in stimulation of osteoblastogenesis by 25(OH)D3. A) After 7 days treatment, Ketoconazole (10 μM) blocked the stimulation of ALP activity by 25(OH)D3 (10 nM) in hMSCs (p<0.05, 25(OH)D3 vs. control; p<0.001, 25(OH)D3 vs. 25(OH)D3 & Ketoconazole; NS, not significant, Ketoconazole vs. 25(OH)D3 & Ketoconazole; n=4, ANOVA). B) Electrophoretogram shows a dose-dependent decrease in VDR expression by Scriptaid in hMSCs from a 61-year-old man (24 hours). C) Stimulation of ALP by 25(OH)D3 was blocked by Scriptaid (10 μM), a histone deacetylase inhibitor (p<0.05, 25OHD3 vs. control; NS, Scriptaid vs. 25OHD3 & Scriptaid; n=4, ANOVA).
3.3 Histone deacetylase is required for 25(OH)D3 to stimulate osteoblastogenesis in hMSCs
We tested the effect of Scriptaid, an inhibitor of histone deacetylase (HDAC), on constitutive VDR gene expression in hMSCs from a 61-year-old man. There was a dose-dependent down-regulation of the VDR by Scriptaid (Fig. 2B).
In an experiment on regulation of osteoblast differentiation, hMSCs were cultured for 7 days in osteoblastogenic medium ± 25(OH)D3 (10 nM) ± Scriptaid. Stimulation of ALP activity by 25(OH)D3 (158% relative to control, p<0.05) was blocked by Scriptaid (Fig. 2C).
3.4 Histone deacetylation mediates the synergistic stimulation by PTH (1-34) and 25(OH)D3 on osteoblastogenesis in hMSCs
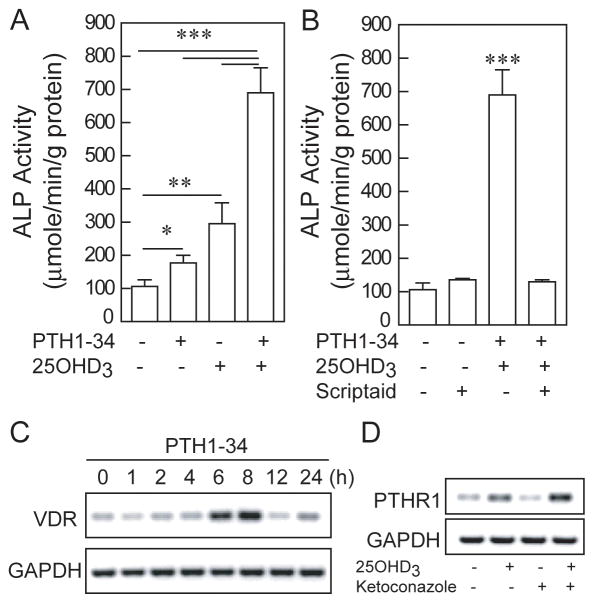
To test the effect of simultaneous treatment of hMSCs with PTH and 25(OH)D3 on osteoblastogenesis, we treated hMSCs from a 76-year-old man with PTH (1-34) (100 nM) and/or 25OHD3 (100 nM) in osteoblastogenic medium for 7 days (Fig. 3A). There was 170% more ALP activity with PTH (1-34) (p<0.05, compared with control) and 280% more with 25(OH)D3 (p<0.01). The simultaneous combination of 25(OH)D3 and PTH (1-34) resulted in 650% of control levels of ALP, with statistical significance compared with all the other groups (p<0.001).
Fig. 3.
Role of histone deacetylation in the synergistic effects of 25(OH)D3 and PTH1-34 on osteoblastogenesis in hMSCs from elders. A) There was stimulation of Alkaline Phosphatase activity in hMSCs from a 76-year-old man by PTH (1-34) (100 nM, *p<0.05) and by 25(OH)D3 (100 nM, **p<0.01, and with greater stimulation by the simultaneous combination of PTH and 25(OH)D3 than with either alone (***p<0.001). B) Treatment with Scriptaid (10 μM) inhibited the stimulation of 25(OH)D3 & PTH on osteoblastogenesis. Electrophoretograms show C) that PTH (1-34) (100 nM) upregulated VDR mRNA at 6–8 hours of treatment, and D) that 25(OH)D3 (10 nM) upregulated PTHR1 mRNA and that ketoconazole (10 μM) did not blocked the upregulation of PTHR1 by 25(OH)D3 in hMSCs from a 72-year-old woman (24 hours).
Blocking histone deacetylases with Scriptaid inhibited the synergistic stimulation of PTH and 25(OH)D3 on osteoblastogenesis (Fig. 3B). These findings indicate that chromatin deacetylation is required for their synergistic effect on osteoblastogenesis in hMSCs.
Further studies on interactions between 25(OH)D3 and PTH revealed that PTH (1-34) upregulated VDR gene expression (Fig. 3C) and that 25(OH)D3 (10 nM) upregulated PTHR1 gene expression (Fig 3D). The effect of 25(OH)D3 on PTHR1 mRNA appeared to be a direct effect that did not require CYP27B1; the cytochrome P450 inhibitor ketoconazole (10 μM) did not inhibit the up- regulation of PTHR1 by 25(OH)D3. These data about interactions of 25(OH)D3 and PTH with their mutual receptors add information about the mechanisms of their synergy in promoting osteoblastogenesis in hMSCs from elders.
4. Discussion
These studies indicate 1) that the steroid hormone nuclear VDR is required for the pro-differentiation and anti-proliferative actions of 1α, 25(OH)2D3 in hMSCs and 2) that histone deacetylation mediates the rejuvenation of osteoblastogenesis by a synergizing combination of 25(OH)D3 and PTH. Studies with differentiated osteoblasts have shown that many [13] but not all actions of 1α, 25(OH)2D3 on them are mediated via genomic actions of the classical VDR [14]. Many rapid effects of 1α, 25(OH)2D3 and, in fact, 25(OH)D3 occur via membrane-associated receptors [15].
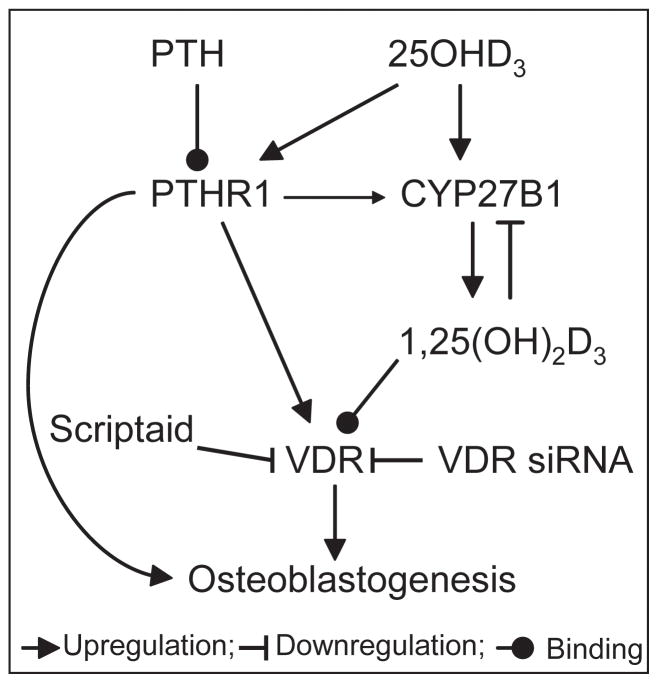
We previously determined that pretreatment of hMSCs with PTH (1-34) upregulated CYP27B1 and rendered hMSCs from elders responsive to the pro-osteoblastogenic effects of 25(OH)D3 [4]. We show herein that simultaneous treatment with PTH and 25(OH)D3 had a synergistic effect on stimulating osteoblastogenesis and that histone deacetylation was required for that rejuvenation. The VDR may be central in this epigenetic regulation (Fig. 4), as suggested by Scriptaid’s inhibition of VDR and inhibition of stimulation of osteoblastogenesis by 25(OH)D3.
Fig. 4.
A summary scheme proposing the role of VDR in the interacting effects of PTH and 25(OH)D3 on osteoblastogenesis in hMSCs.
These studies add further support for the view that vitamin D metabolism in hMSCs serves an autocrine/paracrine role in osteoblast differentiation. The first lines of evidence showed that 1α-hydroxylation was required for the effects of 25(OH)D3 [3] and that PTH promoted the biosynthesis of 1α, 25(OH)2D3 in hMSCs from elders [4]. This study shows that osteoblast differentiation with hMSCs from elders was stimulated by simultaneous treatment with 25(OH)D3 and PTH, with each hormone stimulating the receptor for the other. The pro-osteoblastogenic effects of 25(OH)D3 alone and when combined with PTH were prevented by Scriptaid, which also had a direct effect to down-regulate VDR gene expression.
There is other information about in vivo interactions between PTH and vitamin D metabolism. Samadfam et al. found greater increases in bone density by PTH in mice that had an intact 1α, 25(OH)2D-synthesizing system [16]. The concept that PTH and vitamin D interact to potentiate osteoblast differentiation is supported by an analysis of factors associated with heterogeneity in skeletal response to clinical PTH therapy for osteoporosis [17]. Of all variables examined, only the change in serum 1α, 25(OH)2D explained larger gains in bone density in response to PTH. More information about the molecular mechanisms of the osteoanabolic effects of PTH may help to reduce the variability seen in its clinical efficacy to increase bone density [18].
These studies demonstrate that histone deacetylation was required for the synergistic effect of 25(OH)D3 and PTH on osteoblastogenesis in hMSCs and begin to unravel mechanisms of skeletal aging. Epigenetic regulation of the VDR may be central to rejuvenating osteoblastogenesis.
Highlights.
The pro-differentiation and anti-proliferative effects of 1α, 25(OH)2D in human marrow stromal cells (MSCs) required VDR.
With age there are declines in osteoblast differentiation of human MSCs and in its stimulation by 25(OH)D.
25(OH)D3 and PTH act synergistically to stimulate osteoblastogenesis in MSCs from elders.
Histone deacetylation is required for the synergistic effect of 25(OH)D3 and PTH.
Epigenetic regulation of VDR may rejuvenate osteoblastogenesis in hMSCs of elders.
Acknowledgments
These studies were supported by grants from the National Institutes of Health R01 AG025015 and R01 AG028114 (J.G.), the China Scholarship Council (S.G.), the American Federation for Aging Research grant A09052 (S.Z.), and the BWH-BRI Fund (S.Z.). Discarded marrow was obtained and studied with approval and annual review from the Partners Human Research Committee.
Abbreviations
- 1α
25(OH)2D3, 1α,25-dihydroxyvitamin D3
- 25(OH)D3
25-hydroxyvitamin D3
- hMSCs
Human Marrow Stromal Cells
- PTH
Parathyroid Hormone
- VDR
Vitamin D Receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shuanhu Zhou, Email: szhou@rics.bwh.harvard.edu.
Shuo Geng, Email: shuogeng.hmu@gmail.com.
Julie Glowacki, Email: jglowacki@rics.bwh.harvard.edu.
References
- 1.Liu P, Oyajobi BO, Russell RG, Scutt A. Regulation of osteogenic differentiation of human bone marrow stromal cells: interaction between transforming growth factor-α and 1,25(OH)2 vitamin D3 in vitro. Calcif Tissue Int. 1999;65:173–180. doi: 10.1007/s002239900678. [DOI] [PubMed] [Google Scholar]
- 2.Zhou S, LeBoff MS, Glowacki J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinol. 2010;151:14–22. doi: 10.1210/en.2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geng S, Zhou S, Glowacki J. Effects of 25-hydroxyvitamin D3 on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1α-hydroxylase. J Bone Mineral Res. 2011;26:1145–1153. doi: 10.1002/jbmr.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geng S, Zhou S, Glowacki J. Age-related decline in 1α-hydroxylase/CYP27B1 and osteoblasto-genesis in human mesenchymal stem cells; Stimulation by PTH. Aging Cell. 2011;10:962–971. doi: 10.1111/j.1474-9726.2011.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–590. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 6.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Mineral Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 8.Zhou S, Glowacki J, Kim SW, Hahne J, Geng S, Mueller SM, Shen L, Bleiberg I, Leboff MS. Clinical characteristics influence in vitro action of 1,25-dihydroxyvitamin D(3) in human marrow stromal cells. J Bone Mineral Res. 2012 May 10; doi: 10.1002/jbmr.1655. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou S, Bueno EM, Kim SW, Amato I, Shen L, Hahne J, Bleiberg I, Morley P, Glowacki J. Effects of age on parathyroid hormone signaling in human marrow stromal cells. Aging Cell. 2011;10:780–788. doi: 10.1111/j.1474-9726.2011.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scacheri PC, Rozenblatt-Rosen O, Caplen NJ, Wolfsberg TG, Umayam L, Lee JC, Hughes CM, Shanmugam KS, Bhattacharjee A, Meyerson M, Collins FS. Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proc Natl Acad Sci USA. 2004;101:1892–7. doi: 10.1073/pnas.0308698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khojasteh SC, Prabhu S, Kenny JR, Halladay JS, Lu AY. Chemical inhibitors of cytochrome P450 isoforms in human liver microsomes: a re-evaluation of P450 isoform selectivity. Eur J Drug Metab Pharmacokinet. 2011;36:1–16. doi: 10.1007/s13318-011-0024-2. [DOI] [PubMed] [Google Scholar]
- 12.Sacco RE, Nonnecke BJ, Palmer MV, Waters WR, Lippolis JD, Reinhardt TA. Differential expression of cytokines in response to respiratory syncytial virus infection of calves with high or low circulating 25-hydroxyvitamin D3. PLoS One. 2012;7:e33074. doi: 10.1371/journal.pone.0033074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walters MR, Rosen DM, Norman AW, Luben RA. 1,25-Dihydroxyvitamin D receptors in an established bone cell line. Correlation with biochemical responses. J Biol Chem. 1982;257:7481–7484. [PubMed] [Google Scholar]
- 14.Willems HM, van den Heuvel EG, Carmeliet G, Schaafsma A, Klein-Nulend J, Bakker AD. VDR dependent and independent effects of 1,25-dihydroxyvitamin D3 on nitric oxide production by osteoblasts. Steroids. 2012;77:126–131. doi: 10.1016/j.steroids.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Menegaz D, Mizwicki MT, Barrientos-Duran A, Chen N, Henry HL, Norman AW. Vitamin D receptor (VDR) regulation of voltage-gated chloride channels by ligands preferring a VDR-alternative pocket (VDR-AP) Mol Endocrinol. 2011;25:1289–1300. doi: 10.1210/me.2010-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samadfam R, Xia Q, Miao D, Hendy GN, Goltzman D. Exogenous PTH and endogenous 1,25-dihydroxyvitamin D are complementary in inducing an anabolic effect on bone. J Bone Mineral Res. 2008;23:1257–1266. doi: 10.1359/jbmr.080318. [DOI] [PubMed] [Google Scholar]
- 17.Sellmeyer DE, Black DM, Palermo L, Greenspan S, Ensrud K, Bilezikian J, Rosen CJ. Heterogeneity in skeletal response to full-length parathyroid hormone in the treatment of osteoporosis. Osteoporosis Int. 2007;18:973–979. doi: 10.1007/s00198-007-0336-x. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz P, Jorgensen NR, Mosekilde L, Vestergaard P. Effects of increasing age, dosage, and duration of PTH treatment on BMD increase--a meta-analysis. Calcif Tissue Int. 2012;90:165–173. doi: 10.1007/s00223-011-9564-3. [DOI] [PubMed] [Google Scholar]