Abstract
Objectives
To determine the gene expression profile of pelvic ganglia neurones after bilateral cavernosal nerve resection (BCNR) and subsequent treatment with sildenafil in relation to neurotrophic-related pathways.
Materials and methods
Fisher rats aged 5 months were subjected to BCNR or sham operation and treated with or without sildenafil (20 mg/kg body-weight in drinking water) for 7 days.
Total RNA isolated from pelvic ganglia was subjected to reverse transcription and then to quantitative reverse transcriptase-polymerase chain reaction (PCR) with the RAT-neurotrophic array.
Results were corroborated by real-time PCR and western blotting.
Another set of animals were injected with a fluorescent tracer at the base of the penis, 7 days before BCNR or sham operation, and were sacrificed 7 days after surgery.
Sections of pelvic ganglia were used for immunohistochemistry with antibodies against neurturin, neuronal nitric oxide synthase, tyrosine hydroxylase and glial cell line-derived neurotrophic factor receptor α2.
Results
A down-regulation of the expression of neuronal nitric oxide synthase accompanied by changes in the level of cholinergic neurotrophic factors, such as neurturin and its receptor glial cell line-derived neurotrophic factor receptor α2, artemin, neurotrophin-4 and cilliary neurotrophic factor, was observed 7 days after BCNR in pelvic ganglia neurones.
Treatment with sildenafil, starting immediately after surgery, reversed all these changes at a level similar to that in sham-operated animals.
Conclusions
Sildenafil treatment promotes changes in the neurotrophic phenotype, leading to a regenerative state of pelvic ganglia neurones.
The present study provides a justification for the use of phosphodiesterase 5 inhibitors as a neuroprotective agent after BCNR.
Keywords: bilateral cavernosal nerve resection, GFR-α2, main pelvic ganglia, neurturin, nNOS, sildenafil
Introduction
Erectile dysfunction and urinary incontinence are common complications for patients undergoing surgery for prostate, bladder and colorectal cancers, as a result of damage to the nerves associated with the major pelvic ganglia [1–4]. Despite nerve-sparing techniques [5], it takes many months or years for patients to regain continence and erectile function, and a full recovery is not always achieved. Although peripheral nerves have the ability to regenerate [6], the recovery very often is inadequate.
The neurones of the major pelvic ganglia (MPG) innervate urogenital organs and components of the lower bowel [7]. The cavernosal nerves, involved in the motor innervation of the penis, originate from the MPG and contain parasympathetic and sympathetic fibres. The main neurotransmitter released by the cavernosal nerves is nitric oxide (NO), which is synthesized by neuronal nitric oxide synthase (nNOS) located in the nerve terminals [8]. NO increases the production of cGMP in smooth muscle fibres and is recognized as the most important activator for local relaxation of penile smooth muscle [8].
The success of functional reinnervation of target organs depends on the capacity of the neurones to survive and switch towards a regenerative phenotype [6]. A variety of neurotrophic factors have been reported to stimulate axonal regeneration and neuronal survival, indicating that not only one factor, but also a mixture of different neurotrophic factors and neurotropic molecules [9] is involved in potentiating the regenerative capacity of axotomized neurones.
Neurturin (NRTN), a parasympathetic neurotrophic factor, is a member of the glial cell line-derived neurotrophic factor (GDNF) family that is structurally related to the transforming growth factor-b proteins [10,11]. Other members of the family are persephin [12] and artemin [13]. Biological actions of the GDNF family members are mediated via the GDNF family receptors (i.e. glial cell line-derived neurotrophic factor receptor [GFR]-α1–4) and the signal transducing receptor tyrosine kinase Ret [14,15]. NRTN promotes the survival and maintenance of parasympathetic neurones via the activation of GFR-α2 and the transmembrane tyrosine kinase Ret. NRTN and its receptor, GFR-α2, are widely expressed in the penile corpora cavernosa and neurones of the MPG, and are responsible for the maintenance of terminal fields in urogenital organs [16] in men. In addition, Bella et al. [17] have shown that treatment with NRTN at the site of cavernous nerve crush injury facilitates the recovery of the erectile function, suggesting that NRTN could be used as a neuroprotective or neuroregenerative agent to facilitate functional recovery after cavernosal or other pelvic autonomic nerve injuries
Emerging evidence indicates that NO and cGMP favour a neuroprotective outcome in the central nervous system. Sildenafil induces neurogenesis in neurospheres in vitro [18] by increasing cGMP levels, which plays an important role in modulating the fate of neural stem cell differentiation [19]. Reduced levels of cGMP result in a reduced formation of neurones and an increased formation of non-neuronal cells [19]. The activation of the NO/cGMP pathway protects against the striatal degeneration induced by 3-nitropropionic acid neurotoxicity by promoting the activation survival pathways [20].
It was previously shown that treatment with phosphodiesterase (PDE)5 inhibitors such as sildenafil [21], vardenafil [22] and tadalafil [23] preserves penile corporal smooth muscle and ameliorates the fibrotic degeneration normally seen after bilateral cavernosal nerve resection (BCNR) in rats. This effect is mediated by the down-regulation of genes related to fibrosis and the up-regulation of genes related to smooth muscle preservation [24]. However, little is still known about the changes in pelvic ganglia neurones after BCNR, nor whether PDE5 inhibitors can exert a neuroprotective effect by switching the axotomized pelvic ganglia neurones into a regenerative state possibly promoting nerve regeneration.
The present study aimed to investigate changes in the gene expression profile of neurotrophin-related pathways in pelvic ganglia neurones after BCNR and subsequent treatment with sildenafil.
Materials and methods
Animal treatment
Fisher 344 rats (Harlan Sprague Dawley, San Diego, CA, USA), aged 5 months, were treated with an Institutional Animal Care and Use Committee-approved protocol, and divided (n = 8 per group) as: sham-operated; BCNR; and BCNR+sildenafil (BCNR+SIL); and then sacrificed 7 days after surgery. Selection of the time point was based on the findings of a previous study where a manifested fibrotic degeneration in the penile corpora cavernosa was observed [25]. BCNR was performed as described previously [21–25]. In the sham-operated group, both cavernosal nerves were identified but not resected. In the BCNR and BCNR+SIL groups, the main cavernosal nerves and ancillary branches were resected by removing a 5-mm segment. Sildenafil (Bayer, West Haven, CT) was dissolved in drinking water (0.3 mg/mL), as described previously [24]. The drinking volume was determined daily and body-weight was recorded weekly. The daily sildenafil dose (20 mg/kg body-weight), corrected for differences in total body surface area [24], was more or less equivalent to a single 200-mg tablet daily dose in men, which was shown to be effective for treating previous sildenafil non-responder patients [26].
After the corresponding treatments, rats were sacrificed by CO2 inhalation. The pelvic ganglia (n = 8 animals per group) were dissected out and immersed in RNAlater (Ambion, Foster City, CA) for RNA preservation.
RT2 Profiler™ PCR array analysis of neurotrophic and nitric oxide-related target genes
For total RNAs, pelvic ganglia were pooled and isolated by homogenization with Trizol Reagent (Invitrogen, Carlsbad, CA, USA). Total RNAs were purified using Rneasy minicolumns (Qiagen, Valencia, CA, USA) and stored at −80 °C until further use. RNA samples were subjected to reverse transcription, and the resulting cDNA was analyzed by the Rat Neurotrophin and Receptors RT2 Profiler™ PCR Array (SABiosciences Corp., Frederick, MD, USA), which contains a panel of 84 primer sets related to genes involved in neuronal cell growth, differentiation, neuronal regeneration and survival.
Real-time PCRs comprised: melting for 10 min at 95 °C, 40 cycles of two-step PCR including melting for 15 s at 95°C, and annealing for 1 min at 60 °C. The raw data were analyzed using the ΔΔCt method in accordance with the manufacturer’s instructions (SABiosciences Corp.) [24,27]. Only genes that expressed more than 1.8-fold changes were considered to be significant compared to the respective controls.
Real-time PCR by TaqMan®
The levels of expression of NRTN and nNOS encoding transcripts in the pelvic ganglia in sham-operated, BCNR and BCNR+SIL individual animals were measured by quantitative real-time PCR using custom TaqMan® primers and FAM-labelled TaqMan® Minor Groove Binder probes (Applied Biosystems, Foster City, CA, USA). Real-time PCR reactions were set up by combining 10 μL of TaqMan® Universal Master Mix II no UNG (Applied Biosystems), 1 μL of forward and reverse primers (previously diluted to 10 μM) and 9 μL of 50 ng/μL life-cycle and isolate-specific cDNA, giving a total volume of 20 μL. Triplicate reactions were set up for each sample, isolate and life-cycle stage combination in a MicroAmp™ Optical 96-well plate sealed with MicroAmp™ Optical adhesive film (Applied Biosystems). All real-time PCR runs were carried out on an ABI 7000 sequence detection system (Applied Biosystems) using the conditions: 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s. Collection of data occurred during the final phase of each cycle. Triplicate real-time PCR runs were carried out for each gene under test. The cycle threshold (Ct) values were recalculated by setting the baseline and threshold to identical values for each of the three runs to enable direct comparison among the runs. Relative quantification of the gene expression level was carried out using the comparative Ct (ΔΔCt) method [28] and determined as the difference between the Ct for a specific mRNA gene and the Ct for a reference mRNA, normalized to ribosomal protein, large 1/3 threshold expression.
Western blot analysis
Pelvic ganglia proteins were extracted from Trizol fractions in accordance with the manufacturer’s instructions. Protein aliquots (30–50μg) were subjected to western blotting [24,27] by 4–15% Tris–HCl PAGE (Bio-Rad, Hercules, CA, USA) in running buffer (Tris/glycine/SDS). Proteins were transferred to nitrocellulose membranes in transfer buffer (Tris/glycine/methanol) for 3 h. Subsequently, the non-specific binding was blocked by immersing the membranes into 5% non-fat dried milk and 0.1% (v/v) Tween-20 in phosphate-buffered saline (PBS) (× 1) overnight at 4 °C. After several washes with washing buffer (PBS Tween 0.1%), the membranes were incubated with the primary antibodies for 3 h at room temperature: (i) mouse monoclonal nNOS (dilution 1 : 500) (BD Biosciences Pharmingen, San Diego, CA, USA); (ii) rabbit polyclonal neurturin (dilution 1 : 500) (Abcam, Cambridge, MA, USA); (iii) a polyclonal antibody GDNF family receptor, GFR-α2 (dilution 1 : 500) (ProSci Inc., Poway, CA, USA); and (iv) glyceraldheyde 3-phosphate dehydrogenase (GAPDH) (dilution 1 : 10 000) (Chemicon International, Temecula, CA, USA). The washed membranes were incubated for 1.5 h at room temperature with a 1 : 2000 dilution of secondary antibodies, anti-mouse or anti-rabbit, respectively, linked to horseradish peroxidase. After several washes, the immunoreactive bands were visualized using the ECL Plus Western Blotting System (Amersham Biosciences, Piscataway, NJ, USA). The densitometric analysis of the bands was performed using ImageJ (NIH, Bethesda, MD, USA). A positive control was run for all gels for each antibody to standardize with respect to variation in exposures and staining intensities. Negative controls were performed omitting the primary antibody. Band intensities were determined by densitometry and the respective intensities were corrected by GAPDH, a housekeeping protein, upon reprobing the same membrane.
Retrograde labelling of pelvic ganglion cells
Pelvic ganglia neurones that specifically innervate the penis were traced by pressure injection of the fluorescent tracer Fluorogold (FG; Fluorochrome Inc., Engelwood, CO, USA), to the penis, 1 week before any experimental manipulation. Briefly, under general isoflurane anesthesia, rats were injected 7 days before sham operation or BCNR surgery with 4% FG into the cavernosal space of the penis at multiple sites (2 μL each, maximum of 8–10 μL) using a 10-μL Hamilton syringe and a 30-gauge needle. Overflow of tracer solutions was rinsed with sterile saline, and the injection sites were dried and coated with Dermabond (Ethicon Inc., Somerville, NJ, USA) to prevent any spread of dye [29]. To show the specificity of the retrograde tracing, a group of animals were injected with FG into the peritoneal cavity as a negative control. Animals were sacrificed 14 days after FG injection to allow the dye to reach the spinal cord.
After treatment, rats were deeply anaesthetized with pentobarbital (100 mg/kg bodyweight), perfused with saline followed by 4% p-formaldehyde. The pelvic ganglia were dissected out, postfixed in 4% p-formaldehyde overnight and transferred to 25% sucrose until sectioning.
Immunohistochemistry and immunofluorescence
The detection of neurturin, nNOS and GFR-α2 by immunohistochemistry was carried out on 16-μm frozen sections [30]. Sections were washed with PBS, quenched for endogenous peroxidase activity, blocked with 10% normal goat serum in PBS in 0.15% Triton X-100, and incubated overnight in a humidified chamber at 4 °C with the primary antibodies: anti-nNOS (dilution 1 : 400) (BD Biosciences Pharmingen), anti-neurturin (dilution 1 : 250) (Abcam) and anti-GFR-α2 (dilution 1 : 250) (ProSci Inc.) as described above. Biotinylated goat anti-rabbit or horse anti-mouse secondary antibody (dilution 1 : 200) (Vector Laboratories, Burlingame, CA, USA) were applied and incubated for 40 min, followed by 30 min with ABC peroxidase complex (Vector Laboratories) and 3,3′-diaminobenzidine as a chromogen. Sections were dehydrated and cover slips were mounted. Negative controls were made by replacing the first antibody with non-immune IgG. Each slide assayed had a negative control. Sections were counterstained with haematoxylin, except for GFR-α2 immunostaining.
Colocalization of FG positive neurones with nNOS, tyrosine hydroxylase (TH), neurturin and GFR-α2 by immunofluorescence
Frozen sections were pre-incubated with 10% goat serum or horse serum and subsequently with a 1 : 400 dilution of the anti-neurturin polyclonal antibody, or a 1 : 200 dilution of GFR-α2, or a 1 : 200 dilution of anti-nNOS monoclonal antibody, and a 1 : 400 dilution of anti-TH polyclonal antibody followed by a 1 : 200 dilution of anti-rabbit biotinylated secondary antibody or anti-mouse biotinylated secondary antibody (Vector Laboratories), respectively. Sections were then incubated in 15 mg/mL of streptavidin-Texas red or streptavidin-fluorescein isothiocyanate (Vector Laboratories). Sections were mounted in Prolong Anti-Fade [35] (Molecular Probes, Eugene, OR, USA) and examined under an Olympus microscope (Olympus, Tokyo, Japan). For the visualization of FG positive neurones, a wide ultraviolet filter was employed.
Quantitative image analysis
Quantitative image analysis was performed using ImagePro Plus, version 7.0 (Media Cybernetics, Silver Spring, MD, USA) [21–27]. For NRTN and nNOS staining, the number of positive neurones vs the total number of neurones per field was analyzed per tissue section. For determination of GFR-α2, the total integrated optical density was used to determine changes in expression. After the images are calibrated for background lighting, the integrated optical density is proportional to the concentration of antigen assayed. In all cases, at least four matched sections per animal and eight animals per group were analyzed in each of the groups [23–27].
Statistical analysis
Values were expressed as the mean (SEM). The normality distribution of the data was established using the Wilk–Shapiro test. Multiple comparisons were analyzed by one-way one-way ANOVA followed by post-hoc comparisons with Tukey’s test using GraphPad Prism, version 5.0 (GraphPad Software Inc., San Diego, CA, USA). P < 0.05 was considered statistically significant.
Results
Characterization of pelvic ganglia neurones that innervate the penis
To characterize the pelvic ganglia neurones that innervate the penis, FG was injected into the corpora cavernosa of sham-operated animals. Immunohistochemistry for non-adrenergic, non-cholinergic-parasympathetic pathway nNOS and sympathetic pathway TH showed that all FG-positive cells co-localized with nNOS and not with TH (Fig. 1A), indicating that the parasympathetic innervation arises from the pelvic ganglia. Moreover, no changes in the expression of TH and co-localization with FG were observed after BCNR and sildenafil treatment in the pelvic ganglia, although a decrease in the labelling of FG positive neurones was observed after BCNR (Fig. 1B).
FIG. 1.
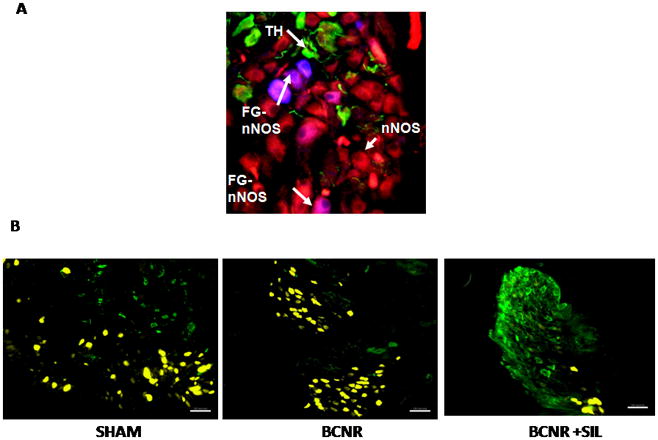
Top: characterization of the pelvic ganglia neurones that innervate the penis. Sections of sham-operated pelvic ganglia were immunostained with antibodies against nitric oxide synthase (nNOS) and antibodies against tyrosine hydroxylase (TH) and double co-localized with Fluorogold (FG)-labelled neurones. FG was injected in the penile corpora cavernosa 7 days before surgery and animal treatment. Blue: FG; red: nNOS; green: TH. Magnification × 400. Bottom: TH expression is not affected by bilateral cavernosal nerve resection (BCNR) and BCNR+sildenafil (BCNR+SIL). Sections of the pelvic ganglia from sham-operated (SHAM), BCNR and sildenafil animals 7 days after treatment were immunostained with anti-TH antibody and by biotin secondary antibody followed by streptavidin-fluorescein isothiocyanate. Green: TH positive neurones; yellow: FG positive neurones. Magnification × 100. Scale bar = 50μm.
BCNR and sildenafil treatment promotes changes in the gene expression of neurotrophic factors and receptors
The effect of BCNR and BCNR+SIL treatment on the gene expression profile of neurotrophins and receptors in the pelvic ganglia was studied 7 days after surgery. The profile of the fold difference between BCNR and sham-operated animals showed a decrease in the expression of five genes related to neurotrophic factors and receptors. Table 1 shows the list of these genes with their respective fold changes that were down-regulated by BCNR in comparison with sham-operated animals. A reduction in the gene expression of neurotrophic factors such as cilliary neurotrophic factor (CNTF) and neurotrophin-4 (NTF-4), and GDNF family members such as artemin and neurturin (NRTN) with its respective receptor GFR-α2, were observed 7 days after BCNR.
TABLE 1.
Changes in the gene expression profile of neurotrophic-related pathways determined by quantitative reverse transcriptase-polymerase chain reaction (PCR) array 7 days after sham operation, bilateral cavernosal nerve resection (BCNR) and BCNR+sildenafil (BCNR+SIL) treatment
| Symbol | Description | Sham vsBCNR | BCNR vs BCNR+SIL |
|---|---|---|---|
| Artn | Artemin | −3.21 | 3.79 |
| Cntf | Ciliary neurotrophic factor | −2.38 | 2.17 |
| Gfra2 | GDNF family receptor α2 | −1.92 | 2.05 |
| nNOS | Neuronal nitric oxide synthase | −1.59 | 2.11 |
| Nrtn | Neurturin | −1.46 | 2.80 |
| Ntf-4 | Neurotrophin 4 | −2.48 | 2.4 |
Pooled major pelvic ganglia total RNA from sham-operated (Sham), BCNR and BCNR+SIL animals after 7 days was subjected to qRT-PCR array applying the Rat Neurotrophin and Receptors RT2 Profiler™ PCR Array. A list is provided of genes that are up- or down-regulated by BCNR in comparison with Sham and BCNR+SiL in comparison with BCNR. The ratios between the Sham vs BCNR and BCNR vs BCNR+SIL were normalized by ribosomal protein, large 1; ribosomal protein, large 3; and lactate dehydrogenase housekeeping genes, and were calculated for the assays performed in quadruplicate. Genes that were +1.8-fold up-regulated are shown underlined; genes down-regulated by −1.8-fold are shown in bold. Genes regulated by less than 1.8-fold are shown in normal text. Genes that show 1.8-fold changes or more are considered as significant.
Sildenafil treatment starting immediately after surgery reversed this process by up-regulating the expression of the same genes described above (Table 1). A significant up-regulation of NTF-4, CNTF, NRTN and GFR-α2 was observed after BCNR+SIL treatment. The comparison between sham-operated and BCNR+SIL animals did not show significant changes, indicating that sildenafil is exerting a more neuroprotective effect on the pelvic ganglia neurones rather than a neurotrophic effect after nerve damage.
The changes observed by PCR array in the pooled samples were confirmed by real-time PCR using TaqMan®. mRNA levels from selected genes were evaluated in each experimental animal. A 75% reduction in NRTN mRNA expression with respect to sham operation was found after BCNR. Sildenafil reversed this effect by maintaining the same level as the sham-operated animals.
At the protein level, the expression of the parasympathetic neurotrophic factor, NRTN, was studied by western blotting and immunohistochemistry. Figure 2B shows that the expression of NRTN was decreased after BCNR by 60% compared to sham operation. Sildenafil treatment promoted a significant, up-regulation of NRTN expression compared to BCNR, reaching a level similar to that in sham-operated animals. To determine the cellular localization of NRTN, its expression was assessed by immunohistochemistry and quantified by image analysis. NRTN was observed to be expressed in neurones. BCNR reduced NRTN expression by 80%, although 7 days of sildenafil treatment after BCNR maintained the same level of expression as in sham-operated animals (Fig. 2C)
FIG. 2.
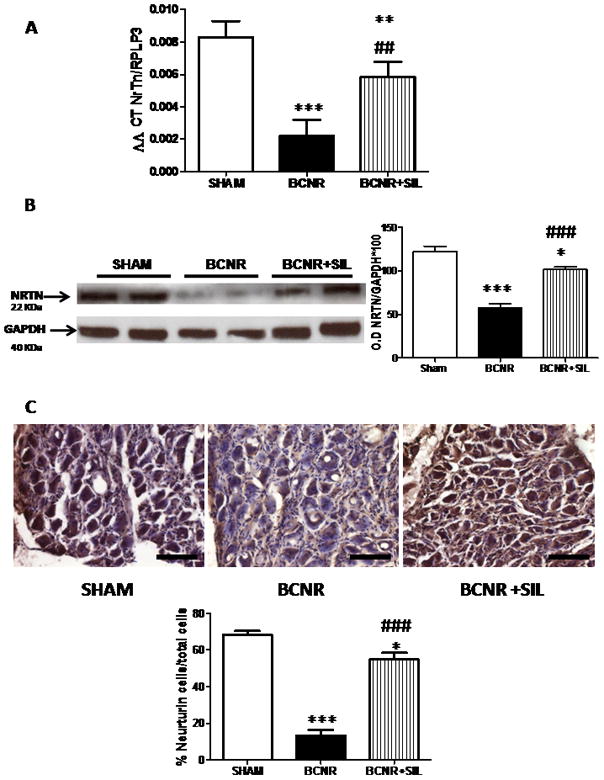
Treatment with sildenafil increases the expression of neurturin in pelvic ganglia neurones after bilateral cavernosal nerve resection (BCNR). A, Total RNA was extracted from sham-operated (SHAM), BCNR and BCNR+sildenafil (BCNR+SIL) animals after 7 days and subjected to real-time PCR by TaqMan® for neurturin (NRTN) (Applied Biosystems), normalized by ribosomal protein, large 3 (RPLP3) housekeeping gene. B, Pelvic ganglia protein extracts were subjected to western blot analysis for NRTN (Abcam), normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) with the correspondent densitometric analysis. C, Frozen pelvic ganglia sections from Sham, BCNR and BCNR+SIL animals after 7 days were immunostained for NRTN and subsequent detection by 3,3′-diaminobenzidine, and counterstained with haematoxylin. Representative images (magnification × 100; scale bar = 50 μm). Bottom: image analysis determined by the percentage of positive NRTN cells over the total number of cells in the pelvic ganglia. *P < 0.05, **P < 0.01 and ***P < 0.001 with respect to Sham; ###P < 0.001 and ##P < 0.01 with respect to BCNR.
The changes in the expression of NRTN after BCNR and sildenafil treatment were also corroborated by immunofluorescence. Figure 3 shows the localization of NRTN (green) and FG (yellow) positive neurones with its respective composites. Decreased NRTN expression by BCNR was not only confined to FG positive neurones, but also mostly in all pelvic ganglia neurones. Sildenafil treatment restored the levels of NRTN, especially in FG positive neurones, with an increased density close to the boundary of the cells.
FIG. 3.
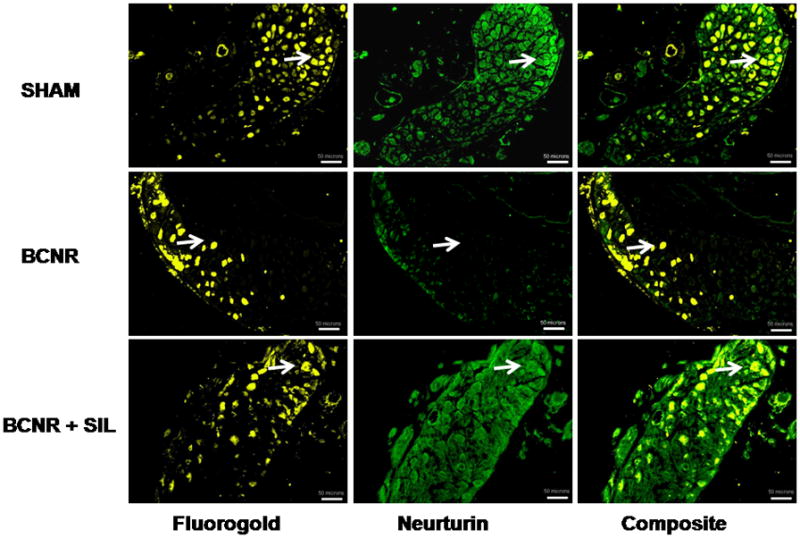
Co-localization of neurturin (NRTN) and Fluorogold (FG) is up-regulated by sildenafil (SIL) treatment after bilateral cavernosal nerve resection (BCNR). Frozen pelvic ganglia sections (as in Fig. 1) were immunostained with a polyclonal antibody against NRTN followed by a biotinylated secondary antibody and streptavidin conjugated with fluorescein isothiocyanate. Green: NRTN; yellow: FG. Magnification × 100. Scale bar = 100 μm. Arrows indicate FG and NRTN positive neurones. SHAM, sham-operated.
The expression of NRTN receptor, GFR-α2, was also evaluated by western blotting. Figure 4A shows that the expression of GFR-α2 was decreased 7 days after BCNR and preserved by sildenafil treatment. Densitometry analysis showed a 57% decrease compared to sham operation, whereas levels were maintained by sildenafil treatment.
FIG. 4.
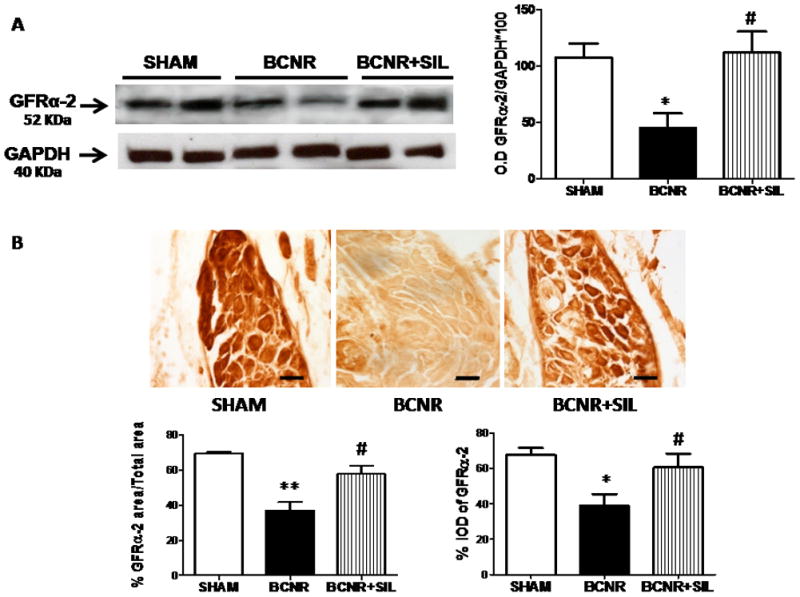
Sildenafil (SIL) modulates the expression of glial cell line-derived neurotrophic factor receptor α2 (GFR-α2) in the pelvic ganglia after bilateral cavernosal nerve resection (BCNR). A, Protein extracts (as in Fig. 2) were subjected to western blot analysis for GFR-α2 and normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) with the correspondent densitometric analysis. B, Frozen pelvic ganglia sections from sham-operated (SHAM), BCNR and BCNR+SIL animals after 7 days were immunostained for GFR-α2 and subsequent detection by 3,3′-diaminobenzidine with no counterstaining. Representative pictures (magnification × 200; scale bar = 50 μm). Bottom: image analysis determined by the percentage of GFR-α per area and by the percentage total integrated optical density per cell. *P < 0.05 and **P < 0.01 with respect to Sham; #P < 0.05 with respect to BCNR.
The localization of GFR-α2 was determined by immunohistochemistry followed by image analysis. The analysis showed a 30% decrease in the expression of GFR-α2 in BCNR but subsequent up-regulation by sildenafil treatment (Fig. 4B). To determine whether the changes in the expression of GFR-α2 were only confined to the penile-projecting neurones, co-localization with FG was performed. Figure 5 shows that the expression of GFR-α2 is located in the membrane of the cells and decreased expression is only observed in the affected neurones.
FIG. 5.
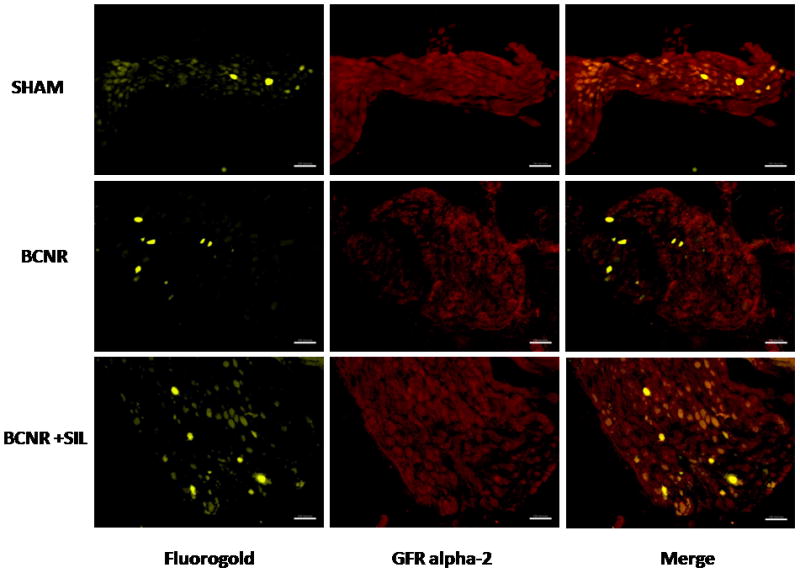
The expression of glial cell line-derived neurotrophic factor receptor α2 (GFR-α2) in neurones that project to the penis is modulated by sildenafil (SIL) after bilateral cavernosal nerve resection (BCNR). Frozen pelvic ganglia sections (as in Fig. 1) were immunostained with a polyclonal antibody against GFR-α2 followed by a biotinylated secondary antibody and streptavidin conjugated with Texas red. Red: GFR-α2; yellow: Fluorogold (FG). Magnification × 100. Scale bar = 100 μm. SHAM, sham-operated.
nNOS expression is modulated by BCNR+SIL treatment
Table 1 shows (by PCR array) that the expression of nNOS in the pelvic ganglia was reduced by BCNR compared to sham operation but then up-regulated in expression by sildenafil compared to BCNR. To confirm these results, the levels of RNA were determined by TaqMan® real-time PCR in individual samples. The nNOS mRNA levels in BCNR decreased by 78% but were then significantly up-regulated by sildenafil, confirming our PCR array studies (Fig. 6A).
FIG. 6.
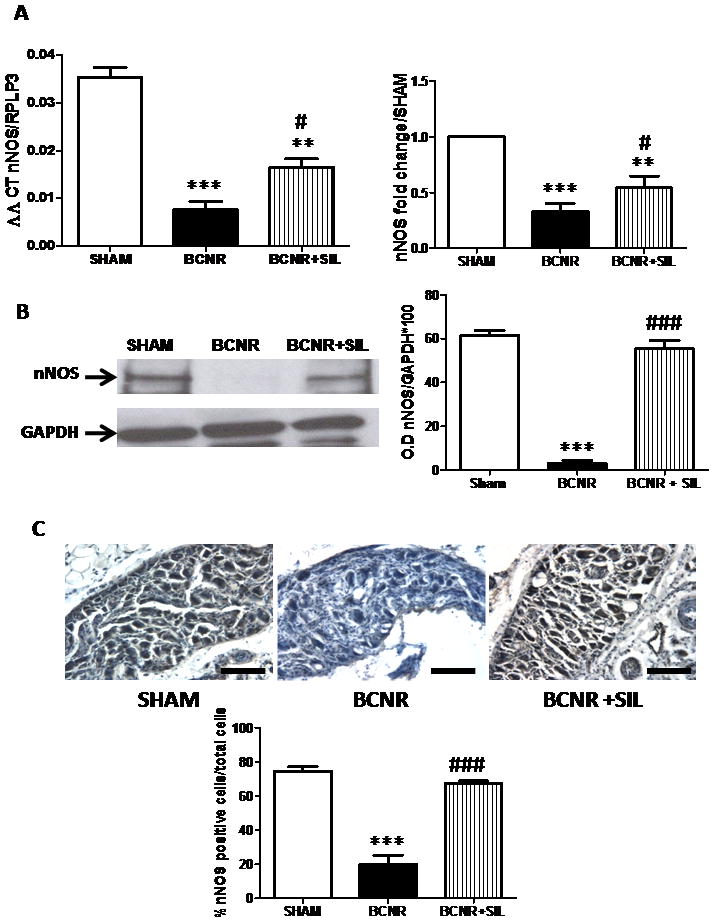
The expression of nitric oxide synthase (nNOS) in pelvic ganglia neurones is modulated by bilateral cavernosal nerve resection (BCNR) and sildenafil. A, Total RNA was extracted from sham-operated (SHAM), BCNR and BCNR+sildenafil (BCNR+SIL) animals after 7 days and subjected to real-time PCR by TaqMan® for nNOS and normalized by the ribosomal protein, large 3 (RPLP3) housekeeping gene (as in Fig. 2). B, Pelvic ganglia protein extracts were subjected to western blot analysis for nNOS employing a monoclonal antibody normalized by GAPDH with the correspondent densitometric analysis. C, Frozen pelvic ganglia sections from Sham, BCNR and BCNR+SIL animals after 7 days were immunostained for nNOS employing a polyclonal antibody and subsequent detection by 3,3′-diaminobenzidine, and were counterstained with haematoxylin. Representative images are shown. Magnification × 100. Scale bar = 100 μm. Bottom: image analysis determined by the percentage of positive nNOS cells over total number of cells in the pelvic ganglia *P < 0.05; **P < 0.01 and ***P < 0.001 with respect to sham operation; #P < 0.05 and ###P < 0.001 with respect to BCNR.
To corroborate that changes in steady-state mRNA levels were also observed at the protein level, the expression of nNOS was determined by western blotting and immunohistochemistry. Figure 6B shows that nNOS expression was reduced by BCNR and preserved by sildenafil treatment. Densitometric analysis showed a significant reduction of 95% by BCNR and up-regulation by sildenafil treatment at a level similar to that in sham-operated animals. Figure 6C shows nNOS expression as assessed by immunohistochemistry, confirming that the expression of nNOS was dramatically reduced after BCNR, and also that sildenafil treatment preserved nNOS expression at a similar level to that in sham-operated animals.
Discussion
The results obtained in the present study show that treatment with PDE5 inhibitors ameliorates the impact of cavernosal nerve damage on pelvic ganglia neurones by promoting a neuroprotective microenvironment that may favour nerve regeneration. It is shown that cavernosal nerve damage triggered a cascade of events in axotomized pelvic ganglia neurones. This included changes in the level of neurotransmitters, neurotrophic factors and cytokine production (not shown), all of which were reversed by sildenafil treatment.
The results obtained in the present study show that, 7 days after cavernosal nerve damage, the pelvic ganglia neurones had undergone dramatic changes that included a general down-regulation of the expression of nNOS, as well as changes in the level of cholinergic neurotrophic factors such as neurturin, its receptor GFR-α2, NTF-4, artemin and CNTF specifically in axotomized neurones. Remarkably, treatment with sildenafil starting immediately after surgery reversed all these changes to a level similar to that in sham-operated animals.
The neurturin-GFR-α2 pathway is mainly implicated in the development of the parasympathetic neurones of many pelvic organs [16,31]. Neurturin is considered to be one of the neurotrophic factors responsible for the survival and maintenance of nNOS-positive neurones [32] and its expression is modulated by cavernosal nerve damage [33,34]. The results obtained in the present study indicate that BCNR down-regulates the expression of NRTN and GFR-α2 in both axotomized neurones and non-axotomized neurones, showing that cavernosal nerve damage affects the whole organ. Sildenafil promotes an up-regulation of the level of the neurotrophic factor and its receptor similar to that in sham-operated animals, indicating that sildenafil is involved in preserving the survival of pelvic ganglia neurones. The results obtained in the present study are in agreement with those reported by Palma et al. [35] who showed a down-regulation of the expression of neurturin and GFR-α2 after axotomy. An up-regulation of the expression of GFR-α2 co-localized with nNOS was found in the contralateral MPG after nerve damage, implicating an involvement of GFR-α2 in the maintenance and recovery of erectile function after nerve damage.
The impact of peripheral nerve damage on nNOS expression is controversial because it may depend on localization of the nerve injury and the type of injured neurones. Some studies have indicated that peripheral nerve damage in adult animals results in a dramatic up-regulation of NOS in certain types of central and peripheral neurones normally lacking or rarely expressing the enzyme, and this may contribute to neuronal degeneration and neuropathic pain [36–38]. The results obtained in the present study show that the expression of nNOS in the pelvic ganglia was dramatically reduced by BCNR. This is in agreement with our previous study reporting that, at 7 days (and even more pronounced at 30 days), a down-regulation of the expression of nNOS was observed at the nerve terminals in the penis [25]. The results obtained in the present study also show, for the first time, an effect of sildenafil with respect to maintaining the expression of nNOS in the pelvic ganglia after nerve damage. However, up-regulation of the expression of nNOS in the penile corporal nerve terminals was reported using the PDE5 inhibitor, Icarisid II, in a model of diabetes-associated erectile dysfunction [39].
There is strong evidence that neuronal NO contributes to neuroplasticity-associated protein expression through the activation of cGMP, protein kinase G and extracellular signal-regulated kinase (ERK) [40]. Moreover, increased cGMP levels as a result of the inhibition of cGMP-hydrolyzing phosphodiesterases have been shown to reverse deficits in long-term potentiation and long-term depression in neurodegenerative disease models [41,42]. In addition, it was previously reported that sildenafil exerts a protective effect on cardiomyocytes after ischaemia reperfusion through the activation of the ERK1/2 and p38 mitogen-activated protein kinase pathway [43]. Accordingly, we postulate that sildenafil would promote neuroplasticity of the MPG neurones by preserving nNOS levels and also by modulating cGMP levels, which in term would activate the mitogen-activated protein kinase/ERK signalling pathway and induce the up-regulation of neurotrophic factors and receptors such as neurturin and GFR-α2.
In conclusion, the results obtained in the present study indicate that treatment with PDE inhibitors can up-regulate the expression of beneficial factors in pelvic ganglia neurones that may favour nerve regeneration. However, further studies are needed to determine whether nerve regeneration is achieved after long-term treatment with PDE5 inhibitors. The results obtained in the present study provide a rationale for the administration of PD5 inhibitors for erectile dysfunction-associated neuropathies.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke and the National Institute of General Medicine NINDS/NIGMS SC1NS064611 (M.G.F.), the National Center for Research Resources (NCRR) 1U54RR026138 (J.N.A.) and the Research Centers in Minority Institutions, Translational Research Network (RTRN) U54RR022762 (J.N.A.). We thank Jessica Escobedo from Axis (U54RR026138) for editing the manuscript.
Abbreviations
- BCNR
bilateral cavernosal nerve resection
- CNTF
cilliary neurotrophic factor
- ERK
extracellular signal-regulated kinase
- FG
Fluorogold
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GDNF
glial cell line-derived neurotrophic factor
- GFR-α2
glial cell line-derived neurotrophic factor receptor α2
- IMM
immunohistochemistry
- MPG
major pelvic ganglia
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NRTN
neurturin
- NTF-4
neurotrophin-4
- PDE
phosphodiesterase
- SIL
sildenafil
References
- 1.Hatzimouratidis K, Burnett AL, Hatzichristou D, McCullough AR, Montorsi F, Mulhall JP. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. Eur Urol. 2009;55:334–47. doi: 10.1016/j.eururo.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 2.El-Sakka AI. Alleviation of post-radical prostatectomy cavernosal fibrosis: future directions and potential utility for PDE5 inhibitors. Expert Opin Investig Drugs. 2011;20:1305–9. doi: 10.1517/13543784.2011.609315. [DOI] [PubMed] [Google Scholar]
- 3.Peterson AC, Chen Y. Patient reported incontinence after radical prostatectomy is more common than expected and not associated with the nerve sparing technique: results from the center for prostate disease research (CPDR) database. Neurourol Urodyn. 2012;31:60–3. doi: 10.1002/nau.21189. [DOI] [PubMed] [Google Scholar]
- 4.Nitori N, Hasegawa H, Ishii Y, Endo T, Kitajima M, Kitagawa Y. Sexual function in men with rectal and rectosigmoid cancer after laparoscopic and open surgery. Hepatogastroenterology. 2008;55:1304–7. [PubMed] [Google Scholar]
- 5.Walsh PC, Mostwin JL. Radical prostatectomy and cystoprostatectomy with preservation of potency. Results using a new nerve-sparing technique. Br J Urol. 1984;56:694–7. doi: 10.1111/j.1464-410x.1984.tb06149.x. [DOI] [PubMed] [Google Scholar]
- 6.Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell BS. Morphology and neurochemistry of the pelvic, and paracervical ganglia. Histol Histopathol. 1993;8:761–73. [PubMed] [Google Scholar]
- 8.Giuliano F, Rampin O. Neural control of erection. Physiol Behav. 2004;83:189–201. doi: 10.1016/j.physbeh.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HY, Jin XB, Lue TF. Three important components in the regeneration of the cavernous nerve: brain-derived neurotrophic factor, vascular endothelial growth factor and the JAK/STAT signaling pathway. Asian J Androl. 2011;13:231–5. doi: 10.1038/aja.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotzbauer PT, Lampe PA, Heuckeroth RO, et al. Neurturin, a relative of glial-cell-line-derived neurotrophic factor. Nature. 1996;384:467–70. doi: 10.1038/384467a0. [DOI] [PubMed] [Google Scholar]
- 11.Golden JP, Baloh RH, Kotzbauer PT, et al. Expression of neurturin, GDNF, and their receptors in the adult mouse CNS. J Comp Neurol. 1998;398:139–50. doi: 10.1002/(sici)1096-9861(19980817)398:1<139::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Milbrandt J, de Sauvage FJ, Fahrner TJ, et al. Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron. 1998;20:245–53. doi: 10.1016/s0896-6273(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 13.Baloh RH, Tansey MG, Lampe PA, et al. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron. 1998;21:1291–302. doi: 10.1016/s0896-6273(00)80649-2. [DOI] [PubMed] [Google Scholar]
- 14.Paratcha G, Ledda F. GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–91. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Ernsberger U. The role of GDNF family ligand signalling in the differentiation of sympathetic and dorsal root ganglion neurons. Cell Tissue Res. 2008;333:353–71. doi: 10.1007/s00441-008-0634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan H, Keast JR. Neurturin regulates postnatal differentiation of parasympathetic pelvic ganglion neurons, initial axonal projections, and maintenance of terminal fields in male urogenital organs. J Comp Neurol. 2008;507:1169–83. doi: 10.1002/cne.21593. [DOI] [PubMed] [Google Scholar]
- 17.Bella AJ, Fandel TM, Tantiwongse K, et al. Neurturin enhances the recovery of erectile function following bilateral cavernous nerve crush injury in the rat. J Brachial Plex Peripher Nerve Inj. 2007;2:5. doi: 10.1186/1749-7221-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang RL, Zhang Z, Zhang L, Wang Y, Zhang C, Chopp M. Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. J Neurosci Res. 2006;83:1213–9. doi: 10.1002/jnr.20813. [DOI] [PubMed] [Google Scholar]
- 19.Gómez-Pinedo U, Rodrigo R, Cauli O, et al. cGMP modulates stem cells differentiation to neurons in brain in vivo. Neuroscience. 2010;165:1275–83. doi: 10.1016/j.neuroscience.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 20.Puerta E, Hervias I, Barros-Miñones L, et al. Sildenafil protects against 3-nitropropionic acid neurotoxicity through the modulation of calpain, CREB, and BDNF. Neurobiol Dis. 2010;38:237–45. doi: 10.1016/j.nbd.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Kovanecz I, Rambhatla A, Ferrini M, et al. Long-term continuous sildenafil treatment ameliorates corporal veno-occlusive dysfunction (CVOD) induced by cavernosal nerve resection in rats. Int J Impot Res. 2008;20:202–12. doi: 10.1038/sj.ijir.3901612. [DOI] [PubMed] [Google Scholar]
- 22.Ferrini MG, Davila HH, Kovanecz I, Sanchez SP, Gonzalez-Cadavid NF, Rajfer J. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology. 2006;68:429–35. doi: 10.1016/j.urology.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Kovanecz I, Rambhatla A, Ferrini MG, et al. Chronic daily tadalafil prevents the corporal fibrosis and veno-occlusive dysfunction that occurs after cavernosal nerve resection. BJU Int. 2008;101:203–10. doi: 10.1111/j.1464-410X.2007.07223.x. [DOI] [PubMed] [Google Scholar]
- 24.Sirad F, Hlaing S, Kovanecz I, Artaza JN, Garcia LA, Rajfer J, Ferrini MG. Sildenafil promotes smooth muscle preservation and ameliorates fibrosis through modulation of extracellular matrix and tissue growth factor gene expression after bilateral cavernosal nerve resection in the rat. J Sex Med. 2011;8:1048–60. doi: 10.1111/j.1743-6109.2010.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrini MG, Kovanecz I, Sanchez S, Umeh C, Rajfer J, Gonzalez-Cadavid NF. Fibrosis and loss of smooth muscle in the corpora cavernosa precede corporal veno-occlusive dysfunction (CVOD) induced by experimental cavernosal nerve damage in the rat. J Sex Med. 2009;6:415–28. doi: 10.1111/j.1743-6109.2008.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMahon CG. High dose sildenafil citrate as a salvage therapy for severe erectile dysfunction. Int J Impot Res. 2002;14:533–8. doi: 10.1038/sj.ijir.3900936. [DOI] [PubMed] [Google Scholar]
- 27.Garcia LA, King KK, Ferrini MG, Norris KC, Artaza JN. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology. 2011;152:2976–86. doi: 10.1210/en.2011-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Micevych P, McDonald J, Rapkin A, Chaban V. Inflammation in the uterus induces phosphorylated extracellular signal-regulated kinase and substance P immunoreactivity in dorsal root ganglia neurons innervating both uterus and colon in rats. J Neurosci Res. 2008;86:2746–52. doi: 10.1002/jnr.21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrini MG, Magee TR, Vernet D, Rajfer J, González-Cadavid NF. Penile neuronal nitric oxide synthase and its regulatory proteins are present in hypothalamic and spinal cord regions involved in the control of penile erection. J Comp Neurol. 2003;458:46–61. doi: 10.1002/cne.10543. [DOI] [PubMed] [Google Scholar]
- 31.Stewart AL, Anderson RB, Kobayashi K, Young HM. Effects of NGF, NT-3 and GDNF family members on neurite outgrowth and migration from pelvic ganglia from embryonic and newborn mice. BMC Dev Biol. 2008;8:73. doi: 10.1186/1471-213X-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hisasue S, Kato R, Suetomi T, Kato K, et al. Age-related alteration of neurturin receptor GFRa2 and nNOS in pelvic ganglia. Neurobiol Aging. 2006;27:1524–30. doi: 10.1016/j.neurobiolaging.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Nangle MR, Keast JR. Deafferentation and axotomy each cause neurturin-independent upregulation of c-Jun in rodent pelvic ganglia. Exp Neurol. 2009;215:271–80. doi: 10.1016/j.expneurol.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Bella AJ, Lin G, Lin CS, Hickling DR, Morash C, Lue TF. Nerve growth factor modulation of the cavernous nerve response to injury. J Sex Med. 2009;6 (Suppl 3):347–52. doi: 10.1111/j.1743-6109.2008.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palma CA, Keast JR. Structural effects and potential changes in growth factor signalling in penis-projecting autonomic neurons after axotomy. BMC Neurosci. 2006;7:41. doi: 10.1186/1471-2202-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei IH, Tu HC, Huang CC, Tsai MH, Tseng CY, Shieh JY. (−)-Epigallocatechin gallate attenuates NADPH-d/nNOS expression in motor neurons of rats following peripheral nerve injury. BMC Neurosci. 2011;12:52. doi: 10.1186/1471-2202-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu W. Expression of nitric-oxide synthase (NOS) in injured CNS neurons as shown by NADPH diaphorase histochemistry. Exp Neurol. 1993;120:153–9. doi: 10.1006/exnr.1993.1050. [DOI] [PubMed] [Google Scholar]
- 38.Jia YS, Wang XA, Ju G. Nitric oxide synthase expression in vagal complex following vagotomy in the rat. Neuroreport. 1994;5:793–6. doi: 10.1097/00001756-199403000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Wang YB, Ma CG, et al. Icarisid II, a PDE5 inhibitor from Epimedium wanshanense, increases cellular cGMP by enhancing NOS in diabetic ED rats corpus cavernosum tissue. Andrologia. 2011 Jul 6; doi: 10.1111/j.1439-0272.2010.01144.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Gallo EF, Iadecola C. Neuronal nitric oxide contributes to neuroplasticity-associated protein expression through cGMP, protein kinase G, and extracellular signal-regulated kinase. J Neurosci. 2011;31:6947–55. doi: 10.1523/JNEUROSCI.0374-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puzzo D, Staniszewski A, Deng SX, et al. Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer’s disease mouse model. J Neurosci. 2009;29:8075–86. doi: 10.1523/JNEUROSCI.0864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Picconi B, Bagetta V, Ghiglieri V, et al. Inhibition of phosphodiesterases rescues striatal long-term depression and reduces levodopa-induced dyskinesia. Brain. 2011;134:375–87. doi: 10.1093/brain/awq342. [DOI] [PubMed] [Google Scholar]
- 43.Das A, Salloum FN, Xi L, Rao YJ, Kukreja RC. ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury inmice. Am J Physiol Heart Circ Physiol. 2009;296:H1236–43. doi: 10.1152/ajpheart.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


