Abstract
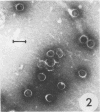
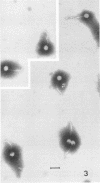
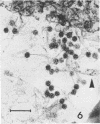
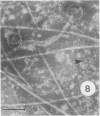
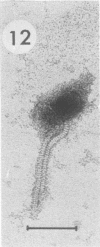
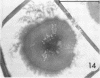
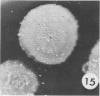
Bacillus medusa was found to carry three phages or phagelike structures named phi med-1, phi med-2, and phi med-3. phi med-1 is a minute, 25-nm-diameter particle without a tail. It was extracted from the sporulation lysate of a phi med-2-minus strain of B. medusa and purified by differential centrifugation. The nucleic acid from this structure was shown to be orcinol positive, alkali sensitive, RNase sensitive, and DNase resistant. An RNase-resistant core of nucleic acid was not found, indicating that it was single-stranded RNA. A host strain has not yet been found for phi med-1. Phage phi med-3 was induced with mitomycin C or UV light and consisted of empty heads of 57 nm in diameter, whereas phi med-2 induced with mitomycin was a phage of 60-nm head diameter and 220-nm tail length. The sporulation sequence proceeded faster in those mutants lacking phi med-2, and when the phage was reintroduced to B. medusa the extended wild-type sporulation sequence was observed. B. thuringiensis var. schwetzova was sensitive to phi med-2 and yielded small turbid plaques. B. medusa produced small numbers of phi med-2 during growth. The other phage may be produced at the same time but were not detected. Phi med-1 was found in sporulating cells by electron microscopy techniques. Its release from these was demonstrated by both electron microscopy techniques and a radioactive assay. It appears to participate in the formation of a surface layer on the parasporal inclusion of B. medusa.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F., Hull R. Comparative virology of the small RNA viruses. J Gen Virol. 1973 Jun;20(Suppl):43–60. doi: 10.1099/0022-1317-20-Supplement-43. [DOI] [PubMed] [Google Scholar]
- DAVISON P. F., FREIFELDER D. The physical properties of the deoxyribonucleic acid from T7 bacteriophage. J Mol Biol. 1962 Dec;5:643–649. doi: 10.1016/s0022-2836(62)80092-8. [DOI] [PubMed] [Google Scholar]
- FRASCA J. M., PARKS V. R. A ROUTINE TECHNIQUE FOR DOUBLE-STAINING ULTRATHIN SECTIONS USING URANYL AND LEAD SALTS. J Cell Biol. 1965 Apr;25:157–161. doi: 10.1083/jcb.25.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry G. S., Fitz-James P. C. Characteristics of phi T, the temperate bacteriophage carried by Bacillus megaterium 899a. J Virol. 1974 Feb;13(2):494–499. doi: 10.1128/jvi.13.2.494-499.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry G. S., Fitz-James P. C. Disruptive action of potassium phosphotungstate on phiT, the temperate bacteriophage of Bacillus megaterium 899a. J Virol. 1974 Feb;13(2):529–531. doi: 10.1128/jvi.13.2.529-531.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry G. S., Fitz-James P. C. Exclusion of induced bacteriophage from cells of a lysogenic Bacillus megaterium committed to sporulation. J Bacteriol. 1974 Apr;118(1):295–303. doi: 10.1128/jb.118.1.295-303.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Marmur J. Characterization of inducible bacteriophages in Bacillus licheniformis. J Virol. 1970 Feb;5(2):237–246. doi: 10.1128/jvi.5.2.237-246.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S. M., Fitz-James P. C. Sporulation of a cortexless mutant of a variant of Bacillus cereus. J Bacteriol. 1971 Jan;105(1):339–348. doi: 10.1128/jb.105.1.339-348.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabussay D., Zillig W., Herrlich P. Characterization of the Bacillus stearothermophilus phage phimu-4 and its DNA. Virology. 1970 May;41(1):91–100. doi: 10.1016/0042-6822(70)90057-7. [DOI] [PubMed] [Google Scholar]
- Rogoff M. H., Yousten A. A. Bacillus thuringiensis: microbiological considerations. Annu Rev Microbiol. 1969;23:357–386. doi: 10.1146/annurev.mi.23.100169.002041. [DOI] [PubMed] [Google Scholar]
- Shafia F., Thompson T. L. Isolation and preliminary characterization of bacteriophage phi-mu-4. J Bacteriol. 1964 May;87(5):999–1002. doi: 10.1128/jb.87.5.999-1002.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN C., BORST P. DOUBLE-STRANDED RIBONUCLEIC ACID FORMATION IN VITRO BY MS 2 PHAGE-INDUCED RNA SYNTHETASE. Science. 1963 Nov 29;142(3596):1188–1191. doi: 10.1126/science.142.3596.1188. [DOI] [PubMed] [Google Scholar]