Abstract
Collectively, the data in both humans and murine models of human primary biliary cirrhosis (PBC) suggest that activated T cells, particularly CD8 T cells, play a critical role in biliary cell destruction. Under physiological conditions, T cell activation involves two critical signals that involve the MHC and a set of co-stimulatory molecules which include a receptor on T cells coined cytotoxic T lymphocyte antigen 4 (CTLA-4). Germane to the studies reported herein, signaling via CTLA-4 has the potential to modulate co-stimulation and induce inhibitory signals. In this study we have taken advantage of our well-defined murine model of PBC in which mice are immunized with 2-octynoic acid coupled to BSA, leading to the production of high titer anti-mitochondrial autoantibodies and portal cellular infiltrates. To investigate the potential of CTLA-4 Ig as an immunotherapeutic agent, we treated mice both before and after induction of autoimmune cholangitis. Firstly, we demonstrate that CTLA-4 Ig treatment begun one day before 2-OA-BSA immunization, completely inhibits the manifestations of cholangitis, including AMA production, intra-hepatic T cell infiltrates and bile duct damage. However, and more critically, treatment with CTLA-4 Ig initiated after the development of autoimmune cholangitis in previously immunized mice, also resulted in significant therapeutic benefit, including reduced intra-hepatic T cell infiltrates and biliary cell damage, although AMA levels were not altered. These data suggest that an optimized regimen with CTLA-4 Ig has the potential to serve as an investigative therapeutic tool in patients with PBC.
Keywords: Primary biliary cirrhosis, cytotoxic T lymphocyte antigen 4, Abatacept autoimmunity, cholangitis
Introduction
Primary biliary cirrhosis (PBC) is a chronic autoimmune liver disease characterized by non-suppurative destructive cholangitis and cholestasis, as well as progressive development of fibrosis, cirrhosis, leading eventually to liver failure (1). Previous studies have suggested a critical involvement of autoreactive T cells in the pathogenesis of human PBC (2-4). Using various animal models, we have demonstrated that CD8 T cells play a critical role in the pathogenesis of PBC (5-7). Therefore we submit that successful therapy of PBC utilizing immunological approaches requires control of T cell activation and proliferation, their recruitment to the liver and the secretion of pro-inflammatory cytokines by these cellular infiltrates (8-12).
Under physiological conditions, T cell activation involves two critical signals, one being the presentation of a peptide epitope in the context of class I or class II MHC by an antigen-presenting cell (APC) to the T cell receptor (13-15). The second signal is delivered by the co-stimulators CD80/CD86 on the APC to their receptor CD28 on the T cell (16, 17) which is required to sustain the APC-T cell formation of an immunological synapse leading to significant enhancement of T cell activation. In contrast, cytotoxic T lymphocyte antigen 4 (CTLA4), another receptor expressed by T cells is associated with inhibitory properties (18, 19). CTLA4 binds to CD80/CD86 with a much higher affinity than CD28 (20), and functions to attenuate T cell activation by inhibiting co-stimulation and transmitting inhibitory signals to T cells. This results in decreased cytokine production, inhibition of cell cycle progression, down-modulation of TCR signaling, and decreased activation of B cells and macrophages (21-26).
These properties of CTLA-4 prompted us to explore the potential of CTLA4-based therapy for PBC utilizing our murine model in which mice immunized with 2-octynoic acid conjugated BSA (2OA-BSA) develop a PBC-like cholangitis (7, 27, 28). CTLA4-Ig is a soluble recombinant human fusion protein comprised of the extracellular domain of human CTLA4 linked to a modified portion of the Fc domain of human IgG-1 in which the sequences involved in FcR binding and complement activation have been eliminated preventing ADCC and complement mediated activity (Abatacept, Bristol-Myers Squibb, Clinton, NY)(29-31). CTLA4-Ig reversibly binds to both human and murine CD80/86 via its CTLA4 portion, thereby preventing CD80/86 from interacting with CD28 and thereby inhibiting the delivery of the second signal required for full T-cell activation. We herein demonstrate that CTLA4-Ig treatment started one day before 2OA-BSA immunization prevents the clinical and histological manifestations of cholangitis including the inhibition of serum anti-mitochondrial antibody (AMA), intrahepatic inflammatory change and bile duct damage. Of importance is our finding that CTLA4-Ig treatment initiated after the onset of established disease in previously 2OA-BSA-immunized mice led to a significant reduction in levels of intrahepatic infiltrating pathogenic effector CD4 and CD8 T cells as well as the severity of biliary cell damage. Our data suggests that an optimized regimen with CTLA4-Ig commercially known as “Abatacept” is a potential therapeutic candidate for patients with PBC.
Materials and Methods
Mice
Female C57BL/6J (B6) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained in ventilated cages under specific pathogen-free conditions at the animal facilities of the University of California at Davis. All studies conducted with the use of these animals were approved by the Animal Care and Use Committee at the University of California at Davis.
Induction of cholangitis
Two-octynoic acid (2OA, a synthetic chemical mimic of lipoic acid-lysine located within the inner domain of PDC-E2) was coupled to bovine serum albumin (2OA-BSA) as previously described (7). For the induction of autoimmune cholangitis, 100 μg 2OA-BSA conjugate (in 50 μl PBS) were emulsified with 50 μl of Complete Freund's Adjuvant (CFA; containing 1mg/mL of Mycobacterium tuberculosis strain H37RA, Sigma-Aldrich, St. Louis, MO) and injected intraperitoneally (I.P.) into 6 week-old female B6 mice. Additionally, mice received 100 ng of pertussis toxin (List Biological Laboratories, Campbell, CA) in 100 μl PBS by I.P. at the time of and two days after the initial immunization with 2OA-BSA. After 2 weeks, the mice were re-boosted with 100 μg 2OA-BSA in 50 μl PBS emulsified with 50 μl of Incomplete Freund's Adjuvant (IFA; Sigma-Aldrich) administered intraperitoneally.
CTLA4-Ig treatment
Abatacept (BMS-188667, CTLA4-Ig), utilized for the studies reported herein, was a gift from Bristol Meyers-Squibb (New York, NY). Each vial of CTLA4-Ig (250 mg/vial) for injection was reconstituted with 10 ml of sterile water to yield a concentration of 25 mg/ml, and further diluted with PBS to 4 mg/ml. Mice were injected I.P. 3 times a week at a dosage of 20 mg/kg body weight. Preventative treatment was started one day before the first immunization with 2OA-BSA and continued 3× weekly for 8 weeks. Therapeutic treatment was started 8 weeks after the first immunization with 2OA-BSA and continued 3× weekly for 4 weeks.
Detection of anti-PDC-E2 antibodies
Serum was collected every two weeks after 2OA-immunization and stored at −80°C. Levels of anti-PDC-E2 autoantibodies in serum were measured by enzyme-linked immunosorbent assay (ELISA) as previously described (7, 32), using purified recombinant PDC-E2 to coat the ELISA plates. The serum samples were tested at a dilution of 1:250.
Cell Preparation and Flow Cytometry Analysis
Livers and spleens were harvested immediately following sacrifice. Livers were first perfused with PBS containing 0.2% BSA (0.2% BSA/PBS), passed through a 100-μm nylon cell strainer (BD Bioscience) and re-suspended in 0.2% BSA/PBS. Hepatocytes were removed as pellets after centrifugation at 75 g for 1 min and the remaining suspended cells were collected. Spleens were disrupted between two glass slides and suspended in 0.2% BSA/PBS. Mononuclear cells (MNCs) from the liver and spleen were isolated by gradient centrifugation using Histopaque-1.077 (Sigma-Aldrich, St Louis, MO, USA). For flow cytometry, cells were first incubated with mAb against Fc receptor for blocking non-specific binding (BioLegend, San Diego, CA, USA) for 10 min at 4°C and then stained with combinations of fluorochrome- conjugated antibodies, including fluorescein isothiocyanate (FITC)-conjugated anti-CD44 (BioLegend), phycoerythrin (PE)-conjugated anti-CD62L (BioLegend), allophycocyanin (APC)-conjugated anti-T cell receptor (TCR)-β (eBioscience, San Diego, CA, USA), PerCP-conjugated anti-CD8α (Biolegend) and APC-Cy7-conjugated anti-CD4 (BioLegend) for phenotypic analysis of T cell subsets. Multicolor flow cytometric analyses were performed using a FACScan flow cytometer (BD Biosciences) upgraded by Cytec Development (Fremont, CA, USA) to allow for five-color analysis. Acquired data were analyzed with CellQuest software (BD Biosciences).
Histopathologic staining
Livers and spleens were harvested immediately after sacrifice and aliquots fixed in 10% buffered formalin at room temperature for 2 days, embedded in paraffin, and cut into 4 μm sections for routine hematoxylin (DakoCytomation, Carpinteria, CA) and eosin (American Master Tech Scientific, Lodi, CA) (H&E) staining. Standard pathologic evaluation utilizing light microscopy was performed and the relative levels of biliary cell damage were recorded on coded H&E-stained sections. Each section was scored as either 0 = no pathologic change, 1 = minimal, 2 = mild, 3 = moderate, or 4 = severe pathology by a pathologist as previously described (7, 33, 34).
Statistical Analysis
Statistical differences between groups were determined using a two-tail unpaired t test and a Chi-squared test for independence. All results were expressed as mean ± standard error (SEM). The Prism statistical package (GraphPad Software Inc, La Jolla, CA) was used. A p-value of < 0.05 was considered statistically significant.
Results
CTLA4-Ig treatment prior to immunization with 2OA-BSA
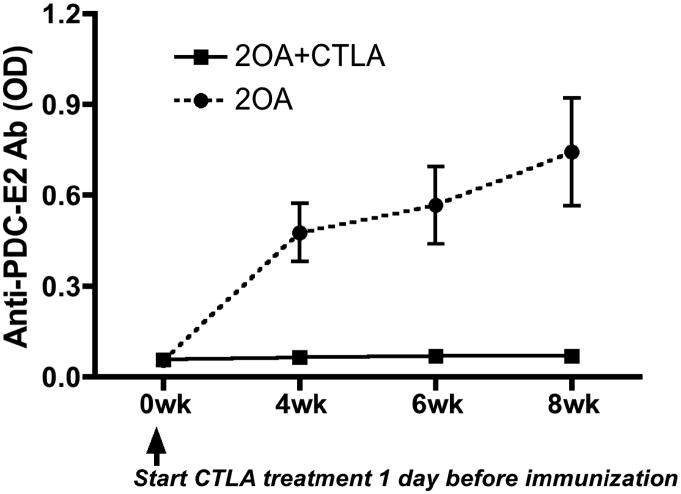
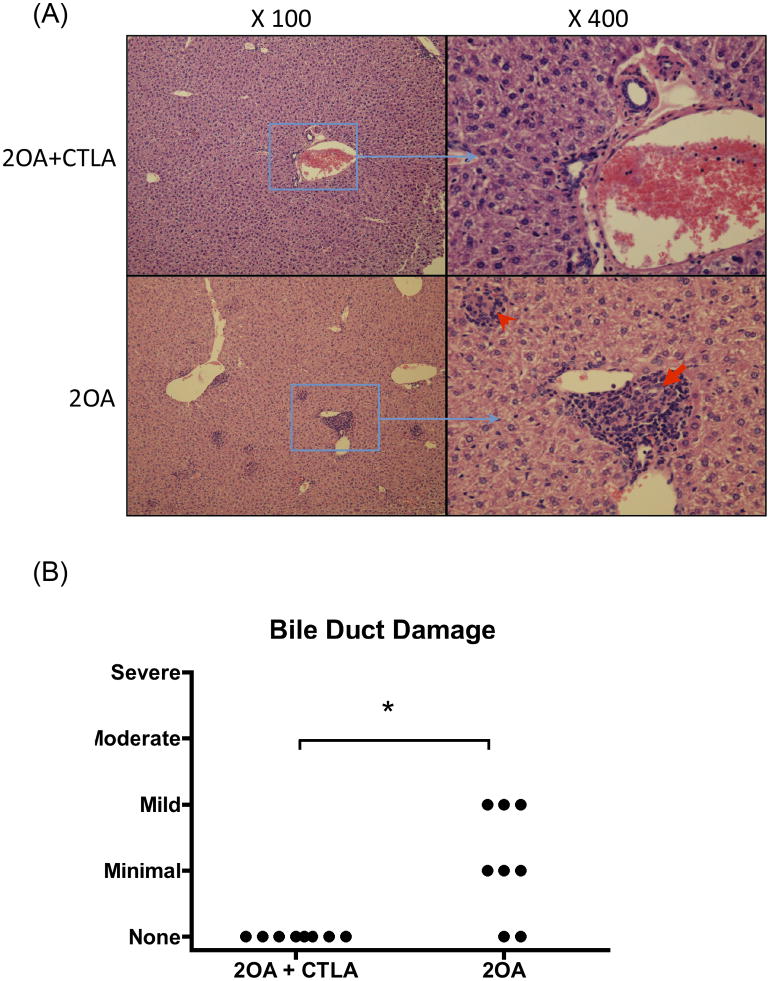
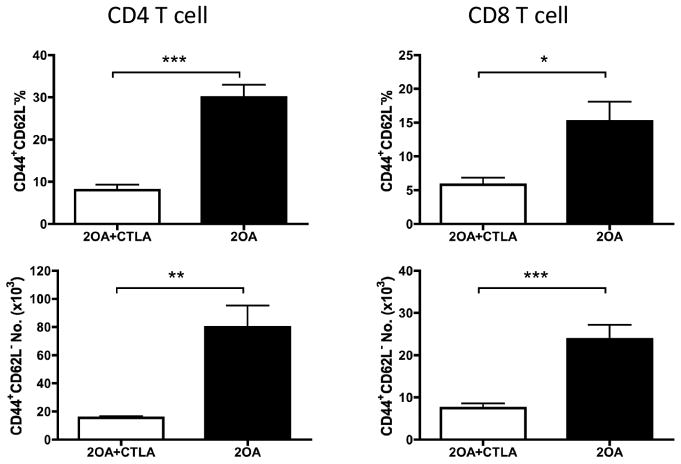
In efforts to investigate the effects of CTLA4-Ig treatment on preventing 2OA-BSA-induced autoimmune cholangitis, groups of B6 mice were untreated (controls) or treated with CTLA4-Ig prior to immunization with 2OA-BSA. As expected, control mice developed significant levels of serum autoantibodies to PDC-E2. In contrast, anti-PDC-E2 autoantibodies were not detected in serum from mice treated with CTLA4-Ig (Figure 1), indicating that the administration of CTLA4-Ig before 2OA-BSA immunization prevented production of AMAs. Coincidentally, while cholangitis exemplified by lympho-plasmacytic infiltration and bile duct damage was readily found in the liver of control mice at eight weeks following 2OA immunization, liver tissues from mice pre-treated with CTLA4-Ig showed a significant decrease in the extent of liver inflammation (Figure 2A). Furthermore, interlobular bile duct damage was not observed in any of the treated mice (Figure 2B). Of note, administration of CTLA4-Ig alone without immunization with 2OA-BSA did not induce any detectable pathologic changes in mouse liver (data not shown). Finally, flow cytometric assisted analysis was also performed on intra-hepatic MNCs isolated from the liver of control and CTLA4-Ig treated mice at 8 weeks post 2OA-BSA immunization. Results of such analysis revealed that while the former showed marked increases (p < 0.001) in the frequency and absolute numbers of effector (CD44+CD62L−) CD4 and CD8 T cells, the latter demonstrated marked decreases in such infiltrates (see Figure 3). These data indicate that CTLA4-Ig treatment started prior to 2OA-BSA immunization completely prevents loss of tolerance and subsequent autoimmune cholangitis.
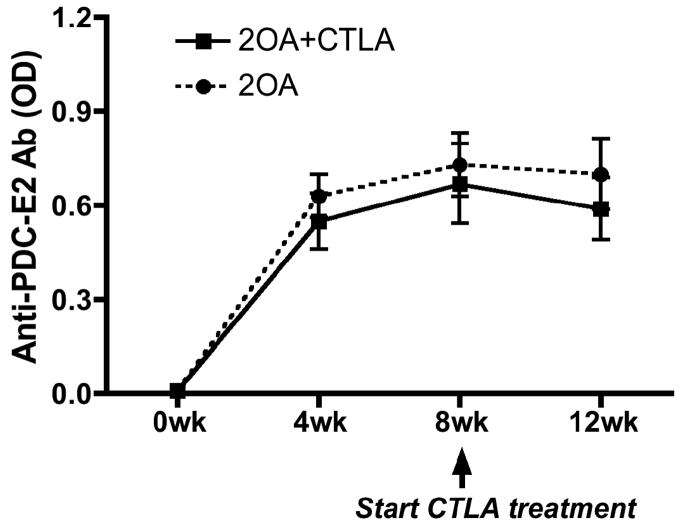
Figure 1.
CTLA4-Ig treatment inhibited production of PDC-E2-specific autoantibodies induced by 2OA-BSA immunization. CTLA4-Ig treatment was started 1 day before initial 2OA-BSA immunization and continued for 8 weeks (2OA+CTLA). Untreated mice (2OA) were utilized as a control group. Serum samples were collected at different time points, diluted 1:250 and tested for anti-PDC-E2 reactivity by ELISA assay. OD, optical value. Each group included 8 mice.
Figure 2.
CTLA4-Ig treatment protected mice against 2OA-BSA-induced cholangitis. CTLA4-Ig treatment was started 1 day before initial 2OA-BSA immunization and continued for 8 weeks. (A) H&E–stained representative liver sections from 2OA-BSA-immunized mice treated with CTLA4-Ig compared to controls. Note, epithelioid granuloma (red arrowhead) and interlobular bile duct damage (red arrow). (B) Bile duct damage was evaluated as described on H&E stained liver sections. Each group included 8 mice. *, p < 0.05 (Chi-squared test for independence).
Figure 3.
CTLA4-Ig treatment prevented intrahepatic infiltration of effector T cells. CTLA4-Ig treatment was started 1 day before initial 2OA-BSA immunization. The frequency of the effector (CD44+CD62L−) population in CD4 and CD8 T cells as well as number of these populations in liver were analyzed by flow cytometry. Each group included 8 mice. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (two-tailed unpaired t test).
Therapeutic efficacy of CTLA4-Ig in 2OA-BSA-induced cholangitis in mice
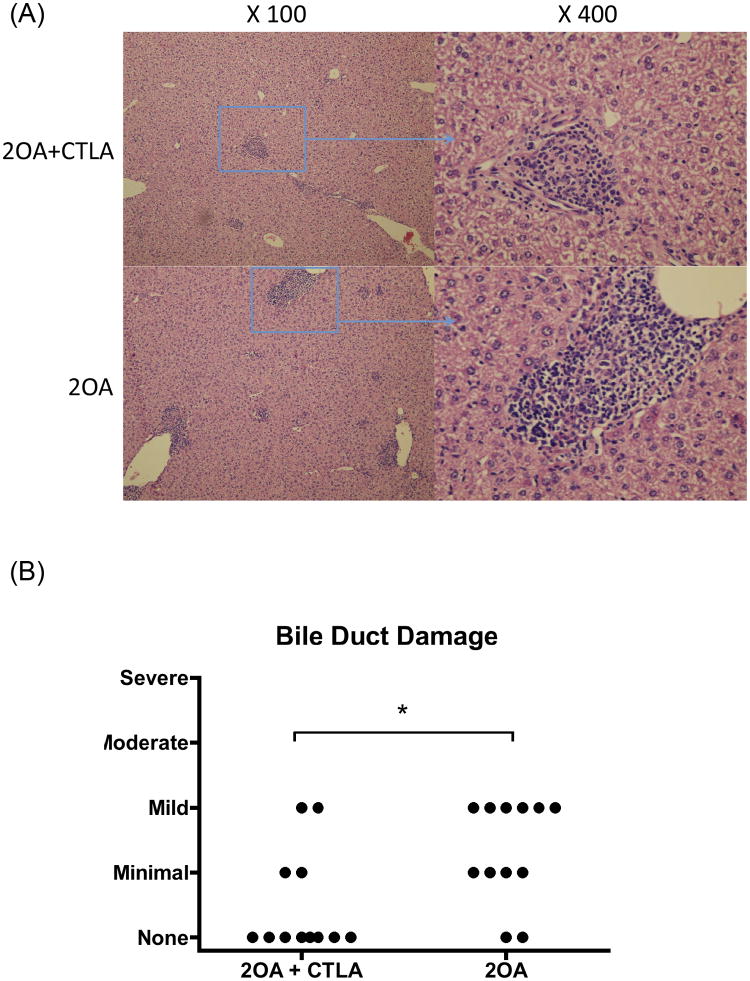
In efforts to determine the therapeutic efficacy of CTLA4-Ig, we utilized our standard protocol to induce cholangitis in groups of mice by 2OA-BSA immunization. As seen in Figure 2, since the disease onset was detected at 8 weeks after the initial immunization, consistent with our previously data (7, 27, 28), we started treatment with CTLA4-Ig at that time point. After 4 weeks of treatment, mice were sacrificed and examined for liver pathology. As shown in Figure 4A, CTLA4-Ig treatment significantly reduced the extent and severity of liver inflammation and the extent of biliary cell damage (Fig. 4B) as compared to untreated mice. Flow cytometric analysis of the intra-hepatic cellular infiltrates reflect a marked diminution in the frequency and absolute numbers of CD44+CD62L− effector CD4 and CD8 T cells in CTLA4-Ig treated mice as compared with control mice (Fig. 5). Analysis of serum collected pre- and post CTLA4-Ig treatment from these mice for levels of PDC-E2-specific autoantibodies did not show any detectable difference in titers (Fig. 6).
Figure 4.
Effects of CTLA4-Ig treatment on 2OA-BSA-induced cholangitis. 2OA-BSA immunized mice were treated with CTLA4-Ig for 4 weeks, started 8 weeks after initial immunization (2OA+CTLA). 2OA-BSA immunized mice without CTLA treatment were utilized as control group (2OA). (A) H&E-stained representative liver sections. (B) Scores of intrahepatic bile duct damage in 2OA-immunized mice with or without CTLA4-Ig treatment. Each group included 12 mice. *, p < 0.05 (Chi-squared test for independence).
Figure 5.
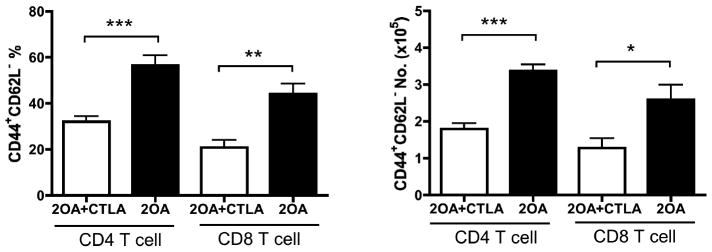
CTLA4-Ig treatment reduced intrahepatic infiltration of effector T cells. 2OA-immunized mice were treated with CTLA4-Ig for 4 weeks, started 8 weeks after initial immunization. Frequency of the effector (CD44+CD62L−) population in CD4+ and CD8+ T cells as well as the number of these populations in liver were analyzed by flow cytometry. Each group included 8 to 12 mice. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (unpaired t-test).
Figure 6.
CTLA4-Ig treatment started after onset of cholangitis did not affect serum levels of PDC-E2-specific autoantibodies. CTLA4-Ig treatment was started 8 weeks after initial 2OA-BSA immunization and continued for 4 weeks. Each group included 6 to 12 mice.
Discussion
CTLA4-Ig (Abatacept) is a soluble recombinant human fusion protein comprised of the extracellular domain of human CTLA4, linked to a modified portion of the Fc domain of human IgG-1 which is devoid of FcR binding and complement activation activity (29-31). There is considerable conservation between murine and human CTLA4, including in studies of the effects of graft-versus-host disease across the major histocompatibility complex barrier in mice (35).
This agent mimics the action of native CTLA4 by binding to CD80/CD86 on APCs and competitively inhibits the essential CD28:CD80/CD86 co-stimulatory signal required for T cell activation resulting in down regulation of subsequent immune effector mechanisms (31, 36, 37). CTLA4-Ig similarly inhibits the interaction between murine CD28 and its ligand CD80/CD86. CTLA4-Ig is an FDA approved drug for patients with rheumatoid arthritis and juvenile idiopathic arthritis (38, 39). CTLA4-Ig has also been shown to ameliorate autoimmunity in vivo during collage-induced arthritis (40), experimental autoimmune encephalomyelitis (41), psoriatic arthritis (42), systemic lupus erythematosus (43), ankylosing spondylitis (44) and shown to be effective in the prolongation of allograft and xenograft survival (45-48).
In the current study we used our animal model of xenobiotic-induced cholangitis to investigate the potential effects of CTLA4-Ig both as a preventative and as a therapeutic agent. Our data demonstrated that the development of both anti-PDC-E2 and liver pathology was successfully prevented in mice treated with CTLA4-Ig one day before immunization with 2OA. Since CTLA4 inhibits co-stimulation of T cells required for T cell activation, our results demonstrate the critical role of T cell activation in the development of 2OA-induced autoimmune cholangitis in which T cells serve as a pathogenic effector, as well as in the autoreactive B cell response in which the T cell help is also essential. We thus proceeded to treat mice with established cholangitis at 8 weeks following 2OA-BSA immunization. Treatment with CTLA4-Ig in this group not only resulted in reduced histological inflammatory changes in liver and biliary cell damage but also reduced levels of intrahepatic pathogenic effector CD4 and CD8 T cells, indicating the potential therapeutic effect of CTLA4-Ig.
Although CTLA4-Ig treatment did not result in complete resolution of established cholangitis in our mouse model, the reduced frequency and absolute numbers of liver infiltrating pathogenic T cells and improved liver histology are important initial therapeutically beneficial findings. These data are consistent with the view that CTLA4-Ig treatment inhibits activation of naïve T cells but not previously primed autoreactive memory T cells since the later have a lower threshold of activation and are less dependent on co-stimulatory signals for activation. However, it is important to keep in mind that activated T cells also need interaction with co-stimulatory molecules to provide survival signals for sustained effector T cell responses. Thus, while the results of the current studies show significant therapeutic benefit, the studies only address the acute effects of CTLA4-Ig therapy. Studies to determine if discontinuing treatment will result in relapse of cholangitis, and if a prolonged CTLA4-Ig regimen (>4 weeks) will improve its therapeutic efficacy need to be performed.
Although treatment with CTLA4-Ig post immunization reduced intra-hepatic effector T cell infiltration and biliary cell damage, these mice continued to manifest AMAs at levels comparable with control mice. The serum AMA is produced by long-lived plasma cells, which can be replenished from previously primed memory B cells with a lower threshold of activation (49, 50). This would explain the lack of effect of CTLA4-Ig treatment on serum AMA levels in this group of mice. The differential response to CTLA4-Ig treatment on T cells versus AMAs, as well as its impact on the therapeutic efficacy for PBC, should also be evaluated in future studies.
It is becoming increasingly clear that besides CTLA4 there are a number of other inhibitory receptors that function to control immune responses which include TIGIT (T cell immunoreceptor with immunoglobulin and ITIM domains), PD-1 (programmed death-1), and TIM-3 (T cell immunoglobulin and mucin domain containing molecule-3). It is not clear at present whether there is a hierarchy amongst these molecules in terms of an ordered sequence by which these molecules act to regulate immune responses. In light of our previous studies on the role of inflammatory cytokines, such as IFNγ, IL-6, IL-12 and IL-23 (51-53), in the pathogenesis of PBC (including autoantibody production and biliary cell damages), it is reasonable to assume that CTLA4 could influence the effect of these downstream inflammatory cytokines. However, it is clear that they do serve at different check points along the T cell activation pathway and it is likely that an optimized therapeutic approach may require a combination of therapeutic inhibitors to maintain long term inhibition of autoimmune effector mechanisms (54) without compromising anti-viral and tumor surveillance mechanisms.
With regards to CTLA4, despite its extensive experimental and clinical use for therapy of autoimmune disease and transplant rejection, there is remarkably a paucity of data on precisely how and when CTLA4-Ig mediates its effect on T cells in vivo. In addition, the effect of CTLA4-Ig on T-dependent B cell responses are poorly understood and/or characterized. Although Abatacept has been demonstrated to have a significant therapeutic benefit in patients with rheumatoid arthritis (55, 56), and increased risk of infection, malignancy and autoimmune events have been reported (57, 58). We reason that elucidation of the precise mechanisms by which CTLA4-Ig mediate its effect will aid in defining optimum therapeutic application of this unique immunomodulatory drug while minimizing in vivo toxicity and clinically non-beneficial effects.
Acknowledgments
The authors thank Yugo Ando, Chen-yen Yang, Kazuhito Kawata and Hajime Tanaka for technical support in this experiment. We also thank Ms. Nikki Phipps for support in preparing this article.
Financial support provided by a grant from the National Institutes of Health, DK067003.
Abbreviations
- PBC
Primary biliary cirrhosis
- CTLA4
cytotoxic T lymphocyte antigen 4
- APC
antigen-presenting cell
- 2OA-BSA
2-octynoic acid conjugated BSA
- CFA
Complete Freund's Adjuvant
- IFA
Incomplete Freund's Adjuvant
- MNCs
Mononuclear cells
- OD
optical value
- AMA
anti-mitochondrial antibody
Contributor Information
Amy Dhirapong, Email: adhirapong@ucdavis.edu.
Guo-Xiang Yang, Email: gxyang@ucdavis.edu.
Steven Nadler, Email: steven.nadler@bms.com.
Weici Zhang, Email: ddzhang@ucdavis.edu.
Koichi Tsuneyama, Email: ktsune@med.u-toyama.ac.jp.
Patrick Leung, Email: psleung@ucdavis.edu.
Stuart Knechtle, Email: Stuart.Knechtle@emoryhealthcare.org.
Aftab A. Ansari, Email: pathaaa@emory.edu.
Ross L. Coppel, Email: ross.coppel@monash.edu.
Fu-Tong Liu, Email: fliu@ucdavis.edu.
Xiao-Song He, Email: xiaosong@stanford.edu.
References
- 1.Gershwin ME, Ansari AA, Mackay IR, Nakanuma Y, Nishio A, Rowley MJ, Coppel RL. Primary biliary cirrhosis: an orchestrated immune response against epithelial cells. Immunol Rev. 2000;174:210–225. doi: 10.1034/j.1600-0528.2002.017402.x. [DOI] [PubMed] [Google Scholar]
- 2.Kita H, Lian ZX, Van de Water J, He XS, Matsumura S, Kaplan M, Luketic V, et al. Identification of HLA-A2-restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. The Journal of experimental medicine. 2002;195:113–123. doi: 10.1084/jem.20010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J, Coppel RL, et al. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. The Journal of clinical investigation. 2002;109:1231–1240. doi: 10.1172/JCI14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, Lake J, et al. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. The Journal of clinical investigation. 1998;102:1831–1840. doi: 10.1172/JCI4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang GX, Lian ZX, Chuang YH, Moritoki Y, Lan RY, Wakabayashi K, Ansari AA, et al. Adoptive transfer of CD8(+) T cells from transforming growth factor beta receptor type II (dominant negative form) induces autoimmune cholangitis in mice. Hepatology. 2008;47:1974–1982. doi: 10.1002/hep.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang GX, Wu Y, Tsukamoto H, Leung PS, Lian ZX, Rainbow DB, Hunter KM, et al. CD8 T cells mediate direct biliary ductule damage in nonobese diabetic autoimmune biliary disease. J Immunol. 2011;186:1259–1267. doi: 10.4049/jimmunol.1001597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakabayashi K, Lian ZX, Leung PS, Moritoki Y, Tsuneyama K, Kurth MJ, Lam KS, et al. Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology. 2008;48:531–540. doi: 10.1002/hep.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 9.Green JM, Noel PJ, Sperling AI, Walunas TL, Gray GS, Bluestone JA, Thompson CB. Absence of B7-dependent responses in CD28-deficient mice. Immunity. 1994;1:501–508. doi: 10.1016/1074-7613(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 10.Guerder S, Picarella DE, Linsley PS, Flavell RA. Costimulator B7-1 confers antigen-presenting-cell function to parenchymal tissue and in conjunction with tumor necrosis factor alpha leads to autoimmunity in transgenic mice. Proc Natl Acad Sci U S A. 1994;91:5138–5142. doi: 10.1073/pnas.91.11.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orabona C, Grohmann U, Belladonna ML, Fallarino F, Vacca C, Bianchi R, Bozza S, et al. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol. 2004;5:1134–1142. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- 12.Green JM, Noel PJ, Sperling AI, Walunas TL, Lenschow DJ, Stack R, Gray GS, et al. T cell costimulation through the CD28 receptor. Proc Assoc Am Physicians. 1995;107:41–46. [PubMed] [Google Scholar]
- 13.Koulova L, Clark EA, Shu G, Dupont B. The CD28 ligand B7/BB1 provides costimulatory signal for alloactivation of CD4+ T cells. J Exp Med. 1991;173:759–762. doi: 10.1084/jem.173.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young JW, Koulova L, Soergel SA, Clark EA, Steinman RM, Dupont B. The B7/BB1 antigen provides one of several costimulatory signals for the activation of CD4+ T lymphocytes by human blood dendritic cells in vitro. J Clin Invest. 1992;90:229–237. doi: 10.1172/JCI115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-Sancho R, Walse B, Stuart DI, et al. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 17.Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunological reviews. 2011;241:180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wing K, Yamaguchi T, Sakaguchi S. Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends in immunology. 2011;32:428–433. doi: 10.1016/j.it.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 21.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 22.Greene JL, Leytze GM, Emswiler J, Peach R, Bajorath J, Cosand W, Linsley PS. Covalent dimerization of CD28/CTLA-4 and oligomerization of CD80/CD86 regulate T cell costimulatory interactions. J Biol Chem. 1996;271:26762–26771. doi: 10.1074/jbc.271.43.26762. [DOI] [PubMed] [Google Scholar]
- 23.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 25.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 26.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 27.Leung PS, Park O, Tsuneyama K, Kurth MJ, Lam KS, Ansari AA, Coppel RL, et al. Induction of primary biliary cirrhosis in guinea pigs following chemical xenobiotic immunization. J Immunol. 2007;179:2651–2657. doi: 10.4049/jimmunol.179.4.2651. [DOI] [PubMed] [Google Scholar]
- 28.Wakabayashi K, Yoshida K, Leung PS, Moritoki Y, Yang GX, Tsuneyama K, Lian ZX, et al. Induction of autoimmune cholangitis in non-obese diabetic (NOD).1101 mice following a chemical xenobiotic immunization. Clin Exp Immunol. 2009;155:577–586. doi: 10.1111/j.1365-2249.2008.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozelle AL, Genovese MC. Efficacy results from pivotal clinical trials with abatacept. Clin Exp Rheumatol. 2007;25:S30–34. [PubMed] [Google Scholar]
- 30.Teng GG, Turkiewicz AM, Moreland LW. Abatacept: a costimulatory inhibitor for treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2005;5:1245–1254. doi: 10.1517/14712598.5.9.1245. [DOI] [PubMed] [Google Scholar]
- 31.Davis PM, Abraham R, Xu L, Nadler SG, Suchard SJ. Abatacept binds to the Fc receptor CD64 but does not mediate complement-dependent cytotoxicity or antibody-dependent cellular cytotoxicity. J Rheumatol. 2007;34:2204–2210. [PubMed] [Google Scholar]
- 32.Oertelt S, Lian ZX, Cheng CM, Chuang YH, Padgett KA, He XS, Ridgway WM, et al. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655–1660. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 33.Lleo A, Bowlus CL, Yang GX, Invernizzi P, Podda M, Van de Water J, Ansari AA, et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52:987–998. doi: 10.1002/hep.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambrosini YM, Yang GX, Zhang W, Tsuda M, Shu S, Tsuneyama K, Leung PS, et al. The multi-hit hypothesis of primary biliary cirrhosis: polyinosinic-polycytidylic acid (poly I:C) and murine autoimmune cholangitis. Clin Exp Immunol. 2011;166:110–120. doi: 10.1111/j.1365-2249.2011.04453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blazar BR, Taylor PA, Linsley PS, Vallera DA. In vivo blockade of CD28/CTLA4: B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility complex barrier in mice. Blood. 1994;83:3815–3825. [PubMed] [Google Scholar]
- 36.Davis PM, Nadler SG, Stetsko DK, Suchard SJ. Abatacept modulates human dendritic cell-stimulated T-cell proliferation and effector function independent of IDO induction. Clin Immunol. 2008;126:38–47. doi: 10.1016/j.clim.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Graca L. CTLA4Ig and the therapeutic potential of T cell co-stimulation blockade. Acta Reumatol Port. 2008;33:267–276. [PubMed] [Google Scholar]
- 38.Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Perez N, Silva CA, Abud-Mendoza C, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372:383–391. doi: 10.1016/S0140-6736(08)60998-8. [DOI] [PubMed] [Google Scholar]
- 39.Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Perez N, Silva CA, Abud-Mendoza C, et al. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis and rheumatism. 2010;62:1792–1802. doi: 10.1002/art.27431. [DOI] [PubMed] [Google Scholar]
- 40.Knoerzer DB, Karr RW, Schwartz BD, Mengle-Gaw LJ. Collagen-induced arthritis in the BB rat. Prevention of disease by treatment with CTLA-4-Ig. J Clin Invest. 1995;96:987–993. doi: 10.1172/JCI118146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cross AH, Girard TJ, Giacoletto KS, Evans RJ, Keeling RM, Lin RF, Trotter JL, et al. Long-term inhibition of murine experimental autoimmune encephalomyelitis using CTLA-4-Fc supports a key role for CD28 costimulation. J Clin Invest. 1995;95:2783–2789. doi: 10.1172/JCI117982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mease P, Genovese MC, Gladstein G, Kivitz AJ, Ritchlin C, Tak PP, Wollenhaupt J, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis and rheumatism. 2011;63:939–948. doi: 10.1002/art.30176. [DOI] [PubMed] [Google Scholar]
- 43.Merrill JT, Burgos-Vargas R, Westhovens R, Chalmers A, D'Cruz D, Wallace DJ, Bae SC, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis and rheumatism. 2010;62:3077–3087. doi: 10.1002/art.27601. [DOI] [PubMed] [Google Scholar]
- 44.Song IH, Heldmann F, Rudwaleit M, Haibel H, Weiss A, Braun J, Sieper J. Treatment of active ankylosing spondylitis with abatacept: an open-label, 24-week pilot study. Annals of the rheumatic diseases. 2011;70:1108–1110. doi: 10.1136/ard.2010.145946. [DOI] [PubMed] [Google Scholar]
- 45.Bonham CA, Peng L, Liang X, Chen Z, Wang L, Ma L, Hackstein H, et al. Marked prolongation of cardiac allograft survival by dendritic cells genetically engineered with NF-kappa B oligodeoxyribonucleotide decoys and adenoviral vectors encoding CTLA4-Ig. J Immunol. 2002;169:3382–3391. doi: 10.4049/jimmunol.169.6.3382. [DOI] [PubMed] [Google Scholar]
- 46.Lin H, Rathmell JC, Gray GS, Thompson CB, Leiden JM, Alegre ML. Cytotoxic T lymphocyte antigen 4 (CTLA4) blockade accelerates the acute rejection of cardiac allografts in CD28-deficient mice: CTLA4 can function independently of CD28. J Exp Med. 1998;188:199–204. doi: 10.1084/jem.188.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rehman A, Tu Y, Arima T, Linsley PS, Flye MW. Long-term survival of rat to mouse cardiac xenografts with prolonged blockade of CD28-B7 interaction combined with peritransplant T-cell depletion. Surgery. 1996;120:205–212. doi: 10.1016/s0039-6060(96)80289-3. [DOI] [PubMed] [Google Scholar]
- 48.Mirenda V, Golshayan D, Read J, Berton I, Warrens AN, Dorling A, Lechler RI. Achieving permanent survival of islet xenografts by independent manipulation of direct and indirect T-cell responses. Diabetes. 2005;54:1048–1055. doi: 10.2337/diabetes.54.4.1048. [DOI] [PubMed] [Google Scholar]
- 49.Tangye SG, Avery DT, Deenick EK, Hodgkin PD. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. Journal of immunology. 2003;170:686–694. doi: 10.4049/jimmunol.170.2.686. [DOI] [PubMed] [Google Scholar]
- 50.Good KL, Tangye SG. Decreased expression of Kruppel-like factors in memory B cells induces the rapid response typical of secondary antibody responses. Proceedings of the National Academy of Sciences of the United States of America; 2007; pp. 13420–13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang W, Tsuda M, Yang GX, Tsuneyama K, Rong G, Ridgway WM, Ansari AA, et al. Deletion of interleukin-6 in mice with the dominant negative form of transforming growth factor beta receptor II improves colitis but exacerbates autoimmune cholangitis. Hepatology. 2010;52:215–222. doi: 10.1002/hep.23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshida K, Yang GX, Zhang W, Tsuda M, Tsuneyama K, Moritoki Y, Ansari AA, et al. Deletion of interleukin-12p40 suppresses autoimmune cholangitis in dominant negative transforming growth factor beta receptor type II mice. Hepatology. 2009;50:1494–1500. doi: 10.1002/hep.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang CY, Leung PS, Yang GX, Kenny TP, Zhang W, Coppel R, Norman GL, et al. Epitope-specific anti-nuclear antibodies are expressed in a mouse model of primary biliary cirrhosis and are cytokine-dependent. Clin Exp Immunol. 2012;168:261–267. doi: 10.1111/j.1365-2249.2012.04577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joller N, Peters A, Anderson AC, Kuchroo VK. Immune checkpoints in central nervous system autoimmunity. Immunol Rev. 2012;248:122–139. doi: 10.1111/j.1600-065X.2012.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buch MH, Boyle DL, Rosengren S, Saleem B, Reece RJ, Rhodes LA, Radjenovic A, et al. Mode of action of abatacept in rheumatoid arthritis patients having failed tumour necrosis factor blockade: a histological, gene expression and dynamic magnetic resonance imaging pilot study. Ann Rheum Dis. 2009;68:1220–1227. doi: 10.1136/ard.2008.091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choy EH. Selective modulation of T-cell co-stimulation: a novel mode of action for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2009;27:510–518. [PubMed] [Google Scholar]
- 57.Schiff M. Abatacept treatment for rheumatoid arthritis. Rheumatology (Oxford) 2011;50:437–449. doi: 10.1093/rheumatology/keq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bluestone JA, St Clair EW, Turka LA. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24:233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]