Abstract
Background
Past findings on gene-by-environment (G × E) effects on depression have been mixed, leading to a debate of the plausibility of such mechanisms and methodological considerations that warrant attention. A developmental systems perspective postulates that complex, multi-level G × E effects are likely contributors to depression.
Methods
Participants from families experiencing low-income status at birth were followed over 28 years. Maltreatment was recorded prospectively using multiple means and sources. Depression was measured repeatedly using well-validated interviews in middle childhood, through adolescence, and into adulthood.
Results
Findings support a G × E effect where the less efficient form of the promoter region of the serotonin transporter gene (5-HTTLPR) contributes to a vulnerability to depressogenic aspects of maltreatment in childhood and adolescence. The presence of less efficient forms of the 5-HTTLPR polymorphism and maltreatment together raised risk for depression. This G × E effect was present generally and also among those who reported clinical levels of depression at only one point in time.
Limitations
This study used a low-income sample which limits generalizability to other populations. Sample size and rates of different forms of depression and depression at individual developmental stages supported general analyses, but limited the sorts of specific sub-analyses that were possible.
Conclusions
These findings support the plausibility of G × E effects on depression during childhood, adolescence, and early adulthood, key periods for the development of depression. Ongoing debates about the presence of G × E effects would be well served by additional work that was theoretically informed and employed prospective, longitudinal methodologies with well-validated measures of key constructs.
Keywords: Gene-by-environment interaction, Child maltreatment, 5-HTTLPR, Depression, Childhood, adolescence, and adulthood
1. Introduction
This study tests for a gene by environment interaction effect (G × E) where the effective short form of an insertion/deletion polymorphism of the promoter region of the serotonin transporter gene (5-HTTLPR; SLC6A4) contributes to a vulnerability to depressogenic aspects of child maltreatment. Consistent with a developmental-systems perspective, we expected maltreatment during childhood and adolescence would be especially linked to depression among individuals with functionally less efficient serotonin-related genotypes given that these are periods of rapid development and plasticity. This study involves 157 low-income participants followed longitudinally from birth through age 28 years. The methods and analyses address concerns with past research on G × E effects through careful and repeated assessment of both child maltreatment and depression, thereby minimizing multiple forms of reporter bias. We extend the literature through greater attention to key periods of childhood, adolescence, and early adulthood, important in explanations of the development of depression.
Depression is a relatively prevalent disorder characterized by mood dysfunction, biased cognitions and attributions, and somatic symptoms, often in response to experiencing life stress (Hankin, 2006). Depressive disorders have been conceptualized in many ways, including as a perturbation of stress response systems. Earlier thought on the canalization of behavioral characteristics, like depression, followed various forms of genetic determinism whereby the individual’s developmental pathway unfolds as a product of their genetic endowment to produce an eventual phenotype (see Scarr-Salapatek, 1976; Waddington, 1942). Contemporary developmental science has largely abandoned deterministic approaches in favor of a systems perspective whereby developmental pathways and the resulting phenotypes are probabilistically influenced by factors across many levels of analysis, including genetics and molecular mechanisms, physiological structures and functioning, psychological abilities, family/systems, and the broader context (Bronfenbrenner, 1976; Gottlieb, 1991).
Systems approaches acknowledge that genetic factors coact, transact, and interact with nongenetic factors over time to influence the functioning of stress response systems and the manifestation of depression. Repeated or prolonged exposure to stress, especially child maltreatment, is a risk factor for depression and for lasting disruptions in related physiological stress response systems (Cicchetti and Rogosch, 2001; Cicchetti et al., 2010a,b, 2011a,b; Gunnar and Vazquez, 2006; Heim et al., 2000). The stress–depression link appears to be particularly salient for stress during periods of rapid physiological development, such as during childhood and adolescence.
Child maltreatment represents a failure of the caregiving system accompanied by adverse and stressful relationship experiences (Belsky, 1993; Cicchetti et al., 2010a,b; Cicchetti and Valentino, 2007). Furthermore, children who experience maltreatment tend to develop in contexts of relationships that lack the warmth, structure, support, and nurturance that promote robust coping and other adaptive resources, placing them at risk for a number of poor outcomes over the lifespan (Cicchetti and Valentino, 2007). Following, childhood maltreatment has been associated with clinical levels of depression in childhood (Toth et al., 1992), adolescence (Hussey et al., 2006), and adulthood (Brown et al., 1999). This is in line with a developmental perspective where earlier experiences and developmental competence (or failure) initiate pathways that probabilistically lead to later adaptation or maladaptation as part of a complex interplay of factors across levels of the individual and her context (Cicchetti and Toth, 1998; Cicchetti and Valentino, 2007; Gottlieb, 1991; Yates et al., 2003). While maltreatment increases the risk for depression, most maltreated children do not become depressed. A systems perspective accounts for this heterogeneity since factors at other levels of analysis (e.g., genotype) contribute in complex fashion to the overall likelihood of an individual’s positive adaptation or maladaptation.
Differences in the promoter region of the serotonin transporter gene (5-HTTLPR) have been conceptualized as a marker for a stress-vulnerable phenotype through the contribution of 5-HTTLPR on serotonin functioning. There are 2 forms of a functional insertion/deletion polymorphism that have been studied with respect to psychiatric outcomes: a 16 unit repeat “long” (L) form and a 14 unit repeat “short” (S) form. The short variant has been linked to decreased transcriptional efficiency and reduced levels of serotonin (Heils et al., 1996). There is also an A/G single-nucleotide polymorphism in the long form (denoted LG; rs25531) that results in functioning similar to the short form (Hu et al., 2005). Given the links with the serotonin system, and links between reduced serotonin functioning and depression, 5-HTTLPR polymorphisms are a potential candidate gene for etiological models of major depression. Nevertheless, past work testing direct effects of the 5HTTLPR S-polymorphism on depression outcomes have produced mixed findings (Collier et al., 1996; Lesch, 2003; Mendlewicz et al., 2004).
Gene-by-environment interaction approaches that incorporate both 5-HTTLPR variation and psychosocial stress have emerged as a promising avenue, given that stress has been robustly linked to depression for many, but not deterministically so for all. When found, variation in 5-HTTLPR genotype confers increased risk for depression among individuals who also experience high levels of stress, particularly childhood maltreatment (Caspi et al., 2010, 2003; Karg et al., 2011; Uher and McGuffin, 2010). However, G × E approaches have produced inconsistent findings including both affirmative and negative meta-analyses (Duncan and Keller, 2011; Karg et al., 2011; Munafo et al., 2009, 2010; Risch et al., 2009; Uher and McGuffin, 2010). This inconsistency has led to greater scrutiny of G × E findings and renewed questions about the plausibility of G × E mechanisms in the etiology of depression. For example, in a widely cited meta-analysis Risch et al. (2009) failed to support a G × E effect for 5-HTTLPR variation and life stress. However, their effort has been criticized for combining studies that employed a range of methodological approaches (e.g., 8 of 14 studies considered were cross-sectional versus 6 prospective designs) with different populations, few considering depression before adulthood (3 of 15 studies), and none involving children (Caspi et al., 2010; Dunn et al., 2011; Rutter et al., 2009). Other reviews and meta-analyses have generally confirmed the G × E effect when attention is paid to differences in study methods (e.g., Uher and McGuffin, 2010) and when focusing on children, adolescents, and young adults (e.g., Dunn et al., 2011). This has led to a general call for additional G × E work that involves theoretically informed perspectives, recognition of potential differences for children and youth versus adults, and increased attention to measurement issues in the assessment of stress across the lifespan (Caspi et al., 2010; Dunn et al., 2011; Rutter et al., 2009; Uher and McGuffin, 2010).
In particular, researchers have repeatedly called attention to potential sources of bias and imprecision in the assessment of stress and other aspects of the environment (Caspi et al., 2010; Rutter et al., 2009; Uher and McGuffin, 2010). Two issues have come to the fore: (1) potential biases of omission when brief self-report measures of life stress are used, and (2) enhanced recall biases for negative events among depressed versus nondepressed individuals (Caspi et al., 2010; Uher and McGuffin, 2010). These issues become exacerbated when individuals are asked to make retrospective accounts that span many years or decades. In support of this point of view, G × E effects are more robust among studies considering more objective sources of major childhood stress, such as child maltreatment, when compared to less objective or less reliable assessments of life stress (Caspi et al., 2010; Karg et al., 2011).
Recent work has also suggested that the G × E effect for child maltreatment and 5-HTTLPR genotype applies only to certain patterns of depression over time. Using two large longitudinal datasets, Uher et al. (2011) found that the G × E effect emerged only for depression that was persistent or involved multiple episodes. The effect was not found for single-episode or nonpersistent presentations. Other work has cast chronic or persistent depression as having a higher heritability (McGuffin et al., 1996) and being more strongly linked to childhood maltreatment (Brown and Harris, 2008; Wiersma et al., 2009). Together, these findings further support the notion of a differential effect for single-episode versus persistent forms of depression, especially when considering genetic contributions and the impact of childhood maltreatment.
The current study tested for a G × E effect for depression in childhood, adolescence, and early adulthood while addressing methodological concerns identified in past work. First, we focused on prospectively measured child maltreatment as a more objective indicator of depressogenic environmental stress. Maltreatment status was observed through frequent and repeated observations, interviews, and record reviews elicited prospectively, further reducing subjective bias and the possibility of omitted records or recollections that are more likely with a follow-back or recall design. We also employed repeated measures of depression around key transitions during middle childhood, adolescence, and emerging adulthood. These measures included “gold standard” self-report clinical interviews during late adolescence and adulthood that show high reliability and validity.
We hypothesized that the experience of childhood maltreatment would moderate 5-HTTLPR genotype to increase risk for depression across childhood, adolescence, and early adulthood. The hypothesis followed an additive genetic risk model such that individuals who are effectively homozygous for the effective short allele (SS) and experienced maltreatment have the highest likelihood of developing depression, followed by those who have been maltreated and are heterozygous (LS), and those who are maltreated and effectively homozygous for the long allele (LL) will show the lowest risk among these three groups. We describe findings in an exploratory fashion for depression at each individual developmental period: middle childhood (age 8), late childhood through adolescence (age 8–17.5), and the transition to early adulthood (age 18–28).
As a secondary hypothesis, we hypothesized that the G × E effect is specific to persistent or recurrent depression, and such an effect does not apply to depression that is brief and unique to one development period (Brown and Harris, 2008; Uher et al., 2011). If such a stance is true, no GxE effect should be evident among individuals who report clinical levels of depression at only one point in time. We described findings separately for those with only one instance of depression and for those who reported repeated episodes of depression or depression at more than one assessment.
2. Methods
A subsample of participants from the Minnesota Longitudinal Study of Risk and Adaptation contributed data to the current study (Sroufe et al., 2005). This ongoing study originally recruited 267 primiparous women receiving public assistance from 1975 through 1977. Children continued to participate through childhood, adolescence, and early adulthood. Parents/Guardians consented to participation at each assessment when children were under 18 years old. Individuals gave assent to participate beginning in adolescence and provided informed consent as adults. Methods were approved by the Institutional Review Board of the University of Minnesota as required at each assessment. One hundred fifty eight participants were located and agreed to contribute genetic data at age 32 years. 5-HTTLPR genotype could not be obtained from one collected sample, resulting in a final sample of 157 participants for analysis. This represents 59% of the original pre-birth sample (157 out of 267). The most prominent reason for non-inclusion in this sub-sample was an inability to locate the individual for the assessment at age 32 when genotyping occurred. Most of this attrition occurred before the participants were 18-months old (77 participants; 28.8% of the original sample; 70.6% of non-included participants). Only 8 (3.0%) participants had participated after age 18-months and could not be located at age 32. Some participants refused to provide a biological sample (14.7% of non-included participants). Ten individuals (9.2% of non-included participants) were known to have died since the start of the study. The analysis subsample predominantly comprised white participants (66.9%), 19.7% multiracial, 8.9% African American, 3.2% Native American, and less than 1% were Hispanic or Asian American. The subsample was roughly half female (51.6%). Descriptive statistics for the analysis subsample are provided in Table 1.
Table 1.
Demographic statistics and rates of depression and childhood maltreatment.
| Count | Percentage | |
|---|---|---|
| Race/ethnicity | ||
| White | 105 | 66.88 |
| African American | 14 | 8.92 |
| American Indian | 5 | 3.18 |
| Hispanic | 1 | 0.64 |
| Asian | 1 | 0.64 |
| Multiracial | 31 | 19.75 |
| Sex | ||
| Male | 76 | 48.41 |
| Female | 81 | 51.59 |
| 5-HTTLPR genotype (effective) | ||
| S/S | 39 | 24.84 |
| L/S | 74 | 47.13 |
| L/L | 44 | 28.03 |
| Maltreatment | ||
| Age 0–4.5 years | 36 | 22.93 |
| Age 4.5–17.5 years | 50 | 31.85 |
| Ever | 64 | 40.76 |
| Depression | ||
| Age 8 | 25 | 15.92 |
| Age 8–17.5 years | 38 | 24.20 |
| Age 18–28 years | 35 | 22.29 |
| Ever | 73 | 46.50 |
| Persistent/recurrent | 26 | 16.56 |
2.1. Measures
Participants completed measures of depression at age 8 years (middle childhood), age 17.5 (late adolescence), and age 28 (early adulthood). Childhood maltreatment was assessed through age 17.5. Demographic characteristics (sex, age) were determined through birth records.
2.1.1. Age 8 depression
Participants completed the Children’s Depression Rating Scale (CDRS) as a self-report measure at the end of third grade, around the time they were 8 years old. This is a 14-item semi-structured interview that asked about depression symptomatology. Items covering different depression symptoms were rated for severity in a standard way using Likert scales (Poznanski et al., 1979). We used an established cutoff of greater or equal to a total score of 40 to denote clinical levels of depression symptoms. The CDRS has been shown to have good psychometric properties, including high reliability and validity in identifying children who qualify for depression diagnoses (Poznanski et al., 1983, 1985).
2.1.2. Age 17.5 depression
Participants completed a version of the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS), a semi-structured interview that is widely used and shows good psychometric properties when identifying clinical levels of depression symptoms in line with criteria from the Diagnostic and Statistical Manual of Mental Disorders, third edition, revised (DSM-III-R; Ambrosini et al., 1989). The KSADS includes a screening portion which indicates the administration of a more comprehensive depression module. Participants also reported the onset of symptoms and whether the symptoms occurred in the past or were present at the time of the interview. Participants were coded as showing clinical levels of depression for this measure if they reported any current or past clinical levels of symptoms since age 8 years relative to DSM-III-R criteria.
2.1.3. Age 28 depression
The Structured Clinical Interview for DSM Disorders (SCID) measured depression from age 18 through 28 years. The SCID is a widely-used, semi-structured interview with good psychometric properties for identifying clinical levels of psychiatric disorder symptoms using criteria from the fourth edition of the DSM (DSM-IV; First et al., 1997; Segal et al., 1994; Ventura et al., 1998). Participants reported on current or past depression symptoms since the age of 18, including whether they experienced multiple episodes of symptoms. Responses were compared to DSM-IV criteria for major depressive disorder (all single- and recurrent-episode diagnoses) and depressive disorder not otherwise specified, producing two variables: any depressive disorder (1=present) and nature of episodes (none; single; recurrent).
2.1.4. Aggregation of depression variables
We coded participants as having ever experienced depression (0=absent, 1=endorsed) if they indicated clinical levels of symptoms at age 8 via the CDRS, from age 8 through 17.5 on the KSADS, and/or from age 18 to 28 on the SCID. Furthermore, we divided participants into three mutually-exclusive groups: those who never evidenced clinical levels of symptoms, those who reported clinical levels at only one point in time, or those who reported persistent or recurrent clinical levels of depression symptoms (either through clinical levels at more than one assessment, or reporting more than one episode of clinical levels between assessments).
2.1.5. Childhood maltreatment
We defined maltreatment as any of the following: sexual abuse (genital contact with a person who was at least 5 years older), physical abuse (parental acts that result in physical harm to the child such as bruises, cuts, or burns), or neglect (insufficient or irresponsible management of day-to-day care, inadequate nutrition, or inadequate supervision by a caregiver). Maltreatment classification (Maltreated versus No Maltreatment) was determined across regular childhood and adolescent assessments using information from a number of sources around the time the maltreatment was occurring. Classification was determined through direct observation, caregiver interviews, reviews of child protection and medical records when available, and teacher interviews. This procedure produced two maltreatment classification variables covering the period of early childhood (birth to age 5 years) and later childhood through adolescence (age 5 through 17.5 years). Reliability of maltreatment classification was excellent (e.g., K’s range: 0.81–0.89 by maltreatment type). More information on procedures for the determination of child maltreatment are available elsewhere in the literature (Egeland, 1997; Shaffer et al., 2008; Yates et al., 2008). Maltreatment status was coded dichotomously for the purposes of the current analyses (0=absent, 1=experienced maltreatment at any point).
2.1.6. 5-HTTLPR Genotype
Participants provided buccal cells for genotyping in concert with a follow-up assessment at age 32 years. DNA was extracted using the conventional method with the Epicenter Buccal/Amp DNA extraction Kit to prepare DNA for polymerase chain reaction (PCR) amplification. Genotyping was conducted following previously published protocols (Cicchetti et al., 2011a,b).
DNA was whole-genome amplified using the Replig kit (Qiagen, Chatsworth, CA., Catalog no. 150043) per the kit instructions to ensure the availability of data over the long term for this valuable sample. Amplified samples were then diluted to a working concentration. The 5-HTTLPR samples were genotyped for fragment length polymorphisms of 5-HTTLPR with Hot Star Taq PCR Mix (Qiagen, Catalog no. 203205), and previously described primers (Gelernter, Kranzler, & Cubells, 1997), followed by fragment analysis using a CEQ8000 (Beckman-Colter, Inc., Fullerton, CA). Although genotypes with one or two short (S) alleles of the 5-HTTLPR gene are generally associated with lower transcription and function of 5-HTT protein in vitro (Bevilacqua and Goldman, 2011) than genotypes with two long (L) alleles, research identifying an A>G substitution in a SNP upstream from the promoter region has shown that LG functions more similarly to the S allele than the LA in its expression and binding potential (Praschak-Rieder et al., 2007; Reimold et al., 2007). Therefore, we used an effective triallelic approach to categorizing genotypes according to their relative efficiency in functioning: homozygous for the functionally long allele LA LA (28.0%; herein denoted as LL); an aggregated grouping of functionally heterozygous genotypes (47.1%; herein denoted as LS), specifically 7.6% LA LG, and 39.5% LAS; and an aggregated grouping of genotypes that are functionally homozygous for the short allele (24.8%; herein denoted as SS), specifically 0.6% LG LG, 4.5% LGS, 19.7% SS (Mileva-Seitz et al., 2011).
If a genotype could not be determined after the first run, then it was repeated up to four times. If the null result persisted, then a genotype was not assigned to that individual. The call rate for 5-HTTLPR was 99.4%. The genotype distribution for the Effective Triallelic 5-HTTLPR was in Hardy-Weinberg Equilibrium X2 (3, N=157)=1.47, β=39, ns.
DNA samples were genotyped in duplicate for quality control. Human DNA from cell lines was purchased from Coriell Cell Repositories for all representative genotypes in duplicate, and genotypes were confirmed by sequencing using DTCS chemistry on an ABI 3130 × 1. These and a no template control were run alongside study samples representing 9% of the total data output. Any samples that were not able to be genotyped to a 95% or greater confidence level were repeated under the same conditions.
2.2. Statistical analyses
Data were assumed to be Missing at Random (MAR; Schafer and Graham, 2002). Eighteen (11.5%) participants in the subsample lacked the age 8 depression score, 16 (10.2%) the age 17.5 assessment, 17 (10.8%) the age 28 assessment, and 21 (13.7%) were missing maltreatment classification. No participant lacked information on race or sex. Missing values were estimated using multiple imputation techniques using SPSS version 20. Twenty datasets were created using fully conditional specification, an iterative Markov Chain Monte Carlo method. Data from the entire original cohort (N=267) were included in the imputation procedure to produce the most robust estimation of missing values, but hypothesis testing involved only individuals with observed 5-HTTLPR genotype (n=157). Analyses were completed for each imputed dataset and results were combined in the fashion described by Rubin (1987).
Hypotheses were tested using separate binary logistic regressions. Terms representing child maltreatment and 5-HTTLPR genotype were entered in independent models (without the other variable nor the interaction term) predicting any episode of depression to describe respective main effects, and then a third model contained each main effect and their interaction. Follow-up binary logistic regressions elaborated on the significant interaction term to understand the nature of the effect. All significance tests evaluated directional hypotheses and were compared against critical values for 1-tailed alphas. We also reported coefficients for significance tests involving individual assessment points and for analyses involving only individuals who experienced persistent or recurrent depression. However, those coefficients should be interpreted with great caution because of concerns related to low statistical power and the possibilities of Type I and Type II error.
Hypotheses were tested in line with an additive model of genetic influence: genotype was represented by the number of effective triallelic S-alleles. Coefficients are also provided for alternative models of genetic influence: dominant sensitivity (effective triallelic SS and LS versus LL) and recessive sensitivity (effective triallelic SS versus LS and LL) effects. Reporting results from these alternative approaches is recommended in the literature and justified as none of the genetic models has been shown to outperform the others (Caspi et al., 2010; Uher et al., 2011; Uher and McGuffin, 2008).
3. Results
Variability in 5-HTTLPR genotype and rates of child maltreatment (64 individuals; 40.8%) and depression (73 individuals; 46.5%) were sufficient for analyses and are presented in Table 1. Very few individuals experienced repeated or persistent depression (26 individuals; 16.6%). Specific analyses involving just that subgroup should be interpreted with caution. Maltreatment status and the number of effective S alleles were unrelated suggesting no gene–environment correlation (t=0.55; 2-tailed p=0.58).
We first evaluated main effects of childhood maltreatment and 5-HTTLPR genotype predicting any clinical level of depression from age 8 through 28, controlling for sex and racial minority status. Main effects were tested in separate models. When predicting depression at any time, childhood maltreatment was not a significant predictor, nor was 5-HTTLPR genotype (all p’s>0.05). Follow-up analyses of depression at each age revealed that childhood maltreatment predicted clinical levels of depression at age 8 (Exp(B)=2.74; 95% CI: 1.05–7.14; p=0.02). There was a significant effect of 5-HTTLPR genotype on Persistent/Recurrent depression in additive (Exp(B)=1.98; 95% CI: 1.02–03.87; p=0.02) and recessive genetic models (Exp(B)=3.59; 95% CI: 1.34–9.62; p=0.006). See Table 2.
Table 2.
Independent main effects of maltreatment and of 5-HTTLPR genotype on depression.
| Maltreatment | 5-HTTLPR Genotype
|
|||
|---|---|---|---|---|
| Additive | Dominant | Recessive | ||
| Any depression | 1.39 (0.68–2.84) | 1.35 (0.84–2.19) | 1.44(0.65–3.19) | 1.58 (0.72–3.46) |
| Age 8 | 2.74 (1.05–7.14)* | 1.55 (0.80–3.02) | 1.25 (0.41–3.78) | 2.42 (0.93–6.28)* |
| Age 8–17.5 | 1.39 (0.61–3.19) | 1.59 (0.91–2.81) | 1.66 (0.64–4.30) | 2.14 (0.89–5.10)* |
| Age 18–28 | 1.38 (0.62–3.09) | 1.46 (0.84–2.53) | 1.72 (0.66–4.47) | 1.64 (0.69–3.90) |
| Persistent/recurrent | 1.94 (0.74–5.10) | 1.98 (1.02–3.87)* | 1.56 (0.50–4.84) | 3.59 (1.34–9.62)** |
| Single-episode | 1.16 (0.50–2.67) | 1.00 (0.56–1.76) | 1.23 (0.51–3.00) | 0.76 (0.27–2.12) |
Note: Coefficients represent odds ratios (95% CI); 5-HTTLPR genotype: for Dominant Genetic Model, 0=L/L, 1=L/S or S/S; for Recessive Genetic Model, 0=L/L or L/S, 1=S/S;
p (1-tailed)<0.05;
p (1-tailed)<0.01.
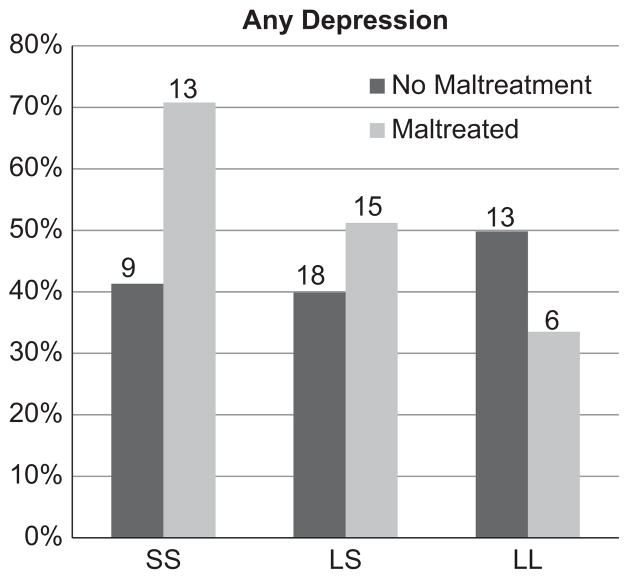
We tested an interaction term of childhood maltreatment and 5-HTTLPR genotype on experiencing depression at any point. The interaction term was a significant predictor (Additive Model: Exp(B)=2.66; 95% CI: 0.99–7.18; p=0.03; Dominant Model: Exp(B)=4.35; 95% CI: 0.89–21.32; p=0.04; Recessive Model: Exp(B)=3.28; 95% CI: 0.61–17.65; p=0.08). See Table 3 and Fig. 1. Follow-up analyses confirmed an effect of the number of short alleles on depression among individuals who experienced childhood maltreatment (Additive Model: Exp(B)=2.29; 95% CI: 1.06–4.95; p=0.02) but not among those who had not been maltreated (Additive Model: Exp(B)=0.92; 95% CI: 0.47–1.78; ns).
Table 3.
Maltreatment × 5-HTTLPR genotype interaction effects on depression.
| Additive | Dominant | Recessive | |
|---|---|---|---|
| Any depression | 2.66 (0.99–7.18)* | 4.35 (0.89–21.32)* | 3.28 (0.61–17.65) |
| Age 8 | 2.16 (0.53–8.89) | 1.04 (0.11–9.59) | 6.77 (0.57–81.03) |
| Age 8–17.5 | 1.76 (0.55–5.60) | 4.22 (0.52–34.33) | 1.24 (0.21–7.28) |
| Age 18–28 | 1.08 (0.37–5.74) | 1.20 (0.18–7.95) | 1.08 (0.19–6.04) |
| Persistent/recurrent | 1.46 (0.37–5.74) | 1.62 (0.16–16.84) | 2.19 (0.28–17.32) |
| Single-episode | 3.25 (0.92–11.54)* | 8.34 (1.11–62.86)* | 2.93 (0.31–27.99) |
Note: Coefficients represent odds ratios (95% CI); 5-HTTLPR genotype: for Dominant Genetic Model, 0=L/L, 1=L/S or S/S; for Recessive Genetic Model, 0=L/L or L/S, 1=S/S;
p (1-tailed)<0.05.
Fig. 1.
Maltreatment by 5-HTTLPR genotype and proportion of individuals who were depressed at any age.
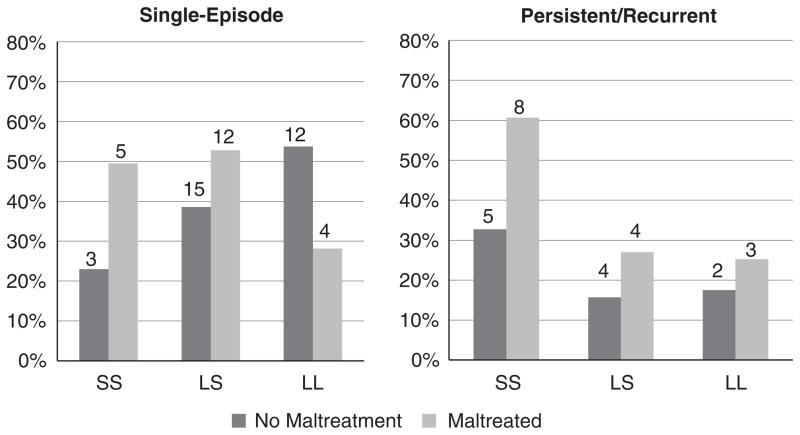
We tested for effects of G × E moderation on single-episode depression, excluding individuals who reported any recurrent-episode depression or depression in more than one developmental period. The interaction term predicted single-episode depression or depression at only one point in time compared to no depression in the additive and dominant models (Additive Model: Exp(B)=3.25; 95% CI: 0.92–11.54; p=0.03; Dominant Model: Exp(B)=8.34; 95% CI: 1.11–62.86; p=0.02; Recessive Model: Exp(B)=2.93; 95% CI: 0.31–27.99; p=0.18). Follow-up analyses revealed a more pronounced effect of 5-HTTLPR genotype among those who experienced childhood maltreatment (Additive Model: Exp(B)=1.99; 95% CI: 0.77–5.15; p=0.08). The effect of 5-HTTLPR genotype was non-significant among those who did not experience childhood maltreatment (Additive Model: Exp(B)=0.64; 95% CI: 0.28–1.48; ns). See Table 3 and Fig. 2. Coefficients for analyses testing for a G × E effect among individuals who experienced persistent/recurrent depression are also presented in Table 3. However, those coefficients should be interpreted with caution because of the possibilities of Type I and Type II error.
Fig. 2.
Maltreatment by 5-HTTLPR genotype and single-episode and persistent/recurrent depression.
4. Discussion
The current findings support a gene-by-environment interaction that contributes to the development of depression across childhood, adolescence, and early adulthood within this low-income sample. The short-version of the 5-HTTLPR polymorphism increased the risk for ever experiencing clinical levels of depression for those who had experienced maltreatment. In an additive genetic model, each version of the short 5-HTTLPR polymorphism increased the odds by about 129% that a maltreated child or adolescent would show clinical levels of depression. Meanwhile, each version of the short 5-HTTLPR polymorphism did not meaningfully influence the odds that non-maltreated individuals would show clinical depression. This affirms that a portion of the risk for developing depression operates as a function of both the presence or absence of maltreatment experiences and 5-HTTLPR genotype.
The expected gene-by-environment interaction also was present for individuals who reported only single-episode depression or depression at only one point in time. Among maltreated individuals, each copy of the short 5-HTTLPR polymorphism increased the odds of a single-episode of depression by about 99%, but 5-HTTLPR genotype did not meaningfully affect the odds for individuals who had not been maltreated. Parallel analyses for individuals reporting persistent or recurrent episodes of depression were non-significant (this is contrary to the extant literature), but should be interpreted with caution because of concerns related to small sample size. However, 5-HTTLPR genotype produced a main effect when predicting persistent or recurrent forms of depression, thereby possibly affirming the role of this genotype in the expression of more persistent forms of depression, albeit in a different way.
The gene-by-environment interaction for single-episode depression contrasts with recent work by Uher et al. (2011) who found a different pattern: the interaction effect emerged only for persistent/recurrent forms of depression and not among those with single-episode manifestations. The Uher analyses considered adult depression measured at relatively frequent intervals, raising the possibility that the longer duration between assessments in the current study may have contributed to recall errors and misclassification of individuals with depression that would have been considered persistent in the Uher study. Alternatively, and perhaps more likely, the interplay between genes and stressful environments may differ for children and adolescents, as well as differences for low-income groups. These views find support elsewhere in developmental behavior genetics (Thapar and McGuffin, 1996; Turkheimer et al., 2003). Similarly, family-environment factors predicted childhood depression symptoms in past analyses of the same longitudinal study where genotype was not considered, but maternal depression and suboptimal early care predicted adolescent depression symptoms (Duggal et al., 2001). Future work should include more frequent measures of depression in childhood, adolescence, and adulthood to better understand these potential differences.
This study adds to the debate around the existence of a gene-by-environment interaction effect in the development of depression. Conflicting meta-analyses (e.g., Caspi et al., 2010; Karg et al., 2011; Risch et al., 2009; Uher and McGuffin, 2010) have resulted in greater attention to methodological detail for measurement, analyses, and approaches organized by a theoretical basis. Strengths of the current study address most of these concerns. First, key variables were measured prospectively from birth using established assessments at regular intervals: maltreatment was recorded through multiple methods (interviews with parents and teachers, direct observation, and review of administrative child welfare and medical records); depression was measured using established self-report measures during multiple developmental transitions implicated in the onset of depression. The pattern of clinical levels of symptoms in the current sample coincides with a developmental increase in adolescence and young adulthood (Costello et al., 2003; Hankin et al., 1998). High overall levels of symptoms are likely attributable, in part, to the fact that this study involved an exclusively low-income sample (Duggal et al., 2001). Appropriate quantitative techniques produced reliable results despite attrition that occurred in this ongoing 28-year longitudinal study.
Study limitations include sample size and distributions of depression symptoms which presented a barrier to fully testing hypotheses related to G × E effects for persistent/recurrent forms of depression. The presence of G × E effects on single-episode depression is an important contribution as it is contrary to findings of G × E effects for only persistent/recurrent depression. The sample size was too small to adequately test for G × E effects on more chronic forms of depression. Similarly, rates of depression at each individual follow-up were sufficiently low as to raise similar concerns about Type II error for analyses of depression at each developmental period. The coefficients reported for these sub-analyses can contribute to future meta-analyses as well as understanding of G × E contributions to depression in the context of a priori theory (e.g., Caspi et al., 2010). However, strong conclusions should not be made based on the null-hypothesis significance tests for the under-powered sub-analyses in this paper.
This study supports the plausibility of a gene-by-environment interaction that contributes to the development of depression in childhood, adolescence, and early adulthood. This is consistent with a developmental systems view which acknowledges factors that interact, coact, and transact in complex ways across multiple levels of analysis and over time to influence the likelihood of positive or maladaptive outcomes (Bronfenbrenner, 1976; Gottlieb, 1991; Yates et al., 2003). Maltreatment continues to be an important indicator of risk for poorer developmental outcomes with respect to emotional wellbeing as well as other domains. Childhood maltreatment represents a failure of the caregiving environment and denotes particular experiences for some (e.g., episodes of sexual or physical abuse) and a marker for a broader context of adversity for others (e.g., accompanying poverty, low parental resources; Cicchetti and Lynch, 1995). Meanwhile, increasing knowledge of the genome has revealed important contributors that, when considered with other factors, help to elucidate the processes of risk and resilience leading toward or away from complex disorders such as depression. Undoubtedly, intermediate factors and processes also are important in the development of depression or resilient outcomes (e.g., serotonergic function at the level of the synapse; past successes or failure in other key developmental domains; poor emotion regulation, cognitive bias, and other factors at the psychological level; e.g., Han et al., 2012; Masten et al., 2005; Stockmeier et al., 1998), and development itself represents a confluence of innumerable factors that operate in such a complex fashion as to suggest the processes are probabilistic at an individual level (Sroufe, 1997). Nevertheless, research efforts should continue to elaborate on this complex picture to further understand the interplay between factors, with the promissory note that such knowledge will not only help to further understand the disorder, but also help inform policy and interventions aimed at promoting resilience.
Acknowledgments
Role of funding source
This research was supported by a National Institute of Mental Health (NIMH) grant to Byron Egeland (R01MH40864-09), by a grant from the Spunk Fund, Inc. to Dante Cicchetti, by a National Institute of Child Health and Human Development (NICHD) grant to W. Andrew Collins (R01HD054850), by predoctoral fellowships awarded to J.J. Cutuli from the Center for Neurobehavioral Development (CNBD), University of Minnesota and the National Institute of Mental Health (NIMH; 5T323MH015755), and by a predoctoral training grant awarded to K. Lee Raby from the National Institute of Mental Health (T32MH015755-33). All interpretations, recommendations, conclusions, and other points of view are those of the authors and do not necessarily reflect those of NIMH, NICHD, or CNBD.
We would like to thank the participants and their families for decades of support, and a special thanks to Judy Cook for her invaluable contributions to this work.
Footnotes
Conflict of interest
The authors have no conflicts of interest to report.
References
- Ambrosini PJ, Metz C, Prabucki K, Lee J. Videotape reliability of the third revised edition of the K-SADS. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28:723–728. doi: 10.1097/00004583-198909000-00013. [DOI] [PubMed] [Google Scholar]
- Belsky J. Etiology of child maltreatment: a developmental-ecological analysis. Psychological Bulletin. 1993;114(3):413–434. doi: 10.1037/0033-2909.114.3.413. http://dx.doi.org/10.1037/0033-2909.114.3.413. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Goldman D. Genetics of emotion. Trends in Cognitive Science. 2011;15:401–408. doi: 10.1016/j.tics.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U. The Ecology of Human Development. William Morrow & Company; Cambridge, MA: 1976. [Google Scholar]
- Brown J, Cohen P, Johnson JG, Smailes EM. Childhood abuse and neglect: specificity of effects on adolescent depression and suicidality. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(12):1490–1496. doi: 10.1097/00004583-199912000-00009. http://dx.doi.org/10.1097/00004583-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Depression and the serotonin transporter 5-HTTLPR polymorphism: a review and a hypothesis concerning gene–environment interaction. Journal of Affective Disorders. 2008;111:1–12. doi: 10.1016/j.jad.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301 (5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Lynch M. Failures in the expectable environment and their impact on individual development: the case of child maltreatment. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology: Risk, disorder, and Adaptation. Vol. 2. Wiley; New York: 1995. pp. 32–71. [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13:783–804. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Development. 2010a;81 (1):252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple M, Toth SL. Interaction of child maltreatment and 5-HTT polymorphisms: suicidal ideation among children from lowses backgrounds. Journal of Pediatric Psychology. 2010b;35(5):536–546. doi: 10.1093/jpepsy/jsp078. http://dx.doi.org/10.1093/jpepsy/jsp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Oshri A. Interactive effects of corticotropin releasing hormone receptor 1, serotonin transporter linked polymorphic region, and child maltreatment on diurnal cortisol regulation and internalizing symptomatology. Development and Psychopathology. 2011a;23:1125–1138. doi: 10.1017/S0954579411000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Toth SL. The effects of child maltreatment and polymorphisms of the serotonin transporter and dopamine D4 receptor genes on infant attachment and intervention efficacy. Development and Psychopathology. 2011b;23:357–372. doi: 10.1017/S0954579411000113. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. The development of depression in children and adolescents. American Psychologist. 1998;53:221–241. doi: 10.1037//0003-066x.53.2.221. http://dx.doi.org/10.1037/0003-066X.53.2.221. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Valentino K. Toward the application of a multiple-levels-of-analysis perspective to research in development and psychopathology. In: Masten AS, editor. Multilevel Dynamics in Developmental Psychology, Minnesota Symposia on Child Development. Vol. 34. Lawrence Erlbaum Associates; Mahwah, NJ: 2007. pp. 243–284. [Google Scholar]
- Collier DA, Stober G, Li T, Helis A, Catalano M, Di Bella D, Lesch KP. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Molecular Psychiatry. 1996;1 (6):453–460. [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Duggal S, Carlson EA, Sroufe LA, Egeland B. Depressive symptomatology in childhood and adoelscence. Development and Psychopathology. 2001;13:143–164. doi: 10.1017/s0954579401001109. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn EC, Uddin M, Subramanian SV, Smoller JW, Galea S, Koenen KC. Research review: gene–environment interaction research in youth depression—a systematic review with recommendations for future research. Journal of Child Psychology and Psychiatry. 2011;52:1223–1238. doi: 10.1111/j.1469-7610.2011.02466.x. http://dx.doi.org/10.1111/j.1469-7610.2011.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland B. Mediators of the effects of child maltreatment on developmental adaptation in adolescence. In: Cicchetti D, Toth SL, editors. Rochester Symposium on Developmental Psychology, vol. 8, The effects of trauma on the developmental process. Vol. 8. 1997. pp. 403–434. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- Gottlieb G. Experiential canalization of behavioral development: Theory. Developmental Psychology. 1991;27 (1):4–13. [Google Scholar]
- Gunnar MR, Vazquez DM. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology: Developmental Neuroscience. 2. Vol. 2. Wiley; Hoboken: 2006. pp. 533–577. [Google Scholar]
- Han G, Klimes-Dougan B, Jepsen S, Ballard K, Nelson M, Houri A, Cullen K. Selective neurocognitive impairments in adolescents with major depressive disorder. Journal of Adolescence. 2012;35:11–20. doi: 10.1016/j.adolescence.2011.06.009. http://dx.doi.org/10.1016/j.adolescence.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL. Adolescent depression: description, causes, and interventions. Epilepsy and Behavior. 2006;8:102–114. doi: 10.1016/j.yebeh.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. Journal of Neurochemistry. 1996;66 (6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of the American Medical Association. 2000;284(5):592–597. doi: 10.1001/jama.284.5.592. http://dx.doi.org/10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Hu Z, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and alcoholism risk. Alcoholism: Clinical and Experimental Research. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. http://dx.doi.org/10.1097/01.ALC.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Hussey JM, Chang JJ, Kotch JB. Child maltreatment in the United States: prevalence, risk factors, and adolescent health consequences. Pediatrics. 2006;118(3):933–942. doi: 10.1542/peds.2005-2452. http://dx.doi.org/10.1542/peds.2005-2452. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP. Neuroticism and serotonin: a developmental genetic perspective. In: Plomin R, DeFries JC, Craig IW, McGuffin P, editors. Behavioral Genetics in the Postgenomic Era. American Psychological Association; Washington, DC: 2003. pp. 389–424. [Google Scholar]
- Masten AS, Roisman GI, Long JD, Burt KB, Obradović J, Riley J, Tellegen A. Developmental cascades: linking academic achievement and externalizing and internalizing symptoms over 20 years. Developmental Psychology. 2005;43:733–746. doi: 10.1037/0012-1649.41.5.733. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Katz R, Watkins S, Rutherford J. A hospital-based twin register of the heritability of DSM-IV unipolar depression. Archives of General Psychiatry. 1996;53:129–136. doi: 10.1001/archpsyc.1996.01830020047006. [DOI] [PubMed] [Google Scholar]
- Mendlewicz J, Massat I, Souery D, Del-Favero J, Oruc L, Nothen MM, Van Broeckhoven C. Serotonin transporter 5HTTLPR polymorphism and affective disorders: no evidence of association in a large European multicenter study. European Journal of Human Genetics. 2004;12:377–382. doi: 10.1038/sj.ejhg.5201149. [DOI] [PubMed] [Google Scholar]
- Mileva-Seitz V, Kennedy J, Atkinson L, Steiner M, Levitan R, Matthews SG, Meaney MJ, Sokolowski MB, Fleming AS. Serotonin transporter allelic variation in mothers predicts maternal sensitivity, behavior and attitudes toward 6-month-old infants. Genes, Brain, and Behavior. 2011;10:325–333. doi: 10.1111/j.1601-183X.2010.00671.x. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biological Psychiatry. 2009;65(3):211–219. doi: 10.1016/j.biopsych.2008.06.009. http://dx.doi.org/10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Durrant C, Lewis G, Flint J. Defining replication: a response to Kaufman and colleagues. Biological Psychiatry. 2010;67 (4):e21–e23. [Google Scholar]
- Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64:442–450. [PubMed] [Google Scholar]
- Poznanski EO, Cook SC, Carroll BJ, Corzo H. Use of the children’s depression rating scale on an inpatient psychiatric population. Journal of Clinical Psychiatry. 1983;44:200–203. [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. Journal of the American Academy of Child and Adolescent Psychiatry. 1985;23:191–197. doi: 10.1097/00004583-198403000-00011. http://dx.doi.org/10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Praschak-Rieder N, Kennedy J, Wilson AA, Hussey D, Boovariwala A, Willeit M, et al. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: a [(11)C] DASB positron emission tomography study. Biological Psychiatry. 2007;62:327–331. doi: 10.1016/j.biopsych.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Reimold M, Smolka MN, Schumann G, Zimmer A, Wrase J, Mann K, et al. Midbrain serotonin transporter binding potential measured with [11C]DASB is affected by serotonin transporter genotype. Journal of Neural Transmission. 2007;114:635–639. doi: 10.1007/s00702-006-0609-0. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K-Y, Eaves L, Hoh J, Ries Merikangas K. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression. Journal of the American Medical Association. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. http://dx.doi.org/10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. J. Wiley and Sons; New York: 1987. [Google Scholar]
- Rutter M, Thapar A, Pickles A. Gene–environment interactions: biological valid pathway or artifact. Archives of General Psychiatry. 2009;66 (12):1287–1289. doi: 10.1001/archgenpsychiatry.2009.167. [DOI] [PubMed] [Google Scholar]
- Scarr-Salapatek S. An evolutionary perspective on infant intelligence: species patterns and individual variations. In: Lewis M, editor. Origins of Intelligence: Infancy and Early Childhood. Plenum; New York: 1976. pp. 165–198. [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychological Methods. 2002;7 (2):147–177. [PubMed] [Google Scholar]
- Segal DL, Hersen M, Hasselt VB. Reliability of the structured clinical interview for DSM-II-R: an evaluative review. Comprehensive Psychiatry. 1994;35:316–337. doi: 10.1016/0010-440x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Shaffer A, Huston L, Egeland B. Identification of child maltreatment using prospective and self-report methodologies: a comparison of maltreatment incidence and relation to later psychopathology. Child Abulse and Neglect. 2008;32:682–692. doi: 10.1016/j.chiabu.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroufe LA. Psychopathology as an outcome of development. Development and Psychopathology. 1997;9:251–268. doi: 10.1017/s0954579497002046. [DOI] [PubMed] [Google Scholar]
- Sroufe LA, Egeland B, Carlson EA, Collins WA. The Development of the Person: The Minnesota Study of Risk and Adaptation From Birth to Adulthood. Guilford Press; New York: 2005. [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli T, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression—postmortem evidence for decreased serotonin activity. The Journal of Neuroscience. 1998;18 (18):7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, McGuffin P. The genetic etiology of childhood depressive symptoms: a developmental perspective. Development and Psychopathology. 1996;8:751–760. http://dx.doi.org/10.1017/S0954579400007409. [Google Scholar]
- Toth SL, Manly JT, Cicchetti D. Child maltreatment and vulnerability to depression. Development and Psychopathology. 1992;4:97–112. http://dx.doi.org/10.1017/S0954579400005587. [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D’Onofrio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychological Science. 2003;14 (6):623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- Uher R, Caspi A, Houts R, Sugden K, Williams B, Poulton R, Moffitt TE. Serotonin transporter gene moderates childhood maltreatment’s effects on persistent but not single-episode depression: replications and implications for resolving inconsistent results. Journal of Affective Disorders. 2011 doi: 10.1016/j.jad.2011.03.010. http://dx.doi.org/10.1016/j.jad.2011.03.010. [DOI] [PMC free article] [PubMed]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Molecular Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. http://dx.doi.org/10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the structured clinical interview for DSM-IV (SCID-I/P) Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Waddington CH. Canalization of development and the inheritance of acquired characters. Nature. 1942;150:563. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- Wiersma JE, Hovens JGFM, van Oppen P, Giltay EJ, van Schaik DJF, Beekman ATF, Penninx BWJH. The importance of childhood trauma and childhood life events for chonicity of depression in adults. The Journal of Clinical Psychiatry. 2009;70(7):983–989. doi: 10.4088/jcp.08m04521. http://dx.doi.org/10.4088/JCP.08m4521. [DOI] [PubMed] [Google Scholar]
- Yates TM, Carlson EA, Egeland B. A prospective study of child maltreatment and self-injurious behavior in a community sample. Development and Psychopathology. 2008;20:651–671. doi: 10.1017/S0954579408000321. http://dx.doi.org/10.1017/S0954579408000321. [DOI] [PubMed] [Google Scholar]
- Yates TM, Egeland B, Sroufe LA. Rethinking resilience: a developmental process perspective. In: Luthar SS, editor. Resilience and Vulnerability: Adaptation in the Context of Childhood Adversities. Cambridge University Press; New York: 2003. pp. 243–266. [Google Scholar]