Abstract
During renin-angiotensin system activation, cyclooxygenase-2 (COX-2)-derived prostaglandins attenuate the pressor and antinatriuretic effects of angiotensin II (AngII) in the renal medulla. The (pro)renin receptor (PRR) is abundantly expressed in the collecting ducts (CD) and its expression is augmented by AngII. PRR overexpression upregulates COX-2 via mitogen-activated kinases (MAPK/ERK1/2) in renal tissues; however, it is not clear if this effect occurs independently or in concert with AngII type 1 receptor (AT1R) activation. We hypothesized that PRR activation stimulates COX-2 expression independently of AT1R in primary cultures of rat renal inner medullary (IM) cells. The use of different cell-specific immunomarkers (aquaporin-2 for principal cells, anion exchanger type-1 for intercalated type-A cells, and tenascin C for interstitial cells) and co-staining for AT1R, COX-2 and PRR revealed that PRR and COX-2 were colocalized in intercalated and interstitial cells while principal cells did not express PRR or COX-2. In normal rat kidney sections, PRR and COX-2 were colocalized in intercalated and interstitial cells. In rat renal IM cultured cells, treatment with AngII (100 nmol/L) increased COX-2 expression via AT1R. In addition, AngII and rat recombinant prorenin (rrPR; 100 nmol/L) treatments increased ERK1/2 phosphorylation, independently. Importantly, rrPR upregulated COX-2 expression in the presence of AT1R blockade. Inhibition of MAPK/ERK1/2 suppressed COX-2 upregulation mediated by either AngII or rrPR. Furthermore, PRR knockdown using PRR-short hairpin RNA blunted the rrPR-mediated upregulation of COX-2. These results indicate that COX-2 expression is upregulated by activation of either PRR or AT1R via MAPK/ERK1/2 in rat renal IM cells.
Keywords: Cyclooxygenase 2, (pro)renin receptor, collecting duct, MAPK, ERK1/2
Introduction
The (pro)renin receptor (PRR) is a member of the renin-angiotensin system (RAS) initially identified as an accessory protein that co-precipitates with the vacuolar H+-proton adenosine triphosphatase (v-ATPase).1, 2 PRR is expressed in the kidneys, particularly in mesangial cells 3, podocytes 4–6, and intercalated type-A cells of distal nephron segments. 2,7 PRR binds renin and prorenin with affinity in the nanomolar range, 8 which increases the catalytic activity of renin and activates prorenin by exposing its active site. 5 Cell surface binding of prorenin to PRR not only increases renin enzymatic activity, but also triggers the intracellular phosphorylation of mitogen activated protein kinases (MAPK) ERK1/2. 5, 9
Activation of MAPK/ERK1/2 pathway upregulates cyclooxygenase-2 (COX-2) expression in cardiac tissues 10 and renal cortex. 11, 12 In angiotensin (AngII)-infused rats, augmentation of COX-2 expression and activity in isolated glomeruli is associated with the activation of MAPK/ERK1/2 pathways. 13 Transgenic rats over-expressing PRR have increased expression of COX-2 and phosphorylated-ERK1/2 (p-ERK1/2) in macula densa cells with normal renal AngII content, suggesting that PRR-mediated COX-2 upregulation may be independent of AngII. 12, 14 In the renal inner medulla, prostaglandin E2 (PGE2) levels increase in response to AngII infusions which counteract the vasoconstrictor and antinatriuretic effects of AngII. 15 In COX-2 knockout mice chronically infused with AngII, the increases in PGE2 levels in response to AngII are blunted, indicating that the response is mediated by COX-2. 15
We recently reported that chronic AngII-infused Sprague-Dawley rats showed increased levels of PRR mRNA and its soluble form (sPRR) in the renal medulla and urine. 7 This model of experimental hyertension exhibits increased synthesis and secretion of renin and most importantly of prorenin by the principal cells of the collecting duct (CD), which is the natural agonist of PRR. 16, 17, 18 During AngII-dependent hypertension, enhanced renin enzymatic activity in renal inner medulla and urine supports a functional role of the PRR and sPRR for local generation of AngII. 16, 17, 18 However, the effects of PRR activation on COX-2 expression in the renal medulla remain unclear. To test the hypothesis that PRR activation enhances COX-2 expression independently of AT1R in primary cultured inner medullary (IM) cells, we examined the effects of AT1R or PRR activation on the stimulation of ERK1/2 pathway and COX-2 expression. By knocking-down PRR using shRNA technology, we examined the direct role of PRR activation on COX-2 expression in rat renal IM cells.
Materials and Methods
Primary cultures of rat IM cells
Long term primary cultures of rat renal IM cells were prepared as described previously. 17 (see expanded methods at http://hyper.ahajournals.org).
Immunocolocalization of PRR and COX-2
Methanol-fixed cultured rat IM cells were stained with specific antibodies (AT1R, AQP-2, COX-2, PRR, Anion exchanger-2, a marker for intercalated cells and Tenascin C, a marker for interstitial cells) to evaluate cell type population. Non-specific cross-reaction between COX-2 and COX-1 antibody was ruled out by Western blot using a COX-2 protein standard. To further confirm our findings in the cultured cells, we performed immunofluorescence studies in rat kidney sections (3 μm) using anti-PRR and anti-COX-2 antibodies. (see expanded methods at http://hyper.ahajournals.org).
Quantitative real-time RT-PCR
COX-2 mRNA expression levels in primary cultured IM cells were measured using TaqMan PCR system using the following primers: 5′-TCCGTAGAAGAACCTTTTCC -3′ (sense); 5′-GGAGTCTGGAACATTGTGAA -3′ (antisense) and 5′-6-FAM-GGAAATAAGGAGCTTCCTGA -BHQ1–3′ (fluorogenic probe). Data were normalized against β-actin mRNA as previously described 17.
Western blot analysis
Protein quantification was performed using a rabbit COX-2 antibody (Cayman, Ann Arbor, MI), a mouse anti-phospho-p44/42 ERK1/2 antibody (Thr202/Tyr204) and a rabbit anti-total ERK antibody (Cell Signaling Technology, Beverly, MA). Densitometric analyses were performed by normalization against β-actin.
PRR knockdown in primary cultures of rat renal IM cells using short hairpin RNA (shRNA)
IM cells were transfected with 1 μg of plasmids (pGeneClip™ hMGFP Vector) using Lipofectamine LTX (Invitrogen, Carlsbad, CA) for 36 hours. Efficiency of transfection was confirmed by green fluorescent protein (GFP) detection (see expanded methods at http://hyper.ahajournals.org). Scramble shRNA sequence was used as a negative control.
Statistical analyses
An average number of 6 to 8 independent observations were performed for each treatment. Data were evaluated by the Grubb test followed when appropriate by paired and unpaired Student t test or by one-way ANOVA with Tukey post-test. For mRNA and protein data, control levels were defined as 100%. Significance was defined as P<0.05. Results are expressed as mean ± SEM. Non significant difference is represent as “NS”.
RESULTS
PRR and COX-2 are co-expressed in interstitial and intercalated cells
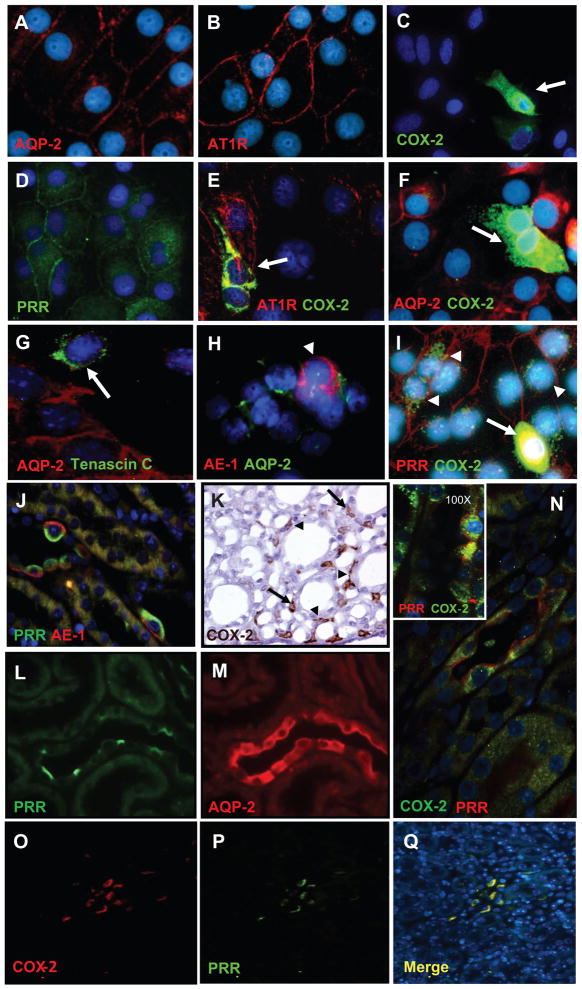
To examine the cell-type specific localization of PRR and COX-2, primary cultured rat IMCD cells and paraffin embedded rat kidney sections (3 μm) were assessed by immunofluorescence using specific antibodies. As shown in Figure 1A–I, in long term primary rat IMCD culture cells (5 days), a sub-population of cells were positive for AQP-2 a marker of principal cells (red; 1-A) while they all expressed AT1R on the cell membrane (red; 1-B). In addition, few cells resulted positive for COX-2 (green; 1-C) and PRR (green, 1-D). Cells expressing COX-2 (green; 1-E) were positive for AT1R (red; 1E) but negative for AQP-2 (red; 1-F), indicating that AT1R co-localizes with COX-2 in interstitial cells (1E), as previously described.19, 20 AQP-2 positive cells (red) did not express Tenascin C (green; 1-G), a well-known marker of interstitial cells; nor anion exchanger type 1 (AE1 in red and AQP-2 in green; 1-H), a marker of intercalated type-A cells. Interestingly, PRR (red) and COX-2 (green) were co-expressed by similar cell-types (1-I). In addition, in the rat kidney sections, PRR was found co-expressed in tubular intercalated type-A cells with AE1 (PRR in green and AE1 in red; 1-J) and with COX-2 in the collecting ducts (PRR in red and COX-2 in green; 1-N) indicating that COX-2 is immunoexpressed by the intercalated cells of the collecting duct. Peroxidase reaction using a COX-2 antibody confirms this observation in inner medullary tissues (1-K). Furthermore, COX-2 (red, 1-O) and PRR (green, 1-P) were co-localized in the interstitial cells of the rat kidney inner medulla (1-Q, merged colors). In true consecutive rat kidney sections (1-L, 1-M) was observed that PRR-positive expressing cells (green, 1-L) were AQP-2-negative (red, 1-M) indicating that PRR is not expressed by the principal cells. As described previously 21, COX-2 and COX-1 colocalize in intercalated cells. Cells stained only for COX-1 were also observed, corresponding to principal cells as described 22 (see supplementary data at http://hyper.ahajournals.org). PRR and COX-2 were coexpressed in tubular (Figure 1M, arrowheads) and interstitial cells (Figure 1M, arrows) in rat kidney sections.
Figure 1.
Characterization of long-term rat inner medullary cells (A-I). IM cells show specific immunoexpression of AQP-2 (red; A), AT1R (red; B), COX-2 (green; C), and PRR (green; D). AT1R (red) co-localizes with COX-2 (green) in the same type of cells (E), indicating that interstitial cells (arrows) strongly stained for COX-2 (green) also co-express AT1R (red) in the plasma membrane, as previously described (E). Cells expressing AQP-2 (red; F), the principal cells, do not co-express COX-2 (arrow, green; F). Tenascin C (arrow, green; G), a marker for interstitial cells, does not co-localize with AQP-2 (red; E). Likewise, anion exchanger type-1 (AE-1; red; H), a known immunomarker for intercalated type-A cells (arrowheads), does not co-localizes with AQP-2 (green; H). Evidence for the presence of PRR (red; I) co-localizing with COX-2 (arrow heads, green; I) is shown in I. Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole; blue). In addition, in normal rat kidney sections (3 μm) immunofluorescence studies demonstrate that PRR (apical green immunoreactivity) and AE-1 (basolateral red immunoreactivity) co-localize in tubular intercalated type-A cells (J). K shows specific immunoreactivity for COX-2 in the interstitial cells (arrows) and some tubular cells (arrowheads) in the rat inner renal medulla (brown chromogen, 3,3′-Diaminobenzidine DAB). In consecutive kidney sections (L and M), is evident that PRR (green; L) is immunoexpressed by negative AQP-2-expressing cells (red; M). Panel N displays examples of the co-localization of PRR (red; cell membrane) with COX-2 (green; intracellular localization) in the intercalated cells of the collecting ducts. In addition, this panel contains a high resolution microphotograph (left upper corner, 100X, oil immersion) for clear details. The lower panels O–Q show microphotographs (20X magnification) of a rat kidney section stained using dual immunofluorescence for COX-2 (red; O) and PRR (green; P) counterstained with DAPI (blue fluorochrome) demonstrate that COX-2 and PRR also co-localized in interstitial cells (merge, Q). Images were visualized using a Nikon Eclipse 50i immunofluorescence microscope and microphotographs were captured using a digital camera Nikon DS-U2/L2.
AngII increases COX-2 expression in cultured rat IM cells via AT1R
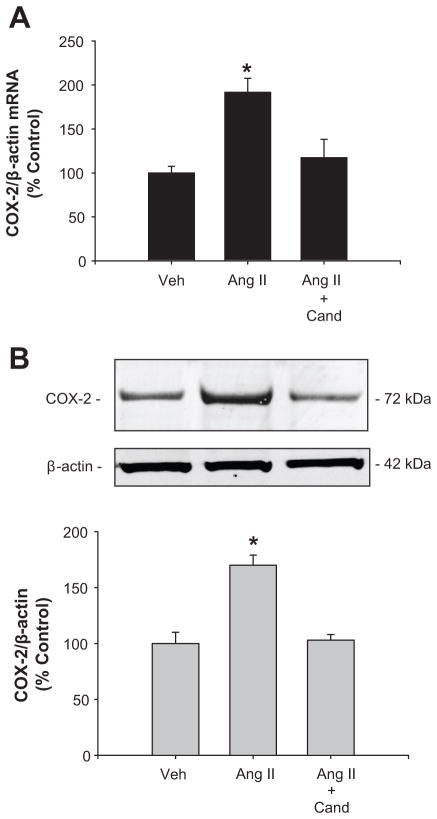
The maximum response for COX-2 protein expression was obtained using a single dose of AngII (100 nmol/L) after 6 hours, while Ang II content in the media was reduced around 20% after 3 to 6 hours. No effects were observed on COX-1 protein levels (see supplementary data at http://hyper.ahajournals.org). AngII (100 nmol/L) increased COX-2 mRNA (AngII: 191 ± 3 % vs. vehicle: 100 ± 4 %; P<0.05; n=8, Figure 2A) and protein levels (AngII: 170 ± 9 % vs. vehicle: 100 ± 10 %; P<0.05; n=8, Figure 2B). These effects were blocked by 1 μM candesartan (AngII + Cand: 103 ± 5 % vs. vehicle: 100 ± 10 %; n=6, P=NS), indicating that AngII-dependent stimulation of COX-2 in IM cells is mediated by AT1R activation.
Figure 2.
COX-2 mRNA (A) and protein levels (B) levels were augmented after 6 h of AngII treatment; candesartan (Cand; 1μM) blocks this effect. n=6–8; *P<0.05
COX-2 expression is stimulated independently by PRR and AT1R via ERK1/2 pathway in rat IM cells
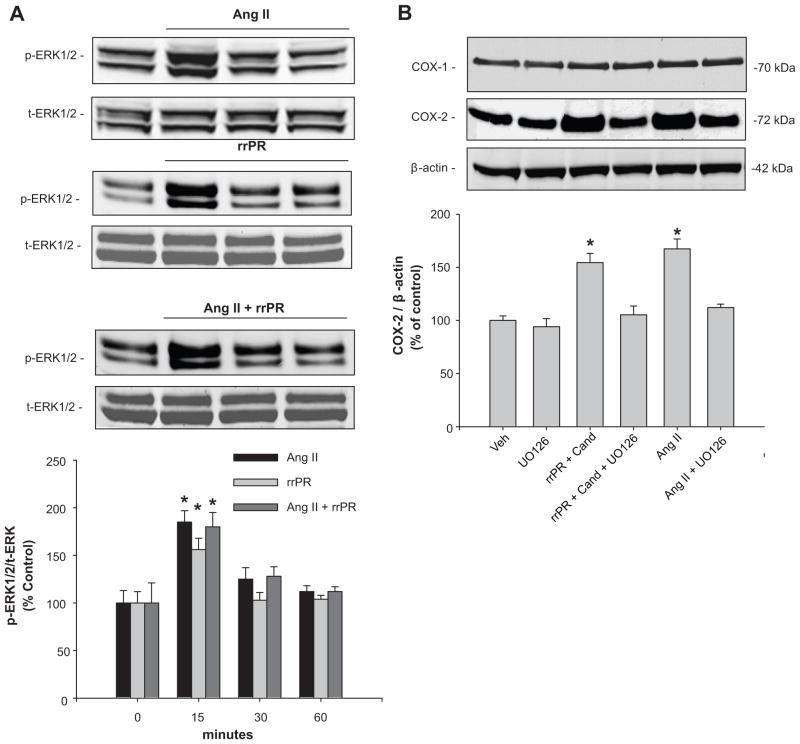
To stimulate PRR in IM cells we used a nanomolar dose of a rat recombinant prorenin (rrPR). 8 As shown in Figure 3A, AngII (100 nmol/L) and rrPR (100 nmol/L) treatments, both independently increased p-ERK1/2 at 15 min (AngII: 185 ± 12 %; rrPR 156 ± 12 % vs. vehicle: 100 ± 13 %; n=6, P<0.05). Combined AngII and rrPR treatments caused a similar response at 15 min, without an additive effect (AngII + rrPR: 180 ± 15 %; n=6, P<0.05). To test if COX-2 expression was upregulated by PRR or AT1R activation via ERK1/2 pathway, IM cells were treated with the ERK1/2 inhibitor UO126 (10 μM). Treatment with rrPR plus candesartan at 1 μM (AT1R blocker), increased COX-2 protein levels (rrPR: 154 ± 8 % vs. vehicle: 100 ± 5 %; n=6, P<0.05) to a similar extent observed in IM cells treated with AngII (AngII: 167 ± 9 % vs. vehicle: 100 ± 5 %; n=6, P<0.05). Importantly, up regulation of COX-2 by rrPR or AngII treatments was prevented by ERK1/2 inhibitor UO126 (rrPR + UO126: 105 ± 8 %; AngII + UO126: 112 ± 3 %; n=6, P=NS; Figure 3B).
Figure 3.
COX-2 protein expression is upregulated by AngII and rat recombinant prorenin (rrPR) via ERK1/2 pathway. A. Representative Western blot showing phosphorylated ERK1/2 (p-ERK1/2) levels in response to AngII + rrPR and AngII + rrPR treatment at 0, 15, 30 and 60 min. *P<0.05 versus control (0 min, n=6). B. Representative Western blot showing that COX-2 protein levels are augmented by rrPR treatment in the presence of AT1R antagonist to avoid the possibility of AngII formation in IM cells. As shown before AngII also increase COX-2 protein levels. The ERK1/2 inhibitor UO126 (10 μM) blunted the effect of both treatments (*P=NS versus vehicle; n=6). No effect was observed on COX-1 protein levels.
PRR knockdown suppresses the PRR-mediated increases in COX-2 expression in rat IM cells
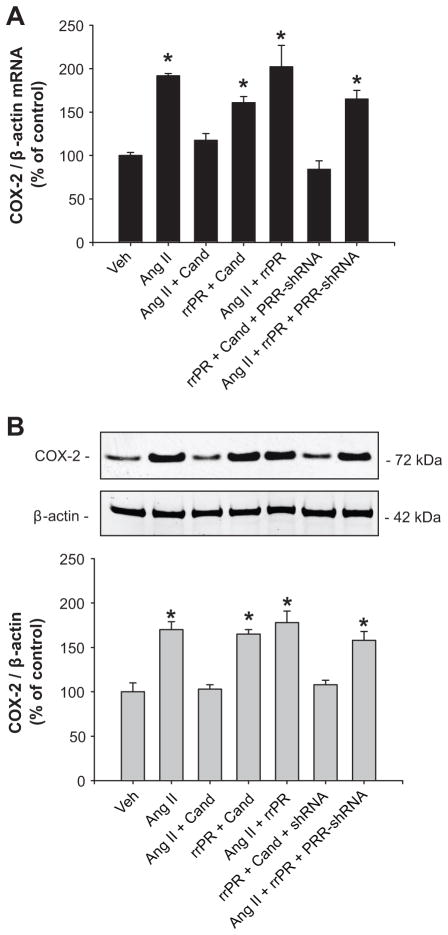
Target cells for transfections were interstitial and epithelial tubular cells and PRR-shRNA reduced PRR protein expression compared to cells transfected with GFP-scramble shRNA (47 ± 4 % vs. vehicle: 100 ± 7 %; n=6, P<0.05; see supplementary data at http://hyper.ahajournals.org). Additional sets of cells were treated using the same dose of rrPR or AngII as previous experiments and compared to cells transfected with PRR-shRNA. As shown in figure Figure 4A and 4B, rrPR treatment in the presence of candesartan increased COX-2 mRNA (160 ± 7% vs. vehicle: 100 ± 4%; n=6, P<0.05) and protein (165 ± 5% vs. vehicle: 100 ± 10%; n=6, P<0.05) levels. No additive effects were observed by combining AngII + rrPR (202 ± 24% vs. vehicle: 100 ± 4%; n=6, P<0.05). AngII stimulation of COX-2 mRNA (191 ± 3% vs. vehicle: 100 ± 4%; n=6, P<005) and protein (170 ± 9% vs. vehicle: 100 ± 10%; n=6, P<0.05) levels was prevented by candesartan. Importantly, PRR-shRNA transfection of IM cells avoids the stimulatory effect of rrPR on COX-2 mRNA (84 ± 10% vs. vehicle: 100 ± 4%; n=6, P=NS) and protein (108 ± 5% vs. vehicle: 100 ± 10%; n=6, P<0.05) levels. PRR-shRNA did not affect the upregulation of COX-2 mediated by Ang II (mRNA: 165 ± 10%; n=6, P<0.05, protein: 158 ± 10%; n=6, P<0.05).
Figure 4.
PRR knockdown suppressed the PRR mediated upregulation of COX-2 in IM cells. COX-2 mRNA (C) and protein (D) levels were augmented by AngII (100 nmol/L) and this effect was suppressed by AT1R blockade with candesartan (Cand; 1 μM). Rat recombinant prorenin (rrPR; 100 nmol/L) plus Cand also upregulates COX-2, demonstrating an independent effect. PRR-mediated upregulation of COX-2 was completely blunted in IM cells previously transfected with PRR-shRNA. *P<0.05 versus vehicle; n=6.
Discussion
The present study demonstrates that COX-2 expression is upregulated in rat kidney IM cells by activation of either PRR or AT1R via MAPK/ERK1/2 pathway. This study also shows that long-term primary cultured rat IM cells are composed of collecting duct epithelial cells and interstitial cells and most importantly the novel finding that, COX-2 and PRR colocalize in interstitial and collecting duct type-A intercalated cells.
Previous studies in rats and mice have shown that AngII stimulates renin and prorenin synthesis and secretion by the principal cells of the CD 16, 17, 23, 24 despite the suppression exerted on juxtaglomerular renin. 18, 25 In diabetic rats the major source of prorenin synthesis and secretion are the principal cells of the CD. 25 We demonstrated that AngII treatment is able to stimulate mainly prorenin synthesis in freshly isolated rat IM cells, 17 and that the urines of AngII-dependent hypertensive rats possess abundant renin and prorenin. 18 PRR can be found in three different molecular forms: the M8.9 which is complexed with the proton V-ATPase; 2, 26 the 28 kDa soluble form (sPRR) which can be detected in plasma 27 and urine 7; and the full length form (37 kDa) located in the cell plasma membrane which is able to trigger the phosphorylation of ERK1/2 after binding of renin or prorenin. 12, 14, 28
Due to the localization of PRR in the mesangium and its ability to increase COX-212, 14, TFG β1, 29 and profibrotic genes such as transforming growth factor β1 (TGF-β1), plasminogen activator inhibitor-1 (PAI-1), collagen and fibronectin. 3, 28 PRR has been implicated in the pathogenesis of chronic kidney disease. 12, 14, 29 PRR activation promotes inflammation, through the activation of MAP/ERK1/2 signaling pathways. 12, 14, 30 Transgenic rats over-expressing PRR develop proteinuria and slowly progressive nephropathy. 12, 14 This model also exhibit increased levels of p-ERK1/2 and COX-2 expression in the renal cortex with normal AngII content, suggesting that COX-2 upregulation is independent of AngII. 12, 14 Furthermore, it has been shown that PRR overexpression in smooth muscle cells leads to elevated blood pressure and high plasma aldosterone levels suggesting a role in circulating AngII formation 30. The pathophysiological AngII-independent effects mediated by PRR may be relevant during conditions in which systemic AngII levels are suppressed or normal, such as occur in diabetic patients, in which high plasma prorenin levels, but not plasma renin activity predict the onset of microvascular complications, and correlate with high COX-2 expression, cell proliferation (short-term activation) and fibrosis (long-term activation). 29, 30, 31
COX-2 is mainly expressed in the interstitial cells, 32 macula densa, 33 and epithelial cells of the thick ascending limb. 34 However, it has been also described in the collecting duct cells 21, 35. In the present study, we demonstrated that in normal rat kidney, COX-2 is detected in PRR positive type-A intercalated cells. Importantly, we found that renal interstitial cells, which abundantly express COX-2, also express specific PRR immunoreactivity. Likewise, in rat renal cultured IM cells, PRR, COX-2 and AT1R colocalized in interstitial and type-A intercalated cells.
COX-2 plays a crucial role in regulating salt and water reabsorption and medullary blood flow 36–38 and its role is known in counterbalancing the effects of Ang II through PGE2 production in medullary tissues. 15 In fact, COX-2 inhibitors cause sodium retention in human subjects with normal kidney function. 39 This effect has been also observed in experimental animals subjected to systemic or selective medullary COX-2 inhibition. 15, 40 Salt loading downregulates COX-2 expression in renal cortex, but upregulates its expression in the renal medulla, 40 however the mechanisms implicated in this events remain unclear. Mineralocorticoid receptor agonism can induce COX-2 in vivo but not in cultured cells, suggesting that COX-2 upregulation is mediated by indirect pathways involving induced electrolyte hypertonicity in the interstitial fluid. 41 This data suggests a complex interaction between signaling pathways in the regulation of COX-2 expression in IM cells. This complexity may reflect a cell type-specific response for example, to hypertonicity or high intrarenal AngII levels as observed in AngII-dependent hypertension. 17
It has been shown that AngII increases glomerular PGE2 production and COX-2 expression via ERK1/2 pathway and that these effects are prevented by the AT1R blockade. 13 Because we previously showed the increased expression of PRR mRNA levels in the CD of AngII infused rats, 7 we further examined if PRR activation upregulates COX-2 independently of AngII. Treatment with rrPR in the presence of the AT1R blocker candesartan to avoid intrinsic activation of AT1R by possible endogenous AngII, increased COX-2 and augmented p-ERK1/2 at 15 min. The same effect was observed by Ang II, indicating that activation of both PRR and AT1R contributed to the phosphorylation of ERK1/2. Lack of an additive effect observed with both treatments (AngII and rrPR) may be explained by the fact that rat renal IM cells were composed by a mixed population of cells, thus the COX-2 stimulation in interstitial and intercalated cell in response to AT1R and PRR activation may differ. Further studies are needed to clarify this issue. These experimental evidence may suggest a key role of PRR in the light of recent evidence showing PRR upregulation by changes in dietary salt 42 implicating that PRR may play a role in renal sodium handling through the activation of intracellular ERK1/2 pathway in renal tubules.
Finally, to test if PRR downregulation can alter COX-2 expression, we further knocked-down the PRR expression using shRNA technology. A 63% reduction in PRR protein expression prevented the upregulation of COX-2 mRNA and protein, supporting our hypothesis of an AngII-independent pathway for COX-2 regulation.
In summary, type-A collecting duct intercalated cells and interstitial cells co-express COX-2, PRR and AT1R. COX-2 expression is upregulated in rat renal IM cells through the independent activation of both PRR and AT1R. These findings provide basis for the critical contributions of PRR and AT1R in the regulation of COX-2 in the renal medulla.
Perspectives
Although previous studies in vivo have shown that AT1R activation in the renal medulla led to COX-2-dependent PGE2 synthesis, our data demonstrates that COX-2 can be also upregulated by PRR activation independently of AngII in rat IM cells. Most of the pathophysiological effects of PRR have been reported in renal cortical tissues particularly in mesangial cells using transgenic models that over-express PRR; however, the present study supports the notion that in the renal inner medulla the activation of PRR contributes to the stimulation of COX-2 via ERK1/2. These findings are of great relevance in the light of recent in vivo evidence demonstrating that during AngII-dependent hypertension there are stimulation of renin and prorenin synthesis and secretion by the collecting duct cells 17, 18 and upregulation of PRR transcript. Clearly, more studies are needed to carefully test if during AngII-dependent hypertension, the activation of PRR in intercalated and interstitial cells by its natural agonists, contribute to buffer the local effects of AngII in the renal medulla by stimulating COX-2 and promoting the synthesis of vasodilator and natriuretic prostanoids.
Supplementary Material
Novelty and significance.
What is New?
This study provides evidence for a new role of the prorenin receptor (PRR) in the regulation of cyclooxygenase-2 (COX-2) in the rat renal medulla via mitogen activated protein kinase/extracellular regulated kinases (ERK1/2). In addition, we provide evidence that the PRR and COX-2 are co-localized in the intercalated cells of the collecting duct and in the interstitial cells, which support further our hypothesis.
What Is Relevant?
Our findings are of critical importance since they support the notion that activation of PRR by upregulating COX-2 via ERK1/2 in the interstitial and intercalated cells, it may increase prostaglandins synthesis, thus contributing to buffer local vasoconstrictor and antinatriuretic effects of AngII.
Summary.
PPR and COX-2 are co-expressed in interstitial cells and intercalated collecting duct cells. Activation of PRR by recombinant prorenin upregulated COX-2 even in the presence of AT1 receptor blockade in rat primary cultured renal inner medullary (IM) cells.
Upregulation of COX-2 by AngII or prorenin was ERK1/2 signaling dependent.
PPR knockdown prevented COX-2 upregulation mediated by prorenin treatment in rat IM cells.
Upregulation of COX-2 in IM cells is mediated by AngII and by the AngII- independent activation of PRR.
Acknowledgments
Sources of Funding
M.C.P. received funds from the National Institutes of Health (NIH) through the Institutional Developmental Award Program of the National Center for Research Resources (P20RR-017659), HL26371; American Heart Association (AHA; 09BGIA2280440) and Eunice Kennedy Shriver National Institute of Child Health & Human Development (K12HD043451). C.P.V. is supported by PFB 12-2007, FONDECYT 1080590, Chile.
Footnotes
Disclosures
None
References
- 1.Ludwig J, Kerscher S, Brandt U, Pfeiffer K, Getlawi F, Apps DK, Schagger H. Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J Biol Chem. 1998;273:10939–10947. doi: 10.1074/jbc.273.18.10939. [DOI] [PubMed] [Google Scholar]
- 2.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension. 2009;542:261–269. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen G, Delarue F, Berrou J, Rondeau E, Sraer JD. Specific receptor binding of renin on human mesangial cells in culture increases plasminogen activator inhibitor-1 antigen. Kidney Int. 1996;50:1897–1903. doi: 10.1038/ki.1996.511. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen G. Renin//prorenin receptors. Kidney Int. 2006;69:1503–1506. doi: 10.1038/sj.ki.5000265. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen G, Contrepas A. Physiology and pharmacology of the (pro)renin receptor. Current Opinion in Pharmacology. 2008;8:127–132. doi: 10.1016/j.coph.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Ichihara A, Sakoda M, Kurauchi-Mito A, Kaneshiro Y, Itoh H. Involvement of (pro)renin receptor in the glomerular filtration barrier. J Mol Med. 2008;86:629–635. doi: 10.1007/s00109-008-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble Form of the (Pro)Renin Receptor Is Augmented in the Collecting Duct and Urine of Chronic Angiotensin II-Dependent Hypertensive Rats. Hypertension. 2011;57:859–864. doi: 10.1161/HYPERTENSIONAHA.110.167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabi AH, Kageshima A, Uddin MN, Nakagawa T, Park EY, Suzuki F. Binding properties of rat prorenin and renin to the recombinant rat renin/prorenin receptor prepared by a baculovirus expression system. Int J Mol Med. 2006;18:483–488. [PubMed] [Google Scholar]
- 9.Nguyen G, Burckle CA, Sraer JD. Renin/prorenin-receptor biochemistry and functional significance. Curr Hypertens Rep. 2004;6:129–132. doi: 10.1007/s11906-004-0088-3. [DOI] [PubMed] [Google Scholar]
- 10.Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem. 1999;274:5038–5046. doi: 10.1074/jbc.274.8.5038. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Barbero A, Dorado F, Velasco S, Pandiella A, Banas B, Lopez-Novoa JM. TGF-beta1 induces COX-2 expression and PGE2 synthesis through MAPK and PI3K pathways in human mesangial cells. Kidney Int. 2006;70:901–909. doi: 10.1038/sj.ki.5001626. [DOI] [PubMed] [Google Scholar]
- 12.Kaneshiro Y, Ichihara A, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Hayashi M, Inagami T. Increased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic rats. Kidney Int. 2006;70:641–646. doi: 10.1038/sj.ki.5001627. [DOI] [PubMed] [Google Scholar]
- 13.Jaimes EA, Tian RX, Pearse D, Raij L. Up-regulation of glomerular COX-2 by angiotensin II: role of reactive oxygen species. Kidney Int. 2005;68:2143–2153. doi: 10.1111/j.1523-1755.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H. Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol. 2007;18:1789–1795. doi: 10.1681/ASN.2006091062. [DOI] [PubMed] [Google Scholar]
- 15.Qi Z, Hao CM, Langenbach RI, Breyer RM, Redha R, Morrow JD, Breyer MD. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest. 2002;110:61–69. doi: 10.1172/JCI14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension. 2011;57:594–599. doi: 10.1161/HYPERTENSIONAHA.110.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Gonzalez AA, McCormack M, Seth DM, Kobori H, Navar LG, Prieto MC. Increased Renin Excretion Associated with Augmented Urinary Angiotensin (Ang) II Levels in Chronic Angiotensin II-infused Hypertensive Rats. Am J Physiol Renal Physiol. 2011;301:F1195–F1201. doi: 10.1152/ajprenal.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuo J, Alcorn D, McCausland J, Mendelsohn FAO. Localization and regulation of angiotensin II receptors in renomedullary interstitial cells. Kidney Int. 1994;46:1483–1485. doi: 10.1038/ki.1994.425. [DOI] [PubMed] [Google Scholar]
- 20.Zhuo JL. Renomedullary interstitial cells: A target for endocrine and paracrine actions of vasoactive peptides in the renal medulla. Clin Exp Pharm Physiol. 2000;27:465–473. doi: 10.1046/j.1440-1681.2000.03277.x. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson S, Hébert RL, Laneuville O. NS-398 upregulates consitutive cyclooxygenase-2 expression in the M-1 cortical collecting duct cell line. J Am Soc Nephrol. 1999;10:2261–2271. doi: 10.1681/ASN.V10112261. [DOI] [PubMed] [Google Scholar]
- 22.Campean V, Theilig F, Paliege A, Breyer M, Bachmann S. Key enzymes for renal prostaglandin synthesis: site-specific expression in rodent kidney (rat, mouse) Am J Physiol Renal Physiol. 2003;285:F19–F32. doi: 10.1152/ajprenal.00443.2002. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, Upchurch GM, Prieto MC, Kobori H, Navar LG. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol. 2010;298:F150–F157. doi: 10.1152/ajprenal.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip goldblatt hypertensive rats. Hypertension. 2008;51:1590–1596. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The Collecting Duct Is the Major Source of Prorenin in Diabetes. Hypertension. 2008;51:1597–1604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327:459–463. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 27.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension. 2009;53:1077–1082. doi: 10.1161/HYPERTENSIONAHA.108.127258. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen G. Increased cyclooxygenase-2, hyperfiltration, glomerulosclerosis, and diabetic nephropathy: put the blame on the (pro)renin receptor? Kidney Int. 2006;70:618–620. doi: 10.1038/sj.ki.5001723. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Matavelli LC, Siragy HM. Renal (pro)renin receptor contributes to development of diabetic kidney disease through transforming growth factor-beta1-connective tissue growth factor signalling cascade. Clin Exp Pharmacol Physiol. 2011;38:215–221. doi: 10.1111/j.1440-1681.2011.05486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burckle CA, Jan Danser AH, Muller DN, Garrelds IM, Gasc JM, Popova E, Plehm R, Peters J, Bader M, Nguyen G. Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension. 2006;47:552–556. doi: 10.1161/01.HYP.0000199912.47657.04. [DOI] [PubMed] [Google Scholar]
- 31.Ichihara A, Itoh H, Inagami T. Critical roles of (pro)renin receptor-bound prorenin in diabetes and hypertension: sallies into therapeutic approach. J Am Soc Hypertens. 2008;2:15–19. doi: 10.1016/j.jash.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Hao C-M, Kömhoff M, Guan Y, Redha R, Breyer M. Selective targeting of cyclooxygenase-2 reveals its role in renal medullary interstitial cell survival. Am J Physiol-Renal Physiol. 1999;277:F352–F359. doi: 10.1152/ajprenal.1999.277.3.F352. [DOI] [PubMed] [Google Scholar]
- 33.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest. 1994;94:2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vio CP, Cespedes C, Gallardo P, Masferrer JL. Renal identification of cyclooxygenase-2 in a subset of thick ascending limb cells. Hypertension. 1997;30:687–692. doi: 10.1161/01.hyp.30.3.687. [DOI] [PubMed] [Google Scholar]
- 35.Yang T, Zhang A, Pasumarthy A, Zhang L, Warnock Z, Schnermann JB. Nitric oxide stimulates COX-2 expression in cultured collecting duct cells through MAP kinases and superoxide but not cGMP. Am J Physiol Renal Physiol. 2006;291:F891–F895. doi: 10.1152/ajprenal.00512.2005. [DOI] [PubMed] [Google Scholar]
- 36.Harris RC. An update on cyclooxygenase-2 expression and metabolites in the kidney. Curr Opin Nephrol Hypertens. 2008;17:64–69. doi: 10.1097/MNH.0b013e3282f1bb7d. [DOI] [PubMed] [Google Scholar]
- 37.Green T, Rodriguez J, Navar LG. Augmented cyclooxygenase-2 effects on renal function during varying states of angiotensin II. Am J Physiol Renal Physiol. 2010;299:F954–F962. doi: 10.1152/ajprenal.00609.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreri NR, An SJ, McGiff JC. Cyclooxygenase-2 expression and function in the medullary thick ascending limb. Am J Physiol. 1999;277:F360–F368. doi: 10.1152/ajprenal.1999.277.3.F360. [DOI] [PubMed] [Google Scholar]
- 39.Kammerl MC, Nusing RM, Schweda F, Endemann D, Stubanus M, Kees F, Lackner KJ, Fischereder M, Kramer BK. Low sodium and furosemide-induced stimulation of the renin system in man is mediated by cyclooxygenase 2. Clin Pharmacol Ther. 2001;70:468–474. doi: 10.1067/mcp.2001.119720. [DOI] [PubMed] [Google Scholar]
- 40.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann J, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol. 1998;274:F481–F489. doi: 10.1152/ajprenal.1998.274.3.F481. [DOI] [PubMed] [Google Scholar]
- 41.Zhang MZ, Hao CM, Breyer MD, Harris RC, McKanna JA. Mineralocorticoid regulation of cyclooxygenase-2 expression in rat renal medulla. Am J Physiol Renal Physiol. 2002;283:F509–F516. doi: 10.1152/ajprenal.00236.2001. [DOI] [PubMed] [Google Scholar]
- 42.Huang J, Siragy HM. Sodium Depletion Enhances Renal Expression of (Pro)Renin Receptor via Cyclic GMP-Protein Kinase G Signaling Pathway. Hypertension. 2012;59:317–323. doi: 10.1161/HYPERTENSIONAHA.111.186056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.